Abstract
Sepsis syndrome develops through enhanced secretion of pro-inflammatory cytokines and the generation of reactive oxygen species (ROS). Sepsis syndrome is characterized by vascular hyperpermeability, hypotension, multiple organ dysfunction syndrome (MODS), and increased mortality, among others. Endotoxemia-derived sepsis is an important cause of sepsis syndrome. During endotoxemia, circulating endotoxin interacts with endothelial cells (ECs), inducing detrimental effects on endothelium function. The endotoxin induces the conversion of ECs into fibroblasts, which are characterized by a massive change in the endothelial gene-expression pattern. This downregulates the endothelial markers and upregulates fibrotic proteins, mesenchymal transcription factors, and extracellular matrix proteins, producing endothelial fibrosis. Sepsis progression is modulated by the consumption of specific nutrients, including ω-3 fatty acids, ascorbic acid, and polyphenolic antioxidant flavonoids. However, the underlying mechanism is poorly described. The notion that gene expression is modulated during inflammatory conditions by nutrient consumption has been reported. However, it is not known whether nutrient consumption modulates the fibrotic endothelial gene-expression pattern during sepsis as a mechanism to decrease vascular hyperpermeability, hypotension, MODS, and mortality. Therefore, the aim of this study was to investigate the impact of the consumption of dietary ω-3 fatty acids, ascorbic acid, and polyphenolic antioxidant flavonoid supplements on the modulation of fibrotic endothelial gene-expression patterns during sepsis and to determine the effects on sepsis outcomes. Our results indicate that the consumption of supplements based on ω-3 fatty acids and polyphenolic antioxidant flavonoids was effective for improving endotoxemia outcomes through prophylactic ingestion and therapeutic usage. Thus, our findings indicated that specific nutrient consumption improves sepsis outcomes and should be considered in treatment.
1. Introduction
Sepsis syndrome is the most prevalent cause of mortality worldwide in critically ill patients admitted to intensive care units (ICU) [1,2]. Sepsis syndrome develops through an overactivation of the immune system, which involves the enhanced secretion of pro-inflammatory cytokines and the generation of reactive oxygen species (ROS) [3,4]. Sepsis syndrome is characterized by decreased blood pressure (hypotension), multiple organ dysfunction syndrome (MODS), and increased mortality, among others [5,6]. Despite numerous basic and clinical studies addressing sepsis syndrome, current therapies for treating it and its sequelae are unsatisfactory [4,5,6]. Endotoxemia-derived sepsis is an important cause of sepsis syndrome and is frequently characterized by the deposition of large amounts of the Gram-negative bacterial endotoxin lipopolysaccharide (LPS) [7,8]. During endotoxemia, the endotoxin circulating in the bloodstream interacts with the endothelial cells (ECs) located in the internal endothelial monolayer of blood vessels, inducing detrimental effects on endothelium function [8,9,10,11].
It is well accepted that endothelial dysfunction is an important factor in the pathogenesis of endotoxemia-derived sepsis syndrome and other inflammatory diseases. Endotoxin is able to induce the conversion of ECs into fibroblasts [12,13,14]. Endotoxin-induced endothelial fibrosis is mediated through a process known as endothelial-to-mesenchymal transition (EndMT) in a similar way to that observed using the best-studied EndMT inducer, tumor growth factor β (TGF-β) [15,16,17,18,19]. Endothelial fibrosis has been detected in septic ICU patients and in several animal septic models, and the pharmacological inhibition of endotoxemia-induced endothelial fibrosis improves organ failure and survival [13,20,21]. Endotoxemia-induced endothelial fibrosis is characterized by a massive change in the endothelial gene-expression profile, which downregulates endothelial markers, including vascular endothelial cadherin (VE-cadherin) and platelet endothelial cell adhesion molecule 1 (PECAM-1). This change also strongly upregulates several other genes, including fibrotic and extracellular matrix (ECM) proteins such as the α۔smooth muscle actin (α-SMA), fibroblast-specific protein 1 (FSP-1), collagen type I and III (Col I/III), and fibronectin (FN), as well as transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), Slug, and Twist. It also upregulates pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and IL-6, as well as oxidative enzymes such as NAD(P)H oxidase (NOX), among several others [15,16,22,23,24,25,26]. Endothelial fibrosis has been associated with the origin and progression of several features of sepsis syndrome, including endothelial monolayer disruption, which promotes vascular hyperpermeability and edema formation, endothelial migration, refractory hypotension, pro-inflammatory cytokine production, MODS, and increased mortality [9,13,20,23,27,28,29,30].
It has been reported that specific nutrient consumption modulates sepsis. The dietary intake of ω-3 fatty acids enhances survival in septic mice, improves sepsis-induced intestinal inflammation and oxidative stress injury, and reduces the mortality rate of sepsis-induced acute respiratory distress syndrome [31,32,33]. Furthermore, dietary ascorbic acid decreases mortality and suppresses the inflammatory response to endotoxemia and sepsis, showing potential action on ECs [34,35]. Moreover, polyphenolic antioxidant flavonoid consumption improves endotoxemia and septic condition. Several types of flavonoids improve MODS and increase survival against sepsis [36,37,38]. The evidence suggests that the nutrient composition of food affects sepsis progression and its outcomes, but the molecular underlying mechanism has not been completely described.
It has been reported that gene expression is modulated under inflammatory conditions by nutrient consumption. The consumption of ω-3 fatty acids reduces inflammation by downregulating pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 [39,40]. Ascorbic acid has the capacity to modulate gene expression, including collagen and fibronectin genes, in response to several stimuli, such as oxidative stress [41,42,43,44]. Interestingly, ascorbic acid regulates extracellular matrix production through collagen and fibronectin gene expression [44,45]. Furthermore, pro-inflammatory cytokine expression decreases as a consequence of flavonoid supplementation [46]. However, it is not known whether specific nutrient consumption modulates the fibrotic endothelial gene-expression pattern during sepsis to inhibit sepsis-induced endothelial fibrosis as a mechanism to decrease vascular endothelial hyperpermeability, hypotension, MODS, and mortality.
Therefore, the aim of this study was to investigate the effect of dietary supplements with ω-3 fatty acids, ascorbic acid, and polyphenolic antioxidant flavonoid on the modulation of fibrotic endothelial gene-expression patterns during sepsis and its effects on improving vascular hyperpermeability, hypotension, MODS, and mortality.
2. Materials and Methods
2.1. Animals, Dietary Supplement Administration, Endotoxemia Induction, and Parameter Recordings
Male Sprague Dawley (SD) rats weighing from 190 to 210 g were used. They were housed in cages with water and food ad libitum, a 12 h light/dark cycle, and 25 ± 1 °C temperature. All experimental procedures in rats were approved by the Commission of Bioethics and Biosafety of Universidad Andres Bello (N°002/2020) and following instruction form the Guide for the Care and Use of Laboratory Animals from the National Research Council and the American Association for Laboratory Animal Science. This research complies with the commonly-accepted 3Rs.
Rats were treated with 0.9% NaCl saline solution (saline-treated condition) or with the endotoxin lipopolysaccharide from E. coli (LPS (Sigma-Aldrich, Saint Louis, MO, USA), 10 mg/kg) (endotoxemia-treated condition) by intraperitoneal (I.P.) injection (50 µL), and animals were followed during the experiment. Saline-treated and endotoxemia-treated rats were subjected to dietary supplementation by means of a prophylactic (4 weeks before and during the 7 days of endotoxemia) and a therapeutic (only during the 7 days of endotoxemia) protocol. The period of prophylactic and therapeutic supplement treatments is compatible with those previously reported [31,47,48,49]. Furthermore, the period for endotoxemia induction is similar to those reported by us and other groups [3,13,30,50,51,52,53].
The composition of the dietary supplementation was as follows: sham supplementation (Sham): 2 mL water; ω-3 fatty acid supplementation (ω-3 FA): 300 mg ω-3 FAs (Sigma-Aldrich) (composed by 180 mg EPA and 120 mg DHA in 1000 mg (1.075 mL) of fish oil); ascorbic acid supplementation (AsA): 600 mg of AsA (Sigma-Aldrich) (2 mL of 300 mg/mL AsA solution in water); flavonoid supplementation (Flav): 6 mg of mixed flavonols, flavanones, isoflavones, flavones, flavan-3-ols, and anthocyanins, which were administered in a 2 mL of total solution (water or oil solvent) (all from Sigma-Aldrich). The dietary supplementation was administered daily by gavage. Twelve rats per group were used.
Rat blood samples were collected in a sodium citrate or lithium heparin-containing blood collection Vacutainer® tube (Becton Dickinson Co., East Rutherford, NJ, USA) through tail-vein puncture or cardiac puncture after anesthetization. The obtained blood was immediately centrifuged at 4000 rpm for 10 min at 4 °C to separate the plasma and immediately stored at −80 °C. Systolic blood pressure (PS) and heart rate (HR) were acquired with a physiological recording system and a pressure tail cuff for a noninvasive blood-pressure-recording system for rats (ML125/R), coupled with an MLT125/R pulse transducer (AD Instruments, Bella Vista, Australia). To perform the recordings of PS and HR, animals were recorded for 1 min several times to obtain reliable values. All transducers were connected to a PowerLab® 8/30 (AD Instruments), and physiological variables were instantaneously displayed through Chart® software (v6.1.1, AD Instruments).
2.2. Primary Rat Mesenteric Endothelial Cell (RMEC) Isolation and mRNA Expression Determination by RT-qPCR
Primary rat mesenteric endothelial cells (RMECs) were isolated from the mesenteric artery. The mesenteric artery was occluded on its distal end and cannulated from its proximal end with a polyethylene tubing connected to a 21-gauge syringe. The mesentery was surgically removed and washed with sterile PBS. For the enzymatic isolation of RMECs, the mesenteric artery was slowly perfused in a culture hood for 5 min with 5 mL M-199 medium supplemented with 40 μL penicillin/streptomycin (10,000 U/mL/10,000 μg/mL), 20 μL Fungizone (250 μg/mL), and 12.5 mg collagenase type II. The cell suspension was centrifuged at 3000 rpm for 7 min; the pellet was reconstituted in 3 mL M-199 medium supplemented with 8 mL/L of Pen/Strep (10,000 U/mL/10,000 μg/mL), 4 mL/L of Fungizone (250 μg/mL), 10% FBS, and 10% CCS. Thereafter, RMECs were subjected immediately to mRNA expression determination. RT-qPCR experiments were performed to measure mRNA expression levels in RMECs. Total RNA was extracted with Trizol according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). DNAse I-treated RNA was used for reverse transcription using the Super Script II Kit (Invitrogen). Equal amounts of RNA were used as templates in each reaction. Quantitative-PCR was performed using the SYBR Green PCR Master Mix (AB Applied Biosystems, Foster City, CA, USA). Assays were run using a RotorGene instrument (Corbet Research, Sydney, Australia). The determination of mRNA expression was normalized relative to 28S mRNA and were expressed relative to sham-supplemented condition.
2.3. In Vivo Permeability Assay and MODS Determination
At the end of experiments, rats were anesthetized with isoflurane and injected with Evans blue dye (EBD (Sigma-Aldrich), 80 mg/kg, intravenous) for 10 min. EBD binds to plasma proteins and extravasates to tissue parenchyma at sites of increased vascular permeability. Then, rats were euthanized and perfused with saline solution via the left ventricle to wash excess EBD. Mesentery, kidney, and liver were removed, washed in cold saline solution, gently dried using a paper towel, weighted, and prepared for EBD extraction. Briefly, organs were weighted and homogenized with 100% trichloroacetic acid in a 1:2 (mg:mL) proportion. Homogenates were centrifuged at 4180× g for 30 min, and the supernatant optical density was determined spectrophotometrically at 630 nm. To assess MODS, plasma levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), and gamma-glutamyl transferase (GGT) were quantified to measure liver function; creatinine (CRE) and blood urea nitrogen (BUN) were quantified to measure kidney function; lactic acid (LAC) and glycemia (GLY) were quantified to measure metabolic function. MODS markers were quantified using a Piccolo Xpress Chemistry Analyzer equipped with General Chemistry 13, MetLyte Plus CRP, and Basic Metabolic Panel Plus panels (Abaxis, Union City, CA, USA), according to the manufacturers’ instructions.
2.4. Statistical Analyses
Differences were considered significant at p < 0.05. Statistical differences were assessed by the Mann–Whitney U-test and one-way analysis of variance (one-way ANOVA) (or Kruskal–Walli’s type), followed by Dunn’s post hoc test and two-way analysis of variance (two-way ANOVA) followed by Tukey post hoc test. See the figure legends for the specific test used. Survival Kaplan–Meier curves were compared by the log-rank (Mantel–Cox) test and the Gehan–Breslow–Wilcoxon test to determine survival rates. Contingency analyses with Fisher’s exact test were used to assess the relative risk of death. Statistical testing was two-sided and used the 5% significance level. The data were analyzed with GraphPad Prism version 9.4 (GraphPad Software, LLC, Boston, MA, USA). Samples used in the study were defined to identify the mean magnitude effect of a ≥2-fold change with standard deviations of 10%. Accordingly, a sample size of 12 rats per group would provide 90% statistical power using a two-sided 0.05 significance level.
3. Results
3.1. Impact in EndMT Gene Expression through Dietary Supplementation Based on ω-3 FA, Ascorbic Acid, or Polyphenolic Antioxidant Flavonoids in Endotoxemic Rats
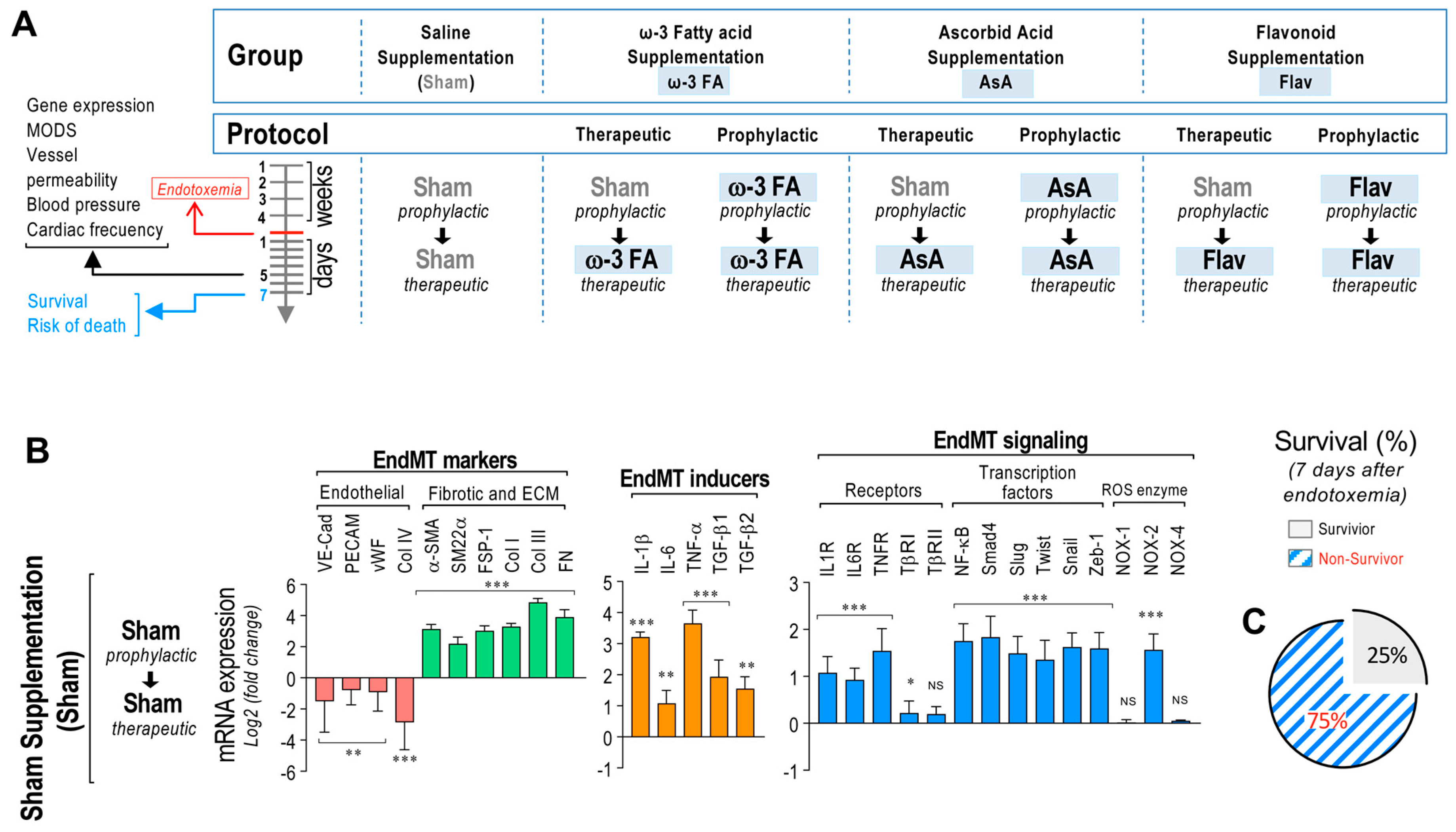
To determine the effects on EndMT-related gene expression and sepsis syndrome, rats were subjected to saline treatment or endotoxemia and fed with ω-3 fatty acid, ascorbic acid, or flavonoid supplementation by means of a prophylactic protocol (4 weeks before and for 7 days of endotoxemia) or therapeutic protocol (only for the 7 days of endotoxemia) (Figure 1A). Firstly, we established the change in endotoxemia-induced mRNA expression profiles for several selected genes involved in EndMT-mediated endothelial fibrosis in endotoxemic rats. For this purpose, we analyzed the mesenteric endothelium expression profile because it is a vascular network that frequently undergoes vascular disease associated with fibrosis and regulates a relevant portion of total blood flow. The mesentery is a vascular area that represents blood vessels in other tissues well. Thus, ECs from the mesentery are an appropriate model for studying dysfunctional endothelial changes and are useful as representatives of the endothelium from internal organs.
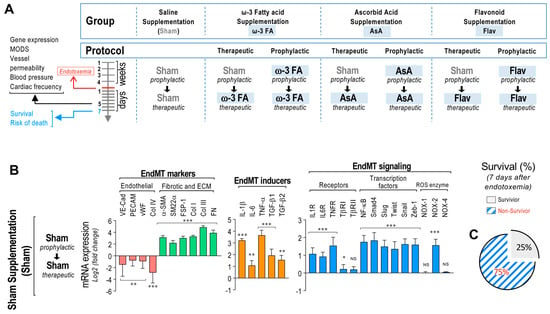
Figure 1.
EndMT gene expression in endotoxemic rats. (A) Scheme of the strategy of saline– and endotoxemia–treated rats subjected to dietary supplementation by means of a prophylactic (4 weeks before and during the 7 days of endotoxemia) and a therapeutic (only during the 7 days of endotoxemia) protocol. Sham supplementation (Sham), ω–3 fatty acid supplementation (ω–3 FA), ascorbic acid supplementation (AsA), and flavonoid supplementation (Flav). (B) The mRNA expression of EndMT markers (endothelial markers (red bars) and fibrotic and ECM markers (green bars)), EndMT inducers (orange bars), and EndMT signaling (blue bars) was detected in rats subjected to endotoxemia. (C) Survival percentage was determined 7 days after endotoxemia induction in rats subjected to endotoxemia. Statistical differences were assessed by a one-way analysis of variance (ANOVA) (Kruskal–Wallis) followed by Dunn’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the saline-treated condition. NS: non-significant. Determination of mRNA expression was normalized relative to 28S mRNA and is expressed relative to sham-supplemented condition. Values are expressed as the mean ± SD. (N = 12).
Primary rat mesenteric endothelial cells (RMECs) were extracted from the rats of all groups (see Figure 1A), and mRNA expression was immediately determined to avoid any possible cell transformation. The results showed that RMECs extracted from endotoxemia-treated rats of all groups exhibited decreased mRNA expression of the endothelial markers VE-Cadherin, PECAM-1, von Willebrand Factor (vWF), and collagen type IV (Col IV) compared to saline-treated rats. However, endotoxemic rats showed increased mRNA expression of the fibrotic and ECM markers alpha smooth muscle actin (α-SMA), smooth muscle protein 22 alpha (SM22α), FSP-1, Col I, Col III, and FN. Furthermore, the mRNA expressions of the EndMT inducers IL-1β, IL-6, TNF-α, transforming growth factor beta-1 (TGF-β1), and transforming growth factor beta-2 (TGF-β2) were also increased in endotoxemic rats. The mRNA expression of the cytokine receptors IL-1R, IL-6R, TNFR, and TβRI were also increased in endotoxemic rats, while TβRI did not show changes compared to saline-treated rats. All transcription factors tested (NF-κB, Smad4, Slug, Twist, Snail, and Zeb-1) showed increased mRNA expression in endotoxemic rats. In addition, mRNA expression of NOX-2 from the endotoxemic condition was increased, whereas mRNA expression of NOX-1 and NOX-4 did not show changes compared to saline-treated rats (Figure 1B). The survival percentage of the endotoxemic rats was 25% after 7 days of endotoxemia challenge (Figure 1C).
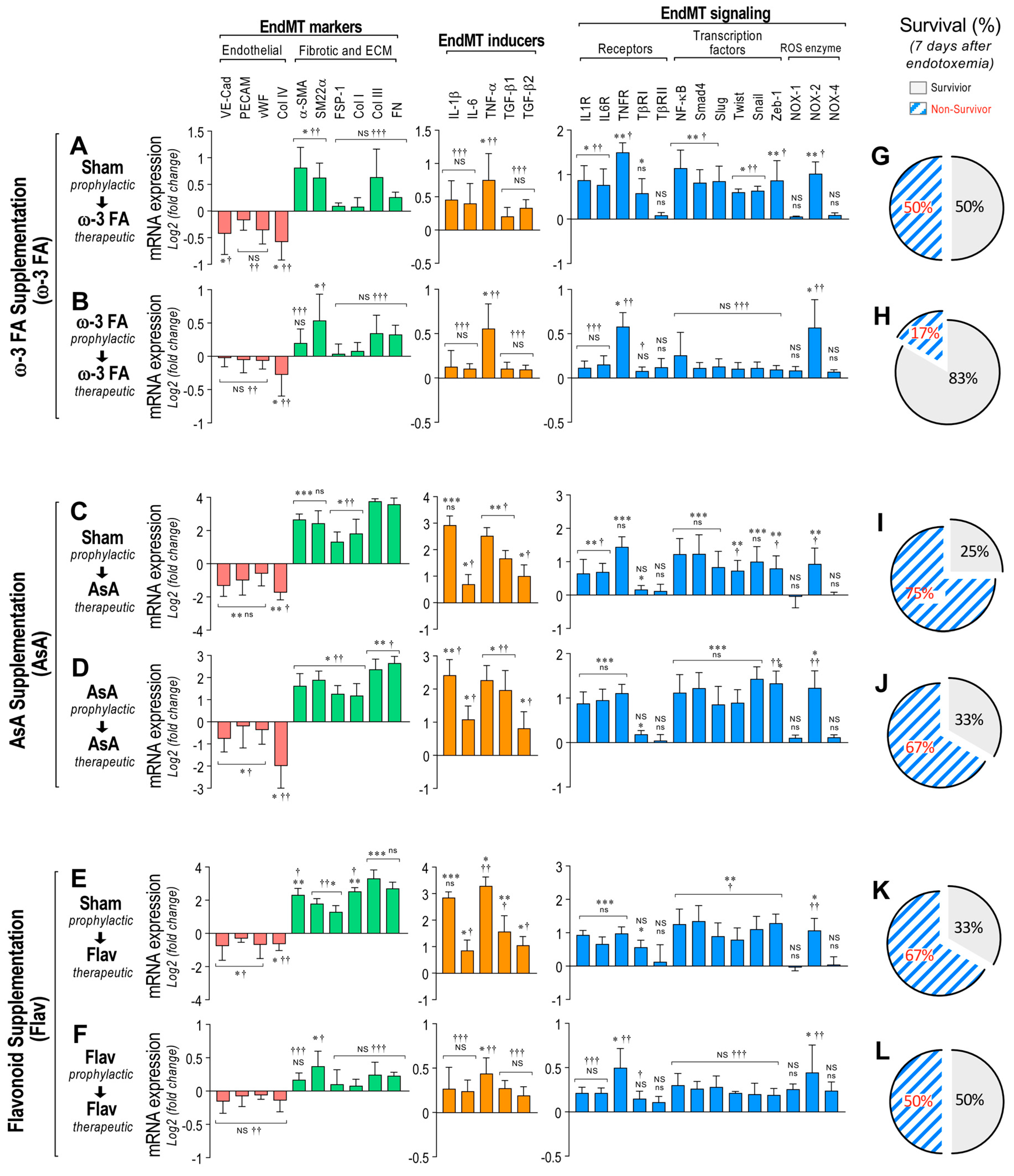
Interestingly, the endotoxemic rats fed with a diet supplemented with ω-3 fatty acid following the therapeutic and prophylactic protocols showed a reduced decrease in mRNA expression of the endothelial markers (Figure 2A,B, left panels). Furthermore, the ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols showed a reduced increase in mRNA expression of the fibrotic and ECM markers, EndMT inducers, cytokine receptors, transcription factors, and NOX-2 enzyme compared with saline-treated rats (Figure 2A,B, middle and right panels). Notably, the ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocol showed a non-significant change in mRNA expression of several EndMT-related genes compared with saline-treated rats (Figure 2A,B), suggesting that the ω-3 fatty acid supplementation efficiently abolished the endotoxemia-induced change in the EndMT expression profile. Similar results were obtained when the supplementation with ascorbic acid (AsA) was used (Figure 2C,D). However, because the non-significant change was almost not detected, it is suggested that AsA supplementation has a minor impact on EndMT-related mRNA expression compared with ω-3 fatty acid supplementation.
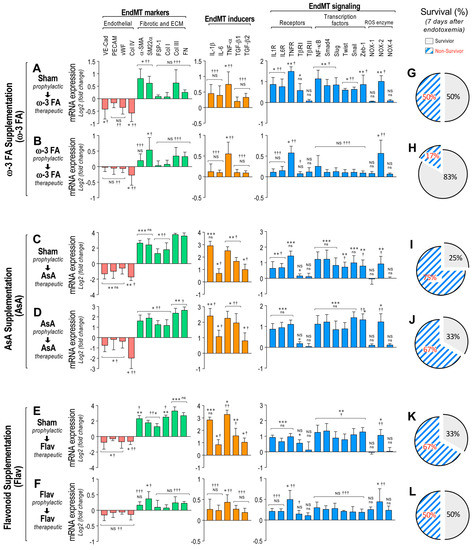
Figure 2.
Impact on EndMT gene expression through dietary supplementation based on ω–3 FA, ascorbic acid, or polyphenolic antioxidant flavonoids in endotoxemic rats. (A–D) The mRNA expression of EndMT markers (endothelial markers (red bars) and fibrotic and ECM markers (green bars)), EndMT inducers (orange bars) and EndMT signaling (blue bars) were detected in rats subjected to endotoxemia and supplemented with ω–3 fatty acid (ω-3 FA, (A,B)), ascorbic acid (AsA, (C,D)), or polyphenolic antioxidant flavonoids (Flav, (E,F)) through a therapeutic (A,C,E) and a prophylactic (B,D,F) protocol. (G–L) Survival percentage was determined 7 days after endotoxemia induction in rats subjected to endotoxemia and supplemented with ω–3 fatty acid (ω–3 F (G,H)), ascorbic acid (AsA, (I,J)), or polyphenolic antioxidant flavonoids (Flav, (K,L)) through a therapeutic (G,I,K) and a prophylactic (H,J,L) protocol. Statistical differences were assessed by a one-way analysis of variance (ANOVA) (Kruskal–Wallis) followed by Dunn’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the saline-treated condition. † p < 0.05, †† p < 0.01, ††† p < 0.001 compared with the mRNA-expression values from endotoxemia-treated rats shown in Figure 1B. NS: non-significant. Determination of mRNA expression was normalized relative to 28S mRNA and are expressed relative to sham-supplemented condition. Values are expressed as the mean ± SD. (N = 12).
Furthermore, similar results to those observed with ω-3 fatty acid supplementation were obtained when the supplementation with flavonoids was assessed (Figure 2E,F). However, a non-significant change in mRNA expression was observed in only the prophylactic treatment of flavonoid supplementation. These results indicate that predominantly in the prophylactic protocol, the ω-3 fatty acid and flavonoid supplementation exhibited similar and strong protection against the endotoxemia-induced EndMT gene-expression change.
Notably, the survival percentages in the endotoxemic rats fed with a diet supplemented with ω-3 fatty acid in the therapeutic and prophylactic protocol were 50% and 83%, respectively, (Figure 2G,H, respectively). This shows protection against endotoxemia compared with the sham-supplemented endotoxemic rats after 7 days of endotoxemic challenge. Supplementation with AsA produced a modest increase in survival (25% to 33%) in only the prophylactic treatment, whereas the therapeutic treatment showed the same survival percentage as that observed in the sham-supplemented endotoxemic rats (Figure 2I,J, respectively). Importantly, the survival percentages in the flavonoid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols were 33% and 50%, respectively, after 7 days of endotoxemia challenge (Figure 2K,L, respectively). This shows protection against endotoxemia compared with the sham-supplemented endotoxemic rats after 7 days of endotoxemic challenge. These results indicated that principally, the ω-3 fatty acid and flavonoid supplementation produced protection against mortality induced by endotoxemia.
3.2. Effects on Vascular Hyperpermeability through Dietary Supplementation Based on ω-3 FA, Ascorbic Acid, or Polyphenolic Antioxidant Flavonoids in Endotoxemic Rats
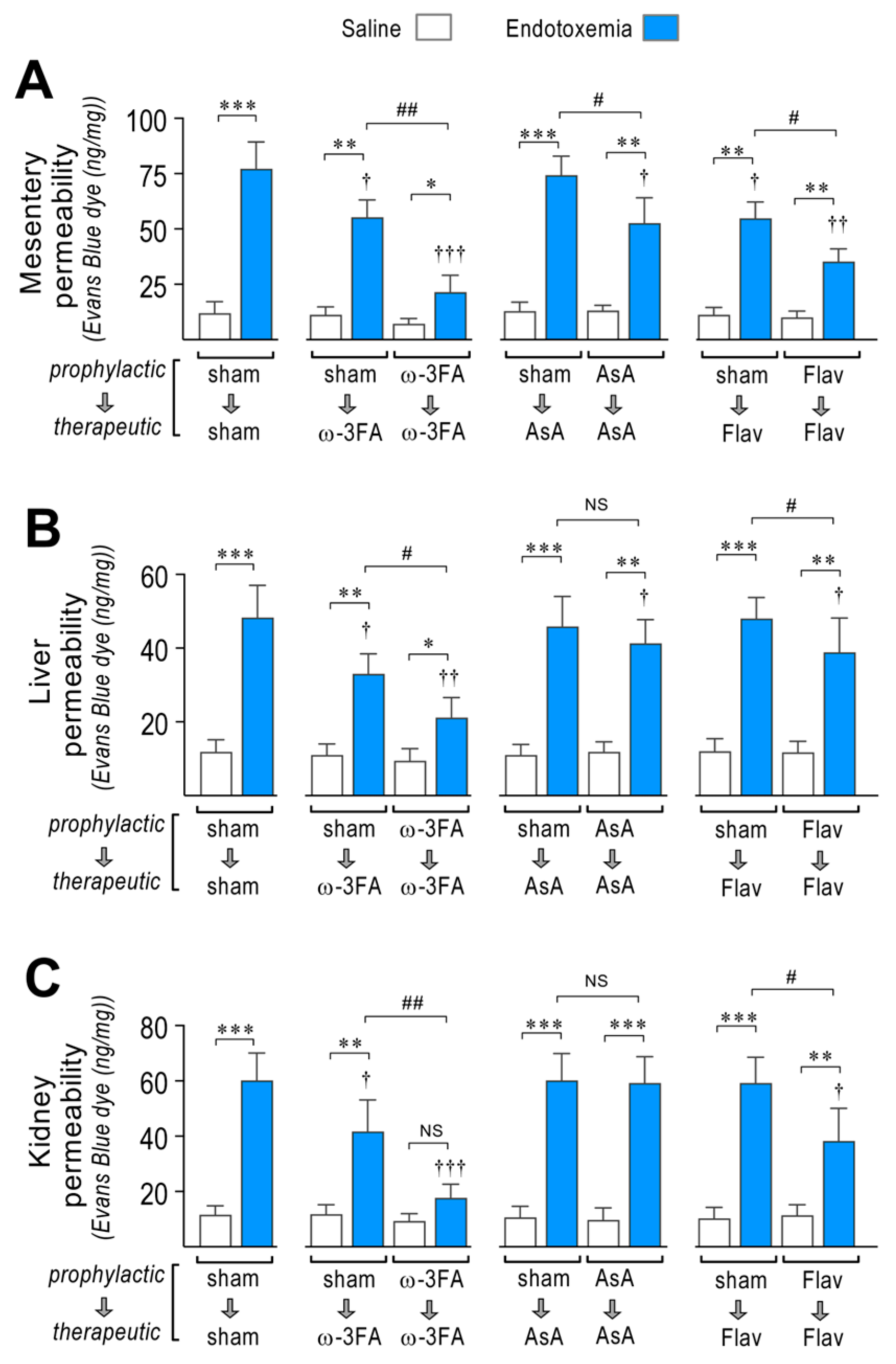
EndMT-induced endothelial fibrosis produces a severe disruption of the vascular endothelium monolayer, which promotes increased permeability and generates edema formation. Considering this, we tested whether the consumption of a diet supplemented with ω-3 fatty acid, ascorbic acid, or flavonoids is able to improve endotoxemia-induced vascular hyperpermeability. To this end, blood vessel hyperpermeability was tested in the mesentery and in two additional relevant organs that are severely affected during sepsis: the liver and the kidney. This was performed using endotoxemic rats by means of the Evans blue dye (EBD) permeability assay.
The mesentery, liver, and kidney extracted from sham-treated endotoxemic rats showed increased EBD accumulation, which indicates that blood vessels leaked to the interstitial compartment in contrast with saline-treated rats (Figure 3A–C, left panel). Interestingly, the ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic protocol and notably in the prophylactic protocol showed a strong decrease in the endotoxemia-induced hyperpermeability in the mesentery, liver, and kidney (Figure 3A–C, middle left panel). AsA-supplemented endotoxemic rats showed decreased endotoxemia-induced hyperpermeability in the mesentery and liver, but not in the kidney (Figure 3A–C, middle right panel) with the prophylactic protocol. The therapeutic protocol did not show any change in endotoxemia-induced hyperpermeability in all tested organs. The flavonoid-supplemented endotoxemic rats in only the prophylactic protocol showed decreased endotoxemia-induced hyperpermeability in all tested organs (Figure 3A–C, right panel).
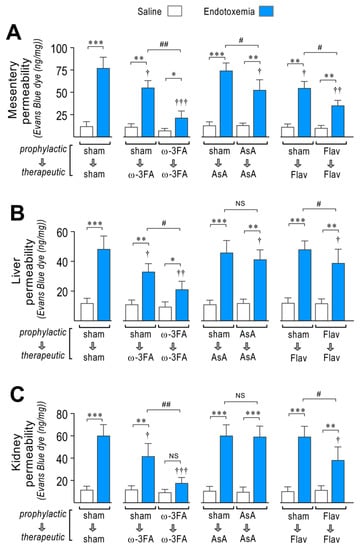
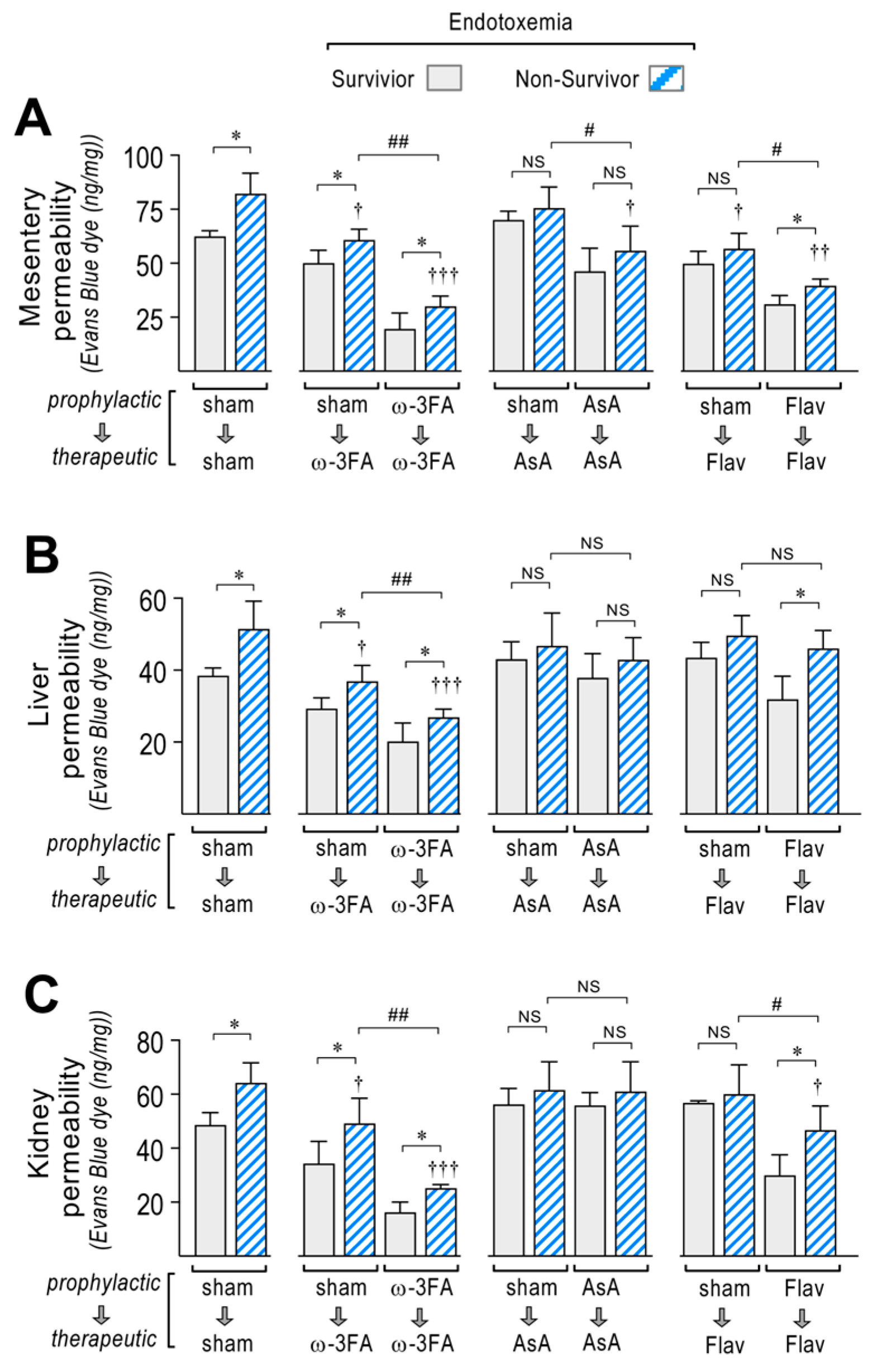
Figure 3.
Effects in vascular hyperpermeability through dietary supplementation based on ω-3 FA, ascorbic acid, or polyphenolic antioxidant flavonoids in endotoxemic rats. After saline (open bars) and endotoxemia (blue bars) treatments that were supplemented with water (sham), ω-3 fatty acid (ω-3 FA), ascorbic acid (AsA), or polyphenolic antioxidant flavonoids (Flav), through a therapeutic and a prophylactic protocol, the mesentery (A), kidney (B), and liver (C) were harvested and processed for EBD extraction to determine blood vessel hyperpermeability. EBD content was normalized to total sample weight. Statistical differences were assessed by two-way ANOVA followed by Dunnett’s post hoc test. * p < 0.05; ** p < 0.01; *** p < 0.001 comparing saline and endotoxemia condition. † p < 0.05, †† p < 0.01, ††† p < 0.001 compared with sham-treated condition. # p < 0.05; ## p < 0.01 comparing non-survivor endotoxic condition between therapeutic and prophylactic protocol. NS: non-significant. Results are expressed as the mean ± SD (N = 12).
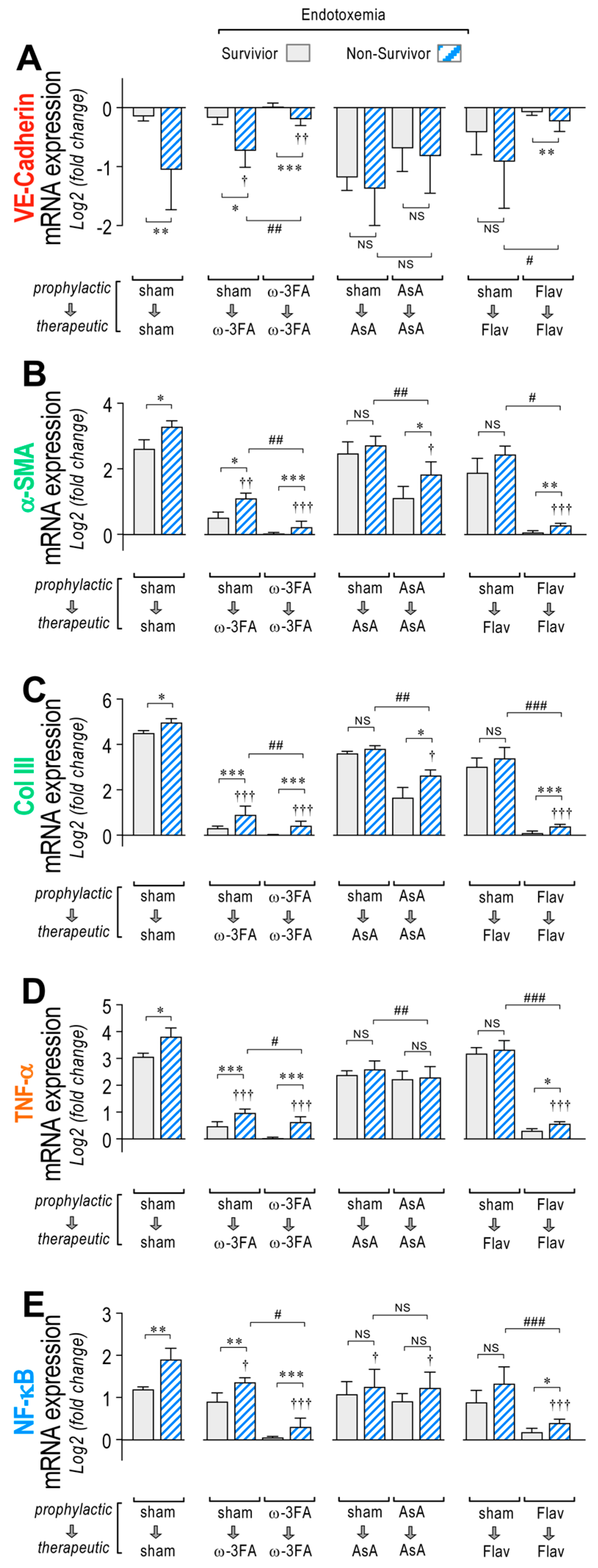
During endotoxemia, vascular hyperpermeability is associated with changes in EndMT gene expression, so correlation analyses were performed between these two factors. The results showed that in the endotoxemic condition, the increased permeability in the mesentery (Supplementary Table S1), liver (Supplementary Table S2), and kidney (Supplementary Table S3) correlates with the change in mRNA expression of most of the EndMT-related genes tested in Figure 2. Interestingly, in all tested organs, VE-Cadherin, α-SMA, Col III, TNF-α, and NF-κB showed correlation coefficients (r2) and p-values (p) higher than 0.8 and 0.01, respectively. The expression of these genes at the protein level was verified and showed concordant results compared to those shown in the mRNA determination (Supplementary Figure S1).
Interestingly, higher changes in mRNA expression of VE-Cad, α-SMA, Col III, TNF-α, and NF-κB were found mainly in the non-surviving endotoxemic rats compared to survivors, as observed in the sham group (Figure 4A–E, left panel, respectively). The ω-3 fatty acid-supplemented endotoxemic rats had decreased hyperpermeability values in both the therapeutic and prophylactic protocols in non-survivors and survivors, compared to the corresponding condition in the sham group, showing higher changes in mRNA expression in the non-surviving ω-3 fatty acid-supplemented endotoxemic rats compared to survivors (Figure 4A–E, middle left panel). However, AsA-supplemented endotoxemic rats showed, to a major extent, non-significant differences in mRNA expression changes between non-surviving and surviving rats (Figure 4A–E, middle right panel). The flavonoid-supplemented endotoxemic rats showed higher changes in mRNA expression in the non-surviving rats subjected to the prophylactic protocol, whereas in the therapeutic protocol, non-significant differences were observed (Figure 4A–E, right panel).
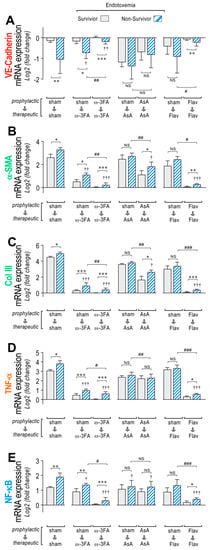
Figure 4.
VE-Cadherin, α-SMA, Col III, TNF-α, and NF-κB mRNA expression through dietary supplementation based on ω-3 FA, ascorbic acid, or polyphenolic antioxidant flavonoids in survivor and non-survivor endotoxemic rats. After endotoxemia treatment supplemented with water (sham), ω-3 fatty acid (ω-3 FA), ascorbic acid (AsA), or polyphenolic antioxidant flavonoids (Flav), through a therapeutic and a prophylactic protocol, the mRNA expression of VE-Cadherin (A), α-SMA (B), Col III (C), TNF-α (D), and NF-κB (E) were determined in survivor (grey bars) and non-survivor (dashed bars) endotoxemic rats. Statistical differences were assessed by two-way ANOVA followed by Dunnett’s post hoc test. * p < 0.05; ** p < 0.01; *** p < 0.001 comparing saline and endotoxemia condition. † p < 0.05, †† p < 0.01, ††† p < 0.001 compared with sham-treated condition. # p < 0.05; ## p < 0.01; ### p < 0.001 comparing non-survivor endotoxic condition between therapeutic and prophylactic protocol. NS: non-significant. Results are expressed as the mean ± SD (N = 12).
Vascular hyperpermeability has detrimental effects on the function of several organs and tissues and promotes mortality, and it is increased during sepsis. As we expected, higher permeability values in the mesentery, liver, and kidney were found in the non-surviving endotoxemic rats compared to survivors, as observed in the sham group (Figure 5A–C, left panel). The ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols had decreased hyperpermeability values and showed the same distribution in the mesentery, liver, and kidney (Figure 5A–C, middle left panel). Higher permeability values were found in the non-surviving ω-3 fatty acid-supplemented endotoxemic rats compared to survivors. Interestingly, AsA-supplemented endotoxemic rats lost this distribution and showed non-significant differences in the permeability values between non-surviving and surviving rats (Figure 5A–C, middle right panel). The flavonoid-supplemented endotoxemic rats showed higher permeability values in the non-surviving rats in the prophylactic protocol, whereas in the therapeutic protocol, non-significant differences were observed (Figure 5A–C, right panel).
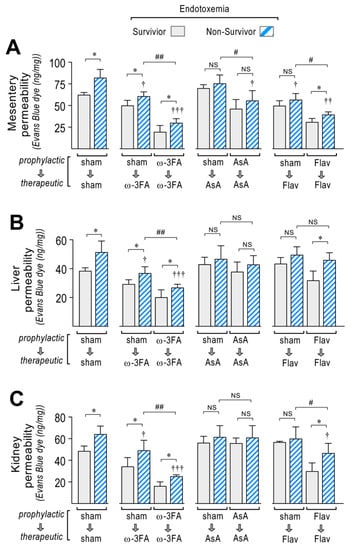
Figure 5.
Vascular hyperpermeability through dietary supplementation based on ω-3 FA, ascorbic acid, or polyphenolic antioxidant flavonoids in survivor and non-survivor endotoxemic rats. After endotoxemia treatment supplemented with water (sham), ω-3 fatty acid (ω-3 FA), ascorbic acid (AsA), or polyphenolic antioxidant flavonoids (Flav), through a therapeutic and a prophylactic protocol, the mesentery (A), kidney (B), and liver (C) were harvested and processed for EBD extraction to determine blood vessel hyperpermeability. EBD content was normalized to total sample weight. Statistical differences were assessed by two-way ANOVA followed by Dunnett’s post hoc test. * p < 0.05 comparing saline and endotoxemia condition. † p < 0.05, †† p < 0.01, ††† p < 0.001 compared with sham-treated condition. # p <0.05; ## p < 0.01 comparing non-survivor endotoxic condition between therapeutic and prophylactic protocol. NS: non-significant. Results are expressed as the mean ± SD (N = 12).
3.3. Effects on Endotoxemia-Induced Hypotension through Dietary Supplementation Based on ω-3 FA, Ascorbic Acid, or Polyphenolic Antioxidant Flavonoids in Endotoxemic Rats
Septic syndrome and endotoxemia are characterized by decreased blood pressure or hypotension. EndMT-induced increased hyperpermeability contributes to hypotension. Thus, we tested whether the consumption of a diet supplemented with ω-3 fatty acid, ascorbic acid, or flavonoids is able to improve endotoxemia-induced hypotension. Systolic pressure monitoring showed that the ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols showed strong improvement of hypotension (Figure 6A, middle left panel) compared to sham-supplemented endotoxemic rats (Figure 6A, left panel). However, AsA-supplemented endotoxemic rats did not show any change in endotoxemia-induced hypotension (Figure 6A, middle right panel). The flavonoid-supplemented endotoxemic rats showed improved hypotension when subjected to the prophylactic protocol, whereas in the therapeutic protocol, no change was detected (Figure 6A, right panel). As compensation for the hypotension, the heart rate was found to be increased in endotoxemia-treated rats (Figure 6B, left panel). The ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols showed decreased tachycardia (Figure 6B, middle left panel). However, AsA-supplemented endotoxemic rats did not show any change in tachycardia (Figure 6B, middle right panel). The flavonoid-supplemented endotoxemic rats showed improved tachycardia in rats subjected to the prophylactic protocol but not in those subjected to the therapeutic protocol (Figure 6B, right panel).
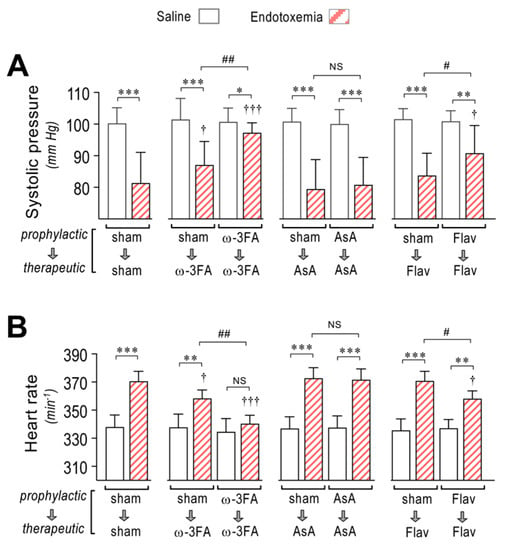
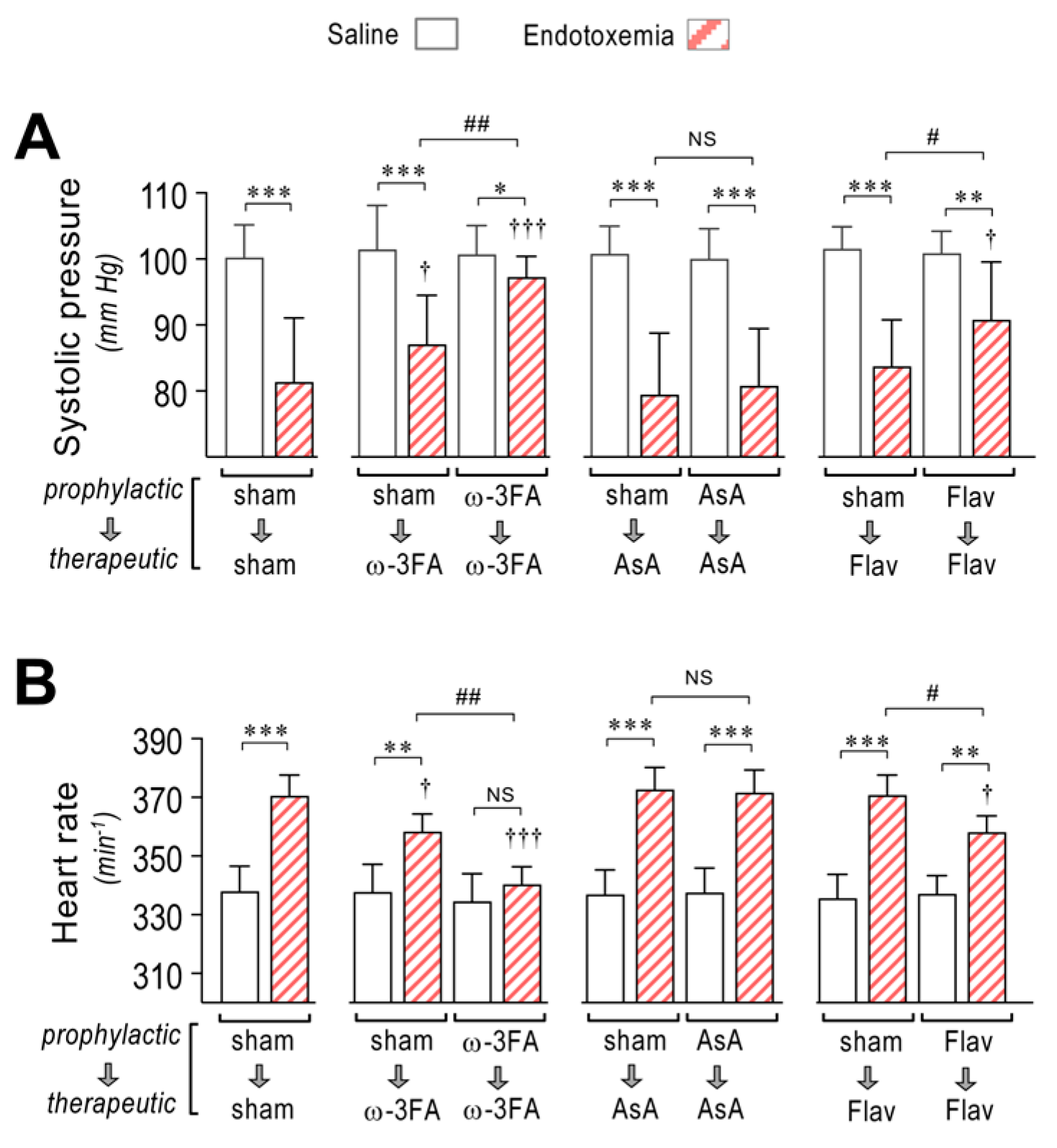
Figure 6.
Effects in systolic blood pressure and cardiac frequency through dietary supplementation based on ω-3 FA, ascorbic acid, or polyphenolic antioxidant flavonoids in endotoxemic rats. After saline (open bars) and endotoxemia (dashed bars) treatments that were supplemented with water (sham), ω-3 fatty acid (ω-3 FA), ascorbic acid (AsA), or polyphenolic antioxidant flavonoids (Flav), through a therapeutic and a prophylactic protocol, the systolic blood pressure (A) and cardiac frequency (B) were determined. Statistical differences were assessed by two-way ANOVA followed by Dunnett’s post hoc test. * p < 0.05; ** p < 0.01; *** p < 0.001 comparing saline and endotoxemia condition. † p < 0.05, ††† p < 0.001 compared with sham-treated condition. # p < 0.05; ## p < 0.01 comparing non-survivor endotoxic condition between therapeutic and prophylactic protocol. NS: non-significant. Results are expressed as the mean ± SD (N = 12).
3.4. Effects on Endotoxemia-Induced MODS through Dietary Supplementation Based on ω-3 FA, Ascorbic Acid, or Polyphenolic Antioxidant Flavonoids in Endotoxemic Rats
MODS is a crucial factor during sepsis syndrome and severely impacts survival, which is promoted by hyperpermeability. Thus, we assessed whether the consumption of a diet supplemented with ω-3 fatty acid, ascorbic acid, or flavonoids is able to improve the endotoxemia-induced MODS by measuring blood markers of liver, renal, and metabolic function.
The ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols showed improved liver function with decreases in the endotoxemia-induced increased blood levels of the liver dysfunction markers alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), and gamma-glutamyl transferase (GGT) (Figure 7A–D, middle left panel), compared to sham-supplemented endotoxemic rats (Figure 7A–D, left panel). AsA-supplemented endotoxemic rats did not show protection against endotoxemia-induced liver dysfunction (Figure 7A–D, middle right panel). The flavonoid-supplemented endotoxemic rats showed protection against liver dysfunction with decreases in endotoxemia-induced increased blood levels of ALT, AST, TBIL, and GGT in rats subjected to the prophylactic protocol, whereas in the therapeutic protocol, no change was detected (Figure 7A–D, right panel).
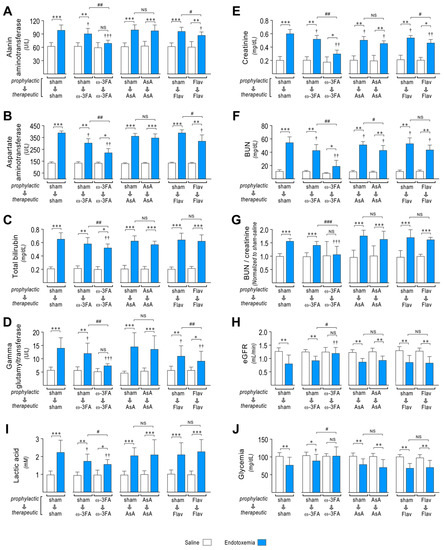
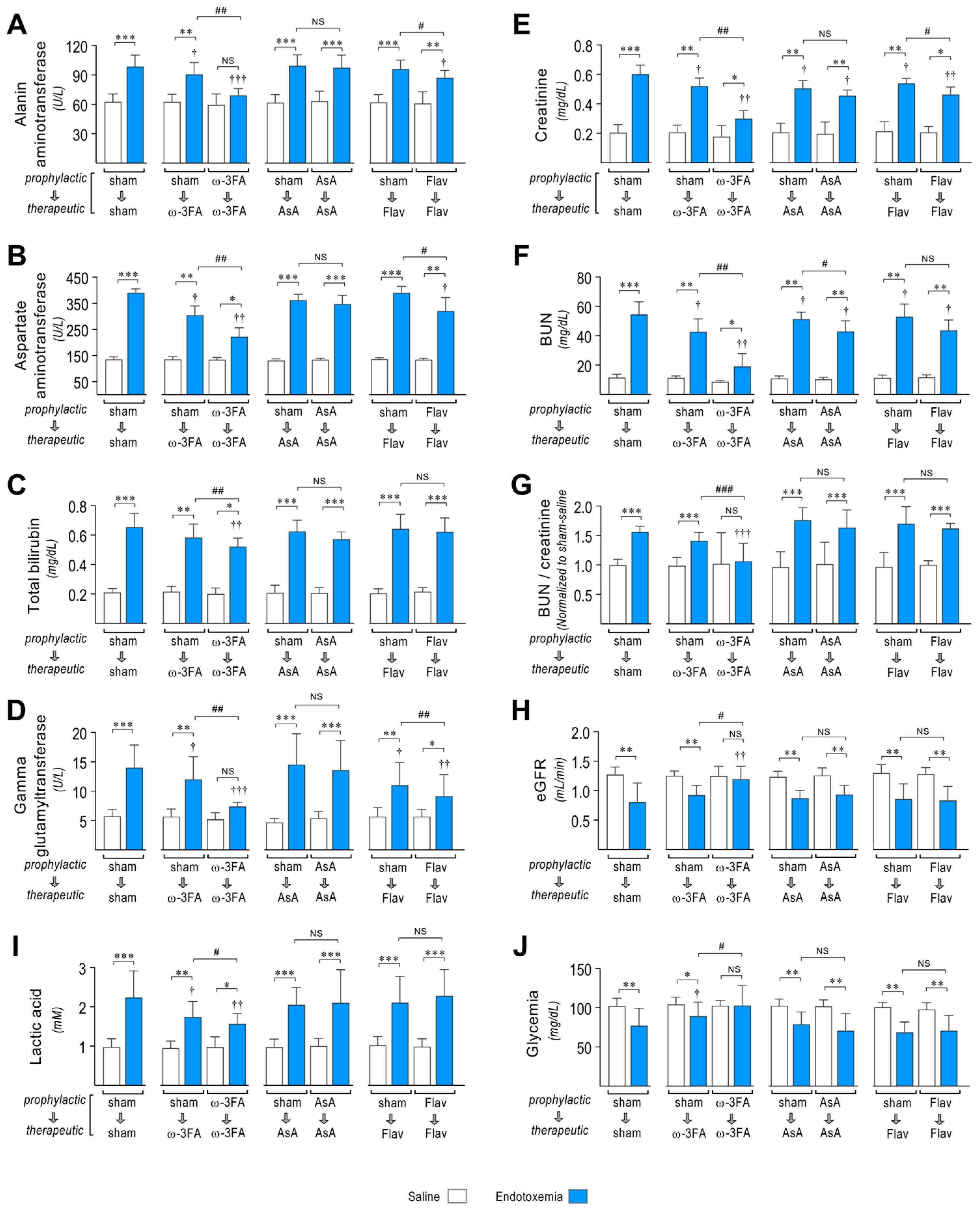
Figure 7.
Effects in MODS through dietary supplementation based on ω-3 FA, ascorbic acid, or polyphenolic antioxidant flavonoids in endotoxemic rats. After saline (open bars) and endotoxemia (blue bars) treatments that were supplemented with water (sham), ω-3 fatty acid (ω-3 FA), ascorbic acid (AsA), or polyphenolic antioxidant flavonoids (Flav), through a therapeutic and a prophylactic protocol, the blood levels of the liver dysfunction markers alanine aminotransferase (ALT, (A)), aspartate aminotransferase (AST, (B)), total bilirubin (TBIL, (C)), and gamma-glutamyl transferase (GGT, (D)); renal dysfunction markers creatinine (CRE, (E)) and blood urea nitrogen (BUN, (F)); BUN/Creatinine ratio (G); estimated GFR (eGFR, (H)); and the metabolic dysfunction markers lactic acid (LAC, (I)) and glycemia (GLY, (J)) were measured. Statistical differences were assessed by two-way ANOVA followed by Dunnett’s post hoc test. * p < 0.05; ** p < 0.01; *** p < 0.001 comparing saline and endotoxemia condition. † p < 0.05, †† p < 0.01, ††† p < 0.001 compared with sham-treated condition. # p < 0.05; ## p < 0.01; ### p < 0.001 comparing non-survivor endotoxic condition between therapeutic and prophylactic protocol. NS: non-significant. Results are expressed as the mean ± SD (N = 12).
Regarding renal function, the ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols showed improved renal dysfunction with decreased blood levels of markers of renal dysfunction creatinine (CRE) and blood urea nitrogen (BUN) (Figure 7E,F, middle left panel) compared to sham-supplemented endotoxemic rats (Figure 7E,F, left panel). Furthermore, the BUN/creatinine ratio, which is an indicator of kidney disease risk, was increased in sham-supplemented endotoxemic rats (Figure 7G, left panel) but was limited in the ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols (Figure 7G, middle left panel). AsA and flavonoid-supplemented endotoxemic rats did not show modification in the BUN/creatinine ratio (Figure 7G, middle right and right panel). We also estimated GFR, which is a typical diagnostic test for chronic kidney disease, by using a validated plasma creatinine and urea-based equation to determine the estimated GFR (eGFR) in male Sprague Dawley rats [54]. The ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols showed improved eGFR (Figure 7H, middle left panel) compared to sham-supplemented endotoxemic rats (Figure 7H, left panel). However, AsA and flavonoid-supplemented endotoxemic rats did not show a change in eGFR (Figure 7H, middle right and right panel).
Next, we investigated the action of nutrient supplementation on endotoxemia-induced metabolic dysfunction. The ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols showed improved metabolic dysfunction. Endotoxemia-induced changes in the blood levels of lactic acid (LAC) and glycemia (GLY), which are markers of metabolic function (Figure 7I,J, middle left panel), were normalized compared to sham-supplemented endotoxemic rats (Figure 6I,J, left panel). However, AsA and flavonoid-supplemented endotoxemic rats did not show protection against endotoxemia-induced metabolic dysfunction (Figure 7I,J, middle right and right panel).
These results indicate that ω-3 fatty acid supplementation and, to a minor extent, flavonoid supplementation are able to preserve organ function during endotoxemia.
3.5. Impact on Survival and Risk of Death through Dietary Supplementation Based on ω-3 FA, Ascorbic Acid, or Polyphenolic Antioxidant Flavonoids in Endotoxemic Rats
To determine whether the consumption of a diet supplemented with ω-3 fatty acid, ascorbic acid, or flavonoids is able to improve survival and risk of death in endotoxemic rats, we analyzed survival curves and contingency analysis.
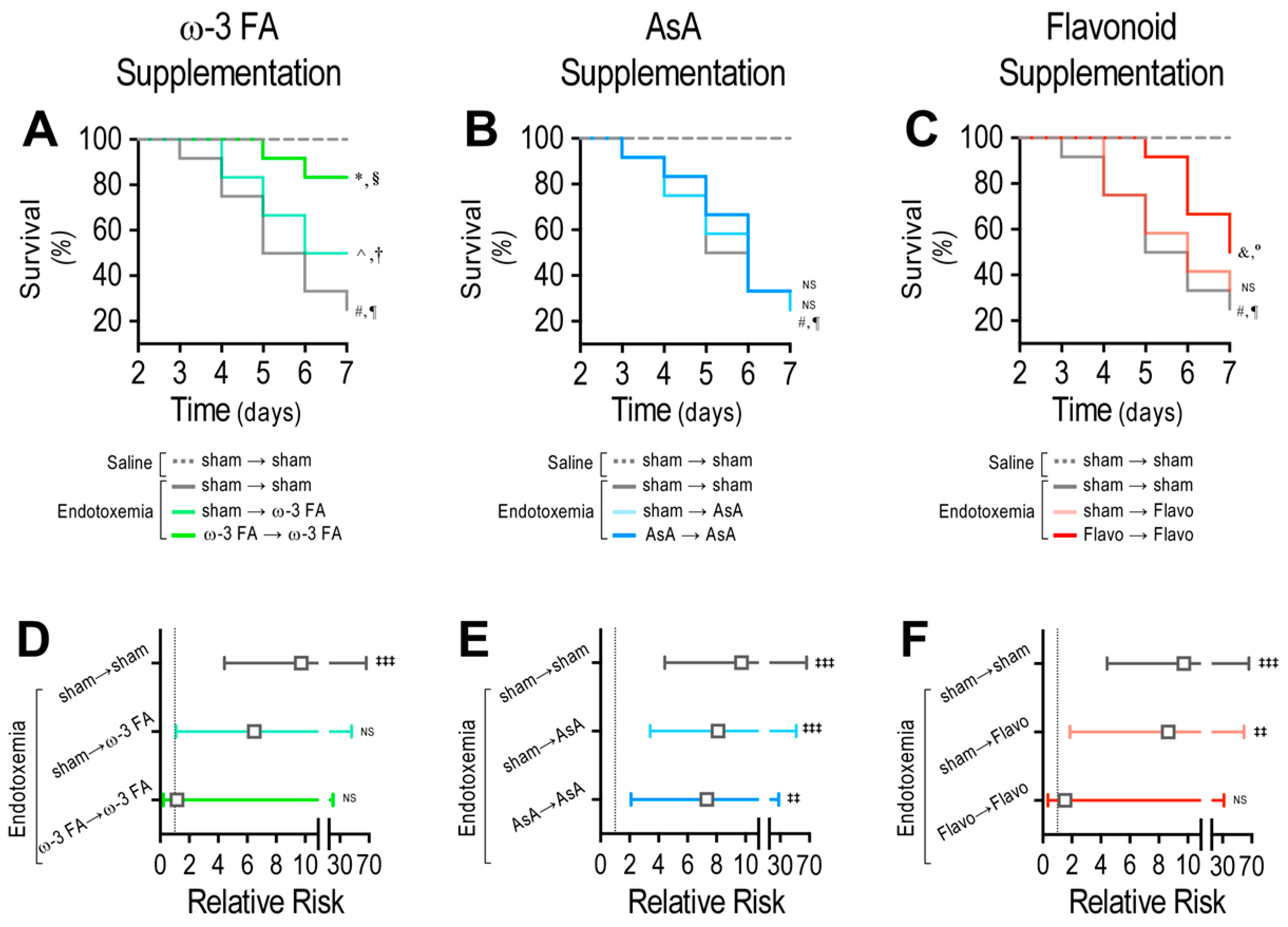
The ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols showed a significant difference compared with the endotoxemia-treated rats, as indicated by the log-rank (Mantel-Cox) test (Figure 8A). To give more weight to deaths at early time points, the Gehan–Breslow–Wilcoxon test was performed, which also showed that the ω-3 fatty acid-supplemented endotoxemic rats in the therapeutic and prophylactic protocols had an increased death incidence (Figure 8A). However, AsA-supplemented endotoxemic rats did not show any change compared with the endotoxemia-treated rats, as indicated by the log-rank (Mantel–Cox) test and the Gehan–Breslow–Wilcoxon test (Figure 8B). The endotoxemic condition in flavonoid-supplemented rats showed a significant difference when compared with the endotoxemic condition in rats subjected to the prophylactic protocol but not in those subjected to the therapeutic protocol (Figure 8C). Next, the contingency analysis showed that the ω-3 fatty acid supplementation decreased the risk of death in endotoxemic rats in both the therapeutic and prophylactic protocols compared with the endotoxemia-treated rats (Figure 8D). However, AsA-supplemented endotoxemic rats did not show any change in the risk of death (Figure 8E). The flavonoid-supplemented endotoxemic rats showed a significant decrease in the risk of death in the endotoxemic rats subjected to the prophylactic protocol but not in those subjected to the therapeutic protocol (Figure 8F).
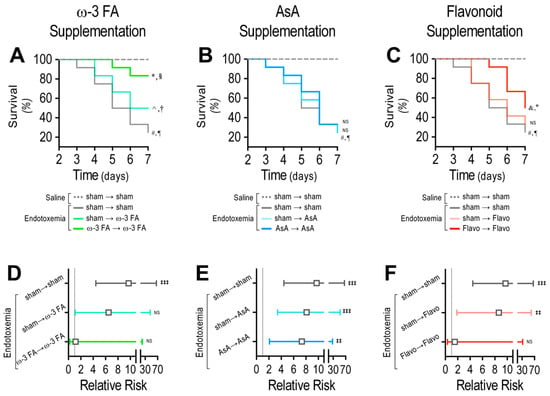
Figure 8.
Survival and risk of death through dietary ω-3 FA, ascorbic acid, or polyphenolic antioxidant flavonoid supplementation in endotoxemic rats. Survival (Kaplan–Meier) curves comparing ω-3 FA (A), ascorbic acid (B), or polyphenolic antioxidant flavonoid (C) supplementation in saline- and endotoxemia-treated rats, through a therapeutic and a prophylactic protocol. *, § = 0.01 and 0.03 (log-rank (Mantel–Cox) test and Gehan–Breslow–Wilcoxon test, respectively) when comparing with endotoxemia-treated condition. ^, † = 0.004 and 0.007 (log-rank (Mantel–Cox) test and Gehan–Breslow–Wilcoxon test, respectively), when comparing with endotoxemia-treated condition. #, ¶ = 0.005 and 0.009 (log-rank (Mantel–Cox) test and Gehan–Breslow–Wilcoxon test, respectively), when comparing with saline-treated condition. &, ° = 0.004 and 0.007 (log-rank (Mantel–Cox) test and Gehan–Breslow–Wilcoxon test, respectively), when comparing with endotoxemia-treated condition. NS: non-significant (N = 12). Contingency analyses performed to determine relative risk in endotoxemic rats supplemented with ω-3 FA (D), ascorbic acid (E), or polyphenolic antioxidant flavonoids (F) in endotoxemia-treated rats, through a therapeutic and a prophylactic protocol. ‡‡ p < 0.01; ‡‡‡ p < 0.001 (Fisher’s exact test) compared to saline-treated condition. Results are expressed as the mean ± 95% confidence interval (CI) NS: non-significant (N = 12).
Taken together, our results showed that the consumption of supplements based on ω-3 fatty acids, ascorbic acid, and polyphenolic antioxidant flavonoids was effective for improving several parameters during endotoxemia outcome through prophylactic ingestion and therapeutic usage (Supplementary Table S4).
4. Discussion
The finding that nutrition can regulate gene expression in healthy and diseased states has been widely studied. Studies have shown that polyunsaturated fatty acids regulate the expression of several genes, including DNA-binding proteins, nutrient-sensitive transcription factors including NF-κB, ROS regulation enzymes, transporters, cell adhesion, and proliferation, among several others [55,56,57,58]. Furthermore, an antioxidant-rich diet also shows modulation of the gene expression in healthy and diseased conditions depending on the antioxidant type used and the oxidative status of the tissues [59,60,61]. Consumption of vitamins C, D, and E also regulates gene expression in several cell types, including immune cells, astrocytes, and osteoblasts [62,63,64,65]. Inflammation-related genes are also differentially expressed in response to a low-calorie diet [66].
Specific nutrient consumption has been associated with gene expression during sepsis and other inflammatory conditions, thus impacting the sepsis outcome. It has been reported that consumption of ω-3 fatty acid reduces expression levels of genes involved in inflammation [39,40,67]. Interestingly, the intake of ω-3 fatty acid regulates the expression of genes involved in membrane architecture that modify intracellular signaling [68]. The beneficial effects of ω-3 fatty acids are principally promoted by eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Treatment with EPA or DHA reduces monounsaturated fatty acids and increases the polyunsaturated fatty acids in primary myometrial and leiomyoma cells [68]. The present study used fish oil containing a combination of EPA and DHA, which had a concentration of 30% relative to the total volume. This natural concentration was effective in improving the outcomes of endotoxemia in rats in this study. This finding opens broad therapeutic possibilities to use higher concentrations of EPA and DHA during sepsis, but further studies are needed to explore this issue. Notably, therapeutical actions of ω-3 fatty acids can be applied and even potentiated by combining them with vitamins, antioxidants, and other elements to alleviate several pathological conditions [69,70]. There is evidence of a beneficial effect of a combination of statins and ω-3 fatty acids on platelet function and other hemostatic mechanisms, as well as the inhibition of the inflammatory process. However, a combined treatment of statins and ω-3 fatty acid on sepsis has not been evaluated yet [71], so further experiments are needed to study this possibility. Dietary supplementation with ω-3 fatty acids also improves survival against sepsis and its complications [31,32,33]. Dietary ω-3 fatty acids increase the survival of Staphylococcus aureus-induced sepsis by reversing the deleterious effect of a high-saturated-fat diet [72]. Acute pancreatitis may cause systemic inflammatory response syndrome, organ failure, and death. The intake of ω-3 fatty acids decreases the risk of organ failure in patients with acute pancreatitis [73]. Dietary ω-3 fatty acids reduced mortality and decreased prostaglandin E2 production in Kupffer cells in a rat model of sepsis [74]. Ascorbic acid has the capacity to modulate gene expression in response to several stimuli, such has oxidative stress [41,42,43]. Interestingly, ascorbic acid induces collagen gene expression in human fibroblasts [44]. Congruently, ascorbic acid is postulated as a key mediator of the extracellular matrix production in trabecular meshwork cells in the eye, mediating the expression of collagen type I, fibronectin, and laminin [45]. Dietary ascorbic acid shows beneficial actions against endotoxemia and sepsis [34,35]. In polymicrobial sepsis, ascorbic acid regulates hepatic vasoregulatory gene expression and reduces hepatic microvascular dysfunction [75]. Interestingly, a phase I trial of intravenous ascorbic acid infusion in patients with severe sepsis showed a positive impact on multiple organ failure, reduced proinflammation biomarkers, and attenuation of vascular endothelial injury [76]. Notably, monocytes challenged with endotoxin and treated with ascorbic acid showed decreased proinflammatory cytokine production [77]. Similar results showed protection against sepsis and acute lung injury associated with sepsis by means of a decrease in the proinflammatory response, improved alveolar fluid clearance, and prevention of sepsis-associated coagulation malfunctions [78]. A polymicrobial sepsis study showed that ascorbic acid infusion diminishes oxidative stress and lipid peroxidation, modulates hepatic vasoregulatory gene expression, and decreases hepatic microvascular dysfunction [75]. A diet containing ascorbic acid combined with hydrocortisone and thiamine improves sepsis features and consequences [79]. The utilization of combined hydrocortisone, ascorbic acid, and thiamine therapy appears to be safe and provides potential benefits for the management of sepsis and septic shock, but some aspects remain controversial since their actions seem to be modest in decreasing mortality [80,81,82]. The consumption of polyphenolic antioxidant flavonoids regulates gene expression. The intake of some flavonoids such as resveratrol and epicatechins has been shown to regulate the gene expression of proteins involved in insulin secretion from β-cells and glucose transport and insulin signaling in adipocytes [83,84]. Supplementation with the flavonoid epicatechin modulates gene-expression profiles of immune cells in humans [85]. The flavonoid polyphenol quercetin upregulates neuron gene expression [86]. The flavonoid rhamnetin decreases organ failure in Gram-negative bacterial sepsis in mice [87]. Similarly, the flavonoids luteolin and icariin increase survival against polymicrobial sepsis [36,37]. The dietary flavonoid fisetin extracted from berries protects against polymicrobial sepsis-induced inflammatory response and multiple organ dysfunction [38]. The flavonoid acacetin exerts protection against sepsis [88]. Oral consumption of the flavonoid kaempferol showed decreased acute lung injury in polymicrobial sepsis [89]. Considering that a combination of several of these flavonoids was used in this study, a massive amount of work remains to clarify the individual contributions of each type of flavonoid in sepsis-induced EndMT gene expression and the deleterious outcomes of sepsis. Importantly, we evaluated the action of dietary supplementation on the expression changes induced by endotoxemia in the mesenterial endothelium because it is a relevant vascular region to study fibrosis and pathological conditions linked to fibrosis [90,91,92]. Moreover, ECs from the mesentery are a suitable and interesting model for studying dysfunctional endothelial changes [93,94].
During sepsis, endothelial dysfunction emerges as a main factor to generate MODS. The endothelium regulates vascular functions such as vascular reactivity, hemostasis, and vascular permeability [95]. During sepsis, endothelial permeability is increased and produces vascular leakage to the interstitium, generating edema [20,29,50,96]. In edema formation, blood volume into the vasculature decreases and produces severe hypotension, which increases the risk of death [20,27,51,97]. Hypotension damages microvascular perfusion and generates massive MODS, which increases mortality and the risk of death [96,98,99]. Thus, the endotoxic-induced EndMT that triggers the endothelial gene-expression profile promotes the decreased expression of the endothelial adhesion proteins and the upregulation of the fibrotic and ECM proteins. This disrupts the endothelial architecture, contributes to the endothelial hyperpermeability, and causes MODS. Endothelial fibrosis has been detected in septic ICU patients, and the expression changes of VE۔cadherin have emerged as an accurate diagnostic tool. In endotoxemic rats, the pharmacological inhibition of endotoxemia-induced endothelial fibrosis improves MODS and survival [13,20,21].
The prophylactic and the therapeutic experimental models are both valuable in a clinical context. ICU patients not affected by sepsis are at risk of undergoing sepsis because ICU non-septic patients often progress into sepsis or septic shock. Prophylactic treatment could be useful in the prevention of the adverse effects of nosocomial infections, such as catheter-induced urinary tract infections, intra-hospital acquired pneumonia, skin infections (mainly in burn patients), bloodstream infections, and surgery-induced infections [100,101]. Enteral nutrition can prevent catabolic states observed in critical illness as well as in intestinal villi atrophy, apoptosis of enterocytes, dysbiosis, and alterations of immune function in the gut [102,103]. Conversely, ω-3 fatty acid supplementation or fish oil nutrition has been used via parenteral nutrition in the septic subject, improving sepsis outcomes [104,105,106]. Further studies are needed to determine the most effective path of administration for each supplement against sepsis.
Sepsis syndrome is the main cause of death in ICU patients, and the current therapy is unsatisfactory. Thus, the results demonstrating that nutrient supplementation inhibited endotoxemia-induced endothelial fibrosis, hypotension, MODS, and mortality during endotoxemia are of great importance. Therefore, specific nutrient consumption could be a therapeutic alternative against deleterious effects of sepsis and other endothelial fibrosis-mediated pathologies.
5. Conclusions
Our results indicate that the consumption of supplements based on ω-3 fatty acids, ascorbic acid, and polyphenolic antioxidant flavonoids can decrease the endotoxemia-induced gene-expression change associated with endothelial fibrosis. Furthermore, endotoxemia-induced mortality was decreased by the consumption of supplements based on ω-3 fatty acids, mainly via prophylactic ingestion as well as in therapeutic usage, and by the consumption of polyphenolic antioxidant flavonoids by means of prophylactic ingestion, while the consumption of supplement of ascorbic acid showed no significant difference. Thus, our findings indicated that specific nutrient consumption improves sepsis outcomes and should be considered in treatment against sepsis and other systemic inflammatory diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12030659/s1, Table S1: Correlation analyses between mRNA expression of EndMT genes and increased permeability in the mesentery in endotoxemic conditions; Table S2: Correlation analyses between mRNA expression of EndMT genes and increased permeability in the liver in endotoxemic conditions; Table S3: Correlation analyses between mRNA expression of EndMT genes and increased permeability in the kidney in endotoxemic conditions; Table S4: Summary of actions of dietary ω-3 fatty acids, ascorbic acid, and polyphenolic antioxidant flavonoid supplements on gene expression, organ failure, and mortality in endotoxemia-induced septic rats; Figure S1: Impact on EndMT protein expression through dietary supplementation based on ω-3 FA, ascorbic acid, or polyphenolic antioxidant flavonoids in endotoxemic rats.
Author Contributions
Conceptualization, Y.P., C.E., C.A.R., C.C.-V., J.F.S. and F.S.; data curation, Y.P., C.E., C.G.F., C.A.R. and F.S.; formal analysis, Y.P., C.E., C.A.R., C.C.-V., J.F.S. and F.S.; funding acquisition, Y.P., C.A.R., C.C.-V., J.F.S. and F.S.; investigation, Y.P., C.E., C.G.F., C.A.R., C.C.-V., J.F.S. and F.S.; methodology, Y.P., C.E., C.G.F. and C.A.R.; project administration, C.G.F., C.C.-V., J.F.S. and F.S.; resources, C.E., C.G.F., C.C.-V., J.F.S. and F.S.; software, Y.P., C.E. and F.S.; supervision, C.E., C.C.-V., J.F.S. and F.S.; validation, C.E., C.C.-V. and F.S.; visualization, Y.P., C.E. and F.S.; writing—original draft, Y.P., C.E., C.A.R. and F.S.; writing—review and editing, Y.P., C.E., C.G.F., C.A.R., C.C.-V., J.F.S. and F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by research grants from Agencia Nacional de Investigación y Desarrollo (ANID)—Fondo Nacional de Desarrollo Científico y Tecnológico FONDECYT Grants 1201039 (F.S.), 1210903 (C.G.F.), 1191300 (C.A.R.), 1200944 (C.C.-V.). ANID—Millennium Science Initiative Program—ICN09_016/ICN 2021_045: Millennium Institute on Immunology and Immunotherapy (ICN09_016/ICN 2021_045; former P09/016-F) (F.S., C.A.R., C.C.-V.). The Millennium Nucleus of Ion Channel-Associated Diseases is a Millennium Nucleus of the Millennium Scientific Initiative, ANID, Ministry of Science, Technology, Knowledge and Innovation, Chile (NCN19_168) (F.S.). BASAL Grant—CEDENNA from the ANID, Government of Chile AFB180001 (C.C.-V.). Ministry of Education, Science and Technological Development of the Republic of Serbia (grant number 451-03-9/2021-14/200015 and 451-03-47/2023-01/200015 (J.F.S.)). ANID-PCHA/Doctorado Nacional 21220694 (Y.P.). ANID-PCHA/Gastos Operacionales proyecto de tesis Doctoral 242220039 (Y.P.).
Institutional Review Board Statement
The experimental protocols were approved by the Commission of Bioethics and Biosafety of Universidad Andres Bello (N° 002/2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet Lond. Engl. 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Gatica, S.; Eltit, F.; Santibanez, J.F.; Varela, D.; Cabello-Verrugio, C.; Simon, F. Expression Suppression and Activity Inhibition of TRPM7 Regulate Cytokine Production and Multiple Organ Dysfunction Syndrome During Endotoxemia: A New Target for Sepsis. Curr. Mol. Med. 2019, 19, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.R. Clinical Studies on Cytokines in Sepsis: Role of Serum Cytokines in the Development of Multiple-Systems Organ Failure. Nephrol. Dial. Transplant. 1994, 9 (Suppl. S4), 94–98. [Google Scholar]
- Remick, D.G. Pathophysiology of Sepsis. Am. J. Pathol. 2007, 170, 1435–1444. [Google Scholar] [CrossRef]
- Riedemann, N.C.; Guo, R.-F.; Ward, P.A. The Enigma of Sepsis. J. Clin. Investig. 2003, 112, 460–467. [Google Scholar] [CrossRef]
- Karima, R.; Matsumoto, S.; Higashi, H.; Matsushima, K. The Molecular Pathogenesis of Endotoxic Shock and Organ Failure. Mol. Med. Today 1999, 5, 123–132. [Google Scholar] [CrossRef]
- Simon, F.; Fernández, R. Early Lipopolysaccharide-Induced Reactive Oxygen Species Production Evokes Necrotic Cell Death in Human Umbilical Vein Endothelial Cells. J. Hypertens. 2009, 27, 1202–1216. [Google Scholar] [CrossRef]
- Sarmiento, D.; Montorfano, I.; Cáceres, M.; Echeverría, C.; Fernández, R.; Cabello-Verrugio, C.; Cerda, O.; Tapia, P.; Simon, F. Endotoxin-Induced Vascular Endothelial Cell Migration Is Dependent on TLR4/NF-ΚB Pathway, NAD(P)H Oxidase Activation, and Transient Receptor Potential Melastatin 7 Calcium Channel Activity. Int. J. Biochem. Cell Biol. 2014, 55, 11–23. [Google Scholar] [CrossRef]
- Dauphinee, S.M.; Karsan, A. Lipopolysaccharide Signaling in Endothelial Cells. Lab. Investig. 2005, 86, 9–22. [Google Scholar] [CrossRef]
- Prado, Y.; Tapia, P.; Eltit, F.; Reyes-Martínez, C.; Feijóo, C.G.; Llancalahuen, F.M.; Riedel, C.A.; Cabello-Verrugio, C.; Stehberg, J.; Simon, F. Sepsis-Induced Coagulopathy Phenotype Induced by Oxidized High-Density Lipoprotein Associated with Increased Mortality in Septic-Shock Patients. Antioxidants 2023, 12, 543. [Google Scholar] [CrossRef]
- Echeverría, C.; Montorfano, I.; Sarmiento, D.; Becerra, A.; Nuñez-Villena, F.; Figueroa, X.F.; Cabello-Verrugio, C.; Elorza, A.A.; Riedel, C.; Simon, F. Lipopolysaccharide Induces a Fibrotic-like Phenotype in Endothelial Cells. J. Cell Mol. Med. 2013, 17, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, A.; Olivares, P.; Gatica, S.; Villegas, V.; Echeverria, C.; Cabello-Verrugio, C.; Simon, F. Endotoxemia-Induced Endothelial Fibrosis Inhibition Improves Hypotension, Tachycardia, Multiple Organ Dysfunction Syndrome, Cytokine Response, Oxidative Stress, and Survival. Lab. Investig. 2019, 99, 1173–1192. [Google Scholar] [CrossRef]
- Echeverría, C.; Montorfano, I.; Hermosilla, T.; Armisén, R.; Velásquez, L.A.; Cabello-Verrugio, C.; Varela, D.; Simon, F. Endotoxin Induces Fibrosis in Vascular Endothelial Cells through a Mechanism Dependent on Transient Receptor Protein Melastatin 7 Activity. PLoS ONE 2014, 9, e94146. [Google Scholar] [CrossRef] [PubMed]
- Medici, D.; Shore, E.M.; Lounev, V.Y.; Kaplan, F.S.; Kalluri, R.; Olsen, B.R. Conversion of Vascular Endothelial Cells into Multipotent Stem-like Cells. Nat. Med. 2010, 16, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Potenta, S.; Xie, L.; Zeisberg, M.; Kalluri, R. Discovery of Endothelial to Mesenchymal Transition as a Source for Carcinoma-Associated Fibroblasts. Cancer Res. 2007, 67, 10123–10128. [Google Scholar] [CrossRef]
- Echeverría, C.; Montorfano, I.; Tapia, P.; Riedel, C.; Cabello-Verrugio, C.; Simon, F. Endotoxin-Induced Endothelial Fibrosis Is Dependent on Expression of Transforming Growth Factors Β1 and Β2. Infect. Immun. 2014, 82, 3678–3686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Montorfano, I.; Becerra, A.; Cerro, R.; Echeverría, C.; Sáez, E.; Morales, M.G.; Fernández, R.; Cabello-Verrugio, C.; Simon, F. Oxidative Stress Mediates the Conversion of Endothelial Cells into Myofibroblasts via a TGF-Β1 and TGF-Β2-Dependent Pathway. Lab. Investig. 2014, 94, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, C.; Montorfano, I.; Cabello-Verrugio, C.; Armisén, R.; Varela, D.; Simon, F. Suppression of Transient Receptor Potential Melastatin 4 Expression Promotes Conversion of Endothelial Cells into Fibroblasts via Transforming Growth Factor/Activin Receptor-like Kinase 5 Pathway. J. Hypertens. 2015, 33, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Tapia, P.; Gatica, S.; Cortés-Rivera, C.; Otero, C.; Becerra, A.; Riedel, C.A.; Cabello-Verrugio, C.; Kalergis, A.M.; Simon, F. Circulating Endothelial Cells from Septic Shock Patients Convert to Fibroblasts Are Associated with the Resuscitation Fluid Dose and Are Biomarkers for Survival Prediction. Crit. Care Med. 2019, 47, 942–950. [Google Scholar] [CrossRef]
- Rojas, M.; Prado, Y.; Tapia, P.; Carreño, L.J.; Cabello-Verrugio, C.; Simon, F. Oxidized High-Density Lipoprotein Induces Endothelial Fibrosis Promoting Hyperpermeability, Hypotension, and Increased Mortality. Antioxidants 2022, 11, 2469. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-Mesenchymal Transition Contributes to Cardiac Fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.; Muñoz-Durango, N.; Riedel, C.A.; Echeverría, C.; Kalergis, A.M.; Cabello-Verrugio, C.; Simon, F. Endothelial-to-Mesenchymal Transition: Cytokine-Mediated Pathways That Determine Endothelial Fibrosis under Inflammatory Conditions. Cytokine Growth Factor Rev. 2017, 33, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Becerra, A.; Rojas, M.; Vallejos, A.; Villegas, V.; Pérez, L.; Cabello-Verrugio, C.; Simon, F. Endothelial Fibrosis Induced by Suppressed STAT3 Expression Mediated by Signaling Involving the TGF-Β1/ALK5/Smad Pathway. Lab. Investig. 2017, 97, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, D.; Montorfano, I.; Cerda, O.; Cáceres, M.; Becerra, A.; Cabello-Verrugio, C.; Elorza, A.A.; Riedel, C.; Tapia, P.; Velásquez, L.A.; et al. Increases in Reactive Oxygen Species Enhance Vascular Endothelial Cell Migration through a Mechanism Dependent on the Transient Receptor Potential Melastatin 4 Ion Channel. Microvasc. Res. 2015, 98, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.; Vallejos, A.; Echeverria, C.; Varela, D.; Cabello-Verrugio, C.; Simon, F. OxHDL Controls LOX-1 Expression and Plasma Membrane Localization through a Mechanism Dependent on NOX/ROS/NF-ΚB Pathway on Endothelial Cells. Lab. Investig. 2019, 99, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Tapia, P.; Chinchón, E.; Morales, D.; Stehberg, J.; Simon, F. Effectiveness of Short-Term 6-Hour High-Volume Hemofiltration during Refractory Severe Septic Shock. J. Trauma Acute Care Surg. 2012, 72, 1228–1238. [Google Scholar] [CrossRef]
- Barrionuevo, N.; Gatica, S.; Olivares, P.; Cabello-Verrugio, C.; Simon, F. Endothelial Cells Exhibit Two Waves of P-Selectin Surface Aggregation Under Endotoxic and Oxidative Conditions. Protein J. 2019, 38, 667–674. [Google Scholar] [CrossRef]
- Echeverria, C.; Eltit, F.; Santibanez, J.F.; Gatica, S.; Cabello-Verrugio, C.; Simon, F. Endothelial Dysfunction in Pregnancy Metabolic Disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1866, 165414. [Google Scholar] [CrossRef]
- Vallejos, A.; Olivares, P.; Varela, D.; Echeverria, C.; Cabello-Verrugio, C.; Pérez-Leighton, C.; Simon, F. Preventive Leptin Administration Protects Against Sepsis Through Improving Hypotension, Tachycardia, Oxidative Stress Burst, Multiple Organ Dysfunction, and Increasing Survival. Front. Physiol. 2018, 9, 1800. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Zhao, Y.; Luo, Y.; Tong, H.; Su, L. Correlation Analysis of Omega-3 Fatty Acids and Mortality of Sepsis and Sepsis-Induced ARDS in Adults: Data from Previous Randomized Controlled Trials. Nutr. J. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.; Schlegel, M.; Theurer, J.; Frohnmeyer, H.; Adolph, M.; Heijink, M.; Giera, M.; Rosenberger, P.; Mirakaj, V. Resolution of Inflammation and Sepsis Survival Are Improved by Dietary Ω-3 Fatty Acids. Cell Death Differ. 2018, 25, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Xie, Y.-J.; Liu, Z.-M.; Chen, W.-B.; Zhang, R.; Ye, H.-X.; Wang, W.; Liu, X.-Y.; Chen, H.-S. Omega-3 Fatty Acids Impair MiR-1-3p-Dependent Notch3 down-Regulation and Alleviate Sepsis-Induced Intestinal Injury. Mol. Med. 2022, 28, 9. [Google Scholar] [CrossRef] [PubMed]
- Kawade, N.; Tokuda, Y.; Tsujino, S.; Aoyama, H.; Kobayashi, M.; Murai, A.; Horio, F. Dietary Intake of Ascorbic Acid Attenuates Lipopolysaccharide-Induced Sepsis and Septic Inflammation in ODS Rats. J. Nutr. Sci. Vitaminol. 2018, 64, 404–411. [Google Scholar] [CrossRef]
- Marik, P.E. Hydrocortisone, Ascorbic Acid and Thiamine (HAT Therapy) for the Treatment of Sepsis. Focus on Ascorbic Acid. Nutrients 2018, 10, 1762. [Google Scholar] [CrossRef]
- Rungsung, S.; Singh, T.U.; Perumalraja, K.; Mahobiya, A.; Sharma, M.; Lingaraju, M.C.; Parida, S.; Sahoo, M.; Kumar, D. Luteolin Alleviates Vascular Dysfunctions in CLP-Induced Polymicrobial Sepsis in Mice. Pharmacol. Rep. 2022, 74, 1054–1068. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, L.; Wang, Z.; Xie, H.; Feng, Y.; Suo, J.; Wang, M.; Shang, W.; Feng, G. Icariin Improves Sepsis-Induced Mortality and Acute Kidney Injury. Pharmacology 2018, 102, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Wu, X.; Guo, Y.; Cheng, W.; Qian, F. Fisetin Alleviates Sepsis-Induced Multiple Organ Dysfunction in Mice via Inhibiting P38 MAPK/MK2 Signaling. Acta Pharmacol. Sin. 2020, 41, 1348–1356. [Google Scholar] [CrossRef]
- Tu, T.; Li, B.; Li, X.; Zhang, B.; Xiao, Y.; Li, J.; Qin, F.; Liu, N.; Sun, C.; Liu, Q.; et al. Dietary ω-3 Fatty Acids Reduced Atrial Fibrillation Vulnerability via Attenuating Myocardial Endoplasmic Reticulum Stress and Inflammation in a Canine Model of Atrial Fibrillation. J. Cardiol. 2022, 79, 194–201. [Google Scholar] [CrossRef]
- Singh, J.E. Dietary Sources of Omega-3 Fatty Acids Versus Omega-3 Fatty Acid Supplementation Effects on Cognition and Inflammation. Curr. Nutr. Rep. 2020, 9, 264–277. [Google Scholar] [CrossRef]
- Belin, S.; Kaya, F.; Burtey, S.; Fontes, M. Ascorbic Acid and Gene Expression: Another Example of Regulation of Gene Expression by Small Molecules? Curr. Genom. 2010, 11, 52–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hitomi, K.; Tsukagoshi, N. Subcellular Biochemistry, Ascorbic Acid: Biochemistry and Biomedical Cell Biology. Subcell. Biochem. 1996, 25, 41–56. [Google Scholar] [PubMed]
- Grano, A.; Tullio, M.C.D. Ascorbic Acid as a Sensor of Oxidative Stress and a Regulator of Gene Expression: The Yin and Yang of Vitamin C. Med. Hypotheses 2007, 69, 953–954. [Google Scholar] [CrossRef] [PubMed]
- Chojkier, M.; Houglum, K.; Solis-Herruzo, J.; Brenner, D.A. Stimulation of Collagen Gene Expression by Ascorbic Acid in Cultured Human Fibroblasts. A Role for Lipid Peroxidation? J. Biol. Chem. 1989, 264, 16957–16962. [Google Scholar] [CrossRef] [PubMed]
- Sawaguchi, S.; Yue, B.Y.; Chang, I.L.; Wong, F.; Higginbotham, E.J. Ascorbic Acid Modulates Collagen Type I Gene Expression by Cells from an Eye Tissue—Trabecular Meshwork. Cell. Mol. Biol. 1992, 38, 587–604. [Google Scholar] [PubMed]
- Martín, M.Á.; Ramos, S. Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases. Nutrients 2021, 13, 850. [Google Scholar] [CrossRef]
- El-Haleim, E.A.A.; Sallam, N.A. Vitamin D Modulates Hepatic MicroRNAs and Mitigates Tamoxifen-induced Steatohepatitis in Female Rats. Fundam. Clin. Pharm. 2022, 36, 338–349. [Google Scholar] [CrossRef]
- Jenks, D.B.; Ehrlich, Y.; Spolnik, K.; Gregory, R.L.; Yassen, G.H. Residual Antibiofilm Effects of Various Concentrations of Double Antibiotic Paste Used during Regenerative Endodontics after Different Application Times. Arch. Oral Biol. 2016, 70, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Khazri, O.; Mezni, A.; Limam, F.; Aouani, E. Bleomycin-Induced Damage in Rat Lung: Protective Effect of Grape Seed and Skin Extract. Dose Response 2022, 20, 15593258221131648. [Google Scholar] [CrossRef]
- Gatica, S.; Villegas, V.; Vallejos, A.; Olivares, P.; Aballai, V.; Lagos-Meza, F.; Echeverria, C.; Cabello-Verrugio, C.; Varela, D.; Simon, F. TRPM7 Mediates Kidney Injury, Endothelial Hyperpermeability and Mortality during Endotoxemia. Lab. Investig. 2019, 100, 234–249. [Google Scholar] [CrossRef]
- Prado, Y.; Pérez, L.; Eltit, F.; Echeverría, C.; Llancalahuen, F.M.; Tapia, P.; González, P.A.; Kalergis, A.M.; Cabello-Verrugio, C.; Simon, F. Procoagulant Phenotype Induced by Oxidized High-Density Lipoprotein Associates with Acute Kidney Injury and Death. Thromb. Res. 2023, 223, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Semmler, A.; Frisch, C.; Debeir, T.; Ramanathan, M.; Okulla, T.; Klockgether, T.; Heneka, M.T. Long-Term Cognitive Impairment, Neuronal Loss and Reduced Cortical Cholinergic Innervation after Recovery from Sepsis in a Rodent Model. Exp. Neurol. 2007, 204, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.B.; Prediger, R.D.; Silva, M.V.; Sousa, J.B.D.; Carneiro, F.P.; Gasbarri, A.; Tomaz, C.; Queiroz, A.J.; Martins, N.T.; Ferreira, V.M. Role of Nicotine on Cognitive and Behavioral Deficits in Sepsis-Surviving Rats. Brain Res. 2013, 1507, 74–82. [Google Scholar] [CrossRef]
- Besseling, P.J.; Pieters, T.T.; Nguyen, I.T.N.; Bree, P.M.D.; Willekes, N.; Dijk, A.H.; Bovée, D.M.; Hoorn, E.J.; Rookmaaker, M.B.; Gerritsen, K.G.; et al. A Plasma Creatinine- and Urea-Based Equation to Estimate Glomerular Filtration Rate in Rats. Am. J. Physiol. Renal Physiol. 2021, 320, F518–F524. [Google Scholar] [CrossRef] [PubMed]
- Roche, H.M. Dietary Lipids and Gene Expression. Biochem. Soc. Trans. 2004, 32, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, A.; Clarke, S.D.; Heird, W.C. Polyunsaturated Fatty Acids and Gene Expression. Curr. Opin. Clin. Nutr. 2004, 7, 151–156. [Google Scholar] [CrossRef]
- Wahle, K.W.J.; Rotondo, D.; Heys, S.D. Polyunsaturated Fatty Acids and Gene Expression in Mammalian Systems. Proc. Nutr. Soc. 2003, 62, 349–360. [Google Scholar] [CrossRef]
- Corral-Jara, K.F.; Cantini, L.; Poupin, N.; Ye, T.; Rigaudière, J.P.; Vincent, S.D.S.; Pinel, A.; Morio, B.; Capel, F. An Integrated Analysis of MiRNA and Gene Expression Changes in Response to an Obesogenic Diet to Explore the Impact of Transgenerational Supplementation with Omega 3 Fatty Acids. Nutrients 2020, 12, 3864. [Google Scholar] [CrossRef]
- Poulsen, H.E.; Jensen, B.R.; Weimann, A.; Jensen, S.A.; Sørensen, M.; Loft, S. Antioxidants, DNA Damage and Gene Expression. Free Radical Res. 2000, 33, S33–S39. [Google Scholar]
- Palmer, H.J.; Paulson, K.E. Reactive Oxygen Species and Antioxidants in Signal Transduction and Gene Expression. Nutr. Rev. 2009, 55, 353–361. [Google Scholar] [CrossRef]
- Feng, N.-H.; Chu, S.-J.; Wang, D.; Hsu, K.; Lin, C.-H.; Lin, H.-I. Effects of Various Antioxidants on Endotoxin-Induced Lung Injury and Gene Expression: MRNA Expressions of MnSOD, Interleukin-1beta and INOS. Chin. J. Physiol. 2004, 47, 111–120. [Google Scholar] [PubMed]
- Abdelgawad, L.M.; Abdelaziz, A.M.; Sabry, D.; Abdelgwad, M. Influence of Photobiomodulation and Vitamin D on Osteoblastic Differentiation of Human Periodontal Ligament Stem Cells and Bone-like Tissue Formation through Enzymatic Activity and Gene Expression. Biomol. Concepts 2020, 11, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Molano, A.; Meydani, S.N. Vitamin E, Signalosomes and Gene Expression in T Cells. Mol. Asp. Med. 2012, 33, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Han, S.N.; Adolfsson, O.; Lee, C.; Prolla, T.A.; Ordovas, J.; Meydani, S.N. Vitamin E and Gene Expression in Immune Cells. Ann. N. Y. Acad. Sci. 2004, 1031, 96–101. [Google Scholar] [CrossRef]
- Kao, P.-F.; Lee, W.-S.; Liu, J.-C.; Chan, P.; Tsai, J.-C.; Hsu, Y.-H.; Chang, W.-Y.; Cheng, T.-H.; Liao, S.-S. Downregulation of Superoxide Dismutase Activity and Gene Expression in Cultured Rat Brain Astrocytes after Incubation with Vitamin C. Pharmacology 2003, 69, 1–6. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Parra, D.; Milagro, F.I.; Goyenechea, E.; Larrarte, E.; Margareto, J.; Martnez, J.A. Differential Expression of Oxidative Stress and Inflammation Related Genes in Peripheral Blood Mononuclear Cells in Response to a Low-Calorie Diet: A Nutrigenomics Study. Omics J. Integr. Biol. 2008, 12, 251–261. [Google Scholar] [CrossRef]
- Ulven, S.M.; Holven, K.B. Metabolomic and Gene Expression Analysis to Study the Effects of Dietary Saturated and Polyunsaturated Fats. Curr. Opin. Lipidol. 2020, 31, 15–19. [Google Scholar] [CrossRef]
- Islam, M.S.; Castellucci, C.; Fiorini, R.; Greco, S.; Gagliardi, R.; Zannotti, A.; Giannubilo, S.R.; Ciavattini, A.; Frega, N.G.; Pacetti, D.; et al. Omega-3 Fatty Acids Modulate the Lipid Profile, Membrane Architecture, and Gene Expression of Leiomyoma Cells. J. Cell. Physiol. 2018, 233, 7143–7156. [Google Scholar] [CrossRef]
- Kemse, N.; Kale, A.; Chavan-Gautam, P.; Joshi, S. Increased Intake of Vitamin B 12, Folate, and Omega-3 Fatty Acids to Improve Cognitive Performance in Offspring Born to Rats with Induced Hypertension during Pregnancy. Food Funct. 2018, 9, 3872–3883. [Google Scholar] [CrossRef]
- Kemse, N.; Sundrani, D.; Kale, A.; Joshi, S. Maternal Micronutrients, Omega-3 Fatty Acids and Gene Expression of Angiogenic and Inflammatory Markers in Pregnancy Induced Hypertension Rats. Arch. Med. Res. 2017, 48, 414–422. [Google Scholar] [CrossRef]
- Makris, G.C.; Geroulakos, G.; Makris, M.C.; Mikhailidis, D.P.; Falagas, M.E. The Pleiotropic Effects of Statins and Omega-3 Fatty Acids against Sepsis: A New Perspective. Expert Opin. Investig. Drugs 2010, 19, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Svahn, S.L.; Ulleryd, M.A.; Grahnemo, L.; Ståhlman, M.; Borén, J.; Nilsson, S.; Jansson, J.-O.; Johansson, M.E. Dietary Omega-3 Fatty Acids Increase Survival and Decrease Bacterial Load in Mice Subjected to Staphylococcus Aureus-Induced Sepsis. Infect. Immun. 2016, 84, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Wolbrink, D.R.J.; Grundsell, J.R.; Witteman, B.; van de Poll, M.; van Santvoort, H.C.; Issa, E.; Dennison, A.; van Goor, H.; Besselink, M.G.; Bouwense, S.A.W.; et al. Are Omega-3 Fatty Acids Safe and Effective in Acute Pancreatitis or Sepsis? A Systematic Review and Meta-Analysis. Clin. Nutr. 2020, 39, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Barton, R.G.; Wells, C.L.; Carlson, A.; Singh, R.; Sullivan, J.J.; Cerra, F.B. Dietary Omega-3 Fatty Acids Decrease Mortality and Kupffer Cell Prostaglandin E2 Production in a Rat Model of Chronic Sepsis. J. Trauma Inj. Infect. Crit. Care 1991, 31, 768–774. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, S.-M. Effect of Ascorbic Acid on Hepatic Vasoregulatory Gene Expression during Polymicrobial Sepsis. Life Sci. 2004, 75, 2015–2026. [Google Scholar] [CrossRef]
- Fowler, A.A.; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I Safety Trial of Intravenous Ascorbic Acid in Patients with Severe Sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef]
- Schmidt, T.; Kahn, R.; Kahn, F. Ascorbic Acid Attenuates Activation and Cytokine Production in Sepsis-like Monocytes. J. Leukoc. Biol. 2022, 112, 491–498. [Google Scholar] [CrossRef]
- Fisher, B.J.; Kraskauskas, D.; Martin, E.J.; Farkas, D.; Wegelin, J.A.; Brophy, D.; Ward, K.R.; Voelkel, N.F.; Fowler, A.A.; Natarajan, R. Mechanisms of Attenuation of Abdominal Sepsis Induced Acute Lung Injury by Ascorbic Acid. Am. J. Physiol.-Lung Cell. 2012, 303, L20–L32. [Google Scholar] [CrossRef]
- Kim, J.; Stolarski, A.; Zhang, Q.; Wee, K.; Remick, D. Hydrocortisone, ascorbic acid, and thiamine therapy decrease renal oxidative stress and acute kidney injury in murine sepsis. Shock 2022, 58, 426–433. [Google Scholar] [CrossRef]
- Ragoonanan, D.; Tran, N.; Modi, V.; Nickelsen, P.M. Unanswered Questions on the Use of Hydrocortisone, Ascorbic Acid, and Thiamine Therapy in Sepsis and Septic Shock. Am. J. Health Syst. Pharm. 2022, 79, 1626–1633. [Google Scholar] [CrossRef]
- Wang, K.; Yin, L.; Song, Y.; Zhang, M.; Lu, Y.; Wang, S. The Use of Hydrocortisone, Ascorbic Acid and Thiamine in Patients with Sepsis and Septic Shock—A Systematic Review. J. Pharm. Pract. 2022, 35, 89719002210971. [Google Scholar] [CrossRef] [PubMed]
- Assouline, B.; Faivre, A.; Verissimo, T.; Sangla, F.; Berchtold, L.; Giraud, R.; Bendjelid, K.; Sgardello, S.; Elia, N.; Pugin, J.; et al. Thiamine, Ascorbic Acid, and Hydrocortisone as a Metabolic Resuscitation Cocktail in Sepsis: A Meta-Analysis of Randomized Controlled Trials with Trial Sequential Analysis. Crit. Care Med. 2021, 49, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Vetterli, L.; Brun, T.; Giovannoni, L.; Bosco, D.; Maechler, P. Resveratrol Potentiates Glucose-Stimulated Insulin Secretion in INS-1E β-Cells and Human Islets through a SIRT1-Dependent Mechanism. J. Biol. Chem. 2011, 286, 6049–6060. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Volti, G.L.; Galvano, F.; et al. Cyanidin-3-O-β-Glucoside and Protocatechuic Acid Exert Insulin-Like Effects by Upregulating PPARγ Activity in Human Omental Adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef]
- Esser, D.; Geleijnse, J.M.; Matualatupauw, J.C.; Dower, J.I.; Kromhout, D.; Hollman, P.C.H.; Afman, L.A. Pure Flavonoid Epicatechin and Whole Genome Gene Expression Profiles in Circulating Immune Cells in Adults with Elevated Blood Pressure: A Randomised Double-Blind, Placebo-Controlled, Crossover Trial. PLoS ONE 2018, 13, e0194229. [Google Scholar] [CrossRef] [PubMed]
- Uzunallı, G.; Bora-Tatar, G.; Dayangaç-Erden, D.; Erdem-Yurter, H. Effects of Flavonoid Quercetin on Survival of Motor Neuron Gene Expression. Cell Biol. Int. 2015, 39, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Krishnan, M.; Kim, M.; Yoon, Y.K.; Kim, Y. Rhamnetin, a Natural Flavonoid, Ameliorates Organ Damage in a Mouse Model of Carbapenem-Resistant Acinetobacter Baumannii-Induced Sepsis. Int. J. Mol. Sci. 2022, 23, 12895. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Rong, Y.; Wang, Y.; Guo, Y.; Shan, L.; Yu, X.; Li, L.; Si, J.; Li, X.; Ma, K. A Systematic Study of the Mechanism of Acacetin Against Sepsis Based on Network Pharmacology and Experimental Validation. Front. Pharmacol. 2021, 12, 683645. [Google Scholar] [CrossRef]
- Rabha, D.J.; Singh, T.U.; Rungsung, S.; Kumar, T.; Parida, S.; Lingaraju, M.C.; Paul, A.; Sahoo, M.; Kumar, D. Kaempferol Attenuates Acute Lung Injury in Caecal Ligation and Puncture Model of Sepsis in Mice. Exp. Lung Res. 2018, 44, 63–78. [Google Scholar] [CrossRef]
- Bösch, F.; Altendorf-Hofmann, A.; Koliogiannis, V.; Ilhan, H.; Jacob, S.; Pretzsch, E.; Nölting, S.; Werner, J.; Klauschen, F.; Auernhammer, C.J.; et al. Immuno-Histochemical Correlation of Fibrosis-Related Markers with the Desmoplastic Reaction of the Mesentery in Small Intestine Neuroendocrine Neoplasms. J. Cancer Res. Clin. 2022, 148, 1–9. [Google Scholar] [CrossRef]
- Chaves, F.J.; Cruz, I.; Lopes, C.; Morais, M.D. Polyposis Coli Associated with Fibrosis of Mesentery, Mesocolon and Retroperitoneal Tissues: A Rare Variant of Gardner’s Syndrome. Am. J. Gastroenterol. 1976, 65, 163–167. [Google Scholar] [PubMed]
- Meng, J.; Mao, Y.; Zhou, J.; Chen, Z.; Huang, S.; Wang, Y.; Huang, L.; Zhang, R.; Shen, X.; Lv, W.; et al. Mesenteric Abnormalities Play an Important Role in Grading Intestinal Fibrosis in Patients with Crohn’s Disease: A Computed Tomography and Clinical Marker-Based Nomogram. Ther. Adv. Gastroenterol. 2022, 15, 17562848221122504. [Google Scholar] [CrossRef] [PubMed]
- Vacek, L.; Bravený, P. Effect of Angiotensin II on Blood Pressure and on Microvascular Beds in Mesentery, Skin, and Skeletal Muscle of the Rat. Microvasc. Res. 1978, 16, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Nagy, J.A.; Manseau, E.J.; Dvorak, H.F. Vascular Permeability Factor/Vascular Endothelial Growth Factor-Mediated Signaling in Mouse Mesentery Vascular Endothelium. Cancer Res. 1998, 58, 1278–1284. [Google Scholar]
- Aird, W.C. Endothelium as an Organ System. Crit. Care Med. 2004, 32, S271–S279. [Google Scholar] [CrossRef]
- Lee, W.L.; Slutsky, A.S. Sepsis and Endothelial Permeability. N. Engl. J. Med. 2010, 363, 689–691. [Google Scholar] [CrossRef]
- Siddall, E.; Khatri, M.; Radhakrishnan, J. Capillary Leak Syndrome: Etiologies, Pathophysiology, and Management. Kidney Int. 2017, 92, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Spicer, A.; Calfee, C.S. Fixing the Leak: Targeting the Vascular Endothelium in Sepsis. Crit. Care 2012, 16, 177. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, D.; Kong, L.; Li, M.; Feng, Z.; Shuai, B.; Wang, L.; Wei, Y.; Li, H.; Wu, S.; et al. Intermedin Protects against Sepsis by Concurrently Re-Establishing the Endothelial Barrier and Alleviating Inflammatory Responses. Nat. Commun. 2018, 9, 2644. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Dickter, J.K. Nosocomial Infections A History of Hospital-Acquired Infections. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 637–652. [Google Scholar] [CrossRef]
- Tan, X.Y.D.; Wiseman, T.; Betihavas, V. Risk Factors for Nosocomial Infections and/or Sepsis in Adult Burns Patients: An Integrative Review. Intensive Crit. Care Nurs. 2022, 73, 103292. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.-C.; Arabi, Y.M.; Berger, M.M.; Casaer, M.; McClave, S.; Montejo-González, J.C.; Peake, S.; Blaser, A.R.; Berghe, G.V.D.; van Zanten, A.; et al. A Guide to Enteral Nutrition in Intensive Care Units: 10 Expert Tips for the Daily Practice. Crit. Care 2021, 25, 424. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.; Hoffman, L. Enteral Nutrition in the Mechanically Ventilated Patient. Nutr. Clin. Pract. 2019, 34, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Gokorsch, S.; Fegbeutel, C.; Hattar, K.; Rosseau, S.; Walmrath, D.; Seeger, W.; Grimminger, F. Parenteral Nutrition with Fish Oil Modulates Cytokine Response in Patients with Sepsis. Am. J. Resp. Crit. Care 2003, 167, 1321–1328. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Yang, E.; Zhang, N.; Cao, S.; Zhou, Y. Fish Oil–Supplemented Parenteral Nutrition Could Alleviate Acute Lung Injury, Modulate Immunity, and Reduce Inflammation in Rats with Abdominal Sepsis. Nutr. Res. 2015, 35, 784–791. [Google Scholar] [CrossRef]
- Sukhotnik, I.; Shany, A.; Bashenko, Y.; Hayari, L.; Chemodanov, E.; Mogilner, J.; Coran, A.G.; Shaoul, R. Parenteral but Not Enteral Omega-3 Fatty Acids (Omegaven) Modulate Intestinal Regrowth After Massive Small Bowel Resection in Rats. JPEN J. Parenter. Enter. Nutr. 2010, 34, 503–512. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).