Different Extraction Procedures Revealed the Anti-Proliferation Activity from Vegetable Semi-Purified Sources on Breast Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
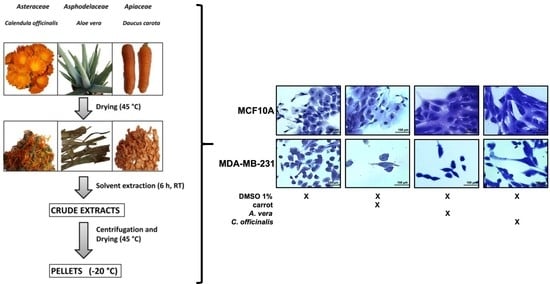
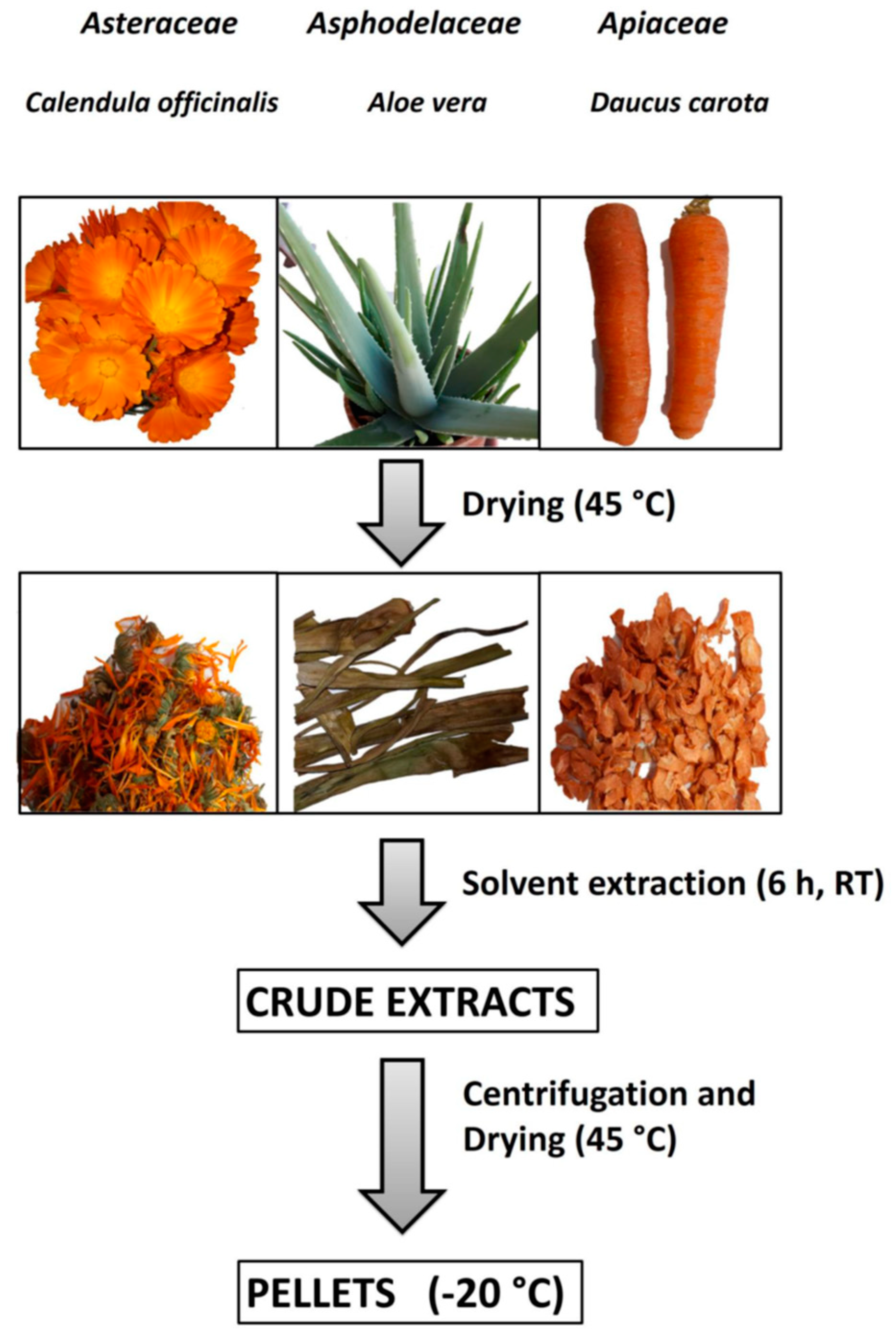
2.2. Sample Preparation and Storage
2.3. Extract Preparation
2.4. Extract Purification
2.5. Chemical Screening for Bioactive Substances
2.6. UHPLC-HRMS and MS/MS Analysis of Semi-Purified Fractions
2.7. Cell culture, Staining, and Proliferation Assay
3. Results
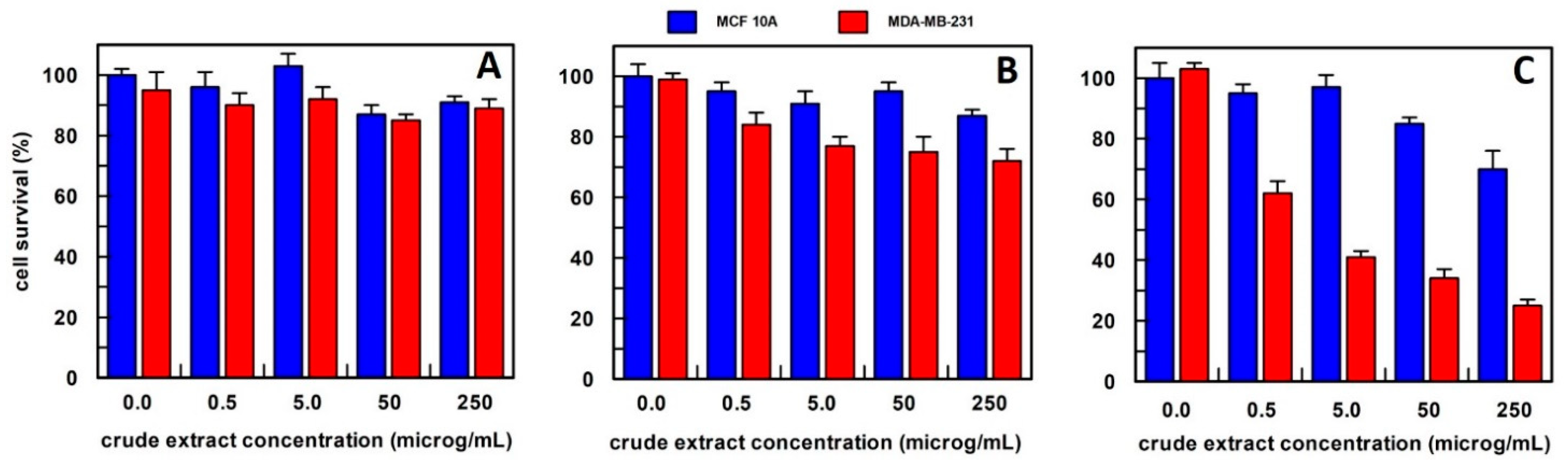
3.1. Extract Preparation and Analysis of Their Biological Activity
3.2. Extract Semi-Purification, Colorimetric Assays, and Chemical Profiling by UHPLC-HRMS
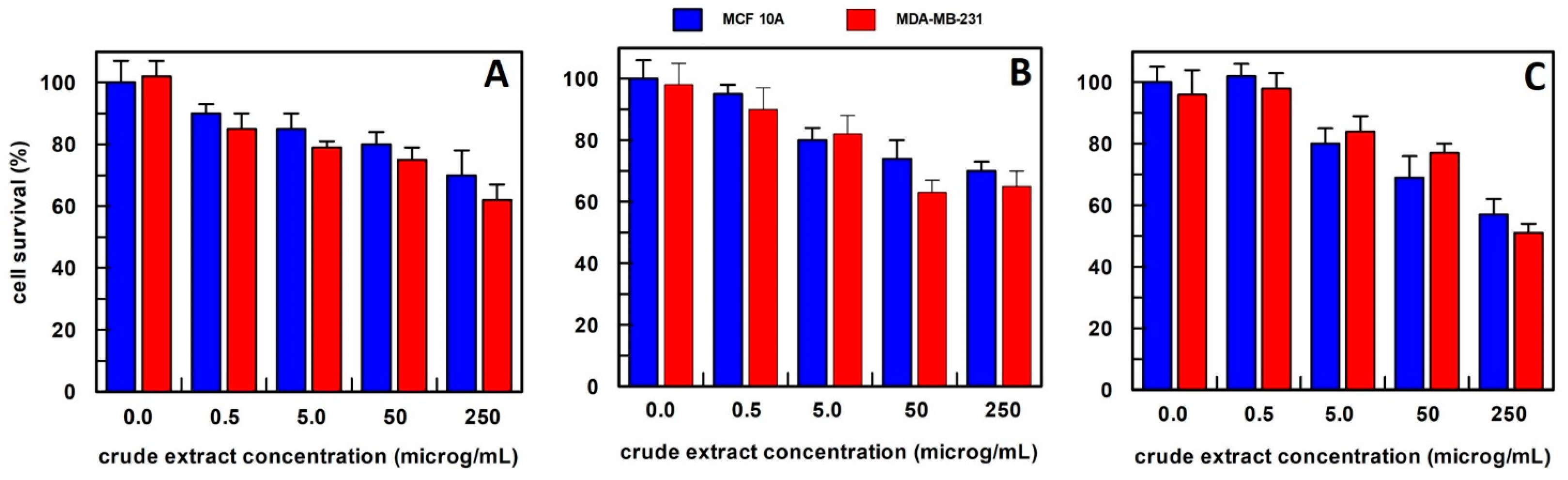
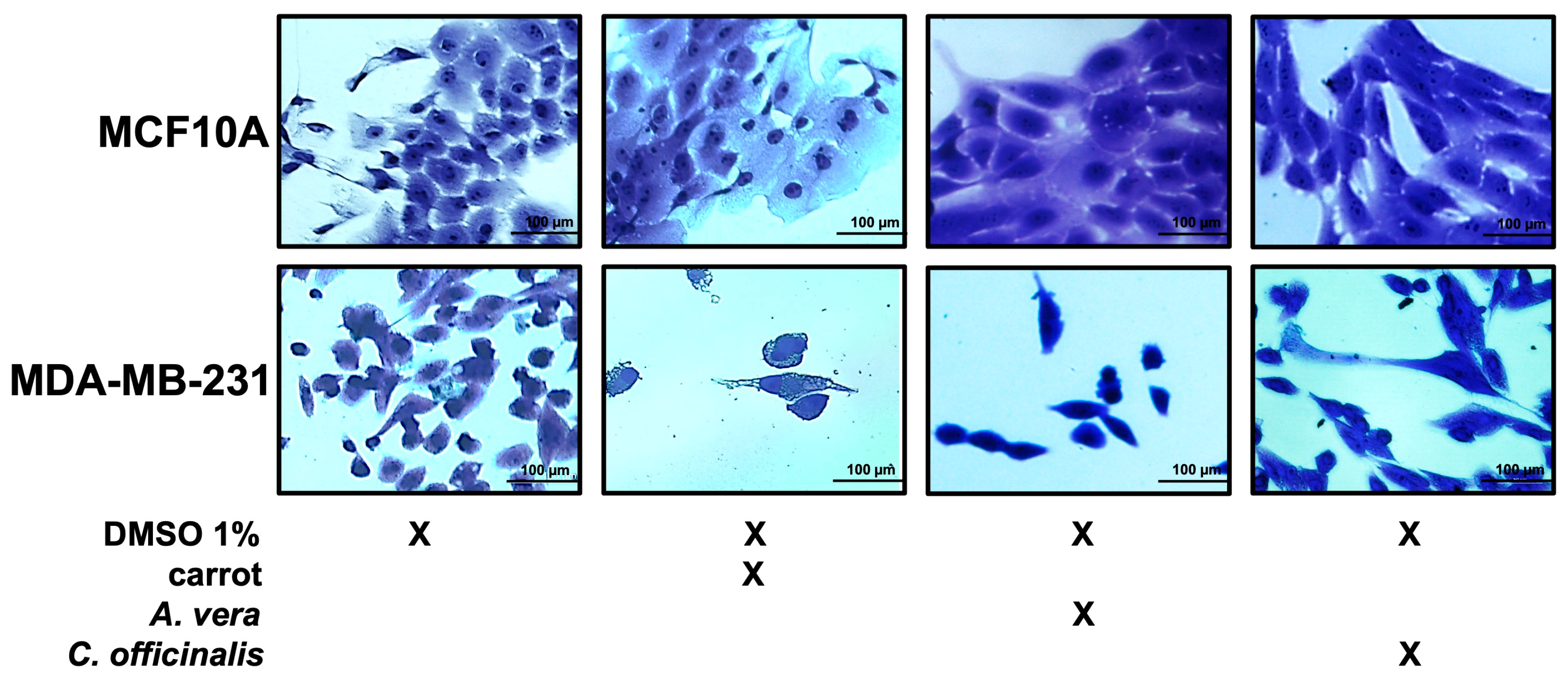
3.3. Semi-Purified Extract Biological Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alkabban, F.M.; Ferguson, T. Breast Cancer. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022; pp. 1–15. [Google Scholar]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17 (Suppl. S3), 43–46. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Drugs: Their Natural, Synthetic, and Biosynthetic Sources. In Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1. Progress in Drug Research; Springer: Berlin/Heidelberg, Germany, 2017; Volume 73. [Google Scholar]
- Rates, S.M.K. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Seidel, V. Plant-Derived Chemicals: A Source of Inspiration for New Drugs. Plants 2020, 9, 1562. [Google Scholar] [CrossRef]
- Gescher, A.; Pastorino, U.; Plummer, S.M.; Manson, M.M. Suppression of tumour development by substances derived from the diet—Mechanisms and clinical implications. Br. J. Clin. Pharmacol. 1998, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.; Lamy, S.; Lord-Dufur, S.; Jackson, J.; Beaulieu, E.; Cotè, M.; Moghrabi, A.; Barrette, S.; Gingras, D.; Beliveau, R. Anti-proliferative and antioxidant activities of common vegetables: A comparative study. Food Chem. 2009, 112, 374–380. [Google Scholar] [CrossRef]
- Soundrarajan, P.; Kim, J.S. Anti-carcinogenic glucosinolates in Cruciferous vegetables and their antagonistic effects on prevention of cancer. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef]
- Mandrich, L.; Caputo, E. Brassicaceae-Derived Anticancer Agents: Towards a Green Approach to Beat Cancer. Nutrients 2020, 12, 868. [Google Scholar] [CrossRef]
- NasriShebaby, W.; El-Sibai, M.; Bodman-Smith, K.; Karam, M.C.; Mroueh, M.; Daher, C.F. The Antioxidant and anticancer effect of wild carrot oil extract. Phytother. Res. 2012, 27, 737–744. [Google Scholar]
- Liu, R.; Choi, H.S.; Kim, S.-L.; Kim, J.-H.; Yun, B.-S.; Lee, D.-S. 6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling. Molecules 2020, 25, 4374. [Google Scholar] [CrossRef]
- Akihisa, T.; Yasukawa, K.; Oinuma, H.; Kasahara, Y.; Yamanouchi, S.; Takido, M.; Kumasi, K.; Tamura, T. Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry 1996, 43, 1255–1260. [Google Scholar] [CrossRef]
- Della Logia, R.; Tubaro, A.; Sosa, A.; Becker, H.; Saar, S.; Isaac, O. The role of triterpenoids in the topical anti-inflammatory activity of Calendula officinalis flowers. Planta Med. 1994, 60, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Kalvatchev, Z.; Walder, R.; Garzaro, D. Anti-HIV activity of extracts from Calendula officinalis flowers. Biomed. Pharmacother. 1997, 51, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Perez-Carreon, J.I.; Cruz-Jimenez, G.; Licea-Vega, J.A.; Arce-Popoca, E.; Fattel-Fazenda, S.; Villa-Trevino, S. Genotoxic and anti-genotoxic properties of Calendula officinalis extracts in rat liver cell cultures treated with diethylnitrosamine. Toxicol. In Vitro 2002, 16, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Medina, E.; Garcia-Lora, A.; Paco, L.; Algarra, I.; Collado, A.; Garrido, F. A new extract of the plant Calendula officinalis produces a dual in vitro effect: Cytotoxic anti-tumor activity and lymphocyte activation. BMC Cancer 2006, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Abutaha, N.; Nasr, F.A.; Al-Zahani, M.; Semlali, A.H.; Al-Mekhlafi, F.A.; Wadaan, M.A. Calendula arvensis L. as an anti-cancer agent against breast cancer cell lines. Mol. Biol. Rep. 2019, 46, 2187–2196. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules 2008, 3, 1599–1616. [Google Scholar] [CrossRef]
- Nejatzadeh-Barandozi, F. Antibacterial activities and antioxidant capacity of Aloe vera. Org. Med. Chem. Lett. 2013, 3, 5. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, D. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 3, 2073–2085. [Google Scholar] [CrossRef]
- Choi, S.; Chung, M.H. A review on the relationship between Aloe vera components and their biologic effects. Semin. Integr. Med. 2003, 1, 53–62. [Google Scholar] [CrossRef]
- Akev, N.; Turkay, G.; Can, A.; Gurel, A.; Yildiz, F.; Yardibi, H.; Ekiz, E.E.; Uzun, H. Tumour preventive effect of Aloe vera leaf pulp lectin (Aloctin I) on Ehrlich ascites tumours in mice. Phytother. Res. 2007, 21, 1070–1075. [Google Scholar] [CrossRef]
- Niciforovic, A.; Adzic, M.; Spasic, S.D.; Radojcic, M.B. Antitumor effects of a natural anthracyclineanalog (Aloin) involve altered activity of antioxidant enzymes in HeLaS3 cells. Cancer Biol. Ther. 2007, 6, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Sharma, C.; Khan, S.; Shah, K.; Haque, S. Aloe vera Inhibits Proliferation of Human Breast and Cervical Cancer Cells and Acts Synergistically with Cisplatin. Asian Pac. J. Cancer Prev. 2015, 16, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Sholz, B.; Liebezeit, G. Chemical Screening for Bioactive Substances in Culture Media of Microalgae and Cyanobacteria from Marine and Brackish Water Habitats: First Results. Pharm. Biol. 2006, 44, 544–549. [Google Scholar] [CrossRef]
- Arnao, M.B.; Casas, J.L.; del Rio, J.A.; Acosta, M.; Garcia-Canovas, F. An enzymatic colorimetric method for measuring naringin using 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) in the presence of peroxidase. Anal. Biochem. 1990, 185, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indolacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.R.; Monteiro, A.F.; do Amaral, F.R.L.; da Silva, A.S. A validated Folin-Ciocolteu method for total phenolics quantification of condensed tannin-rich acai (Euterpe oleacea Mart.) seed extract. J. Food Sci. Technol. 2021, 58, 4693–4702. [Google Scholar] [CrossRef]
- Saptarini, N.M.; Herawaati, I.E.; Permatasari, U.Y. Total flavonoids content in acidified extract of flowers and leaves of gardenia (Gardenia jasminoidesellis). Asian J. Pharm. Clin. Res. 2016, 9, 213–215. [Google Scholar]
- Lellau, T.F.; Liebezeit, G. Alkaloids, saponins and phenolic compounds in salt marsh plants from the Lower Saxonian Wadden Sea. Senckenberg. Marit. 2001, 31, 1–9. [Google Scholar] [CrossRef]
- Tong, X.; Li, M.; Li, D.; Lao, C.; Chen, J.; Xu, W.; Du, J.; Zhang, M.; Yang, X.; Li, J. Aloe vera gel extract: Safety evaluation for acute and chronic oral administration in Sprague-Dawley rats and anticancer activity in breast and lung cancer cells. J. Ethnopharmacol. 2021, 280, 114434. [Google Scholar] [CrossRef]
- Colsch, B.; Fenaille, F.; Warnet, A.; Junot, C.; Tabet, J.-C. Mechanisms governing the fragmentation of glycerophospholipids containing choline and ethanolamine polar head groups. Eur. J. Mass Spectrom. 2017, 23, 427–444. [Google Scholar] [CrossRef]
- Gravina, C.; Fiorentino, M.; Formato, M.; Pecoraro, M.T.; Piccolella, S.; Stinca, A.; Pacifico, S.; Esposito, A. LC-HR/MS Analysis of Lipophilic Extracts from Calendula arvensis (Vaill.) L. Organs: An Unexplored Source in Cosmeceuticals. Molecules 2022, 27, 8905. [Google Scholar] [CrossRef] [PubMed]
- LIPID MAPS® Structure Database (LMSD). Available online: https://lipidmaps.org/databases/lmsd/structure_search (accessed on 30 March 2023).
- Fan, J.J.; Li, C.H.; Hu, Y.J.; Chen, H.; Yang, F.Q. Comparative assessment of in vitro thrombolytic and fibrinolysis activity of four aloe species and analysis of their phenolic compounds by LC–MS. S. Afr. J. Bot. 2018, 119, 325–334. [Google Scholar] [CrossRef]
- Kaparakou, E.H.; Kanakis, C.D.; Gerogianni, M.; Maniati, M.; Vekrellis, K.; Skotti, E.; Tarantilis, P.A. Quantitative determination of aloin, anti-oxidant activity, and toxicity of Aloe vera leaf gel products from Greece. J. Sci. Food Agric. 2021, 101, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Avila, H.; Rivero, J.; Herrera, F.; Fraile, G. Cytotoxicity of a low molecular weight fraction from Aloe vera (Aloe barbadensis Miller) gel. Toxicon 1997, 35, 1423–1430. [Google Scholar] [CrossRef]
- Abedin, M.R.; Barua, S. Isolation and purification of glycoglycerolipids to induce apoptosis in breast cancer cells. Sci. Rep. 2021, 11, 1298. [Google Scholar] [CrossRef]
- Dai, J.; Shen, J.; Pan, W.; Shen, S.; Das, U.N. Effects of polyunsaturated fatty acids on the growth of gastric cancer cells in vitro. Lipids Health Dis. 2013, 12, 71. [Google Scholar] [CrossRef]
- Madhavi, N.; Das, U.N. Effect of n-6 and n-3 fatty acids on the survival of vincristine sensitive and resistant human cervical carcinoma cells in vitro. Cancer Lett. 1994, 84, 31–41. [Google Scholar] [CrossRef]
- Berquin, I.M.; Edwards, I.J.; Kridel, S.J.; Chen, Y.Q. Polyunsaturated fatty acid metabolism in prostate cancer. Cancer Metastasis Rev. 2011, 30, 295–309. [Google Scholar] [CrossRef]
- Lu, X.; Yu, H.; Ma, Q.; Shen, S.; Das, U.N. Linoleic acid suppresses colorectal cancer cell growth by inducing oxidant stress and mitochondrial dysfunction. Lipids Health Dis. 2010, 9, 106. [Google Scholar] [CrossRef]
- Singh, M.; Ding, Y.; Zhang, L.Y.; Song, D.; Gong, Y.; Adams, S.; Ross, D.S.; Wang, J.H.; Grover, S.; Doval, D.C.; et al. Distinct breast cancer subtypes in women with early-onset disease across races. Am. J. Cancer Res. 2014, 4, 337–352. [Google Scholar]




| Vegetables | Purification Level | Colorimetric Methods | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Froth Method (Saponins) | Gelatin-Salt Block Test (Tannins) | ABTS Assay (Antioxidant Activity) | Mayer’s Reagent (Alkaloids) | Salkowski’s Test (Steroids/ Terpenoids) | Folin–Ciocalteu Reagent (Phenols/Flavonoids) | Test Only for Phenols | Shinoda’s Reagent (Flavonoids) | ||
| Daucus carota (carrot) | crude extract | N.D. | N.D. | YES | YES | N.D. | YES | YES | N.D. |
| purified active fractions | N.D. | N.D. | YES | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Calendula officinalis flowers | crude extract | N.D. | N.D. | YES | YES | YES | YES | N.D. | YES |
| purified active fractions | N.D. | N.D. | YES | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Aloe vera leaves | crude extract | N.D. | N.D. | N.D. | YES | YES | YES | N.D. | YES |
| purified active fractions | N.D. | N.D. | N.D. | YES | N.D. | YES | N.D. | YES | |
| Molecular ion (m/z) | Error (Ppm) | Formula | RDB | MS/MS Fragment Ions (m/z) | Tentative Assignment | C.o. (%) | D.c. (%) | A.v. (%) |
|---|---|---|---|---|---|---|---|---|
| 269.0453 | −0.9 | C15H10O5 | 11 | 269.0458; 268.0379; 241.0510; 240.0428; 239.0342; 223.0394; 211.0396; 195.0444; 183.0447 | Aloe-emodin | 74.7 | ||
| 580.3288 [M + FA]− | 5.5 | C27H52NO10P | 3 | 580.3291; 520.3071; 295.2278; 277.2175; 171.1026 | GPC(OH18:2) | 2.9 | ||
| 559.3150 [M + FA]− | 4.7 | C28H48O11 | 5 | 513.3115; 277.2174; 253.0926 | MGMG(18:3) | 7.4 | 3.4 | 7.7 |
| 295.2279 | 0.1 | C18H32O3 | 3 | 295.2281; 277.2173; 195.1396 | Hydroxy-octadecadienoic acid | 23.5 | 92.9 | |
| 699.3803 [M + FA]− | −0.8 | C32H60O16 | 3 | 699.3882; 653.3805; 415.1470; 397.1363; 287.0772; 255.2334; 235.0826 | DGMG(16:0) | 17.6 | ||
| 562.3174 [M + FA]− | 4.2 | C27H50NO9P | 4 | 562.3175; 502.2962; 277.2172; 224.0687 | GPC(18:3) | 13.7 | ||
| 564.3320 [M + FA]− | 2.3 | C27H52NO9P | 3 | 564.3338; 504.3114; 279.2326 | GPC(18:2) isomer 1 | 4.2 | ||
| 564.3323 [M + FA]− | 2.3 | C27H52NO9P | 3 | 564.3345; 504.3124; 279.2336; 224.0693 | GPC(18:2) isomer 2 | 29.7 | ||
| 540.3307 [M + FA]− | 0.0 | C25H52NO9P | 1 | 540.3337; 480.3107; 255.2325; 224.0663 | GPC(16:0) isomer 1 | 2.2 | ||
| 540.3323 [M + FA]− | 3.0 | C25H52NO9P | 1 | 540.3347; 480.3116; 255.2327; 224.0689 | GPC(16:0) isomer 2 | 13.9 | 3.6 | |
| 566.3484 [M + FA]− | 2.0 | C27H54NO9P | 2 | 566.3485; 506.3269; 281.2477; 224.0691 | GPC(18:1) | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandrich, L.; Piccolella, S.; Esposito, A.V.; Costa, S.; Mercadante, V.; Pacifico, S.; Caputo, E. Different Extraction Procedures Revealed the Anti-Proliferation Activity from Vegetable Semi-Purified Sources on Breast Cancer Cell Lines. Antioxidants 2023, 12, 1242. https://doi.org/10.3390/antiox12061242
Mandrich L, Piccolella S, Esposito AV, Costa S, Mercadante V, Pacifico S, Caputo E. Different Extraction Procedures Revealed the Anti-Proliferation Activity from Vegetable Semi-Purified Sources on Breast Cancer Cell Lines. Antioxidants. 2023; 12(6):1242. https://doi.org/10.3390/antiox12061242
Chicago/Turabian StyleMandrich, Luigi, Simona Piccolella, Antonia Valeria Esposito, Silvio Costa, Vincenzo Mercadante, Severina Pacifico, and Emilia Caputo. 2023. "Different Extraction Procedures Revealed the Anti-Proliferation Activity from Vegetable Semi-Purified Sources on Breast Cancer Cell Lines" Antioxidants 12, no. 6: 1242. https://doi.org/10.3390/antiox12061242
APA StyleMandrich, L., Piccolella, S., Esposito, A. V., Costa, S., Mercadante, V., Pacifico, S., & Caputo, E. (2023). Different Extraction Procedures Revealed the Anti-Proliferation Activity from Vegetable Semi-Purified Sources on Breast Cancer Cell Lines. Antioxidants, 12(6), 1242. https://doi.org/10.3390/antiox12061242