Changes in the Bioaccessibility of Antioxidants after Simulated In Vitro Digestion of Bioprocessed Spelt-Enhanced Wheat Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bioprocessing of Spelt Flour
2.3. Bread Preparation
2.4. In Vitro Digestion
2.5. Extraction and Isolation of Phenolics
2.6. Total Phenolics Content
2.7. Phenolic Composition
2.8. Antioxidant Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content
3.2. Phenolic Profile in Undigested and Digested Breads
3.3. Antioxidant Activity of Undigested and Digested Bread Samples
3.4. Bioaccessibility of Phenolics in Bread Samples
3.4.1. Bioaccessibility of Total Phenolics
3.4.2. Bioaccessibility of Individual Phenolics
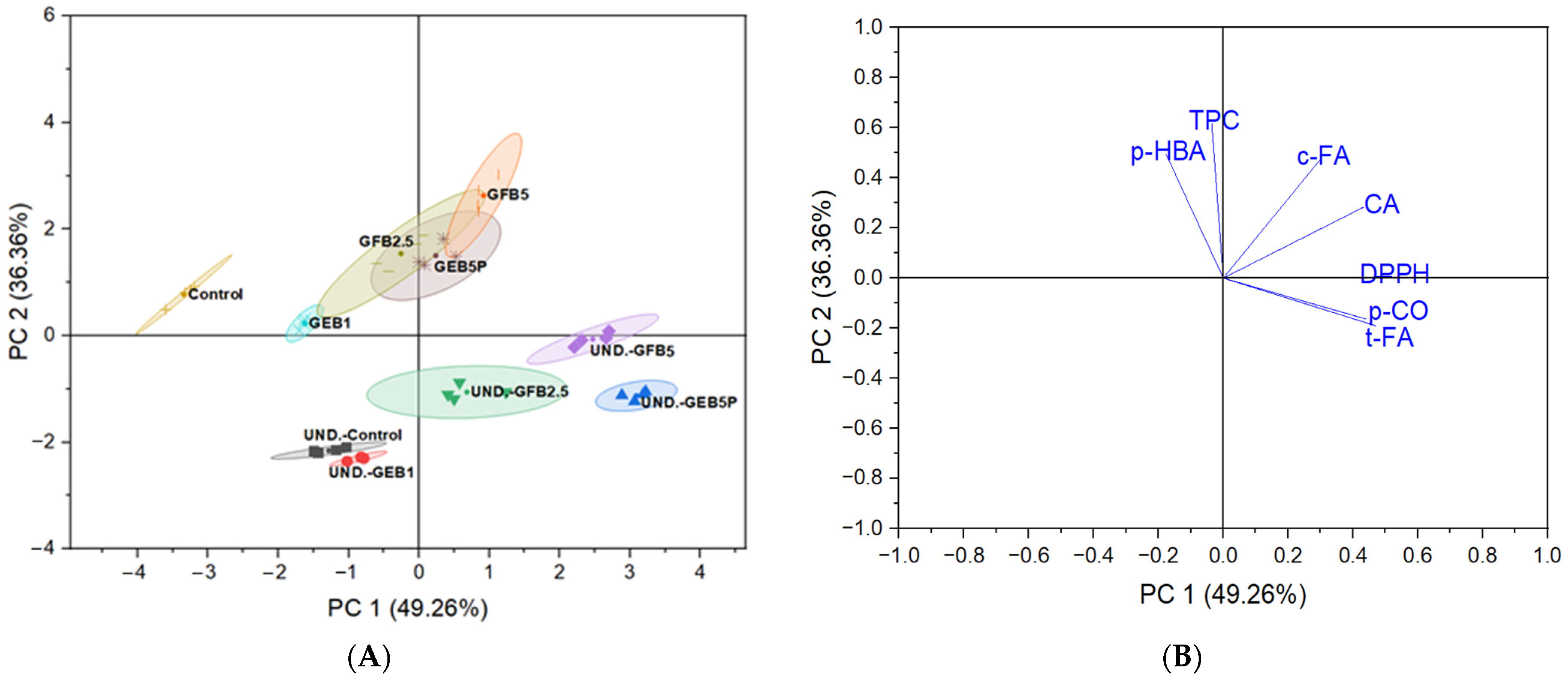
3.5. Correlation Analysis and Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karrar, E.M.A. A Review on: Antioxidant and Its Impact during the Bread Making Process. Int. J. Nutr. Food Sci. 2014, 3, 592. [Google Scholar] [CrossRef]
- Mencin, M.; Jamnik, P.; Mikulič Petkovšek, M.; Veberič, R.; Terpinc, P. Improving Accessibility and Bioactivity of Raw, Germinated and Enzymatic-Treated Spelt (Triticum Spelta L.) Seed Antioxidants by Fermentation. Food Chem. 2022, 394, 133483. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kroon, P.A.; Faulds, C.B.; Ryden, P.; Robertson, J.A.; Williamson, G. Release of Covalently Bound Ferulic Acid from Fiber in the Human Colon. J. Agric. Food Chem. 1997, 45, 661–667. [Google Scholar] [CrossRef]
- Mateo Anson, N.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Ferulic Acid from Aleurone Determines the Antioxidant Potency of Wheat Grain (Triticum Aestivum L.). J. Agric. Food Chem. 2008, 56, 5589–5594. [Google Scholar] [CrossRef]
- Pekkinen, J.; Rosa, N.N.; Savolainen, O.-I.; Keski-Rahkonen, P.; Mykkänen, H.; Poutanen, K.; Micard, V.; Hanhineva, K. Disintegration of Wheat Aleurone Structure Has an Impact on the Bioavailability of Phenolic Compounds and Other Phytochemicals as Evidenced by Altered Urinary Metabolite Profile of Diet-Induced Obese Mice. Nutr. Metab. 2014, 11, 1. [Google Scholar] [CrossRef]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Rio, D.D.; Martini, D. Bioaccessibility and Bioavailability of Phenolic Compounds in Bread: A Review. Food Funct. 2017, 8, 2368–2393. [Google Scholar] [CrossRef]
- Anson, N.M.; Aura, A.-M.; Selinheimo, E.; Mattila, I.; Poutanen, K.; Van Den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioprocessing of Wheat Bran in Whole Wheat Bread Increases the Bioavailability of Phenolic Acids in Men and Exerts Antiinflammatory Effects Ex Vivo1-3. J. Nutr. 2011, 141, 137–143. [Google Scholar] [CrossRef]
- Mencin, M.; Abramovič, H.; Jamnik, P.; Mikulič Petkovšek, M.; Veberič, R.; Terpinc, P. Abiotic Stress Combinations Improve the Phenolics Profiles and Activities of Extractable and Bound Antioxidants from Germinated Spelt (Triticum Spelta L.) Seeds. Food Chem. 2021, 344, 128704. [Google Scholar] [CrossRef]
- Terpinc, P.; Cigić, B.; Polak, T.; Hribar, J.; Požrl, T. LC–MS Analysis of Phenolic Compounds and Antioxidant Activity of Buckwheat at Different Stages of Malting. Food Chem. 2016, 210, 9–17. [Google Scholar] [CrossRef]
- Živković, A.; Polak, T.; Cigić, B.; Požrl, T. Germinated Buckwheat: Effects of Dehulling on Phenolics Profile and Antioxidant Activity of Buckwheat Seeds. Foods 2021, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Mencin, M.; Jamnik, P.; Mikulič Petkovšek, M.; Veberič, R.; Terpinc, P. Enzymatic Treatments of Raw, Germinated and Fermented Spelt (Triticum Spelta L.) Seeds Improve the Accessibility and Antioxidant Activity of Their Phenolics. LWT 2022, 169, 114046. [Google Scholar] [CrossRef]
- Mencin, M.; Mikulič Petkovšek, M.; Veberič, R.; Terpinc, P. Simulated Gastrointestinal Digestion of Bioprocessed Spelt Seeds: Bioaccessibility and Bioactivity of Phenolics. Antioxidants 2022, 11, 1703. [Google Scholar] [CrossRef] [PubMed]
- Anson, N.M.; Selinheimo, E.; Havenaar, R.; Aura, A.-M.; Mattila, I.; Lehtinen, P.; Bast, A.; Poutanen, K.; Haenen, G.R.M.M. Bioprocessing of Wheat Bran Improves in Vitro Bioaccessibility and Colonic Metabolism of Phenolic Compounds. J. Agric. Food Chem. 2009, 57, 6148–6155. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Nordlund, E.; Katina, K.; Mattila, I.; Poutanen, K.; Hanhineva, K.; Aura, A.-M. Effect of Bioprocessing on the In Vitro Colonic Microbial Metabolism of Phenolic Acids from Rye Bran Fortified Breads. J. Agric. Food Chem. 2017, 65, 1854–1864. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Refaey, M.M.; Xu, W. High Spatial and Temporal Variations of Microbial Community along the Southern Catfish Gastrointestinal Tract: Insights into Dynamic Food Digestion. Front. Microbiol. 2017, 8, 1531. [Google Scholar] [CrossRef]
- Tomé-Sánchez, I.; Martín-Diana, A.B.; Peñas, E.; Frias, J.; Rico, D.; Jiménez-Pulido, I.; Martínez-Villaluenga, C. Bioprocessed Wheat Ingredients: Characterization, Bioaccessibility of Phenolic Compounds, and Bioactivity During in Vitro Digestion. Front. Plant Sci. 2021, 12, 790898. [Google Scholar] [CrossRef]
- Xu, K.; She, Z.; Wang, H.; Liu, X.; Zhang, Y.; Chi, C.; Zhang, H. Textural Quality of Sweet Dumpling: Effect of Germination of Waxy Rice. Int. J. Food Sci. Technol. 2021, 56, 4081–4090. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Sęczyk, Ł.; Sugier, D.; Świeca, M.; Gawlik-Dziki, U. The Effect of in Vitro Digestion, Food Matrix, and Hydrothermal Treatment on the Potential Bioaccessibility of Selected Phenolic Compounds. Food Chem. 2021, 344, 128581. [Google Scholar] [CrossRef] [PubMed]
- Skrajda-Brdak, M.; Konopka, I.; Tańska, M.; Czaplicki, S. Changes in the Content of Free Phenolic Acids and Antioxidative Capacity of Wholemeal Bread in Relation to Cereal Species and Fermentation Type. Eur. Food Res. Technol. 2019, 245, 2247–2256. [Google Scholar] [CrossRef]
- Maga, J.A.; Katz, I. Simple Phenol and Phenolic Compounds in Food Flavor. Crit. Rev. Food Sci. Nutr. 1978, 10, 323–372. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on Phenolic Compounds Stability during Microwave-Assisted Extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef]
- Laddomada, B.; Caretto, S.; Mita, G. Wheat Bran Phenolic Acids: Bioavailability and Stability in Whole Wheat-Based Foods. Molecules 2015, 20, 15666–15685. [Google Scholar] [CrossRef]
- Vaher, M.; Matso, K.; Levandi, T.; Helmja, K.; Kaljurand, M. Phenolic Compounds and the Antioxidant Activity of the Bran, Flour and Whole Grain of Different Wheat Varieties. Procedia Chem. 2010, 2, 76–82. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Li, Y. Dough Properties, Bread Quality, and Associated Interactions with Added Phenolic Compounds: A Review. J. Funct. Foods 2019, 52, 629–639. [Google Scholar] [CrossRef]
- Amaya Villalva, M.F.; González-Aguilar, G.; Sández, O.R.; Astiazarán García, H.; Ledesma Osuna, A.I.; López-Ahumada, G.A.; Robles-Sánchez, R.M. Bioprocessing of Wheat (Triticum Aestivum Cv. Kronstad) Bran from Northwest Mexico: Effects on Ferulic Acid Bioaccessibility in Breads. CyTA J. Food 2018, 16, 570–579. [Google Scholar] [CrossRef]
- Tian, W.; Hu, R.; Chen, G.; Zhang, Y.; Wang, W.; Li, Y. Potential Bioaccessibility of Phenolic Acids in Whole Wheat Products during in Vitro Gastrointestinal Digestion and Probiotic Fermentation. Food Chem. 2021, 362, 130135. [Google Scholar] [CrossRef]
- Ti, H.; Zhang, R.; Li, Q.; Wei, Z.; Zhang, M. Effects of Cooking and in Vitro Digestion of Rice on Phenolic Profiles and Antioxidant Activity. Food Res. Int. 2015, 76, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Whole Grain Phytochemicals and Health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Ortega, N.; Macià, A.; Romero, M.-P.; Reguant, J.; Motilva, M.-J. Matrix Composition Effect on the Digestibility of Carob Flour Phenols by an In-Vitro Digestion Model. Food Chem. 2011, 124, 65–71. [Google Scholar] [CrossRef]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.-E. Effect of in Vitro Gastrointestinal Digestion on Encapsulated and Nonencapsulated Phenolic Compounds of Carob (Ceratonia Siliqua L.) Pulp Extracts and Their Antioxidant Capacity. J. Agric. Food Chem. 2017, 65, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated Gastrointestinal Digestion and in Vitro Colonic Fermentation of Carob Polyphenols: Bioaccessibility and Bioactivity. LWT 2020, 117, 108623. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of Polyphenolic Profile Stability and Changes in the Antioxidant Potential of Maqui Berry (Aristotelia Chilensis (Molina) Stuntz) during in Vitro Gastrointestinal Digestion. Ind. Crops Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Doniec, J.; Florkiewicz, A.; Dziadek, K.; Filipiak-Florkiewicz, A. Hydrothermal Treatment Effect on Antioxidant Activity and Polyphenols Concentration and Profile of Brussels Sprouts (Brassica Oleracea Var. Gemmifera) in an In Vitro Simulated Gastrointestinal Digestion Model. Antioxidants 2022, 11, 446. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Lin, M.; Zheng, Y.; Chen, J. Effect of Roasting and in Vitro Digestion on Phenolic Profiles and Antioxidant Activity of Water-Soluble Extracts from Sesame. Food Chem. Toxicol. 2020, 139, 111239. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total Phenolics, Flavonoids, Anthocyanins and Antioxidant Activity Following Simulated Gastro-Intestinal Digestion and Dialysis of Apple Varieties: Bioaccessibility and Potential Uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Y.; Yang, X.; Tao, S.; Ming, J. Phenolic Profiles and Antioxidant Activity of Buckwheat (Fagopyrum Esculentum Möench and Fagopyrum Tartaricum L. Gaerth) Hulls, Brans and Flours. J. Integr. Agric. 2013, 12, 1684–1693. [Google Scholar] [CrossRef]
- Angioloni, A.; Collar, C. Polyphenol Composition and “in Vitro” Antiradical Activity of Single and Multigrain Breads. J. Cereal Sci. 2011, 53, 90–96. [Google Scholar] [CrossRef]
- Hemery, Y.M.; Anson, N.M.; Havenaar, R.; Haenen, G.R.M.M.; Noort, M.W.J.; Rouau, X. Dry-Fractionation of Wheat Bran Increases the Bioaccessibility of Phenolic Acids in Breads Made from Processed Bran Fractions. Food Res. Int. 2010, 43, 1429–1438. [Google Scholar] [CrossRef]
- Lucas-González, R.; Ángel Pérez-Álvarez, J.; Moscaritolo, S.; Fernández-López, J.; Sacchetti, G.; Viuda-Martos, M. Evaluation of Polyphenol Bioaccessibility and Kinetic of Starch Digestion of Spaghetti with Persimmon (Dyospyros Kaki) Flours Coproducts during in Vitro Gastrointestinal Digestion. Food Chem. 2021, 338, 128142. [Google Scholar] [CrossRef] [PubMed]


| TPC | DPPH | |||
|---|---|---|---|---|
| mg TE/g DW | mg TE/g DW | |||
| Sample | Extractable | Bound | Extractable | Bound |
| Undigested | ||||
| Control | 0.83 ± 0.04 a | 3.79 ± 0.72 BC | 0.10 ± 0.01 b | 0.15 ± 0.02 A |
| GEB1 | 1.06 ± 0.03 a | 5.04 ± 0.11 E | 0.12 ± 0.01 c | 0.20 ± 0.02 C |
| GEB5P | 1.66 ± 0.05 b | 5.43 ± 0.39 E | 0.20 ± 0.00 e | 0.24 ± 0.02 D |
| GFB2.5 | 1.54 ± 0.05 b | 4.20 ± 0.14 CD | 0.14 ± 0.01 d | 0.18 ± 0.01 B |
| GFB5 | 2.08 ± 0.04 c | 4.27 ± 0.09 D | 0.19 ± 0.01 e | 0.20 ± 0.01 C |
| Digested | ||||
| Control | 2.92 ± 0.24 e | 3.26 ± 0.13 A | 0.08 ± 0.02 a | 0.33 ± 0.01 E |
| GEB1 | 2.64 ± 0.09 d | 4.10 ± 0.21 CD | 0.09 ± 0.00 ab | 0.38 ± 0.01 F |
| GEB5P | 3.12 ± 0.28 e | 4.24 ± 0.17 D | 0.14 ± 0.01 d | 0.39 ± 0.01 F |
| GFB2.5 | 3.44 ± 0.40 f | 3.24 ± 0.14 A | 0.13 ± 0.02 cd | 0.33 ± 0.01 E |
| GFB5 | 3.87 ± 0.23 g | 3.47 ± 0.12 AB | 0.15 ± 0.01 d | 0.33 ± 0.01 E |
| Phenolic Acids (µg/g DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-Coumaric Acid | trans-Ferulic Acid | cis-Ferulic Acid | Caffeic Acid | p-Hydroxybenzoic Acid | ||||||
| Breads | Extractable | Bound | Extractable | Bound | Extractable | Bound | Extractable | Bound | Extractable | Bound |
| Undigested | ||||||||||
| Control | 0.28 ± 0.01 f | 1.30 ± 0.13 A | 1.72 ± 0.06 c | 49.44 ± 4.20 A | 0.31 ± 0.01 a | 11.27 ± 1.07 A | 0.13 ± 0.01 b | 0.23 ± 0.01 A | 1.12 ± 0.02 c | 2.35 ± 0.13 D |
| GEB1 | 0.23 ± 0.01 d | 2.72 ± 0.09 C | 2.52 ± 0.11 e | 88.15 ± 0.60 D | 0.38 ± 0.01 a | 16.91 ± 0.62 D | 0.13 ± 0.01 b | 0.43 ± 0.02 BC | 0.36 ± 0.01 a | 1.49 ± 0.08 B |
| GEB5P | 0.38 ± 0.01 h | 7.13 ± 0.26 G | 7.85 ± 0.69 h | 125.89 ± 4.87 H | 0.98 ± 0.03 e | 17.14 ± 0.45 D | 0.33 ± 0.01 d | 0.99 ± 0.03 E | 0.51 ± 0.02 b | 2.97 ± 0.10 E |
| GFB2.5 | 0.25 ± 0.02 e | 3.14 ± 0.06 D | 6.07 ± 0.51 f | 75.43 ± 1.86 B | 0.72 ± 0.05 c | 13.88 ± 0.10 B | 0.24 ± 0.02 c | 0.39 ± 0.02 B | 1.25 ± 0.05 d | 1.16 ± 0.02 A |
| GFB5 | 0.30 ± 0.01 g | 3.59 ± 0.03 E | 7.02 ± 0.06 g | 79.35 ± 1.24 BC | 0.79 ± 0.03 d | 15.25 ± 0.37 C | 0.44 ± 0.03 e | 0.68 ± 0.02 D | 1.58 ± 0.03 f | 3.50 ± 0.12 F |
| Digested | ||||||||||
| Control | 0.04 ± 0.01 a | 2.10 ± 0.13 B | 0.47 ± 0.01 a | 80.72 ± 3.02 C | 0.43 ± 0.03 b | 15.77 ± 0.61 C | 0.09 ± 0.01 a | 0.48 ± 0.00 C | 1.81 ± 0.12 g | 2.09 ± 0.10 C |
| GEB1 | 0.09 ± 0.01 b | 3.71 ± 0.35 E | 0.88 ± 0.02 b | 113.38 ± 2.22 G | 0.71 ± 0.02 c | 18.33 ± 0.64 E | 0.26 ± 0.02 c | 0.65 ± 0.03 D | 1.13 ± 0.04 c | 3.01 ± 0.03 E |
| GEB5P | 0.15 ± 0.01 c | 8.15 ± 0.29 H | 2.06 ± 0.14 cd | 143.77 ± 2.15 I | 1.21 ± 0.05 f | 20.29 ± 0.53 F | 0.35 ± 0.02 d | 1.14 ± 0.02 F | 1.55 ± 0.08 f | 3.79 ± 0.02 G |
| GFB2.5 | 0.24 ± 0.01 de | 3.55 ± 0.13 E | 1.87 ± 0.12 c | 96.89 ± 2.52 E | 0.98 ± 0.07 e | 16.78 ± 0.44 D | 0.26 ± 0.01 c | 0.98 ± 0.10 E | 1.45 ± 0.01 e | 3.06 ± 0.09 E |
| GFB5 | 0.30 ± 0.02 g | 5.24 ± 0.18 F | 2.45 ± 0.04 de | 108.43 ± 1.63 F | 1.37 ± 0.09 g | 19.04 ± 0.73 E | 0.35 ± 0.01 d | 1.23 ± 0.06 G | 2.87 ± 0.04 h | 3.67 ± 0.07 G |
| Flavonoids (µg/g DW) | ||||
|---|---|---|---|---|
| Apigenin | Unknown C-glycosyl Derivative | |||
| I | II | III | ||
| Breads | Extractable | Extractable | Extractable | Extractable |
| Undigested | ||||
| Control | 1.22 ± 0.01 c | 0.32 ± 0.02 c | 1.45 ± 0.02 d | 0.22 ± 0.01 c |
| GEB1 | 1.43 ± 0.01 e | 0.48 ± 0.01 e | 1.66 ± 0.03 f | 0.19 ± 0.01 b |
| GEB5P | 1.73 ± 0.03 g | 0.60 ± 0.00 f | 1.72 ± 0.02 g | 0.17 ± 0.00 b |
| GFB2.5 | 1.56 ± 0.10 f | 0.47 ± 0.02 e | 1.57 ± 0.10 e | 0.15 ± 0.01 a |
| GFB5 | 1.76 ± 0.06 g | 0.69 ± 0.02 g | 1.65 ± 0.04 f | 0.14 ± 0.00 a |
| Digested | ||||
| Control | 0.67 ± 0.02 a | 0.15 ± 0.00 a | 1.05 ± 0.03 a | 0.72 ± 0.01 g |
| GEB1 | 0.81 ± 0.02 b | 0.23 ± 0.01 b | 1.08 ± 0.02 a | 0.51 ± 0.01 d |
| GEB5P | 1.35 ± 0.08 d | 0.39 ± 0.02 d | 1.26 ± 0.03 c | 0.54 ± 0.03 e |
| GFB2.5 | 1.19 ± 0.08 c | 0.33 ± 0.01 c | 1.29 ± 0.06 c | 0.70 ± 0.01 f |
| GFB5 | 1.36 ± 0.04 d | 0.38 ± 0.02 d | 1.20 ± 0.02 b | 0.74 ± 0.02 h |
| Bioaccessibility (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breads | TPC | p-Coumaric Acid | trans-Ferulic Acid | cis-Ferulic Acid | Caffeic Acid | p-Hydroxybenzoic Acid | Apigenin | Unknown C-glycosyl Derivative | ||
| I | II | III | ||||||||
| Control | 61.5 b | 2.8 b | 1.3 b | 3.7 a | 26.3 a | 52.0 b | 36.2 b | 28.2 a | 44.5 b | 64.0 e |
| GEB1 | 43.3 a | 3.0 b | 1.0 a | 4.1 b | 46.6 d | 61.2 d | 32.4 a | 29.2 b | 34.6 a | 33.7 b |
| GEB5P | 44.1 a | 2.0 a | 1.5 c | 6.7 c | 26.1 a | 44.5 a | 43.7 c | 31.7 c | 35.9 a | 22.4 a |
| GFB2.5 | 59.9 b | 7.1 c | 2.3 d | 6.7 c | 42.1 c | 60.2 d | 50.2 d | 42.0 d | 46.6 c | 45.3 c |
| GFB5 | 60.9 b | 7.8 c | 2.8 e | 8.5 d | 31.4 b | 56.4 c | 53.4 e | 31.6 c | 43.7 b | 61.8 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mencin, M.; Markanovič, N.; Mikulič-Petkovšek, M.; Veberič, R.; Terpinc, P. Changes in the Bioaccessibility of Antioxidants after Simulated In Vitro Digestion of Bioprocessed Spelt-Enhanced Wheat Bread. Antioxidants 2023, 12, 487. https://doi.org/10.3390/antiox12020487
Mencin M, Markanovič N, Mikulič-Petkovšek M, Veberič R, Terpinc P. Changes in the Bioaccessibility of Antioxidants after Simulated In Vitro Digestion of Bioprocessed Spelt-Enhanced Wheat Bread. Antioxidants. 2023; 12(2):487. https://doi.org/10.3390/antiox12020487
Chicago/Turabian StyleMencin, Marjeta, Nika Markanovič, Maja Mikulič-Petkovšek, Robert Veberič, and Petra Terpinc. 2023. "Changes in the Bioaccessibility of Antioxidants after Simulated In Vitro Digestion of Bioprocessed Spelt-Enhanced Wheat Bread" Antioxidants 12, no. 2: 487. https://doi.org/10.3390/antiox12020487
APA StyleMencin, M., Markanovič, N., Mikulič-Petkovšek, M., Veberič, R., & Terpinc, P. (2023). Changes in the Bioaccessibility of Antioxidants after Simulated In Vitro Digestion of Bioprocessed Spelt-Enhanced Wheat Bread. Antioxidants, 12(2), 487. https://doi.org/10.3390/antiox12020487









