Abstract
One of the main causes of food spoilage is the lipid oxidation of its components, which generates the loss of nutrients and color, together with the invasion of pathogenic microorganisms. In order to minimize these effects, active packaging has played an important role in preservation in recent years. Therefore, in the present study, an active packaging film was developed using polylactic acid (PLA) and silicon dioxide (SiO2) nanoparticles (NPs) (0.1% w/w) chemically modified with cinnamon essential oil (CEO). For the modification of the NPs, two methods (M1 and M2) were tested, and their effects on the chemical, mechanical, and physical properties of the polymer matrix were evaluated. The results showed that CEO conferred to SiO2 NPs had a high percentage of 2,2-diphenyl-l-picrylhydrazyl (DPPH) free radical inhibition (>70%), cell viability (>80%), and strong inhibition to E. coli, at 45 and 11 µg/mL for M1 and M2, respectively, and thermal stability. Films were prepared with these NPs, and characterizations and evaluations on apple storage were performed for 21 days. The results show that the films with pristine SiO2 improved tensile strength (28.06 MPa), as well as Young’s modulus (0.368 MPa) since PLA films only presented values of 27.06 MPa and 0.324 MPa, respectively; however, films with modified NPs decreased tensile strength values (26.22 and 25.13 MPa), but increased elongation at break (from 5.05% to 10.32–8.32%). The water solubility decreased from 15% to 6–8% for the films with NPs, as well as the contact angle, from 90.21° to 73° for the M2 film. The water vapor permeability increased for the M2 film, presenting a value of 9.50 × 10−8 g Pa−1 h−1 m−2. FTIR analysis indicated that the addition of NPs with and without CEO did not modify the molecular structure of pure PLA; however, DSC analysis indicated that the crystallinity of the films was improved. The packaging prepared with M1 (without Tween 80) showed good results at the end of storage: lower values in color difference (5.59), organic acid degradation (0.042), weight loss (24.24%), and pH (4.02), making CEO-SiO2 a good component to produce active packaging.
1. Introduction
For decades, polymers have played an important role in our daily lives, contributing to the improvement and implementation of new technologies in countless applications and fields, one of the most important being the packaging industry. The characteristics of a packaging material have evolved over time, adding new functions that allow the protection and preservation of the contained product. Among these new functions is the so-called active packaging, which is characterized by having an active compound capable of inhibiting the growth of microorganisms and other components that favor food preservation. They are generally manufactured with polymers of petrochemical origin due to their good mechanical, thermal, and physical properties; however, as they are formed by very long chains of carbon and oxygen atoms, it is difficult for them to degrade, so there is a tireless search to try to minimize degradation times and environmental problems generated by the use of these polymers [1,2,3].
One of the most promising options is the use of biodegradable polymers for active packagings, such as polysaccharides, cellulose, chitosan, polypeptides, gelatin, zein, polyhydroxyalkanoates, soy protein isolates, and polylactic acid. For the past 30 years, there has been a gradual growth in research focused on the development of active films based on biodegradable polymers [4]. Polylactic acid (PLA) has been used in the development of active films because it is approved by the Food and Drug Administration (FDA) as a safe material (GRAS) for food and beverage packaging [5]. In addition, it has high mechanical properties, tensile strength (45–65 MPa), and particularly Young’s modulus (5–10 GPa), indicating that it is a good substitute for basic polymers such as polypropylene (PP), polyethylene (PL) and polystyrene (PS) in short-life packaging [6,7].
Some research works have already tested the use of PLA in active films, such as Raduzin et al. (2019) [8], who used garlic extract Allium ursinum at 0.5% w/w, observing that it improved the crystallinity of pure PLA from 7.3 to 13.2% as well as the Tg from 42.6 to 45.9 °C. Roy et al. (2020) [9] made PLA films with curcumin reporting that at low concentrations (0.25 w/w%), the tensile strength and Young’s modulus improved from 46 MPa to 49 MPa and 0.88 to 0.94 GPa, respectively, and Fiore et al., (2021) [10] elaborated PLA films with chitosan-caseinate and rosemary essential oil, showing that the mechanical and barrier properties such as water vapor permeability were improved. As we can see in the references presented, active packaging requires two fundamental elements for its manufacture: polymeric matrix and active component. The latter is the key to active packaging since it must have a high antioxidant and antimicrobial capacity.
The most common active components are natural extracts or essential oils, fungicides, bacteriocins, organic acids, and enzymes. Essential oils are obtained from the extraction of plants by distillation; they are very complex mixtures of terpenes (limonene, sabinene, myrcene, valencene, germacrene, etc.), aromatic compounds (ethylbenzene, eugenol, elemicine, apiole, cinnamaldehyde, etc.), and aliphatic compounds responsible for conferring antimicrobial, antifungal, and organoleptic (flavor and aroma) properties [11,12,13]. Each extract has a component of higher proportion that distinguishes it from the others. In citrus essential oil, its main component is limonene (97%); in rosemary, essential oil is 1,8-cineole (45%); in clove, 76.8% is eugenol, oregano essential oil has carvacrol (70%) and cinnamon essential oil has 5% cinnamaldehyde [14,15,16,17,18].
Their antimicrobial activity depends on their polyphenol content. For example, in [19,20,21], it is found that Cymbopogan citratus reported a MIC of 0.6 µL/mL for Escherichia coli, 0.6 µL/mL for Staphylococcus aureus and 2.5 0.6 µL/mL for Salmonella typhimurium, while Psiada argutia informed a MIC for S. aureus of 0.5 mg/mL, of Enterococcus fecalis of 8 mg/mL and Acinetobacter of 16 mg/mL. Further, Pimenta dioica presented a MIC for E. fecalis of 2.5 mg/mL, S. aureus of 1 mg/mL, and Acinetobacter of 8 mg/mL, and finally, Cinnamomum zeylanicum reported a MIC value of 2 mg/mL for Klebsiella pneumoniae, 4 mg/mL for E. fecalis and 0.5 mg/mL for S. aureus. In the case of cinnamon essential oil, this reported values of 2.0 mg/mL for E. coli, S. aureus, Salmonella Risen, and Pseudomonas fluorescens, and 0.5 mg/mL for Bacillus cereus. Specifically, their inhibition mechanism damages the bacterial cell wall, increasing its permeability due to their lipophilic nature and releasing vital components from inside the cell, leading to cell death. They also apply a reducing action on protein synthesis, intracellular ATP levels, and intracellular pH of bacteria [22,23].
Nowadays, thanks to their properties, essential oils can constitute an alternative to synthetic preservatives and are considered the antibiotics of the future, so we can find in literature several studies of PLA films with essential oils; clove essential oil [24], Oregano essential oil [25], carvacrol and thymol essential oil [26], bergamot, lemongrass, clove and rosemary essential oils [27], cinnamon essential oil [28], Green tea [29] and thyme, rosemary, and oregano essential oils [30]. However, a disadvantage of this semi-crystalline polymer is that its crystallization kinetics is very slow because of the low nucleation capacity. Therefore, this limits its applications since its mechanical properties depend on the degree of crystallinity. Generally, PLA has a crystallization rate in the range of 10 to 20 °C/min at temperatures below 100 °C and a crystallinity of less than 10% due to this poor crystallization ability [31,32]; this is why some material is needed to reinforce and thus improve its crystallinity in order to maintain or even improve its properties.
Nanoparticles of metal oxides are of special interest, and research works have been used for the reinforcement of polymeric matrices, such as titanium dioxide (TiO2) [33,34], zinc oxide (ZnO) [35], magnesium oxide (MgO) and (SiO2) [36] as they have been used as nanofillers in polymeric matrices. In addition, SiO2 nanoparticles are a great option to generate active packaging because, compared to other particles, they are stable, non-toxic, and safe food additives used in food processing and preservation. Negahdari et al. (2020) [35] developed a PLA packaging with Origanum majorana essential oil and ZnO nanoparticles. As a result, they report that the tensile strength decreased by 76% when the essential oil was added, while it increased by 25% when ZnO NPs were added. Water vapor permeability was increased by 3.52 and 1.53 times after the addition of 1.5% essential oil and 1% ZnO NPs, respectively. Their packaging was able to preserve minced meat for 8 days. Alizadeh et al. (2020) [37] performed an active packaging with cellulose nanofibers/serum proteins by adding TiO2 nanoparticles and rosemary essential oil and showed that the antioxidant activity was enhanced by adding rosemary essential oil from 42.2 to 47.3 mg/mL which helped the preservation of meat presenting values of 4.15 log CFU/g for the psychrotrophic bacteria count (PBC) keeping the product below the allowed limit (7 log CFU/g). Compared to other metal nanoparticles, silicon oxide (SiO2) nanoparticles between 20 and 100 nm have low toxicity, are stable, and are used as FDA-approved food additives [38]. Palma et al. (2021) [36] made PLA films with SiO2 nanoparticles (20 nm) at different concentrations (1, 2, 3, 3, 5 wt.%) and found an increase in the percentage of crystallinity from 10 to 77%, as well as the interfacial miscibility was also improved and with it the mechanical properties. Famili Zirak and Tabari (2018) [39] obtained similar results, an increase in the tensile strength from 29 to 43 MPa and Young’s modulus from 2.3 to 3.1 GPa. As can be seen from the above studies, different polymeric matrices, as well as different nanoparticles and essential oils, can be used to produce active packaging.
Considering the above, the present work aims to chemically modify SiO2 nanoparticles with cinnamon essential oil (CEO) to improve the antioxidant and antimicrobial properties of these nanoparticles and can function as a nanofiller to confer these properties to the PLA polymeric matrix. The above with the aim of generating an active component in the preparation of films suitable for food preservation. The casting method was used for the preparation of films as active packaging. The effects on the mechanical, thermal, barrier, physical, and microbiological properties of the films were evaluated, and their effectiveness as active packaging was tested by storing a fruit (apple) for a short period of 21 days.
2. Materials and Methods
2.1. Materials
Silicon dioxide powder SiO2 (size 10–20 nm brand IOLITEC Purity 99%) and Cinnamomum verum essential oil CEO (brand VidaScent purity 99.99%). Ethanol brand CTR Scientific (purity 99.95% density 0.804 g/cm3). Polyethylene glycol sorbitan monooleate Tween 80 (brand Sigma-Aldrich, density 1.07 g/cm3, Oleic acid ≥ 58.0%). Commercial polylactic acid (PLA) filament was used to prepare the films. (brand Inky, white, molecular weight 195,000 g/mol, viscosity 109.76 mPa/s to shear rate between 10–100 s−1, aspect ratio (L/D) approx. 0.5 with an average length of 2000 ± 0.3 µm, an average diameter of 1750 ± 0.2 µm, and a density of 1.24 g/cm3).
2.2. Chemical Modification of SiO2
Two methodologies were used [40] with minor modifications for this work. On the one hand, the first method (M1) consisted of a mixture of CEO with SiO2 in a 1:1 ratio, and 5 mL of ethanol was used for homogenization. The mixture was stirred for 1 h at 30 °C. It is worth mentioning that the CEO is photosensitive; therefore, during the stirring time, the flask was covered with aluminum foil. Finally, the mixture was dried for one day at 50 °C; the modified nanoparticles were labeled as SiO2-M1.
On the other hand, the second method (M2) consisted of that 1 g of SiO2 dissolved in 5 mL of ethanol by stirring, then 5 μL of distilled water and 2.5 μL of tween 80 were added, and finally, 5 μL of basic cinnamon oil were included. The mixture was left under stirring for 30 min at 30 °C. Then, it was dried under the same conditions that M1, and the modified nanoparticles were labeled as SiO2-M2.
2.3. Films Preparation of PLA and SiO2
Films of poly(lactic acid) were prepared by the casting method. Initially, 0.500 g of PLA was dissolved in 20 mL of chloroform under stirring. Then, the modified nanoparticles, either by M1 or M2, were incorporated at a rate of 0.1% w/w regarding the weight of PLA. The mixture was vortexed for 10 min and sonicated for 20 min to eliminate any bubbles and improve the nanoparticles’ dispersion. Finally, the mixture was poured into a petri dish and dried at room temperature. See Scheme 1.
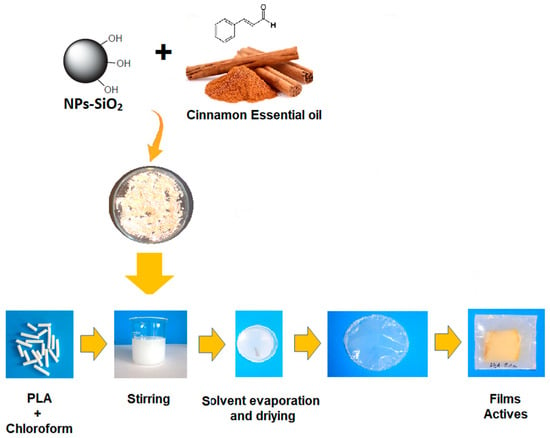
Scheme 1.
Procedure of the preparation of the PLA films with NPs.
2.4. Characterization of the Nanoparticles and Films
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR) and Thermogravimetric Analysis (TGA)
The FTIR spectra of the modified nanoparticles and films were recorded using an Agilent Cary 660 instrument. The analysis was carried out using an Attenuated Total Reflectance (ATR) mode in 4000 to 400 cm−1. The thermogravimetric analysis was carried out with the help of differential scanning calorimeter equipment from TA instruments, model SDT 650, with a simultaneous thermogravimetric analyzer. The analysis was conducted with a 10 °C/min ramp in a temperature range of 25–600 °C under a nitrogen atmosphere.
2.4.2. Antioxidant Activity
The antioxidant activity of modified SiO2 nanoparticles and films was evaluated using the 2,2-diphenyl-l-picrylhydrazyl (DPPH) free radical method [41]. For the different nanoparticles, several concentrations were tested, which corresponded to each number of moles of antioxidant/mole of DPPH. The prepared nanoparticle solutions were dissolved in ethanol up to final values of 4, 8, 12, 16, and 20 mg/mL. Then, the films were first cut into 5 × 5 cm pieces and dissolved in 2 mL of chloroform. Later, a DPPH solution was prepared with ethanol at a concentration of 6 × 10−5 mol/L. An aliquot of 3.9 mL was mixed with 0.1 mL of each nanoparticle solution under stirring. Each solution was incubated in a dark medium at room temperature for 30 min. Finally, the absorbance (abs) of the samples was analyzed in a UV-vis spectrophotometer at 517 nm, and the inhibition % was calculated by Equation (1):
2.4.3. Antimicrobial Activity
Inspection for microbiological activity is essential in food packaging, as the bacteria present are the cause of spoilage, especially in fruits and vegetables. It is known that E. coli can grow in contaminated raw fruits, vegetables, or water. In contrast, S. aureus is a major pathogen found on human skin that may cause infection in several organs during the handling of raw fruits. In order to determine the minimum inhibitory concentration (MIC) of the nanoparticles, the CLSI M7-A7 [42] broth microdilution method was developed. The method consists of inhibiting microbial growth of Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria in a liquid medium. The first stage consisted of preparing suspensions: nanoparticles were suspended in Mueller-Hinton agar, contained in tubes, and then vortexed for 1 min. The second stage was the preparation of the inoculum, for which Escherichia coli and Staphylococcus aureus strains were prepared at a concentration of 2.5 × 108 CFU/mL using the McFarlan scale and diluted for a final concentration of microorganisms of 0.5 × 103–2.5 × 103. Microplates (96 wells) were filled with the prepared suspensions and inoculums, considering negative and positive controls. Subsequently, the microplates were incubated with constant stirring at 120 rpm and a temperature of 35 ± 2 °C. Finally, as a third stage, the absorbance of the 96 wells of each system was measured using a Bio-Rad microplate spectrophotometer at 570 nm. Formula (2) was used to determine the growth:
where S is the absorbance of each sample, is the absorbance of the negative control (without inoculum) and is the absorbance of the positive control (with inoculum).
A drop test was performed to validate the films’ antimicrobial activity. To start the test, an inoculum of each E. coli and S. aureus bacteria was prepared as described previously at a concentration of 2.5 × 108 CFU/mL. Then, each film was prepared at a size of 1 cm × 1 cm and was placed for 5 min under UV light to avoid contamination of the medium. Then, each film was placed in sterile 15 mL Falcon tubes, and 3 mL of the inoculated broth was added. They were left to incubate for 24 h at a temperature of 37 °C. In order to determine the percentage of bacterial inhibition, the absorbance was measured with a UV/VIS U-5100 spectrophotometer for each sample using the following Equation (3):
where A0 corresponds to the absorbance of the control without sample and Am corresponds to the absorbance of each sample.
2.4.4. Cell Viability
Cell viability was determined by Resazurin assay using peripheral blood mononuclear cells seeded in 96-well microplates (Costar, Corning, NY, USA) at a cell density of 3 × 104 cells per well in RPMI-1640 medium (10% fetal bovine serum, 2 mM L-glutamine, 50 U mL−1 of RPMI-1640 medium). The mononuclear cells were exposed to different concentrations (27.78, 50, 100, and 200 ppm for each system and incubated for 24 h at a temperature of 37 °C in a controlled atmosphere (5% CO2 and 95% relative humidity). Before reading the samples by spectrofluorometry, the cells were treated with Resazurin (Sigma-Aldrich, St. Louis, MO, USA) at 30 µg mL−1 diluted in RPMI medium in a final volume of 100 µL per well. Microwells were immediately placed in an incubator at 37 °C and 5% CO2 atmosphere and protected from light. Fluorescence measurements were determined at emission wavelength λ = 590 nm and excitation wavelength λ = 560 nm. Samples were measured with a Synergy H1 spectrofluorimeter (BioTek Instruments Inc. Winooski, VT, USA). Untreated peripheral mononuclear cells were used as the negative control, and cells treated with acetone (10%) (Sigma-Aldrich, St. Louis, MO, USA) were considered the positive control. Cell viability of all systems was determined in triplicate with Equation (4):
where Fs is the fluorescence value of each sample and Fc is the fluorescence of the control.
2.4.5. X-ray Diffraction (XRD) and Scanning Electron Microscopy (SEM)
XRD diffraction spectrograms were obtained using an X-ray diffractometer (D8 ADVANCE, Bruker) using a Cu Kα radiation beam filtered with nickel, with an angular range of 8–80° at a step of 2θ and voltage of 40 kV. Samples were cut into small frames of 1 × 1 cm. The prepared films were analyzed with a Philips/FEI XL30-SFEG high-resolution microscope. A 2 × 2 cm sample was cut out, placed on pins, and coated with a thin layer of silver from the central part of the films. The inspection was made on the surface of each film at an accelerating voltage of 20 kV.
2.5. Physical Properties of the Films
The samples were conditioned 24 h before each measurement in Section 2.5 at a relative humidity of 55% and a temperature of 25 °C.
2.5.1. Film Thickness (FS)
A high-precision (0.001 mm) digital micrometer (ULINE H-7352) was used to determine the thickness of the films. Measurements were taken at 5 different points in each film. The average value of the film thickness was used to determine the mechanical and physical properties of the film with the modified SiO2 nanoparticles.
2.5.2. Water Solubility (WS)
Water solubility can be described as the solubilized dry matter content after 24 h immersion in water. The method for determining water solubility is described as follows: in a laboratory oven, the samples were dried for 24 h at 50 °C until constant weight and then weighed (W1). The samples were then immersed in 40 mL of distilled water for 24 h at room temperature under magnetic stirring. After this time, the film was removed and dried at 110 °C for 24 h until constant weight and was weighed to determine the weight of the final matter (W2) that had not dissolved in water [43]. Formula (5) was used to determine the percentage solubility in water.
2.5.3. Water Vapor Permeability (WVP)
A gravimetric method of ASTM E-96-95 [44,45,46] was chosen to analyze water vapor permeability. First, a solution of 142 mmol/L of sodium ions was prepared with NaCl and mixed with a solution of 2.5 mmol/L of calcium ions, prepared previously with CaCl2. Then, an aliquot of 10 mL of this mixture was taken and poured on a petri dish 5.4 cm in diameter. Finally, a film was placed on the petri dish and adjusted with a rubber band to prevent the film from moving. The weight of the Petri dishes containing the film and the solution was recorded. They were placed in a desiccator containing silica gel.
The desiccator was placed in a temperature-controlled chamber for 6 h at 34 °C (according to the described in the ASTM standard E-96,88), where the weight of each box was recorded every hour. To know the water vapor permeability, Formulas (6) and (7) were used, expressing the water vapor transmission rate as WVTR, which is the water vapor transmission rate Δm/Δt in g and h, respectively; WVP is the water vapor permeability, A is the exposed area of the film in m2, X is the film thickness in m2, P is the saturation vapor pressure of water in Pa at the test temperature (25 °C) finally, R1 is the relative humidity in the desiccator which was 40% and R2 the relative humidity inside the Petri dish.
2.5.4. Contact Angle (CA)
Measurements of water contact angles were performed by observing the intermolecular interactions between the surface of the films and a small droplet (1 µm) placed with a micropipette. Several images were taken through a camera. The contact angles were measured using ImageJ software version 1.54d with the drop analysis function.
2.5.5. Mechanical Properties
In order to estimate the mechanical properties of each film, the method described in ASTM D882-18 [47] was followed with some modifications. The tests of the tensile strength (TS), elongation at break (EB), and Young’s modulus (YM) were evaluated. The samples were customized in 1 × 9 cm films, and the analytical measurements were performed in quintuplicate in a texture analyzer (Texture Pro CT Build 35) under the following conditions: the initial grip of 15 mm and a crosshead speed of 1 mm/s. Tensile strength (MPa), elongation at break (%), and Young’s modulus (MPa) were calculated using the formulas described in Equations (8)–(10), respectively.
where Fmax is the force at break (N), A is the cross-sectional film area (mm2), L0 is the initial film length (mm), and L is the film length at break (mm). Young’s modulus was estimated as the slope of the elastic region in the stress-strain curve. Strain (ε) = (L − L0)/L, stress (σ) = F/A (MPa).
2.5.6. Differential Scanning Calorimetry (DSC)
The thermograms on films were obtained by a Perkin Elmer model 8500 Differential Scanning Calorimeter. The sample was placed in a small, hermetically sealed aluminum capsule containing between 6 and 15 mg. The samples were scanned at 10 °C/min over a temperature range of 25–200 °C. The Tg (glass transition temperature), Tc (crystallization temperature), and Tm (melting temperature) were obtained. The Equation degree of crystallinity (Xc) of the samples was calculated according to the following Equation (11):
where is the enthalpy of fusion, enthalpy of crystallization and (93 J/g) is the heat of fusion for 100% crystallinity of PLA homopolymer [48].
2.6. Storage and Evaluation of Active Packaging in Apple Samples
Golden Delicious apples from Saltillo Coahuila were obtained from a local grocery store. The apples used during the storage test were 2 × 3 cm in size per piece. These apples were packed with properly processed and heat-sealed film. The storage time of the apples was 21 days at 15 °C; in addition, complementary tests of color, pH, acidity, and weight loss were performed weekly to evaluate the behavior of the fruit stored and packed with this active package.
2.6.1. Weight loss
The weight loss, expressed as a percentage, was determined as follows (12):
where Wt is the weight of the apple sample after each week, W0 is the initial weight of the sample.
2.6.2. Total Titratable Acidity (TA)
Titratable acidity (TA) in apple samples was determined following the method described by Velickova et al. [49]. The method consisted of crushing 5 g of apple in a mortar and pestle, adding 50 mL of distilled water, then increasing the room temperature to 80 °C for 30 min without stirring, and finally, filtering. NaOH 0.1 mol/L was then used to titrate the solution to a pH of 8.1. The formula described (13) was used to determine the percentage of titratable acidity.
where V(NaOH) is the volume (mL) of NaOH added, 0.1 is the molarity of the NaOH solution, 0.064 is the conversion factor for malic acid, and m is the mass of the sample.
2.6.3. pH Evaluation
A Hanna Instrument potentiometer was used to determine the pH of the apple samples, which were previously crushed with a mortar and pestle. Then, 50 mL of distilled water was added. In order to avoid pulp residues, the solution was filtered, and the measurement was performed once the equipment was calibrated.
2.6.4. Color Evaluation
The colorimetric test to determine the color of the packaged apple piece was carried out in two stages, the first before packaging (initial value) and the second after 21 days (final value). A color Muse SE pocket colorimeter was used for this test. The main parameters of the Hunter Lab color space, L* (lightness), a* (position between red and green), b* (position between yellow and blue), and the color difference (∆E) obtained by Equation (14) were considered:
where subindex 0 is the initial value and f is the final value.
2.7. Statistical Analysis
An analysis of variance (ANOVA) was used to determine the difference in means of the data obtained, using the Minitab software package for Windows (19.1.1.0) using Tukey multiple range test analysis with a significance level of p < 0.05.
3. Results
3.1. Characterization of Nanoparticles
3.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
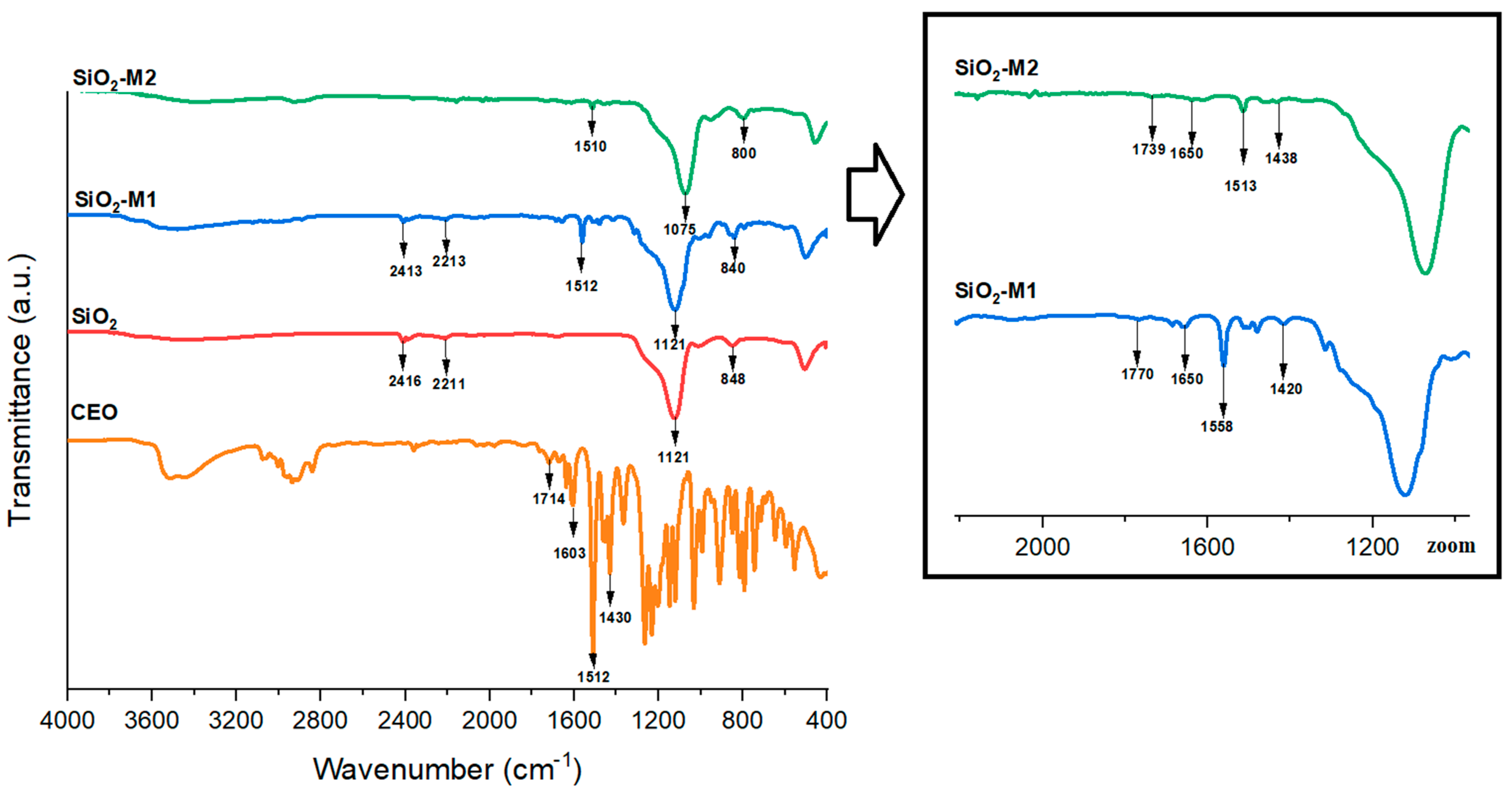
The Fourier transform infrared spectrum provides the necessary information to identify functional groups. With the above in mind, pure and modified SiO2 nanoparticles (M1 and M2) were analyzed to verify that the main compound (cinnamaldehyde, 81.2%) of cinnamon essential oil (CEO) [50] was bonded on SiO2 particles. Firstly, it can be appreciated in Figure 1 the absorption bands for pure SiO2, which appeared at 848 and 1121 cm−1 and are attributed to the symmetric and asymmetric stretching of (Si-O), respectively. The bands at 2416 and 2211 cm−1 correspond to the (-OH) stretching of the hydroxyl groups present on the surface of SiO2 nanoparticles. Previous studies indicate [31,32] that the characteristic bands of SiO2 are found at 1105 cm−1 due to the symmetric (Si-O-Si) stretching and bending mode. The (Si-O) and (O-Si-O) stretching vibrations can be observed between 803 and 450 cm−1. These bands have been observed by Marangoni et al. [33] and Hernández et al. [34].
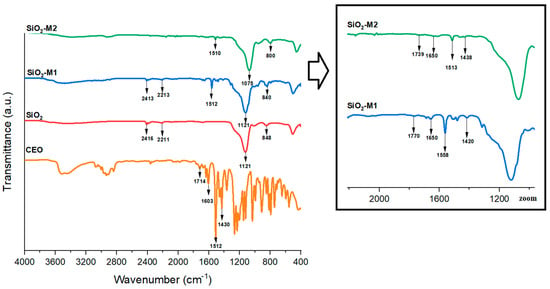
Figure 1.
FTIR spectrum of nanoparticles SiO2 pure, SiO2-M1, SiO2-M2 and CEO.
Figure 1 also illustrates the FTIR spectrum of CEO, which showed the absorption bands at 2938, 2834, 1709, 1603,1505 1503, 1232, 1157, 1031, and 904 cm−1. Several studies mention that the characteristic peaks of CEO are found at 2827–2884 cm−1 and correspond to the (C-H) stretching, while at 1687 cm−1 is found the aldehyde (C=O) stretching, at 1643 cm−1 the alkene (C=C) stretching, and at 1423 cm−1 refers to the aromatic (C-C) ring [50]. In contrast, the bands found in the systems (SiO2-M1 and SiO2-M2) prepared by methods M1 and M2 are located at 1075, 1616, 1510, 1438, 800, and 1770, 1650, 1558, and 1420 cm−1 respectively. It is important to mention that the bands of system M1 were more intense; this could be attributed to a large dipole moment between molecules. In other words, there was a stronger interaction between CEO and SiO2 nanoparticles (SiO2-M1) compared to method two (SiO2-M2) when tween 80 was added, which could be interpreted as the presence of this compound prevents the direct binding of the functional groups of CEO on SiO2 nanoparticles. Compared to the spectrum of pure SiO2, it can be seen that there is no presence of these bands, indicating that the characteristic cinnamaldehyde group of CEO is present only on the modified SiO2 nanoparticles.
3.1.2. Thermal Analysis (TGA)
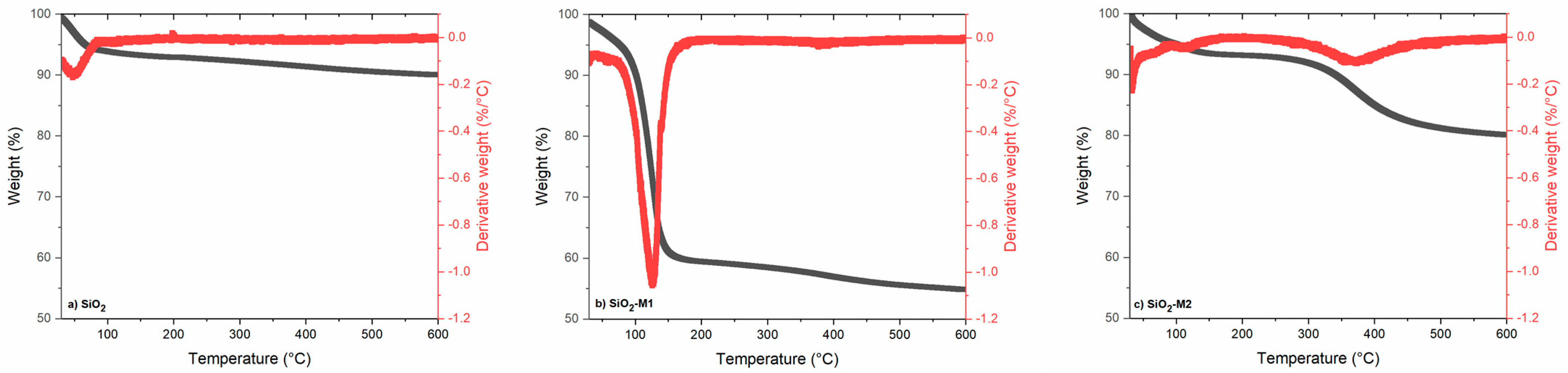
The TGA results are shown in Figure 2, where the thermogram of pure SiO2 nanoparticles can be seen, which remains without weight loss during a temperature range from 0 to 600 °C due to the nature of this compound, i.e., because SiO2 is a pure compound, no curves referring to the loss of other elements were observed; therefore, its graph remains constant. The thermal decomposition of the nanoparticles modified with method two can be observed as the mass loss in two stages, firstly the evaporation of the CEO essential oil occurs with a value of 10%, and in the second stage, there is a loss of 20% when a temperature of 350 °C is reached corresponding to the degradation of Tween 80. Similar results were observed by Cao and Song [51]; Liu et al. [52] and Zhou et al. [53] mention that in this temperature range of 130–369 °C the weight loss in this stage is related to the decomposition of Tween 80 and the degradation of other components. In contrast, it is observed that the nanoparticles of method one possess a higher mass loss of about 40% at a temperature of 50–150 °C due to the deterioration of the volatile/non-volatile components largely present in a cinnamon essential oil [54]. The results of nanoparticles modified with both methodologies demonstrate that the interaction of SiO2 with CEO improves the thermal stability of the oil [55]. Similar results were obtained by Wen et al. [56] when evaluating the thermal stability in the encapsulation of cinnamon essential oil in nanofibrous films for active food packaging, observing that the degradation of CEO occurs at approximately 100 °C, also mentioning that the thermal stability in the PVA nanofibrous film with CEO was improved. Likewise, Zhou et al. [53] evaluated the effect of CEO addition on edible cassava starch films. This gives us an indication that the thermal stability will be maintained in a wide range, and therefore, these nanoparticles can be used in packaging due to this stability and the relationship between the cinnamon essential oil components within the polymeric matrix [57].
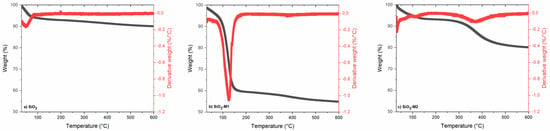
Figure 2.
TGA of the nanoparticles (a) SiO2 pure, (b) SiO2-M1 and (c) SiO2-M2.
3.1.3. Evaluation of Antioxidant Activity of Nanoparticles
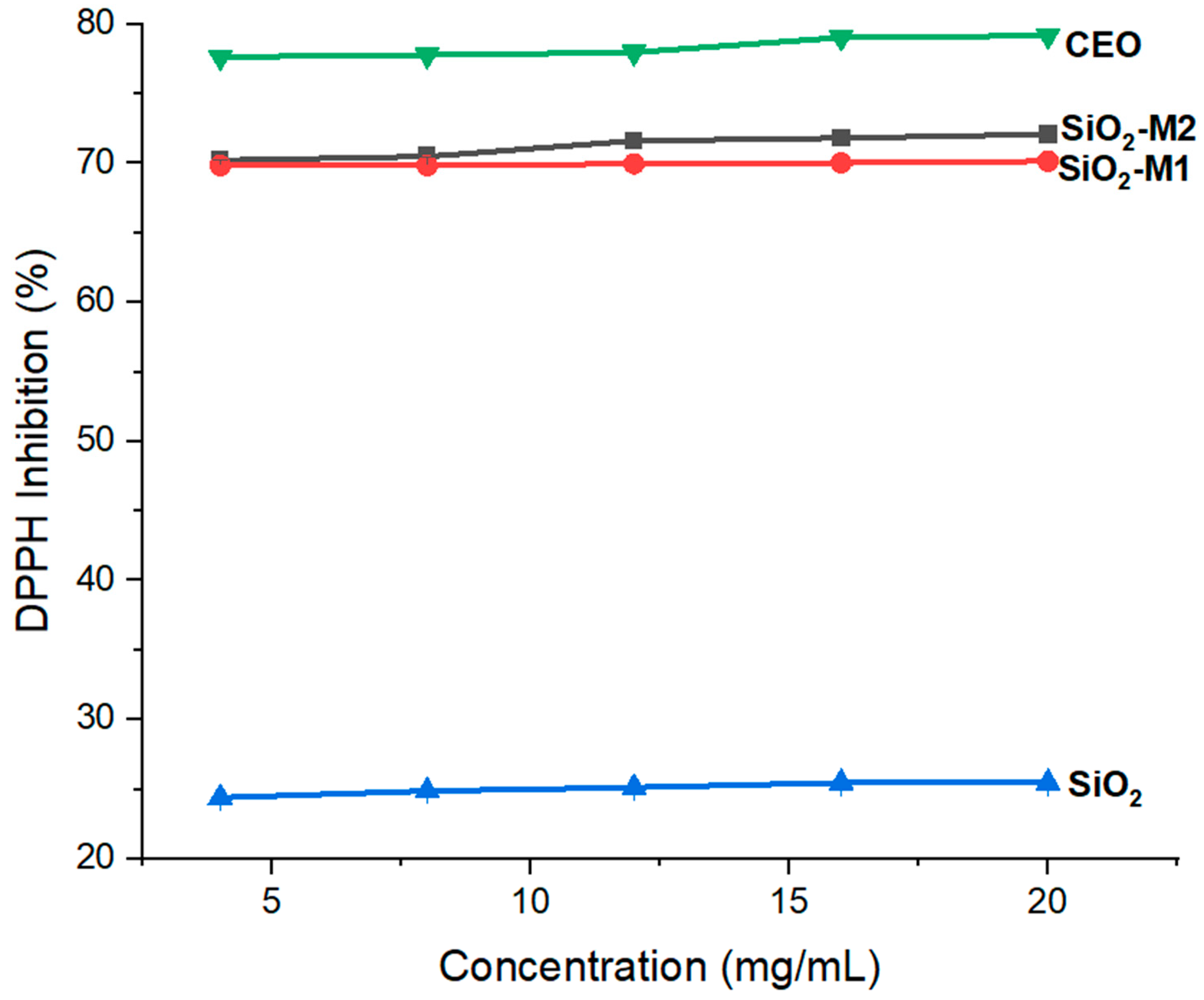
Once it was confirmed that the CEO was present in the SiO2 nanoparticles, its antioxidant power was determined in the nanoparticles modified by both methods (M1 and M2) to know which maintained a higher percentage of free radical inhibition. Figure 3 shows the results obtained for DPPH free radical inhibition. The pure CEO presents a high percentage of inhibition between 77–79%, while the SiO2-M1 and SiO2-M2 modified nanoparticles present values between 69–70% and 70–72%, respectively. These results indicate that the nanoparticles acquired the antioxidant power of CEO at 88% and 91%, respectively. In contrast, it can be observed in Figure 3 that pure SiO2 nanoparticles alone do not present a high antioxidant capacity, reaching only 24–25% inhibition. In this context, it is possible to establish that the phenolic compounds in cinnamaldehyde help inhibit the release of radicals, upon reacting with oxygen, accelerate their biological process, causing autooxidation [58,59]. It is worth mentioning that the percentage of inhibition was measured at different concentrations, so it is observed that the antioxidant capacity depends only on the functional groups present in the particles; This is relevant because, at a minimum concentration (4 mg/mL), the same percentage of inhibition is achieved as at a higher concentration (4 mg/mL). Studies such as that of Guo et al. [60] reported a percentage inhibition of pure CEO between 88.18 and 59.35% using various cinnamon essential oil extraction methods. So far, few previous studies have evaluated CEO antioxidant power in metal oxide nanoparticles. There is only one previous work as a reference [50] where an inhibition percentage of 60% was reported in TiO2 nanoparticles (size 500 nm) lower than that obtained in this study, concluding that the size of the nanoparticles used is an important factor for chemical modification with essential oils.
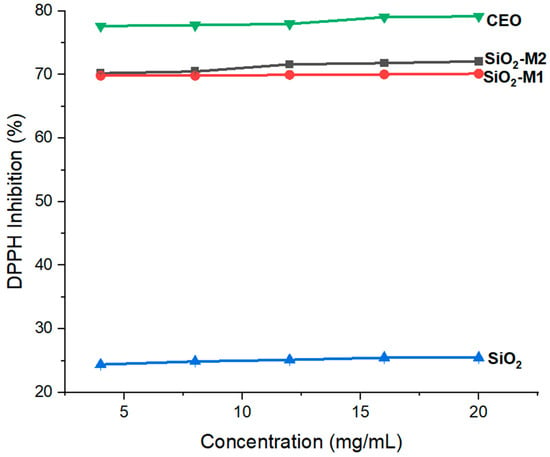
Figure 3.
Antioxidant capacity of nanoparticles SiO2 pure, SiO2-M1 and SiO2-M2.
3.1.4. Microbiological Tests
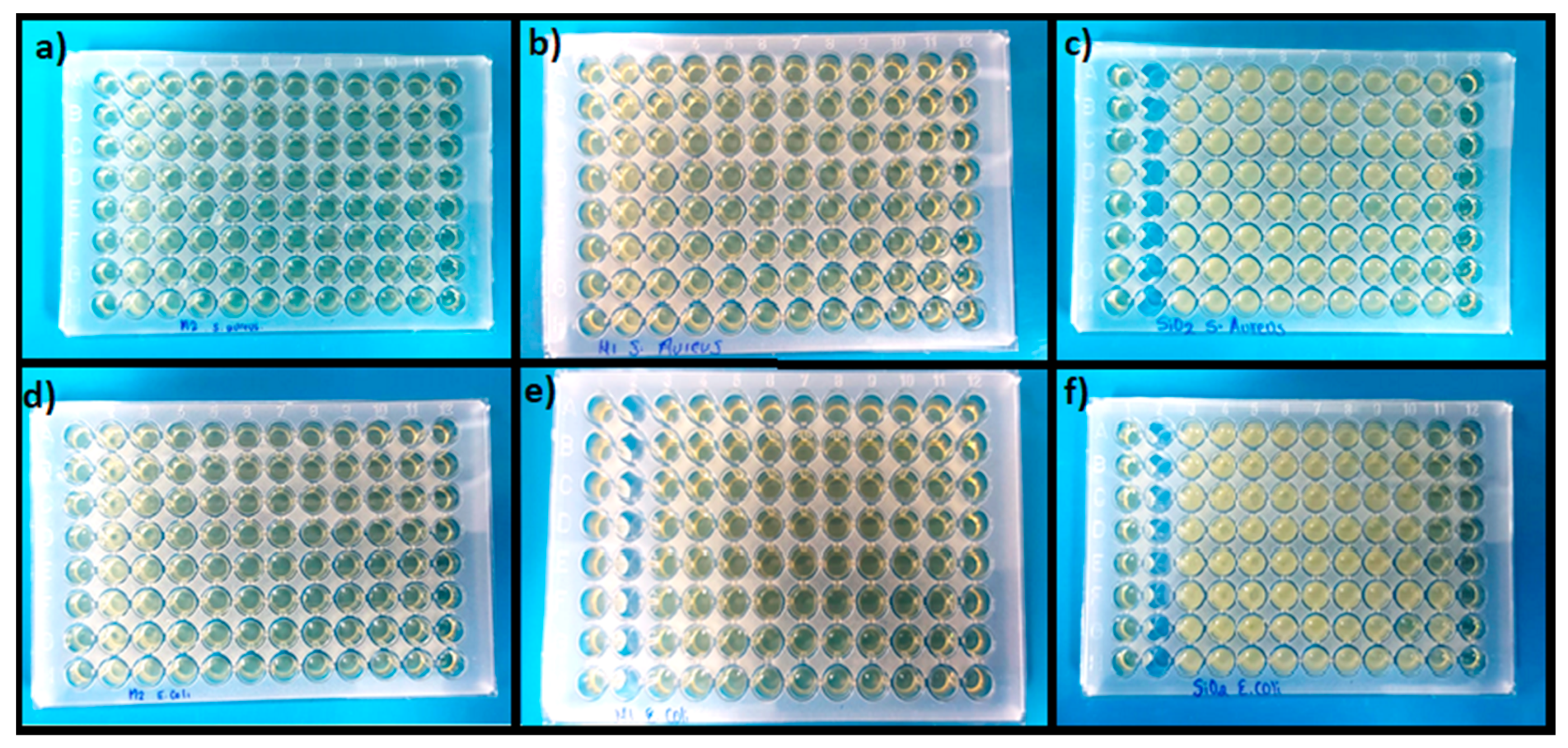
Another property of interest for the manufacture of active packaging is the antimicrobial activity of the active component, so the objective of this assay was to evaluate the minimum inhibitory concentration and its effect on Gram-negative and Gram-positive bacteria. In this context, the antimicrobial capacity of SiO2 nanoparticles modified with the M1 and M2 methods was evaluated against two types of E. coli and S. aureus bacteria using the microdilution technique at different concentrations. In summary, Table 1 shows the results obtained for the minimum inhibitory concentration (MIC) of each system. It is possible to observe in Figure 4c,f that pure SiO2 does not present any antimicrobial capacity since it did not inhibit the growth of bacteria, which is observed as turbidity to the naked eye. In contrast, the wells of the other sections are clear and translucent, which is an indication of microbial inhibition. In contrast, inhibition was observed for the SiO2-M1 system, with a MIC of 45 µg mL−1, while for the SiO2-M2 system, it was 11 µg mL−1 for E. coli. This indicates that the CEO conferred antimicrobial properties to the nanoparticles by inhibiting the growth of Gram-negative bacteria. This is due to the monoterpenes present in the CEO, which are responsible for generating pH alterations in the cell membrane, as well as increasing the permeability of the membrane and causing proteins and nutrients essential for the cells to escape until they die [61]. As for S. aureus bacteria, the MIC for systems M1 and M2 was 90 and 750 µg mL−1, respectively. A higher concentration of nanoparticles was necessary to inhibit growth against this Gram-positive bacterium. The inhibitory capacity of Gram-positive bacteria could be due to the fact that they do not have an outer membrane around the cell wall, which facilitates the diffusion of hydrophobic compounds from the essential oils [62,63]. Some research works, such as Nemattalab et al. [64], mentioned that by fabricating solid lipid nanoparticles loaded with CEO, they obtained a MIC of 65 µg mL−1 for E. coli bacteria compared to nanoparticles without CEO. Jabir et al. [65] fabricated gold nanoparticles modified with glutathione and linalool and reported a MIC for E. coli bacteria of 0.77 µg mL−1 and 1.54 µg mL−1 for S. aureus. Elghobashy et al. [66] elaborated on chitosan nanocomposites with alginate loaded with thyme and essential garlic oil and showed that the bactericidal efficacy of essential oils improved when these were combined, the MIC obtained for E. coli was 15 µg mL−1, while for S. aureus it was 7.5 µg mL−1, and these values were lower than those obtained. We can say that essential oils are effective against the inhibition of Gram-positive and Gram-negative bacteria when transferred to other materials.

Table 1.
Results of the antimicrobial activity expressed as the minimum inhibitory concentration in nanoparticles of SiO2 pure, SiO2-M1 and SiO2-M2.
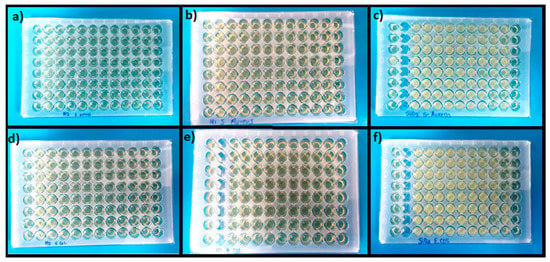
Figure 4.
Microplates for antimicrobial evaluation for S. aureus: (a) SiO2-M2 (b) SiO2-M1 and (c) SiO2 pure; E. coli (d) SiO2-M2 (e) SiO2-M1 and (f) SiO2 pure.
3.1.5. Cell Viability Test
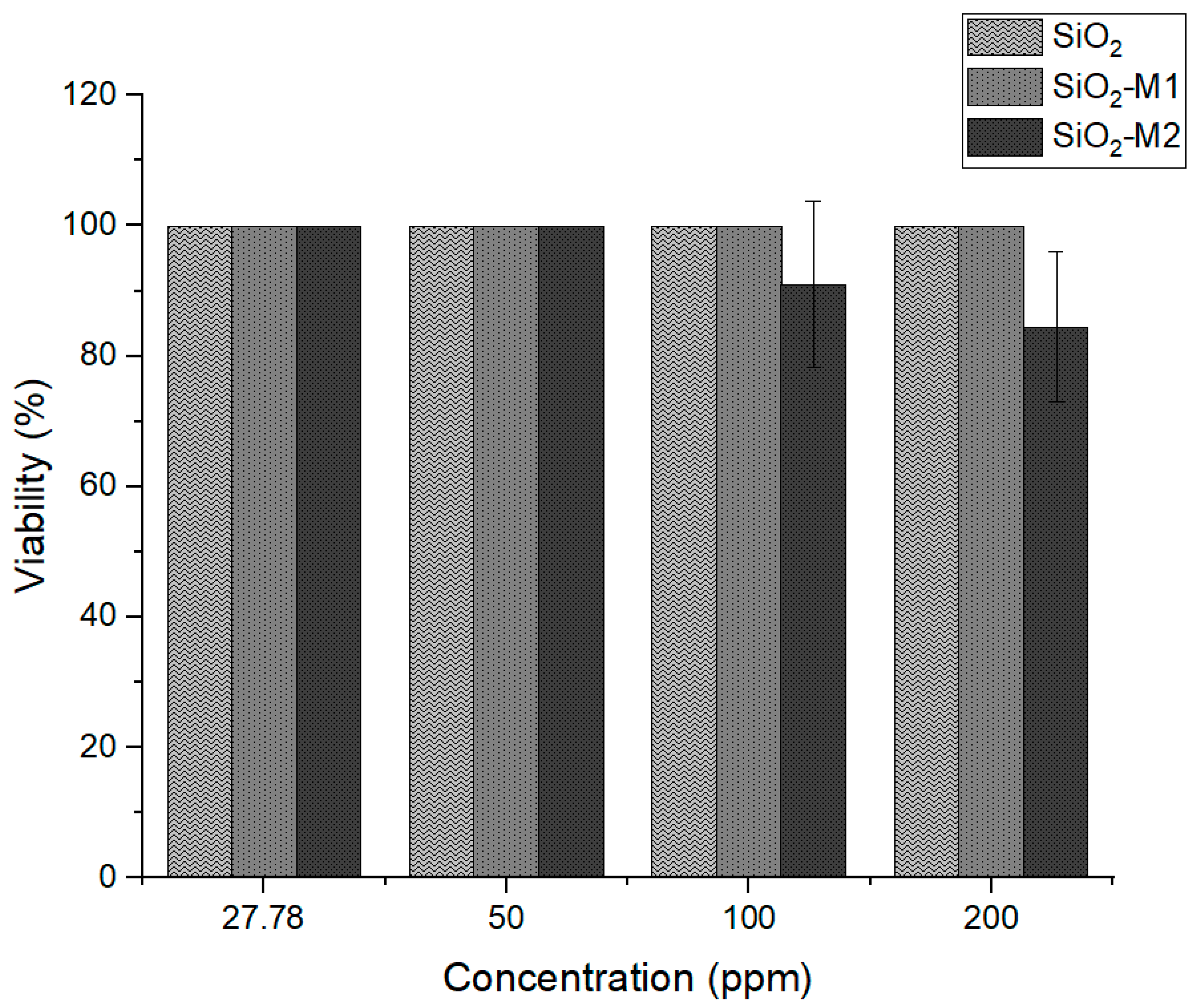
Once the antioxidant and antimicrobial activity of SiO2 nanoparticles modified with essential oil was corroborated, it was necessary to know the suitability of these nanoparticles for their application as active packaging. As they are intended for the food sector, they should not generate a health risk, so their toxicity level was quantified using the cell viability assay. The study results are shown in Figure 5 at an exposure time of 24 h; the nanoparticles treated with method one (SiO2-M1) and pure SiO2 presented 100% cell viability at different concentrations on average. In contrast, nanoparticles treated with method two (SiO2-M2) showed a decrease in cell viability with increasing concentration; however, cell viability remained above the 80% allowed, and according to ISO 10993-5:2009, the material has low toxicity. This may be because the nanoparticles have been treated with Tween 80, unlike methodology 1, so it could be assumed that it is the Tween 80 that causes this effect and not the interaction of the cinnamon essential oil. As expected, the toxicity is low, giving a guideline to develop active packaging for food applications. Similar results have been obtained by Al-Tayyar et al. [67] showing low toxicity in mixtures of PVA nanocomposites with chitosan and ZnO-doped SiO2 particles.
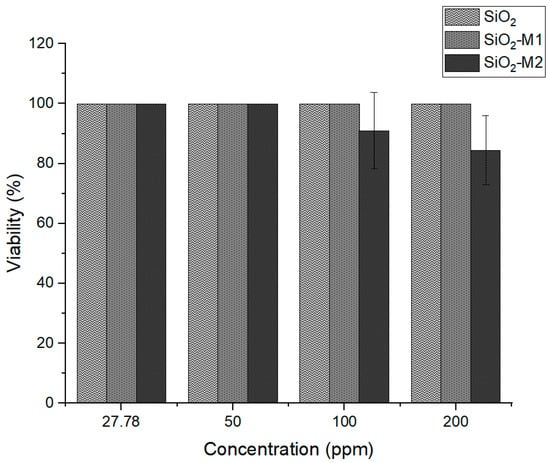
Figure 5.
Percentage of cell viability of the nanoparticles SiO2 pure, SiO2-M1, and SiO2-M2.
3.2. Characterization of the Films
3.2.1. FTIR
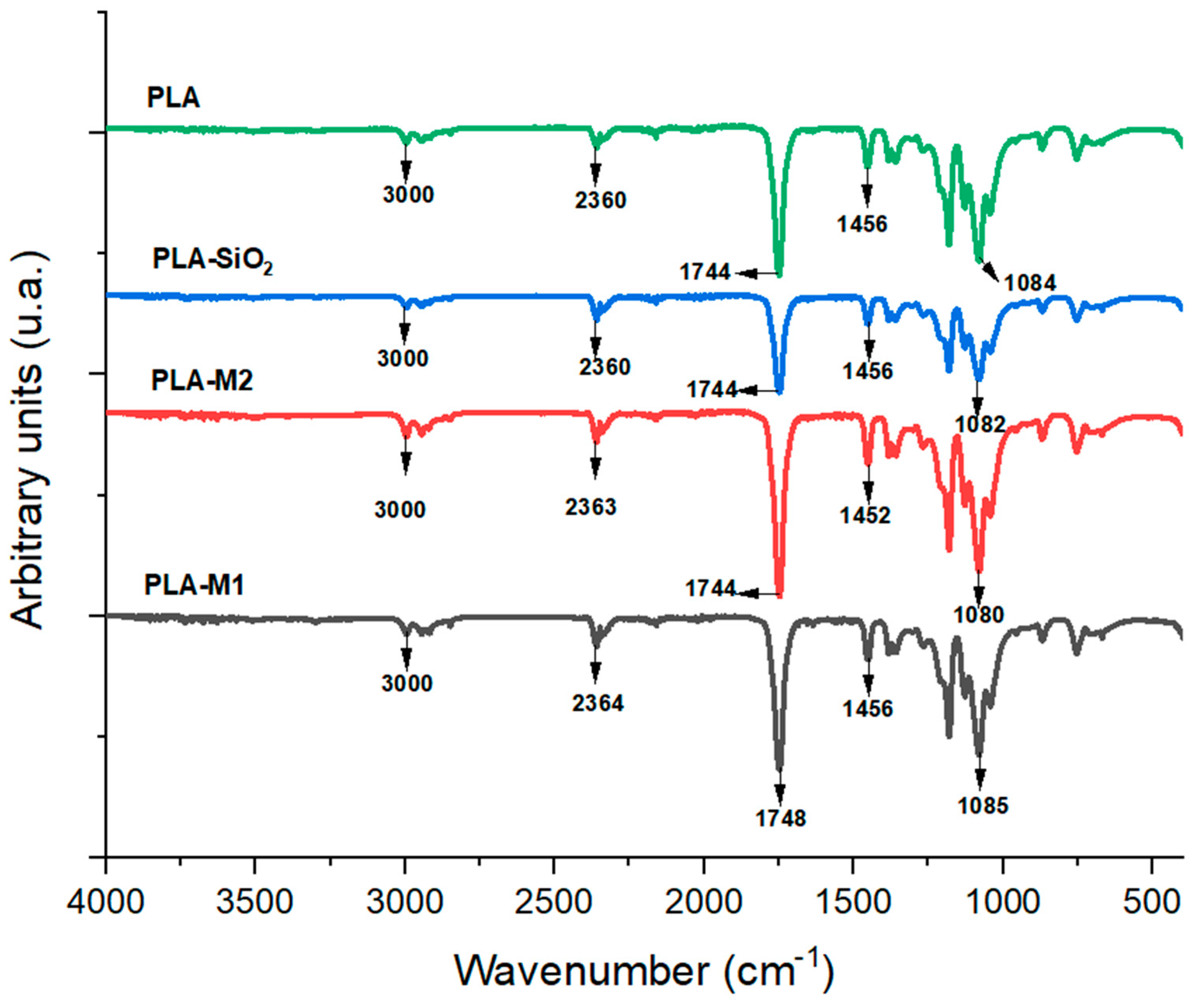
In order to evaluate the modifications in the structure of the PLA polymeric matrix, infrared spectroscopy analysis was performed. Figure 6 illustrates the FTIR spectrum for PLA films and films reinforced with pure SiO2 particles and those modified with the M1 and M2 methods. Absorption signals can be observed at wavelengths of 867 cm−1 and 754 cm−1, corresponding to the PLA amorphous phase and crystalline phase, respectively [68]. Bands also appeared at 1183 and 1081 cm−1, which are attributed to the symmetric and asymmetric stretching of the ether group (C-O-C) [46]. The bands appearing at 1453 and 1358 cm−1 correspond to saturated hydrocarbons’ asymmetric and symmetric stretching vibration (CH3-) [69]. The band at 1744 cm−1 is attributed to the stretching vibration (C=O) of the ester group of PLA molecules [70]. The peaks observed at 2933 cm−1 correspond to the CH3- stretching vibrations, and the bands observed at 1628 and 1506 cm−1 are assigned to the carbonyl and ethylene groups, respectively [71]. In the film samples, no significant changes were observed in the spectra obtained; only slight changes in peak intensities were observed. This indicates that no noticeable changes occurred in the chemical structure of PLA since no new chemical bonds were formed between PLA and pure and modified SiO2 particles. These results were also observed by Lukic et al. [72] and Mohamad et al. [73] on PLA films with essential oils in the polymer matrix, showing that there are no significant chemical changes in the films after the addition of active agents.
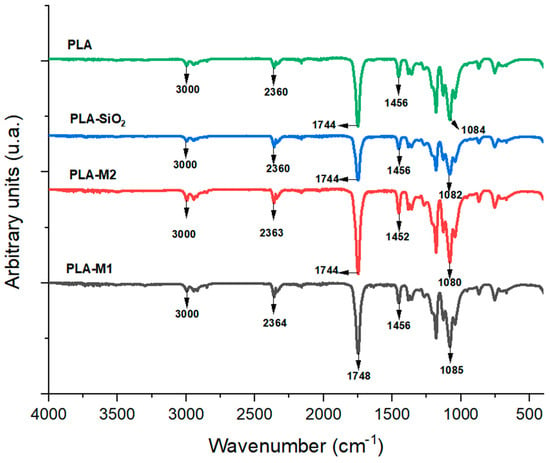
Figure 6.
FTIR spectrum of films PLA pure, PLA-SiO2, PLA-M1, and PLA-M2.
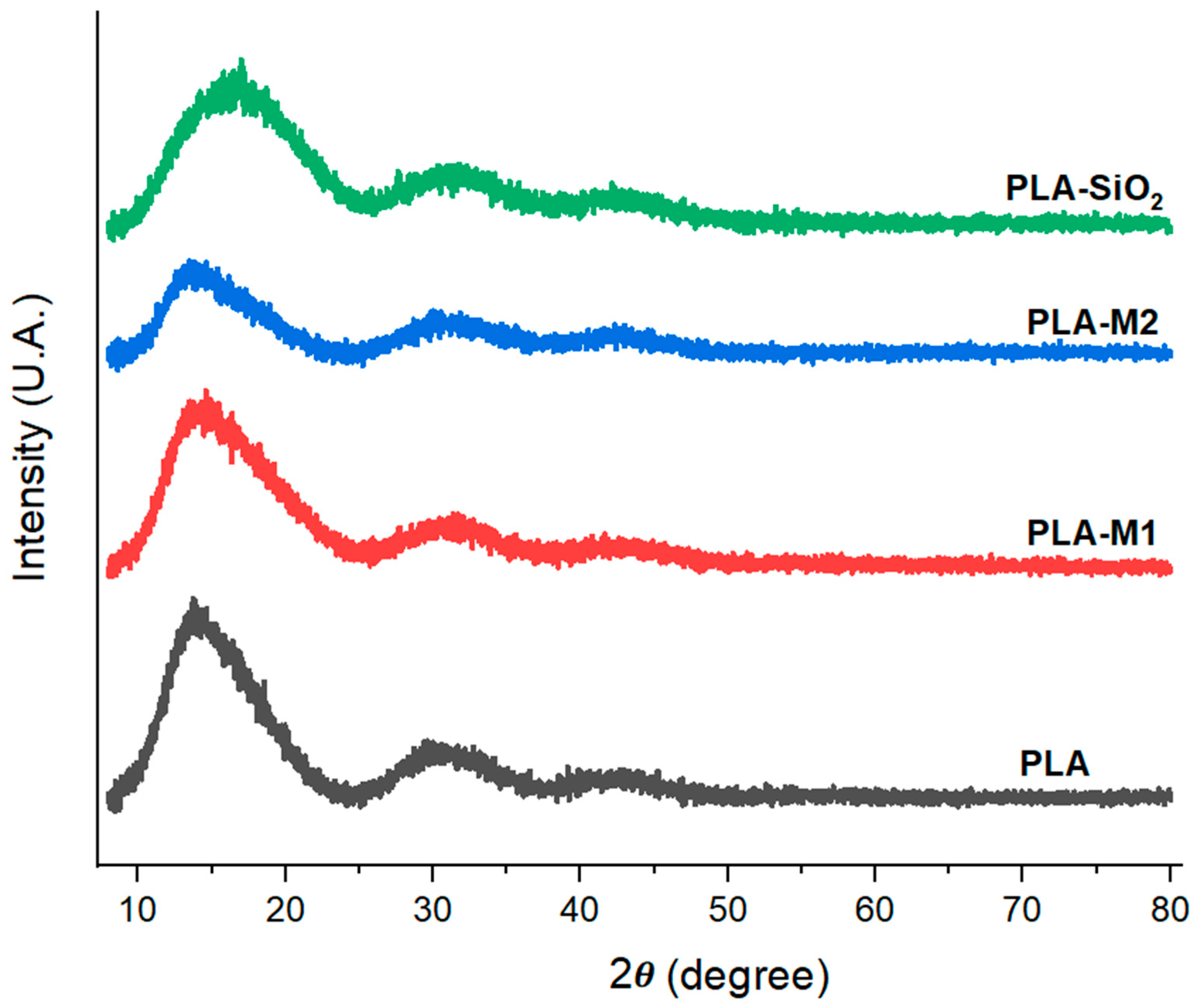
3.2.2. XRD
In order to know the effects of SiO2 with essential oil on the films, the diffractograms were obtained. In Figure 7, where it can be observed that all the films prepared with both pure SiO2 and modified nanoparticles presented similar characteristics. A broad peak of large protrusion with an angle of 2θ = 16.89° is attributed to the semi-crystalline nature of PLA and corresponds to the reflection of the α (110/200) crystalline shape plane [72]. The film formulated with the M1 method showed a slight variation, which can be quantified by calculating the percentage of crystallinity, which was 52.56. At the same time, the pure PLA had a value of 55.01%.
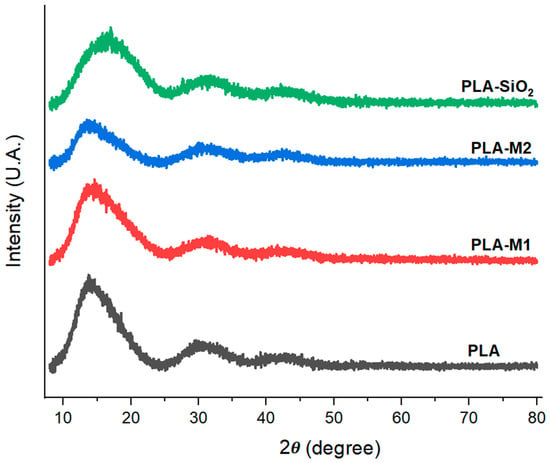
Figure 7.
XRD pattern of films PLA pure, PLA-SiO2, PLA-M1, and PLA-M2.
On the one hand, it was observed that when films were prepared with nanoparticles from the M1 method, the PLA crystallinity was reduced by 4.45%. On the other hand, in the film with nanoparticles modified with method 2, it is observed that this same peak decreases its intensity considerably, indicating that this interaction is increasing the disorder of the polymeric chains by 31% since its percentage of crystallinity was calculated at 37.91%. In contrast, the films formulated with SiO2 show a significant change in curvature due to the presence of these nanoparticles in the matrix, generating a slight broadening of the peak found at 16.89°, in addition to the fact that their crystallinity percentage was 49.97%. This could be because SiO2 is in an amorphous state, which generates, as in the previous case, the disorder of the polymeric chains. It was demonstrated that the effect caused by the addition of CEO in the PLA matrix reduces the crystallinity. This same effect was observed in other investigations. Valenzuela et al. [74] reported that the introduction of sunflower oil in a quinoa protein and chitosan-based film generated a less crystalline structure. Likewise, Ahmed et al. [75] indicated that there were no significant variations in the structure of PLA films when formulated with other materials, such as graphene oxide nanofillers.
3.2.3. SEM
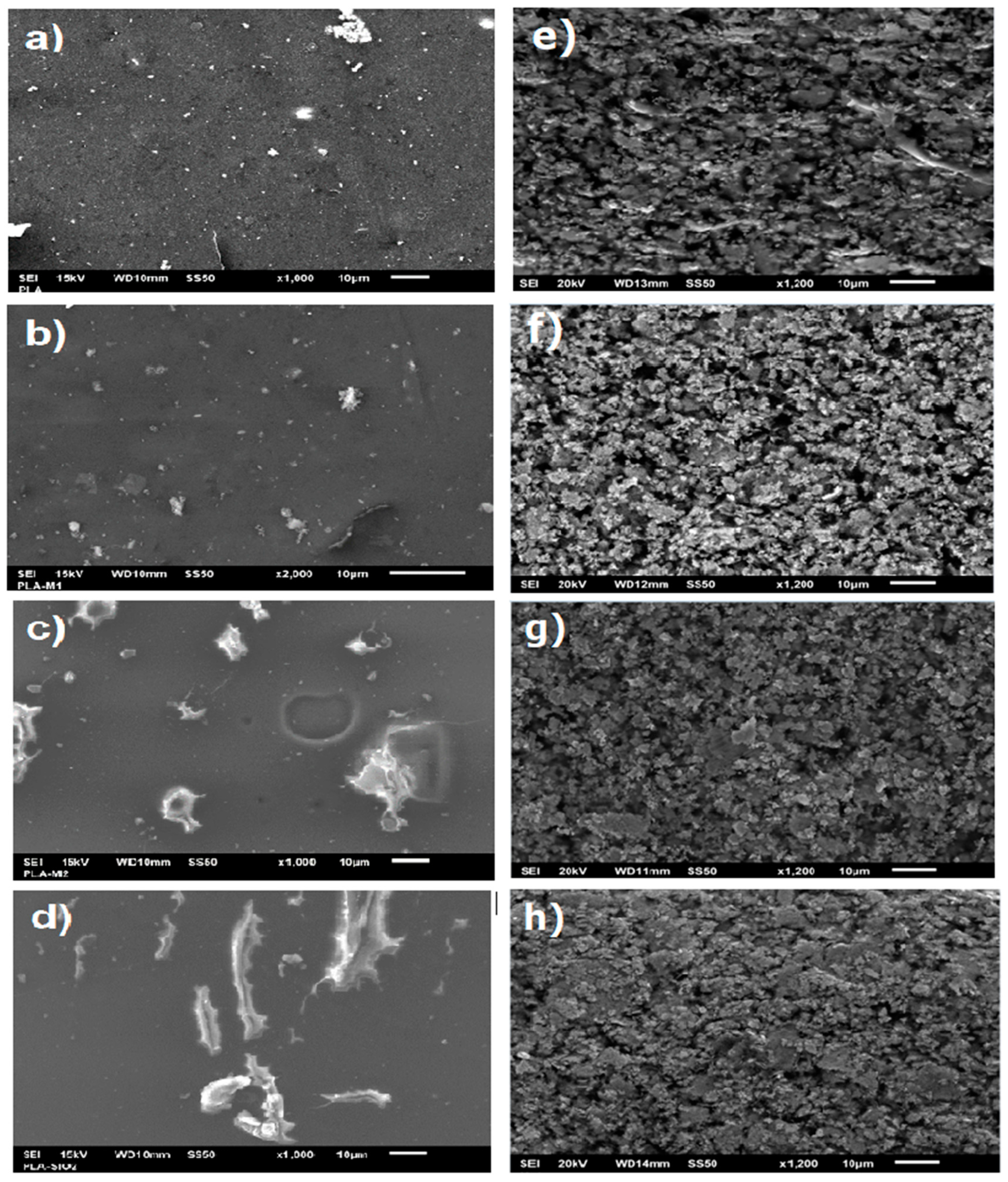
A scanning electron microscopy (SEM) analysis was performed to evaluate the effects on the morphology of the films. Figure 8 shows the SEM micrographs of the films prepared from pure PLA, PLA with pure SiO2, and nanoparticles modified with the essential oils (M1 and M2). In all the films, some areas that could correspond to TiO2 nanoparticles are observed since PLA is fabricated with rutile as a white pigment. In the case of pure PLA films, only these TiO2 nanoparticles are observed; on the contrary, it can be observed that the film containing pure SiO2 nanoparticles did not develop a good interaction of the nanoparticles with the polymeric matrix since agglomerations and non-homogeneous distribution are appreciated. See Figure 8.
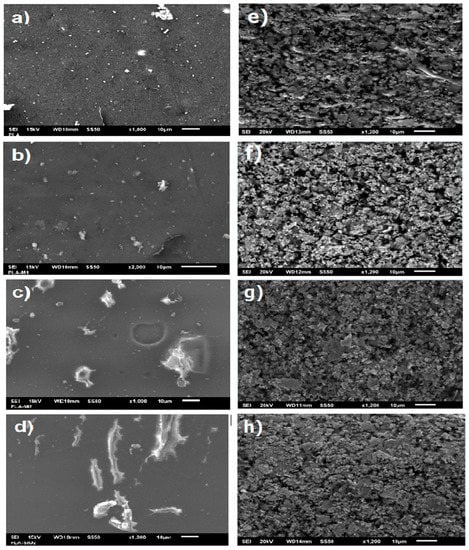
Figure 8.
SEM micrographs of films: (a–d) surface and (e–h) cross-section of (a) PLA, (b) PLA-M1, (c) PLA-M2, and (d) PLA-SiO2.
Similarly, the film with M2 nanoparticles showed small agglomerations, and some scratches, possibly due to the Petri dishes marks; however, there is a better distribution of the nanoparticles compared to pure SiO2. Likewise, it can be observed that the film with M1 nanoparticles has a more homogeneous distribution, is smoother, and does not present scratches or cuts. In the same way, we can observe in the sections (e–h) the cross sections of the films, where a rough texture with holes is observed; in the PLA-M1 film, better dispersion of the nanoparticles is observed, for PLA-M2, a uniform distribution is observed in the same way, but with some agglomerations, while the PLA-SiO2 film shows larger agglomerations that generate rougher films, we can say that the film that had better miscibility with PLA was the one containing nanoparticles modified by method 1. This is the case presented by Mahmood et al. [76] and Yang et al. [77], who developed films with TiO2 as a nanofiller and obtained a heterogeneous dispersion with lumps and rougher fractured surface than the PLA film. On the other hand, the study by Lukic et al. [72] mentions that the interaction of PLA with essential oils without nanofillers generates less smooth films and the formation of large voids on the surface of the films. Given the above, it indicates that the proposed methods improve the morphology of the films.
3.2.4. Antioxidant Activity in Films
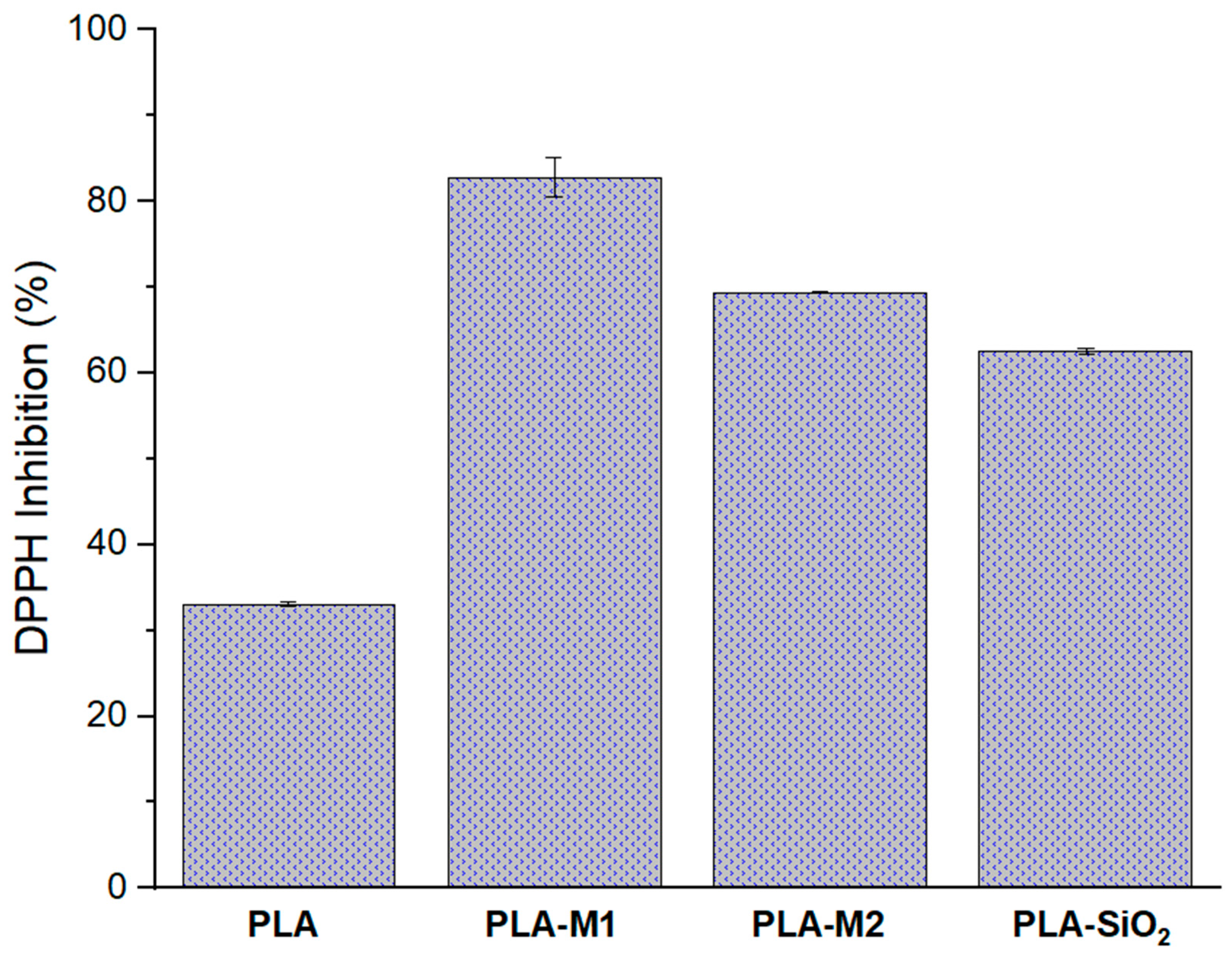
Another important characteristic to evaluate in active packaging is its antioxidant activity in the film. This property allows the stored product to be preserved for longer, maintaining its nutritional properties and appearance since the consumer’s acceptance or rejection of a product depends on this. The results obtained are shown in Figure 9 it can be observed that the films prepared with SiO2 nanoparticles presented a higher percentage of free radical inhibition compared to pure PLA; these values were 82.81%, 69.38%, and 62.55% for the PLA-M1, PLA-M2, and PLA-SiO2 systems, respectively. When these values are compared with those measured for nanoparticles modified with essential oil, it can be observed that this property increased by 15% for the PLA-M1 system, in contrast with the PLA-M2 system, which decreased by approximately 6.94%. Something interesting that can be observed is that the PLA-SiO2 system increased its inhibition percentage by 60% compared to that obtained by the nanoparticles; this could be because the PLA used in white color and has TiO2 in its composition which gives it that coloration, so it is assumed that TiO2 is helping to inhibit free radicals, that is why these increases were observed, Alizadeh et al. [37] reported that at a minimum concentration of 25 mg/mL TiO2 pure is able to inhibit 20% of DPPH. Likewise, Martínez et al. [40] found that at a concentration of 4 mg/mL, TiO2 had a 20% inhibition. Alam et al. [78] reported that at 20 µg/mL, TiO2 presented 14% inhibition to DPPH. Several studies, such as Lukic et al. [72], Kumar et al. [79], and Mariño et al. [80], mention that there are high antioxidant activity values in films containing essential oils, making these active components ideal for use in active packaging.
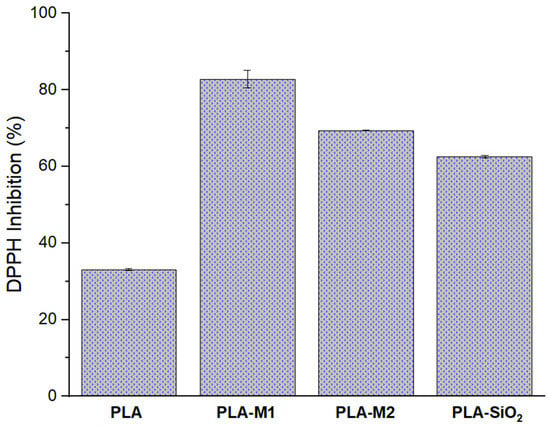
Figure 9.
Antioxidant capacity of PLA films, neat and filled with M1, M2, and SiO2.
3.2.5. Microbiological Analysis of Films
After confirming the antimicrobial activity of the nanoparticles modified with essential oil, a microbiological test was performed on the films to determine if this property could also be transferred to the film, which is an important property in active packaging. In Table 2 it can be observed the drop test results performed on the films. The pure PLA system had practically no inhibition, with values of 0.43% and 0.92% for both E. coli and S. aureus bacteria. In comparison, the systems prepared with nanoparticles modified with the essential oil presented mean values between 1.29 and 3.54% for Gram-positive and Gram-negative bacteria. Such low values could be because the diffusion of the nanoparticles is not so fast, which prevents higher antimicrobial activity in the films [81,82,83,84]. However, this result also indicates that the nanoparticles have well adhered within the polymeric matrix, so there is no migration, which could favor the properties of the active packaging as the slow diffusion could help preserve food for a longer time. Similar to the antimicrobial activity of the nanoparticles, the films followed a behavior of higher inhibition of E. coli bacteria due to the thicker cell wall of S. aureus, which generates a higher force needed to inhibit the bacteria. As can be observed, the PLA-SiO2 system did not have a significant difference between both bacteria, having a very similar value to pure PLA; this could be attributed to the fact that pure SiO2 does not have any inhibitory effect against these bacteria according to what it could be obtained in the microbiological analysis of the nanoparticles. The study by Montero et al. [85] on cellulose nanofiber films loaded with CEO observed the same behavior using the disk diffusion technique, where the films did not show inhibition halos; however, the antimicrobial effect is more visualized when packaging a product inside, they stored strawberries which were kept in good conditions for 15 days.

Table 2.
Results of the antimicrobial activity expressed as % inhibition in the PLA films.
3.3. Evaluation of Physical Parameters in Films
3.3.1. Water Solubility (WS)
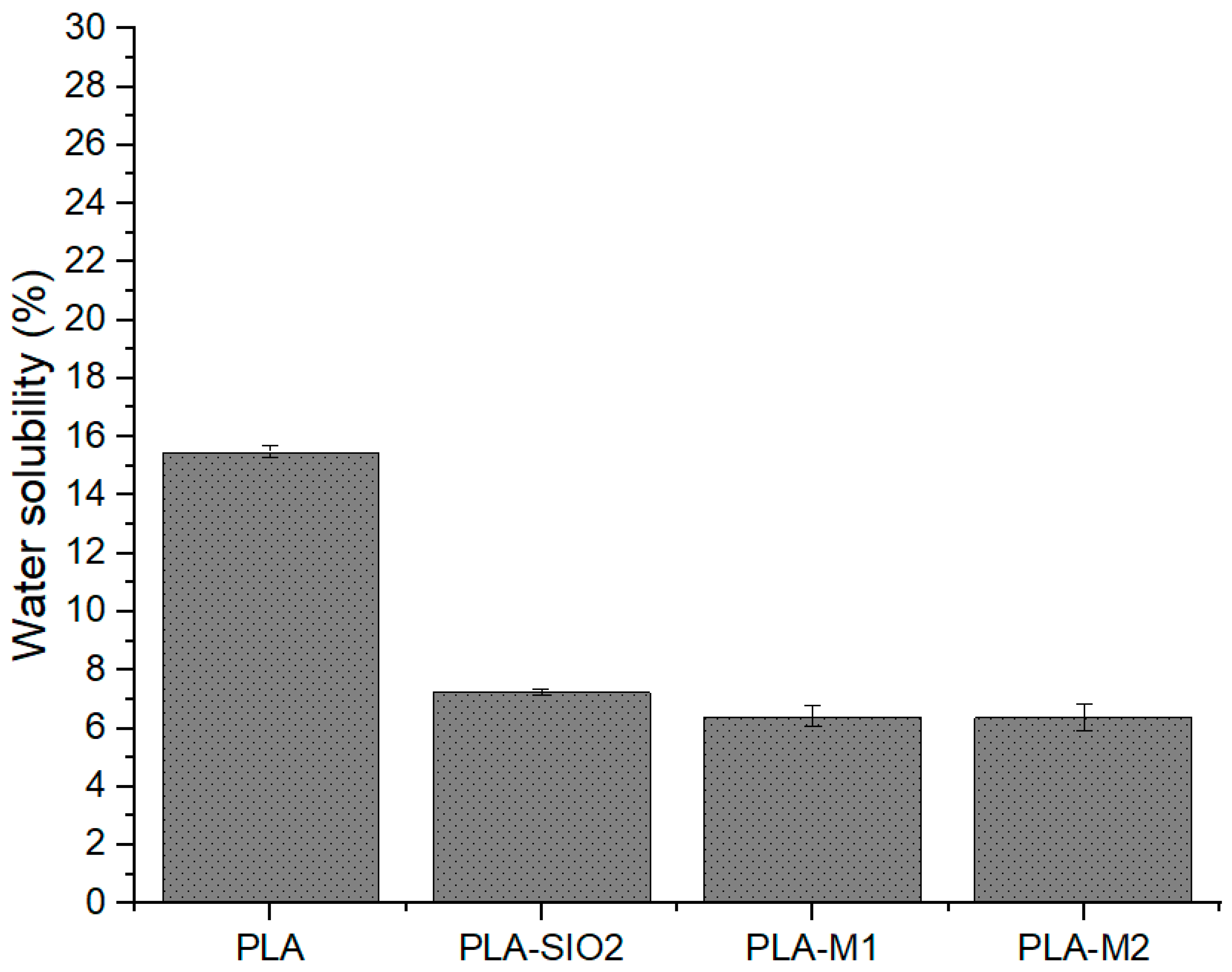
An important factor when choosing a biopolymer is its solubility in water since knowing this parameter can guide the application to food product packaging [86,87,88]. The results obtained are illustrated in Figure 10 and Table 3, which show a significant variation in the solubility of pure PLA of 15% concerning films formulated with SiO2 nanoparticles and those modified with cinnamon essential oils. The films with modified particles maintained low solubility percentages, between 6% and 7%, which indicates that the films are resistant to degradation in aqueous media, making them suitable for food storage at low temperatures with higher percentages of relative humidity. This could be due to the hydrophobic interaction of the essential oils in the polymeric matrix. Previous studies mention that this physical property can be affected positively or negatively, depending on the interaction between the components, for example, increased solubility percentages in studies such as that of Cai et al. [89]. Similar results obtained by Hadi et al. [87] and Cao and Song et al. [51] showed a reduction in water solubility with increasing lemon essential oil (EO) concentration, ranging from 46.2% (control) to 38.7% and 33.4% with the addition of 1% and 2% EO. These results are possibly due to the hydrophobic character of the essential oil in the films and the interaction with the hydroxyl groups of water.
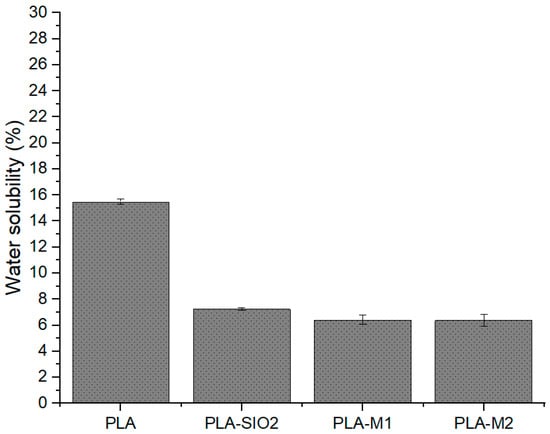
Figure 10.
Percentage solubility in water of films PLA pure, PLA-SiO2, PLA-M1, and PLA-M2.

Table 3.
Water solubility, water vapor permeability, tensile strength, elongation at break, and Young’s module of systems: PLA pure, PLA-SiO2, PLA-M1, and PLA-M2.
3.3.2. Water Vapor Permeability (WVP)
Water vapor permeability (WVP) was determined because this property will serve as an indicator to analyze the stability of the food inside the package. Given the above, the permeability measurement was performed on the films prepared with pure SiO2 and with the particles obtained by methods M1 and M2. The results are summarized in Table 4; the pure PLA film presented the lowest WVP of 3.45 × 10−8 g Pa−1 h−1 m−2 regarding all films. The PLA film with pure SiO2 was the one that presented the highest permeability (8.45 × 10−8 g Pa−1 h−1 m−2), this could be due to the porosity of pure SiO2 that produced an increase in spaces in the polymeric matrix, and this facilitated the transfer of water vapor between the stored food and the external medium. Films prepared with nanoparticles from methods M1 and M2 presented a WVP of 7.93 × 10−8 g Pa−1 h−1 m−2 and 5.50 × 10−8 g Pa−1 h−1 m−2, respectively. With the above information, it can be said that the films are semi-permeable, which could be interpreted as beneficial for use in fresh foods that need to maintain a certain percentage of moisture to preserve their nutritional properties [90].

Table 4.
Results of thermal transitions of PLA and its composites as determined by DSC.
Several studies report increases and decreases in WVP, such as the one performed by Qin et al. [27]; they elaborated PLA films with bergamot (BO), lemongrass (LO), rosemary (RO), and clove (CO) essential oil at 9% wt, and reported that the films containing the essential oils increased the WVP of pure PLA from 1.96 × 10−14 kg m m−2 s−1 Pa−1 to 2.03 × 10−14 kg m m−2 s−1 Pa−1, (BO), 1.69 × 10−14 kg m−2 s−1 Pa−1, (LE), 1.54 × 10−14 kg m m−2 s−1 Pa−1, (RO) and 1. × 10−14 kg m−2 s−1 Pa−1 (CO). Javidit et al. [25] prepared PLA films with oregano essential oil at 1 and 1.5 wt%, finding that the water vapor permeability was reduced for pure PLA from 1.89 × 10−8 kg m m−2 s−1 Pa−1 to 1.25 89 × 10−8 kg m−2 s−1 Pa−1 at 1 wt% concentration, from 1.7289 × 10−8 kg m−2 s−1 Pa−1 at 1.5%wt concentration. Yu et al. [55] prepared PLA films reinforced with acetyl cellulose and ZnO nanoparticles and found that the permeability was reduced in higher proportion when nanoparticles were added at 3% concentration from 1.14 × 10−11 g m−2 Pa−1 s−1 of pure PLA to 0.73 × 10−11 g m−2 Pa−1 s−1. Finally, Praseptiangga et al. [54] prepared carrageenan films reinforced with SiO2 and ZnO nanoparticles, and the WVP of the film was slightly reduced when SiO2 nanoparticles were added, from 1.059 to 1.035 × 10−6 g h−1 m Pa−1; however, when combined at 1:1 SiO2/ZnO ratio an improvement was found to 1.011 × 10−11 g m−2 Pa−1 s−1. Based on the previous studies, the WVP in films depends on intrinsic factors such as the sorption of the molecules present in the matrix as well as their diffusion and the crystallinity of the polymer.
3.3.3. Water Contact Angle (WCA)
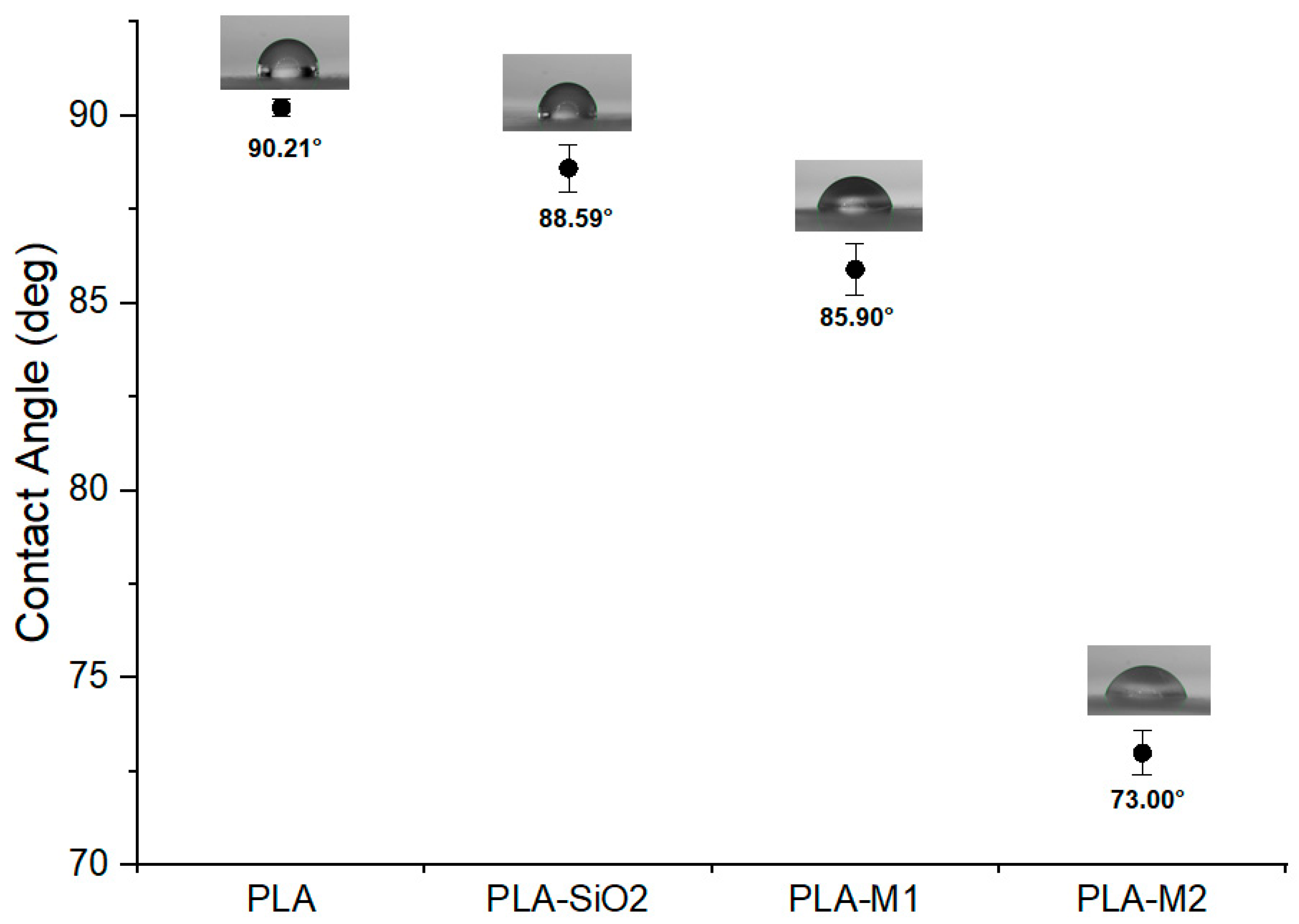
The contact angle indicates the affinity of a surface in contact with a liquid, in this case, water, and is also known as wettability. This property was determined on the films to know the effect of pure and CEO-loaded SiO2 nanoparticles on the wettability property. It is possible to observe in Figure 11 that the pure PLA film had a higher contact angle of 90.21° compared to the other films. The film containing the pure SiO2 nanoparticles and those modified with the M1 and M2 method significantly modified the contact angle (p < 0.05), resulting in values of 88.59°, 85.90°, and 73°, respectively. These values were less than 90°, indicating that the surface of these films is wettable and hydrophilic, so there is good adhesion between the film surface and the aqueous medium, in addition to a high surface free energy. The decrease in contact angle could be generated by pure SiO2 nanoparticles and not by the addition of CEO since previous studies, such as Mengting et al. [91], observed that due to a large number of hydroxyl groups and the high hydrophilicity of SiO2 nanoparticles considerably reduced this property, from 66° to 3° for their chitosan films with sodium phytate. However, Heydari et al. [19,92] mentioned that this property was increased in PLA films containing pure ZnO nanoparticles and peppermint essential oil and multiflora, corroborating that SiO2 nanoparticles in the polymer matrix generate hydrophilic surfaces even when CEO is included. Other studies have reported lower contact angle values, such as Roy et al. [9], who developed PLA films with curcumin as the active component and reported a contact angle for pure PLA of 71.2° while they found a slight increase of 74.3° when curcumin was added at a concentration of 1.5 wt%, indicating that the films had a hydrophobic character. Javidi et al. [25] evaluated PLA films with Origanum vulgare essential oil and reported that the contact angle was higher with increasing oil concentration; pure PLA presented a value of 61.20° and changed to 94.03° with the essential oil at a concentration of 1.5 wt%. Ranjbar et al. [93] prepared PLA/keratin nanofibers reinforced with nanofibrillated natural chitosan and ZnO nanoparticles. The combination of these materials showed that the contact angle decreased from 56° to 41°when the nanoparticles were added. The interaction of the components can help to increase or decrease this property so they do not follow a specific behavior.
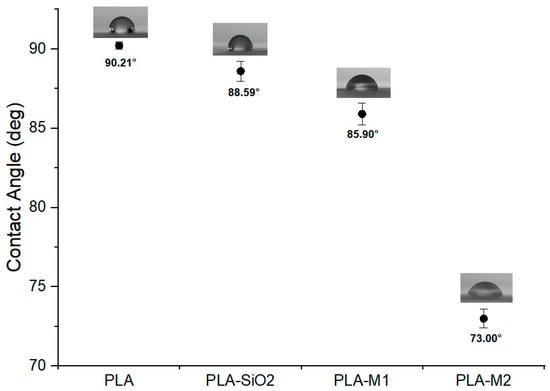
Figure 11.
Contact angle of films PLA, PLA-SiO2, PLA-M1, and PLA-M2.
3.3.4. Mechanical Testing
Mechanical properties are very important parameters to consider when developing active packaging, as they must have good resistance to damage during handling and transportation to ensure that the product remains in good condition [25]. Table 4 shows the results of the mechanical properties, tensile strength (TS), Young’s modulus (YM), and elongation at break (EB) of pure PLA films and nanoparticles modified with the M1 and M2 methods. It can be observed that the TS of pure PLA was 27.06 MPa, and the film with pure SiO2 increased this value by 2.76% since it presented a TS of 28.06 MPa, which is attributed to the hydrogen bonding between the SiO2 nanoparticles and the hydroxyl groups of the polymeric matrix. This behavior was also observed by Singh et al. [94], Palma et al. [36], Casalini et al. [95], Lukic et al. [72], and Liu et al. [96]. However, the films containing CEO decreased the TS by 7.9% and 3.97%, having a minimum value of 25.13 MPa for the PLA-M2 system, generating a more brittle and easily broken film when subjected to a force. This effect could be attributed to the interaction of the CEO in the film network, indicating that discontinuous spaces are being generated, and, therefore, there is a disorder of the polymeric chains, which in turn causes a decrease in stiffness and strength [97]. This effect was observed by Rungsima et al. [98], Radusin et al. [8], and Heydari et al. [92], in which the oil concentration is increased, and hence these properties are reduced.
On the other hand, when evaluating the EB, a low value of 5.08% was obtained for the pure PLA film, indicating that this polymer is not easy to manipulate. In comparison, the films developed with SiO2 showed an increase of 12.3%. However, the films with particles modified with CEO showed a considerable increase; PLA-M2 had 63.77%, while the PLA-M1 system had a more significant increase since its increase was double since the essential oils interacting with the polymers act as plasticizers. This effect has been observed in several studies, where the addition of essential oil increased the spaces in the polymeric matrix, thus reducing the intermolecular forces, and therefore these can move easily without breaking; this property is acceptable for the manufacture of food packaging [8,99,100]. Martins et al. [101] observed a slight increase in elongation when green tea essential acid was added to PLA films, from 3.63 to 4.94%. Celebi et al. [26] observed that %EB increased twofold when thymol essential oil and carvacrol were added from 2.9 to 6.8%. Villegas et al. [102] similarly observed this increase in PLA films with cinnamon essential oil and thymol. A greater increase was observed when thymol was added, increasing the %EB from 10 to 120%.
As expected, Young’s modulus was significantly reduced by 45.98%, especially for the films prepared with the M1 and M2 method. The above indicates that the films can easily undergo deformation when an external force is applied, which is a similar characteristic to those present in food packaging bags. It is possible to notice that the PLA-SiO2 system increased Young’s modulus by 15.38%; this could be attributed to the fact that SiO2 is functioning as a nanofiller in the empty spaces of the polymeric network generating strong interactions that prevent the chains from moving easily and therefore reduce their flexibility [86,103,104,105]. A higher result than that obtained has been reported by Brancewicz et al. [106] of 2.344 Mpa for pure PLA. Torres et al. [107] reported that PLA in fiber form with essential oil with Propolis powder extract Young’s modulus decreased as the extract concentration increased from 36.6 MPa to 7 MPa. Torres et al. [107] report that when they added thymol essential oil to PLA films, Young’s modulus was considerably reduced from 1215 to 45.10 MPa.
3.3.5. DSC
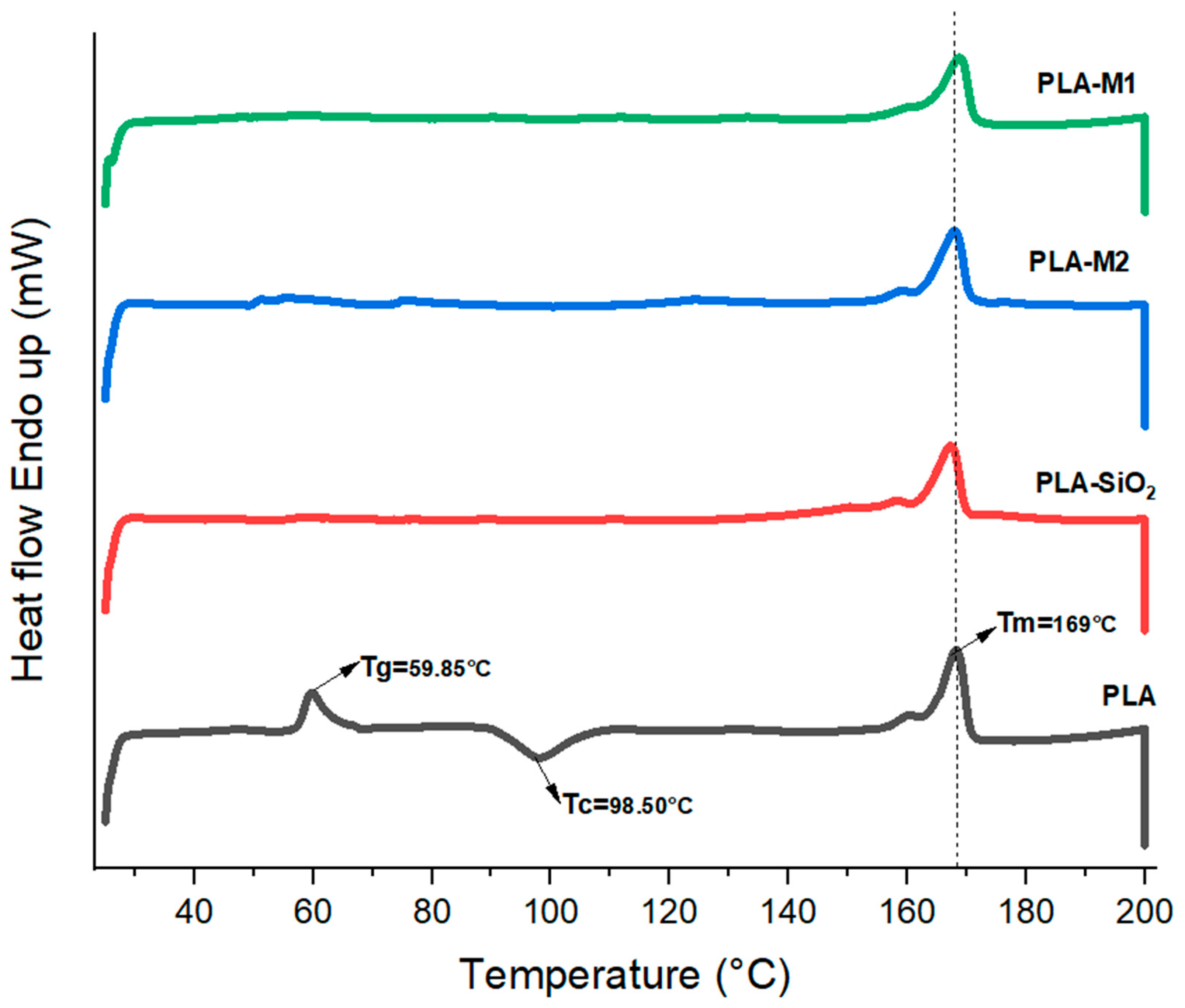
In order to know the thermal transitions of the formulated films, thermograms were obtained by differential scanning calorimetry (DSC). The melting temperature (Tm), crystallization temperature (Tc), and glass transition temperature (Tg) are summarized in Table 4; in addition, these data were plotted in Figure 12. On the one hand, PLA presented a Tg of 59.95 °C, but in the other films prepared with SiO2 (pristine and modified), this transition was not observed, which could be because the nanoparticles reduced the free volume of the polymeric chains, which prevents their movement at temperatures below 100 °C. The crystallization temperature of pure PLA was 98.50 °C, characterized by an exothermic peak, indicating a slow crystallization during the heating, leading to the reorganization of the polymeric chains and their crystallization temperature before reaching their melting temperature, which was 169 °C. This behavior and similar results have been observed in other research works [46,64,77].
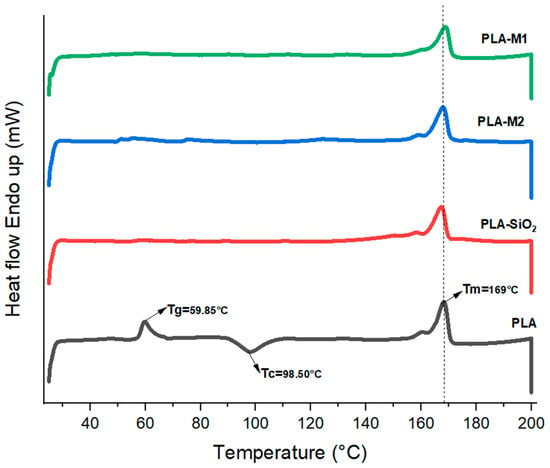
Figure 12.
Thermogram of PLA and composites obtained by DSC. Vertical line indicates the melting temperature of neat PLA.
On the other hand, it can be noticed that the films that had SiO2, SiO2-M1, and SiO2-M2 nanoparticles lacked a crystallization temperature during the heating. This could be due to the fact that the nanoparticles immersed in the polymeric matrix contained CEO and were prepared with chloroform. Therefore, in this analysis, if we detect the crystalline structure of the material prior to cold crystallization, then the crystallization exotherm only reflects what crystallized during film formation by the casting method. The exotherm showed that the melting temperature was similar to that of pure PLA between 166–169 °C. Table 4 also shows the melting enthalpy , crystallization enthalpy , and the degree of crystallization (Xc) obtained from the DSC curve. These results indicate that PLA presented a very low crystallization degree of 10.43%, while the highest degree of crystallization was reached when SiO2 nanoparticles (43.03%) were added. However, when SiO2-M1 and SiO2-M2 nanoparticles were added, these reduced the crystallinity, obtaining 26.25 and 35.89%, respectively.
Some works mention that metal oxide nanoparticles have improved the crystallinity of PLA, as well as its Tg. The study conducted by Zhang et al. [108] mentioned that adding ZnO nanoparticles at 0.25% increased the Tg of pure PLA from 59.04 °C to 63.02 °C, as well as the degree of crystallization from 4.22% to 12.95%. Łopusiewicz et al. [109] made PLA films with fungal melanin as an active component and reported a Tg of 61 °C with a lower Tm than that obtained in the present work of 148 °C and with an increase in the crystallinity from 0.23% to 1.61% with 0.2% of fungal melanin. Cvek et al. [110] elaborated on PLA and propylene carbonate composites loaded with curcumin. When evaluating their effects on thermal properties, they observed that the addition of curcumin did not significantly alter their transition temperatures, Tg (56–59 °C), the Tc (125.5–127.4 °C) and Tm (148.5–148.8 °C); however, the degree of crystallization had a decrease from 1.1 to 0.2%, due to curcumin acts as a plasticizer and therefore reduces the crystallinity in the films; this same effect is observed when essential oils were added.
3.4. Storage Analysis of Apple Samples
3.4.1. Weight Loss
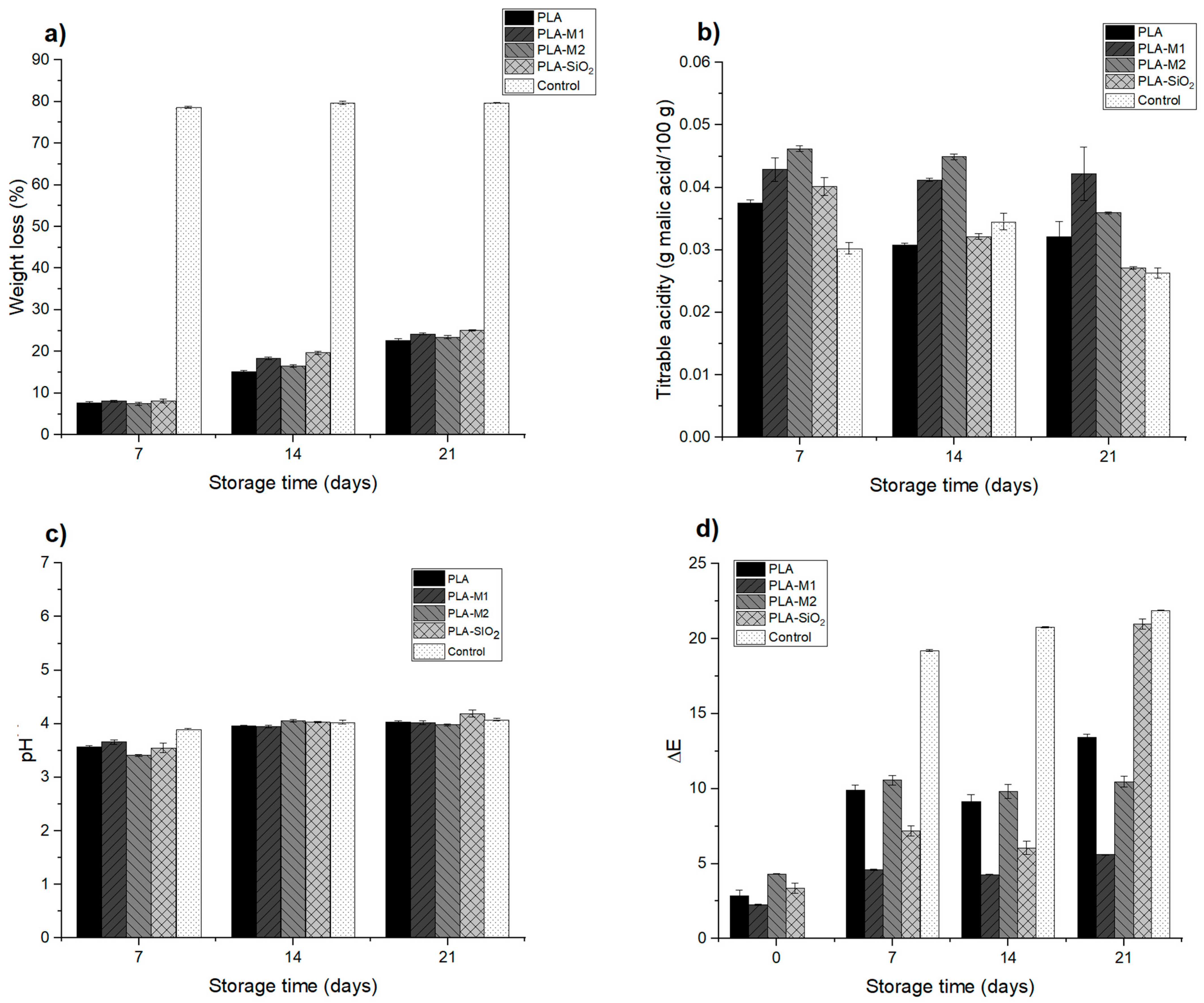
The weight loss of food is influenced by the type of material and storage conditions, as well as by the respiratory rate of some foods. Generally, the packages that maintain weight loss are those in which permeability is minimal since it reduces weight loss in the form of water vapor [111]. A conventional package that has good barrier properties is high-density polyethylene or polypropylene, which has been used in various presentations and has good resistance to shock and impact. This type of material is used to store vegetables, fruits, cereals, nuts, and seeds for more than 21 days. The weight loss in the apple pieces was determined to evaluate the films as suitable packaging to be used in food since their main function is to protect the food from external factors and maintain its properties, in this case, humidity and avoiding water loss in the form of weight [35]. Table 5 shows the final results of storage at 21 days; it can be noticed that there is a large significant weight loss (p < 0.05) for the control sample, which was not stored in packaging; this indicates that it lost a considerable amount of water by 78% compared to the samples stored in the package, which only had a loss of 22.71%, 24.24%, 25.05%, and 23.43% for PLA, PLA-M1, PLA-SiO2, and PLA-M2 systems respectively. Figure 13a shows the evolution of weight loss during storage, observing that this variable increased as the days passed; however, the other samples placed inside the active container maintained a greater amount of water and freshness with a loss of less than 25%. A similar effect was observed in the study by Chen et al. [112] when storing apple slices in films composed of sodium alginate and thymol essential oil, as well as in the study by Sganzerla et al. [113], who elaborated on citrus starch and pectin films for apple storage. The results show that the packaging fulfills its function of protecting the product and, therefore, avoids a greater loss of water by transpiration of the apple during storage, thus prolonging its shelf life.

Table 5.
Weight loss, titratable acidity, color difference, and pH in the samples stored in the active packages at the end of the 21-day storage period.
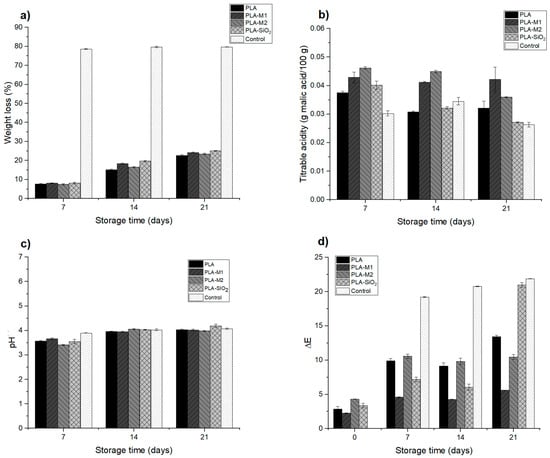
Figure 13.
Physico-chemical evaluation of apple storage: (a) weight loss, (b) titratable acidity, (c) pH, and (d) color difference.
3.4.2. Titratable Acidity and pH
Titratable acidity (TA) is an important quality parameter in foods, especially fruits because they are rich in organic acids. During the storage process, a decrease in this parameter was observed, which could be due to the degradation of organic acids inside the fruit caused by metabolic reactions that trigger modifications in flavor, color, and stability [114,115,116]. Figure 13b and Table 5 show the results at the end of storage. It can be observed that the evolution of the titratable acidity in the control sample decreased considerably. In contrast, the apple samples stored in the packaging after 21 days of storage maintained a TA very close to the initial sample, which was 0.0502; there was only a decrease of 16% (0.042) and 30% (0.035) for the samples in PLA-M1 and PLA-M2 packaging, respectively.
Also, it can be observed that the PLA-SiO2 sample presented a significant decrease of 46% (0.027), very close to that obtained in the control sample that presented a decrease of 48% (0.026), which, as expected, would suffer a greater degradation and loss of nutrients when exposed to the elements. In the previous section, the PLA-SiO2 system presented a high permeability value to water vapor; possibly, this generates a greater transfer of oxygen and components that accelerate the metabolism of organic acids and significantly affect the quality of the apples. PLA-M1 and PLA-M2 packaging slowed down the degradation of malic acid in apples, which could be attributed to the antioxidant properties of CEO. This behavior was observed in the study of Lan et al. [117], who formulated polyvinyl alcohol composite films with polyphenol Tea for strawberry storage. The same occurred in the study of Zhang et al. [86], who developed chitosan films for apple storage.
As for the evaluation of pH, it was determined during the storage of the apple. Each sample was measured during the 21 days, as shown in Figure 13c. Initially, the control sample presented a pH value of 3.09; as the days passed, a slight increase in pH of 4.08 was observed at the storage end; the results are summarized in Table 5. As for the samples deposited in the developed containers, it can be observed that they followed the same behavior with slight increases, for the sample stored in the PLA-M1 system after 7 days presented a pH of 3.66 and at the end of 4.02; likewise, for the PLA-M2 film the sample presented a pH of 3.41 and at the end of 3.98; finally, the sample stored in the PLA-SiO2 film had a significant increase in pH from 3.55 to 4.19. This could be due to the biological processes of metabolism in apples, as the degradation of organic acids and sugars causes effects on pH [118]. This behavior could be related to the TA result obtained in the sample stored in this film.
3.4.3. Color
The measurement of color is another important parameter in food since this property generates the acceptance or rejection of consuming a product. In some foods, the enzyme polyphenoloxidase (PPO) has a visible growth in the so-called enzymatic browning, where fruits that have this enzyme begin to present dark brown spots when they come into contact with air; for example, avocado, banana, pear, and apples to mention a few. In quantitative terms, it is possible to know the shades of the apple samples through three magnitudes (brightness, hue, and saturation) and observe the color difference to find out the effects caused by the enzyme polyphenol oxidase in contact with the air, which causes enzymatic browning.
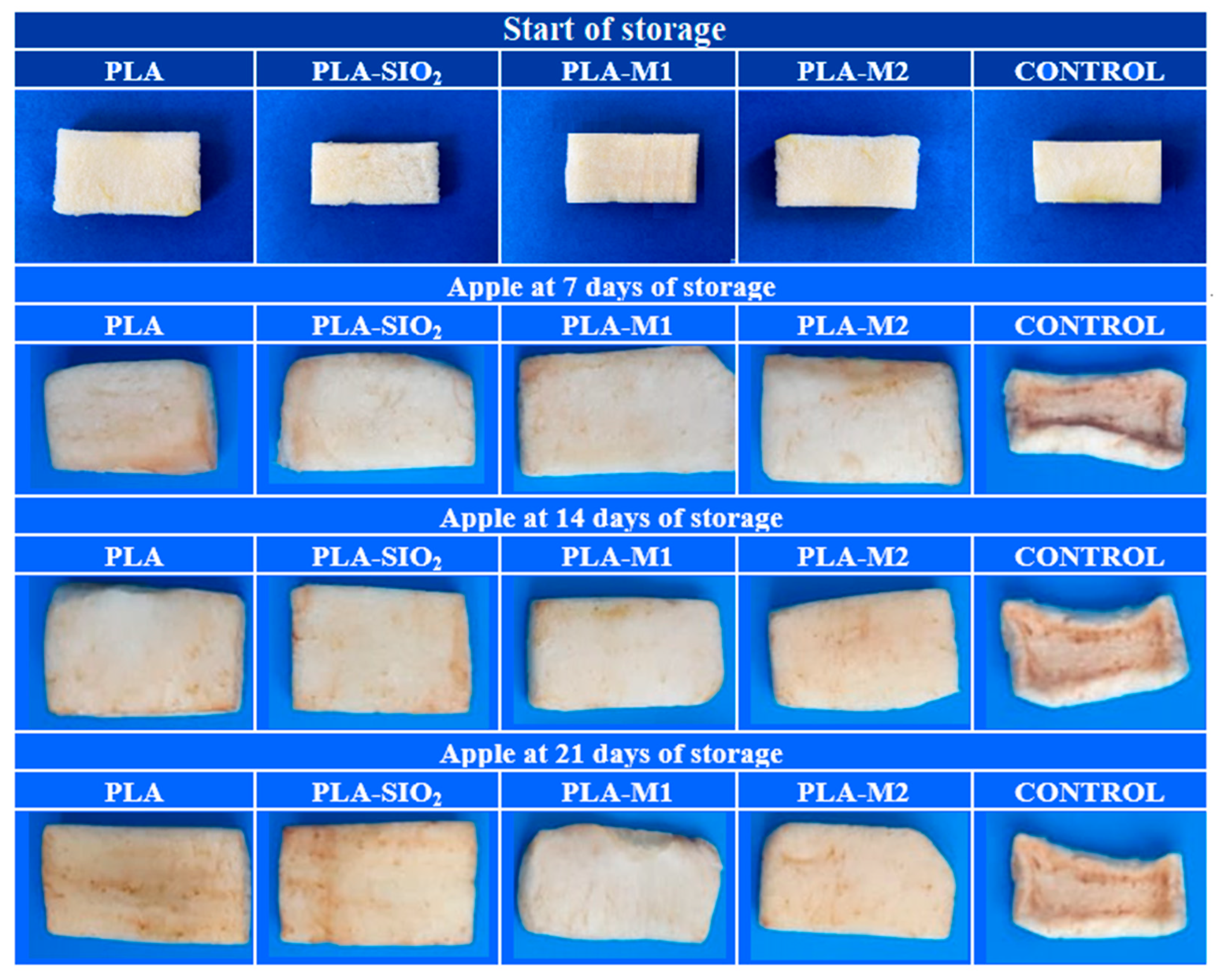
Table 4 summarizes the values obtained for the color difference, which is the greatest interest parameter at the storage end. It is possible to observe that the sample contained in the PLA-SiO2 film had a significant color difference (p < 0.05) of 20.96, very close to the value obtained by the control sample of 21.89, this could be referred that the film did not function as a good barrier between the sample and the outside, which caused a greater oxygen transfer that favored the growth of the polyphenol oxidase enzyme, in addition to the fact that pure SiO2 does not have a good antioxidant capacity to inhibit the growth of the enzyme. In contrast, the samples stored in PLA-M1 and PLA-M2 films had a color difference of 5.59 and 10.47, respectively, these results are very favorable for the preservation of apples, so it is possible to ensure that the essential oil of cinnamon helps to inhibit the growth of the enzyme due to its antioxidant properties that were captured in the SiO2 nanoparticles and maintained in the PLA matrix. In Figure 13d, it is possible to appreciate these results graphically and visually in the photos of Figure 14, where it can be seen the texture and appearance of the samples.
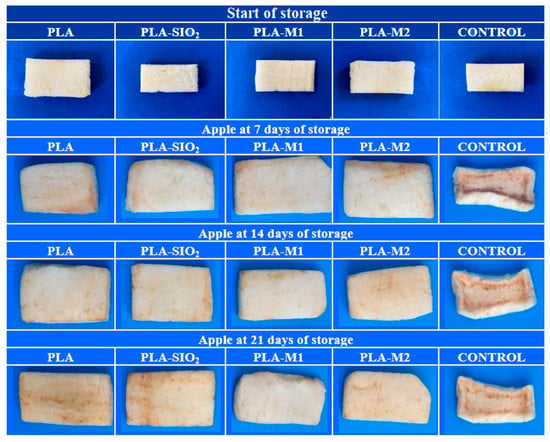
Figure 14.
Visual appearance of apples stored for 21 days.
4. Conclusions
In this study, SiO2 nanoparticles were chemically modified with cinnamon essential oil by applying two methods (M1 and M2) to achieve antioxidant and antimicrobial properties. This was confirmed by FTIR and TGA analysis, which showed the presence of the cinnamaldehyde group, typical of cinnamon essential oil. The modified nanoparticles were found to be non-toxic and to have the ability to inhibit the growth of Gram-positive and Gram-negative bacteria, making them suitable for food applications. With the modified nanoparticles, PLA films were developed, and significant changes were observed; the films became more flexible when SiO2-M1 nanoparticles were added, causing the material to increase its elongation by 50%; this led to low tensile strength and Young’s modulus. The same occurred when SiO2-M2 nanoparticles were added. However, when pure SiO2 nanoparticles were added, the film became more rigid and difficult to handle, demonstrating that cinnamon essential oil functions as a plasticizer. The addition of SiO2, SiO2-M1, and SiO2-M2 nanoparticles decreased the percentage of water solubility of the films, so they may be less susceptible to degradation. In addition, they did not affect the molecular structure of PLA and slightly reduced the percentage of crystallinity of the polymer. As for permeability, slight increases were obtained, making the films more porous, which facilitated the diffusion of water vapor and the wettability of the films, generating contact angles of less than 90°. The PLA- SiO2 film was the most permeable (8.45 × 10–8 g Pa−1 h−1 m−2), which influenced the preservation of the apples by accelerating their deterioration. The SiO2-M1 and SiO2-M2 nanoparticles transferred antioxidant properties to the films, which was useful for the preservation of apples since they maintained a lower loss of weight, color, titratable acidity, and pH during 21 days of storage, so these materials could be used for the preparation of active packaging.
Author Contributions
Conceptualization, V.M.-A. and J.A.G.-C.; Formal analysis, V.M.-A., M.G.P.-J. and P.C.C.-S.; Funding acquisition, A.L.H.-M.; Methodology, V.M.-A. and N.L.F.-M.; Supervision, L.L.-Z., E.D.-A. and E.J.G.-C.; Validation, A.L.H.-M. and J.A.G.-C.; Writing—original draft, V.M.-A.; Writing—review and editing, M.G.P.-J. and J.A.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
J.A. Gonzalez-Calderon thanks CONACYT for supporting the Catedras-Conacyt Program, and Verónica Martinez thanks CONACYT for the Doctoral Fellowship.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Universidad Autónoma de San Luis Potosí (code number 33290, approved date 14 December 2020).
Informed Consent Statement
Informed consent was obtained from author Mariana G. Peña-Juárez who donated her blood for this study.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors acknowledge Claudia Hernández and Rosa Lina Tovar for their support during the XRD and SEM analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic flexible films waste management—A state of the art review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.R.; Pereira, M.F.; Figueiredo, J.L. Towards Controlled Degradation of Poly(lactic) Acid in Technical Applications. J. Carbon Res. 2021, 7, 42. [Google Scholar] [CrossRef]
- Guo, Z.; Boeing, W.J.; Xu, Y.; Borgomeo, E.; Mason, S.A.; Zhu, Y.-G. Global meta-analysis of microplastic contamination in reservoirs with a novel framework. Water Res. 2021, 207, 117828. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Natural antioxidants-based edible active food packaging: An overview of current advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Richard, C.; Kay, K.M.; Song, J. Bioplastics. In Food and Beverage Packaging Technology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; p. 295. [Google Scholar]
- Sun, M.; Zhang, L.; Li, C. Modified cellulose nanocrystals based on SI-ATRP for enhancing interfacial compatibility and mechanical performance of biodegradable PLA / PBAT blend. Polym. Compos. 2022, 43, 3753–3764. [Google Scholar] [CrossRef]
- Yusoff, N.H.; Pal, K.; Narayanan, T.; de Souza, F.G. Recent trends on bioplastics synthesis and characterizations: Polylactic acid (PLA) incorporated with tapioca starch for packaging applications. J. Mol. Struct. 2021, 1232, 129954. [Google Scholar] [CrossRef]
- Radusin, T.; Tomšik, A.; Šari’c, L.; Risti’c, I.; Giacinti Baschetti, M.; Minelli, M.; Novakovi’c, A. Hybrid Pla/wild garlic anti-microbial composite films for food packaging application. Polym. Compos. 2019, 40, 893–900. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of bioactive functional poly(lactic acid)/curcumin composite film for food packaging application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef]
- Fiore, A.; Park, S.; Volpe, S.; Torrieri, E.; Masi, P. Active packaging based on PLA and chitosan-caseinate enriched rosemary essential oil coating for fresh minced chicken breast application. Food Packag. Shelf Life 2021, 29, 100708. [Google Scholar] [CrossRef]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Coutinho, H.D.M.; et al. Combination of essential oils in dairy products: A review of their functions and potential benefits. LWT-Food Sci. Technol. 2020, 133, 110116. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2020, 152, 104620. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Essential Oils: A Natural Alternative to Combat Antibiotics Resistance. In Antibiotic Resistance; Kon, K., Rai, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 227–237. [Google Scholar] [CrossRef]
- Ruiz, B.; Flotats, X. Citrus essential oils and their influence on the anaerobic digestion process: An overview. Waste Manag. 2014, 34, 2063–2079. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical Composition and Antioxidant Properties of Clove Leaf Essential Oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crop. Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Rao, P.V.; Gan, S.H. Cinnamon: A Multifaceted Medicinal Plant. Evidence-Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, F. Antimicrobial, antibiotic potentiating activity and phytochemical profile of essential oils from exotic and endemic medicinal plants of Mauritius. Ind. Crop. Prod. 2015, 71, 197–204. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Guinoiseau, E.; Luciani, A.; Rossi, P.G.; Quilichini, Y.; Ternengo, S.; Bradesi, P.; Berti, L. Cellular effects induced by Inula graveolens and Santolina corsica essential oils on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 873–879. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Ejaz, M.; Mullah, M. Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int. J. Biol. Macromol. 2018, 107, 194–203. [Google Scholar] [CrossRef]
- Javidi, Z.; Hosseini, S.F.; Rezaei, M. Development of flexible bactericidal films based on poly(lactic acid) and essential oil and its effectiveness to reduce microbial growth of refrigerated rainbow trout. LWT 2016, 72, 251–260. [Google Scholar] [CrossRef]
- Celebi, H.; Gunes, E. Combined effect of a plasticizer and carvacrol and thymol on the mechanical, thermal, morphological properties of poly(lactic acid). J. Appl. Polym. Sci. 2018, 135, 45895. [Google Scholar] [CrossRef]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Muller, J.; Quesada, A.C.; González-Martínez, C.; Chiralt, A. Antimicrobial properties and release of cinnamaldehyde in bilayer films based on polylactic acid (PLA) and starch. Eur. Polym. J. 2017, 96, 316–325. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Vidal, J.; Dicastillo, C.; Rodriguez, F.; Guarda, A.; Cruz, R.M.S.; Galotto, M.J. Development of poly(lactic acid) films with propolis as a source of active compounds: Biodegradability, physical, and functional properties. J. Appl. Polym. Sci. 2018, 136, 47090. [Google Scholar] [CrossRef]
- Zeid, A.; Karabagias, I.K.; Nassif, M.; Kontominas, M.G. Preparation and evaluation of antioxidant packaging films made of polylactic acid containing thyme, rosemary, and oregano essential oils. J. Food Process. Preserv. 2019, 43, e14102. [Google Scholar] [CrossRef]
- Kawai, T.; Rahman, N.; Matsuba, G.; Nishida, K.; Kanaya, T.; Nakano, M.; Okamoto, H.; Kawada, J.; Usuki, A.; Honma, N.; et al. Crystallization and Melting Behavior of Poly (l-lactic Acid). Macromolecules 2007, 40, 9463–9469. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-Order Phase Transition and Multiple Melting Behavior of Poly(l-lactide) Investigated by Simultaneous Measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Sharma, S.; Byrne, M.; Perera, K.Y.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Active film packaging based on bio-nanocomposite TiO2 and cinnamon essential oil for enhanced preservation of cheese quality. Food Chem. 2023, 405, 134798. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Z.; Zhang, C.; Zhai, X.; Zhang, X.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; Shi, J. Chitosan-cinnamon essential oil/sodium alginate-TiO2 bilayer films with enhanced bioactive retention property: Application for mango preservation. Int. J. Biol. Macromol. 2022, 222, 2843–2854. [Google Scholar] [CrossRef]
- Negahdari, M.; Partovi, R.; Talebi, F.; Babaei, A.; Abdulkhani, A. Preparation, characterization, and preservation performance of active polylactic acid film containing Origanum majorana essential oil and zinc oxide nanoparticles for ground meat packaging. J. Food Process. Preserv. 2020, 45, e15013. [Google Scholar] [CrossRef]
- Palma-Ramírez, D.; Torres-Huerta, A.; Domínguez-Crespo, M.; Ponce-Hernández, J.; Brachetti-Sibaja, S.; Rodríguez-Salazar, A.; Urdapilleta-Inchaurregui, V. An assembly strategy of polylactic acid (PLA)-SiO2 nanocomposites embedded in polypropylene (PP) matrix. J. Mater. Res. Technol. 2021, 14, 2150–2164. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B. Active Packaging. In Micro and Nano Technologies, Nanomaterials for Food Packaging; Cerqueira, M.Â.P.R., Lagaron, J.M., Castro, L.M.P., de Oliveira Soares Vicente, A.A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 173–202. ISBN 9780323512718. [Google Scholar] [CrossRef]
- Famil Zirak, M.; Tabari, M. PLA-SiO2 nanocomposite films: Morphological and mechanical properties and specific end-use characteristics. Nanomed. Res. J. 2018, 3, 140–145. [Google Scholar] [CrossRef]
- Martínez-Aguilar, V.; Carrillo-Sanchez, P.C.; Del Angel-Monroy, M.; Balderas, G.S.; Flores-Martínez, N.L.; Pérez, E.; González-Calderón, J.A. Chemical modification of TiO2 with essential oils for its application in active packaging. Polym. Bull. 2022, 80, 2753–2778. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Norma ASTM 96-95; Standard Test Methods for Water Vapor Transmission of Materials. American Society for Testing and Materials: Philadelphia, PA, USA, 2009.
- Han, Y.; Wang, L. Sodium alginate/carboxymethyl cellulose films containing pyrogallic acid: Physical and antibac-terial properties. J. Sci. Food Agric. 2017, 97, 1295–1301. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.-F.; Rhim, J.-W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef]
- ASTM D882-18; D882: Standard Test Method for Tensile Properties of Thin Plastic Sheeting. Astm: West Conshohocken, PA, USA, 2018; Volume 14, pp. 1–10.
- Ma, B.; Wang, X.; He, Y.; Dong, Z.; Zhang, X.; Chen, X.; Liu, T. Effect of poly(lactic acid) crystallization on its me-chanical and heat resistance performances. Polymer 2020, 212, 123280. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Boughendjioua, H. Les Plantes Médicinales Utilisées Pour les Soins de la Peau. Composition Chimique, Activité Antioxy-Dante et Antimicrobienne des Huiles Essentielles de Citrus Limon, Cinnamomum Zeylanicum et Thymus Numidicus. Ph.D. Thesis, Université Badji Moktar de Annaba, Annaba, Algeria, 2014. Volume 144. pp. 125–130. [Google Scholar]
- Cao, T.L.; Song, K.B. Effects of gum karaya addition on the characteristics of loquat seed starch films containing oregano es-sential oil. Food Hydrocoll. 2019, 97, 105198. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Zheng, X.; Chen, M.; Tang, K. Soluble soybean polysaccharide/nano zinc oxide antimicrobial nanocomposite films reinforced with microfibrillated cellulose. Int. J. Biol. Macromol. 2020, 159, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, X.; Chen, J.; He, J. Effects of cinnamon essential oil on the physical, mechanical, structural and thermal properties of cassava starch-based edible films. Int. J. Biol. Macromol. 2021, 184, 574–583. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Mufida, N.; Panatarani, C.; Joni, I. Enhanced multi functionality of semi-refined iota carrageenan as food packaging material by incorporating SiO2 and ZnO nanoparticles. Heliyon 2021, 7, e06963. [Google Scholar] [CrossRef]
- Feng, K.; Wen, P.; Yang, H.; Li, N.; Lou, W.Y.; Zong, M.H.; Wu, H. Enhancement of the antimicrobial activity of cinnamon essential oil-loaded electrospun nanofilm by the incorporation of lysozyme. RSC Adv. 2017, 7, 1572–1580. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Wu, H.; Zong, M.-H.; Jing, Y.-R.; Han, S.-Y. Encapsulation of cinnamon essential oil in elec-trospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Xu, T.; Gao, C.; Feng, X.; Huang, M.; Yang, Y.; Shen, X.; Tang, X. Cinnamon and clove essential oils to improve physical, thermal and antimicrobial properties of chitosan-gum arabic polyelectrolyte complexed films. Carbohydr. Polym. 2019, 217, 116–125. [Google Scholar] [CrossRef]
- Amiri, E.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R. Combined antioxidant and sensory effects of corn starch films with nanoemulsion of Zataria multiflora essential oil fortified with cinnamaldehyde on fresh ground beef patties. Meat Sci. 2019, 153, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Arslan, D. Antioxidant effect of essential oils of rosemary, clove and cinnamon on hazelnut and poppy oils. Food Chem. 2011, 129, 171–174. [Google Scholar] [CrossRef]
- Guo, J.; Yang, R.; Gong, Y.; Hu, K.; Hu, Y.; Song, F. Optimization and evaluation of the ultrasound-enhanced subcritical water extraction of cinnamon bark oil. LWT 2021, 147, 111673. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Vertuccio, L.; Vittoria, V.; Pace, B.; Cefola, M.; Quintieri, L.; Bernardo, P.; Clarizia, G. Active packaging for table grapes: Evaluation of antimicrobial performances of packaging for shelf life of the grapes under thermal stress. Food Packag. Shelf Life 2020, 25, 100545. [Google Scholar] [CrossRef]
- Tirado, L.J.P.C.; Ferreira, R.S.B.; Lizárraga, E.; Tapia-Blácido, D.R.; Silva, N.; Angelats-Silva, L.; Siche, R. Bioactive Andean sweet potato starch-based foam incorporated with oregano or thyme essential oil. Food Packag. Shelf Life 2020, 23, 100457. [Google Scholar] [CrossRef]
- Aminzare, M.; Hashemi, M.; Hassanzadazar, H.; Amiri, E.; Abbasi, Z. Antibacterial Activity of Corn Starch Films Incorporated with Zataria multiflora and Bonium persicum Essential Oils. Annu. Res. Rev. Biol. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Nemattalab, M.; Rohani, M.; Evazalipour, M.; Hesari, Z. Formulation of Cinnamon (Cinnamomum verum) oil loaded solid lipid nanoparticles and evaluation of its antibacterial activity against Multi-drug Resistant Escherichia coli. BMC Complement. Med. Ther. 2022, 22, 289. [Google Scholar] [CrossRef]
- Jabir, M.S.; Taha, A.A.; Sahib, U.I. Linalool loaded on glutathione-modified gold nanoparticles: A drug delivery system for a successful antimicrobial therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 345–355. [Google Scholar] [CrossRef]
- Elghobashy, S.A.; Mohammed, A.B.A.; Tayel, A.A.; Alshubaily, F.A.; Abdella, A. Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities. E-Polymers 2022, 22, 997–1006. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial packaging efficiency of ZnO-SiO2 nanocomposites infused into PVA/CS film for enhancing the shelf life of food products. Food Packag. Shelf Life 2020, 25, 100523. [Google Scholar] [CrossRef]
- Wang, N.; Yu, J.; Ma, X. Preparation and characterization of compatible thermoplastic dry starch/poly(lactic acid). Polym. Compos. 2008, 29, 551–559. [Google Scholar] [CrossRef]
- Shameli, K.; Bin, M.; Ahmad WMZWYunus, N.A.; Ibrahim, R.A.; Rahman, M.; Jokar, M.; Darroudi, M. Silver/poly (lactic acid) nanocomposites: Preparation, characterization, and antibacterial activity. Int. J. Nanomed. 2010, 5, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Pan, L.; Tang, C.Y.; Chen, L.; Hao, Z.; Law, W.C.; Wang, X.; Tsui, C.P.; Wu, C. Preparation, optical and thermal properties of CdSe–ZnS/poly(lactic acid) (PLA) nanocomposites. Compos. Part B Eng. 2014, 66, 494–499. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Rheological, morphological, and interfacial properties of compatibilized PLA/PBAT blends. Rheol. Acta 2014, 53, 501–517. [Google Scholar] [CrossRef]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Mohamad, N.; Mazlan, M.M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Fouad, H.; Jawaid, M. Development of active agents filled polylactic acid films for food packaging application. Int. J. Biol. Macromol. 2020, 163, 1451–1457. [Google Scholar] [CrossRef]
- Valenzuela, C.; Abugoch, L.; Tapia, C. Quinoa protein–chitosan–sunflower oil edible film: Mechanical, barrier and structural properties. LWT 2013, 50, 531–537. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.Z.; Vahora, A.; Bher, A.; Auras, R. Polylactide/graphene nanoplatelets composite films: Impact of high-pressure on topography, barrier, thermal, and mechanical properties. Polym. Compos. 2021, 42, 2898–2909. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crop. Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, B.; Wang, J.; Qin, Y. Structural changes and nano-TiO2 migration of poly(lactic acid)-based food packaging film contacting with ethanol as food simulant. Int. J. Biol. Macromol. 2019, 139, 85–93. [Google Scholar] [CrossRef]
- Alam, M.W.; Khalid, N.R.; Naeem, S.; Niaz, N.A.; Mir, T.A.; Nahvi, I.; Souayeh, B.; Zaidi, N. Novel Nd-N/TiO2 Nanoparticles for Photocatalytic and Antioxidant Applications Using Hydrothermal Approach. Materials 2022, 15, 6658. [Google Scholar] [CrossRef]
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple peel extract incorporated poly(vinyl alcohol)-corn starch film for active food packaging: Preparation, characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar] [CrossRef]
- Mariño-Cortegoso, S.; Stanzione, M.; Andrade, M.A.; Restuccia, C.; de Quirós, A.R.-B.; Buonocore, G.G.; Barbosa, C.H.; Vilarinho, F.; Silva, A.S.; Ramos, F.; et al. Development of active films utilizing antioxidant compounds obtained from tomato and lemon by-products for use in food packaging. Food Control 2022, 140, 109128. [Google Scholar] [CrossRef]
- Agustinelli, S.P.; Ciannamea, E.M.; Ruseckaite, R.A.; Martucci, J.F. Migration of red grape extract components and glycerol from soybean protein concentrate active films into food simulants. Food Hydrocoll. 2021, 120, 106955. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. A comprehensive review on the controlled release of encapsulated food ingredients; fundamental concepts to design and applications. Trends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Ortega, F.; Sobral, P.; Jios, J.L.; Arce, V.B.; García, M.A. Starch Nanocomposite Films: Migration Studies of Nanoparticles to Food Simulants and Bio-Disintegration in Soil. Polymers 2022, 14, 1636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, W. Antioxidant and antibacterial chitosan film with tea polyphenols-mediated green synthesis silver nanoparticle via a novel one-pot method. Int. J. Biol. Macromol. 2019, 155, 1252–1261. [Google Scholar] [CrossRef]
- Montero, Y.; Souza, A.G.; Oliveira, E.R.; dos Santos Rosa, D. Nanocellulose functionalized with cinnamon essential oil: A po- 959 tential application in active biodegradable packaging for strawberry. Sustain. Mater. Technol. 2021, 29, e00289. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef]
- Hadi, A.; Nawab, A.; Alam, F.; Zehra, K. Sustainable alginate/aloe vera composite biodegradable films reinforced with carboxymethyl cellulose and hydroxypropyl methylcellulose. Polym. Compos. 2022, 43, 3471–3480. [Google Scholar] [CrossRef]
- Pirouzifard, M.; Yorghanlu, R.A.; Pirsa, S. Production of active film based on potato starch containing Zedo gum and essential oil of Salvia officinalis and study of physical, mechanical, and antioxidant properties. J. Thermoplast. Compos. Mater. 2020, 33, 915–937. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Deng, Y.; Liu, T.; Lu, D. Effect of starch film containing thyme essential oil microcapsules on physicochemical activity of mango. LWT-Food Sci. Technol. 2020, 131, 109700. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Yu, M.; Zhao, S.; Yang, L.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Preparation of a superhydrophilic SiO2 nanoparticles coated chitosan-sodium phytate film by a simple ethanol soaking process. Carbohydr. Polym. 2021, 271, 118422. [Google Scholar] [CrossRef]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M.A.; Hosseini, M. A new active nanocomposite film based on PLA/ZnO nanoparticle/essential oils for the preservation of refrigerated Otolithes ruber fillets. Food Packag. Shelf Life 2019, 19, 94–103. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Shakoori, P.; Arab-Bafrani, Z. Design and characterization of keratin/PVA-PLA nanofibers containing hybrids of nanofibrillated chitosan/ZnO nanoparticles. Int. J. Biol. Macromol. 2021, 187, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Gangil, B.; Ranakoti, L.; Joshi, A. Effect of silica nanoparticles on physical, mechanical, and wear properties of natural fiber reinforced polymer composites. Polym. Compos. 2021, 42, 2396–2407. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Wang, S.; Qin, W.; Zhang, Q. Electrospun Antimicrobial Polylactic Acid/Tea Polyphenol Nanofibers for Food-Packaging Applications. Polymers 2018, 10, 561. [Google Scholar] [CrossRef]
- Shao, P.; Yu, J.; Chen, H.; Gao, H. Development of microcapsule bioactive paper loaded with cinnamon essential oil to improve the quality of edible fungi. Food Packag. Shelf Life 2021, 27, 100617. [Google Scholar] [CrossRef]
- Chollakup, R.; Pongburoos, S.; Boonsong, W.; Khanoonkon, N.; Kongsin, K.; Sothornvit, R.; Sukyai, P.; Sukatta, U.; Harnkarnsujarit, N. Antioxidant and antibacterial activities of cassava starch and whey protein blend films containing rambutan peel extract and cinnamon oil for active packaging. LWT 2020, 130, 109573. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Properties and antimicrobial activity offish protein isolate/fish skin gelatin film containing basil leafessential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014, 41, 265–273. [Google Scholar] [CrossRef]
- Bagheri, F.; Radi, M.; Amiri, S. Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocoll. 2019, 90, 162–171. [Google Scholar] [CrossRef]
- Martins, C.; Vilarinho, F.; Silva, A.S.; Andrade, M.; Machado, A.V.; Castilho, M.C.; Sá, A.; Cunha, A.; Vaz, M.F.; Ramos, F. Active polylactic acid film incorporated with green tea extract: Development, characterization and effectiveness. Ind. Crops Prod. 2018, 123, 100–110. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos. Part B Eng. 2019, 176, 107336. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Castro-Aguirre, E.; Auras, R. Mechanical, structural and thermal properties of Ag–Cu and ZnO reinforced polylactide nanocomposite films. Int. J. Biol. Macromol. 2016, 86, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.L.; Yang, S.Y.; Bin Song, K. Characterization of barnyard millet starch films containing borage seed oil. Coatings 2017, 7, 183. [Google Scholar] [CrossRef]
- Vianna, T.C.; Marinho, C.O.; Júnior, L.M.; Ibrahim, S.A.; Vieira, R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef]
- Brancewicz-Steinmetz, E.; Sawicki, J.; Byczkowska, P. The Influence of 3D Printing Parameters on Adhesion between Polylactic Acid (PLA) and Thermoplastic Polyurethane (TPU). Materials 2021, 14, 6464. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties of polylactic acid films containing thymol incorporated by supercritical impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Zhang, R.; Lan, W.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Liu, Y. Development of polylactic acid/ZnO composite membranes prepared by ultrasonication and electrospinning for food packaging. LWT 2020, 135, 110072. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Jędra, F.; Mizielińska, M. New Poly(lactic acid) Active Packaging Composite Films Incorporated with Fungal Melanin. Polymers 2018, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M.; Paul, U.C.; Zia, J.; Mancini, G.; Sedlarik, V.; Athanassiou, A. Biodegradable Films of PLA/PPC and Curcumin as Packaging Materials and Smart Indicators of Food Spoilage. ACS Appl. Mater. Interfaces 2022, 14, 14654–14667. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Wang, Y.; Li, M.; Jin, Y.; Han, Y.; Dong, T. Application of permselective poly(ε-caprolactone) film for equi-librium-modified atmosphere packaging of strawberry in cold storage. J. Food Process. Preserv. 2017, 41, e13247. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Mi, H. Characterization of sodium alginate-based films in-corporated with thymol for fresh-cut apple packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Rosa, G.B.; Ferreira AL, A.; da Rosa, C.G.; Beling, P.C.; Xavier, L.O.; de Lima Veeck, A.P. Bioactive food packaging based on starch, citric pectin and functionalized with Acca sellowiana waste by-product: Characte-rization and application in the postharvest conservation of apple. Int. J. Biol. Macromol. 2020, 147, 295–303. [Google Scholar] [CrossRef]
- Domene, M.; Rodríguez, M. Parámetros de Calidad Interna en Hortalizas y Frutas en la Industria Agroalimentaria. Neg. Agroaliment. Cooper Cajamar Fichas Transf. 2014, 1, 1–18. Available online: http://www.fundacioncajamar.es/pdf/bd/comun/transferencia/005-calidadinterna1410512030.pdf (accessed on 10 January 2023).
- Ban, S.; Xu, K. Identification of two QTLs associated with high fruit acidity in apple using pooled genome sequencing analysis. Hortic. Res. 2020, 7, 171. [Google Scholar] [CrossRef]
- Xu, K.; Wang, A.; Brown, S. Genetic characterization of the Ma locus with pH and titratable acidity in apple. Mol. Breed. 2011, 30, 899–912. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, R.; Ahmed, S.; Qin, W.; Liu, Y. Effects of various antimicrobial polyvinyl alcohol/tea polyphenol composite films on the shelf life of packaged strawberries. LWT 2019, 113, 108297. [Google Scholar] [CrossRef]
- Kader, A.A.; Ben-Yehoshua, S. Effects of superatmospheric oxygen levels on postharvest physiology and quality of fresh fruits and vegetables. Postharvest Biol. Technol. 2000, 20, 1–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).