Insights into the Structure–Capacity of Food Antioxidant Compounds Assessed Using Coulometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. CDAC Measurement
3. Results
- (i)
- Electron transfer (ET) reactions from the antioxidant to the substrate
- (ii)
- Hydrogen atom transfer (HAT) reactions from the antioxidant to the substrate, which, in aqueous media, can be considered as a proton transfer combined with an electron transfer; it can occur in one step or involve mechanistically distinct ET and proton transfer steps
- (iii)
- Chelation of metal ions by antioxidants, which inhibits the genesis of free radicals.
3.1. CDAC of Ascorbic Acid and Definition of the Electrochemical Ratio (ER)
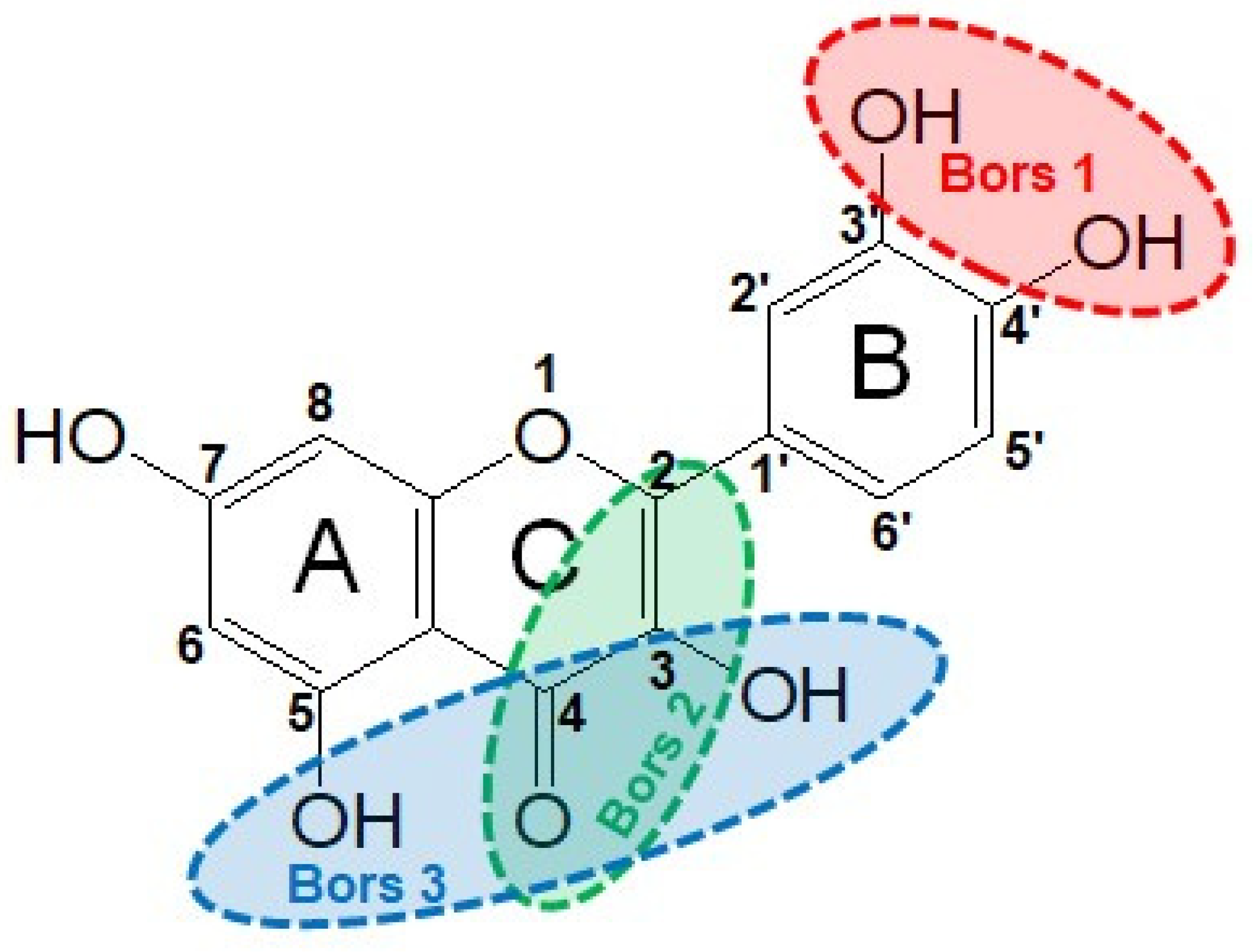
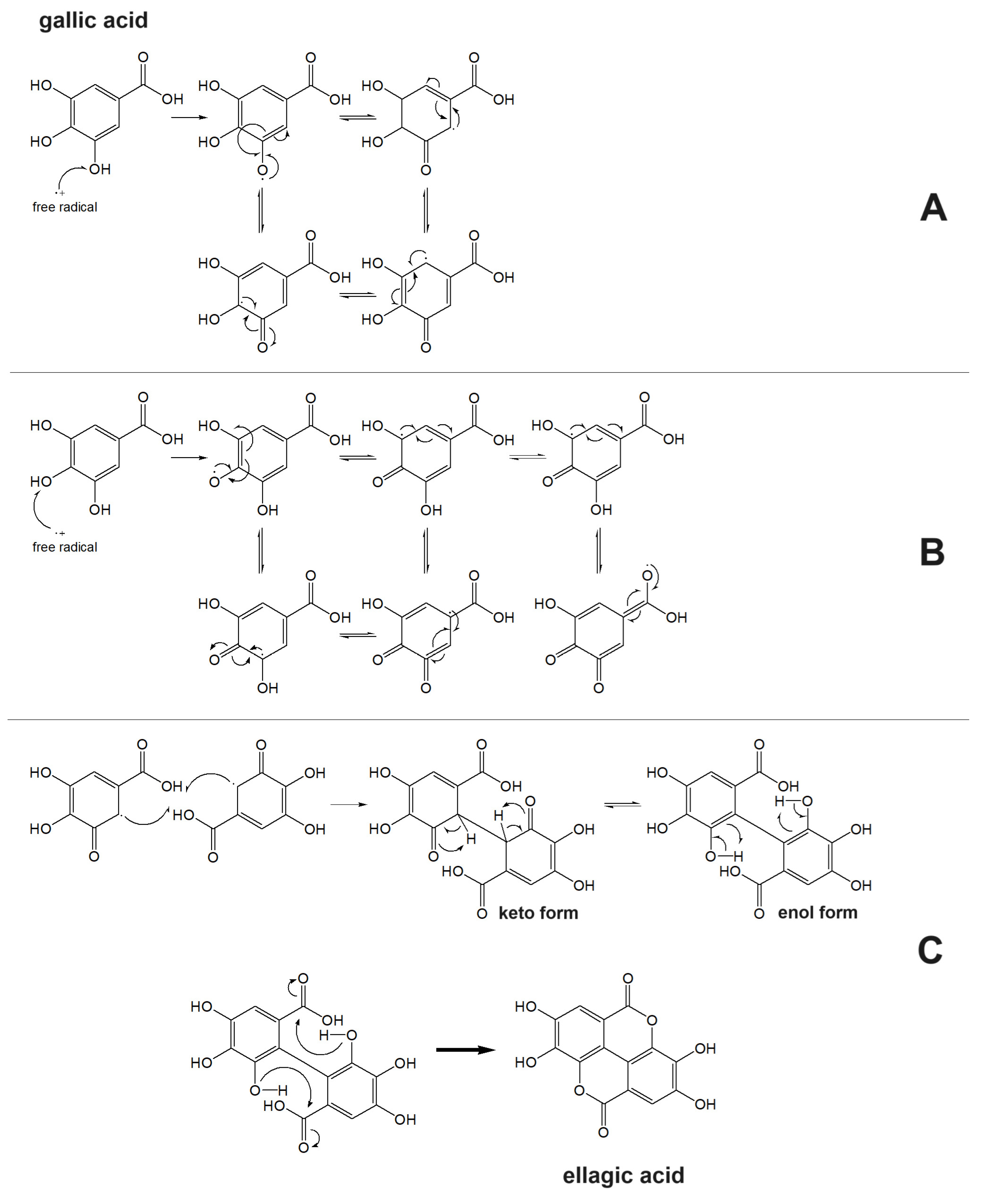
3.2. Structure–Antioxidant Capacity Relationship
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Warraich, U.E.; Hussain, F.; Kayani, H.U.R. Aging-oxidative stress, antioxidants and computational modelling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef]
- Yang, H.Y.; Lee, T.H. Antioxidant enzymes as redox-based biomarkers: A brief review. BMB Rep. 2015, 48, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables–the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar]
- Pellegrini, N.; Vitaglione, P.; Granato, D.; Fogliano, V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: Merits and limitations. J. Sci. Food Agric. 2020, 100, 5064–5078. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; p. 77. [Google Scholar]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Cimpeanu, C.; Predoi, G. Electrochemical methods for total antioxidant capacity and its main contributors determination: A review. Open Chem. 2015, 13, 824–856. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Budnikov, H. Analytical Capabilities of Coulometric Sensor Systems in the Antioxidants Analysis. Chemosensors 2021, 9, 91. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of the antioxidant capacity of food products: Methods, applications and limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. In vitro measurements and interpretation of total antioxidant capacity. Biochim. Biophys. Acta 2014, 1840, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Atalay, V.E.; Atish, I.S.; Shahin, K.F.; Kashikchi, E.S.; Karahan, M. A DFT study: Ranking of antioxidant activity of various candidate molecules. UNEC J. Eng. Appl. Sci. 2022, 2, 33–40. [Google Scholar]
- Uchiyama, S.; Muto, G. Determination of bromination number by controlled potential coulometry with controlled-current coulometric generation of bromine. Anal. Chem. 1984, 56, 2408–2410. [Google Scholar] [CrossRef]
- Abdullin, I.F.; Turova, E.N.; Gaisina, G.K.; Budnikov, G.K. Use of electrogenerated bromine for estimating the total antioxidant capacity of plant raw materials and plant-based medicinal preparations. J. Anal. Chem. 2002, 57, 557–560. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kalmykova, A.; Kupriyanova, O. Constant-Current Coulometry with Electrogenerated Titrants as a Novel Tool for the Essential Oils Screening Using Total Antioxidant Parameters. Antioxidants 2022, 11, 1749. [Google Scholar] [CrossRef]
- Nizamova, A.M.; Ziyatdinova, G.K.; Budnikov, G.K. Electrogenerated Bromine as a Coulometric Reagent for the Estimation of the Bioavailability of Polyphenols. J. Anal. Chem. 2011, 66, 301–309. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C.; Pogorel’tzev, V.I.; Ganeev, T.S. The application of coulometry for total antioxidant capacity determination of human blood. Talanta 2006, 68, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Siano, F.; Picariello, G.; Vasca, E. Coulometrically determined antioxidant capacity (CDAC) as a possible parameter to categorize extra virgin olive oil. Food Chem. 2021, 354, 129564. [Google Scholar] [CrossRef] [PubMed]
- Siano, F.; Picariello, G.; Sammarco, A.S.; Celano, G.; Caruso, T.; Vasca, E. Evaluation of Novel Rapid Analytical Methods to Categorize Extra Virgin Olive Oil Based on the Coulometrically Determined Antioxidant Capacity and on the Spectrophotometric Assessment of Phenolic Compounds. Molecules 2023, 28, 3108. [Google Scholar] [CrossRef] [PubMed]
- Siano, F.; Picariello, G.; Castaldo, D.; Cautela, D.; Caruso, T.; Vasca, E. Monitoring antioxidants by coulometry: Quantitative assessment of the strikingly high antioxidant capacity of bergamot (Citrus bergamia R.) by-products. Talanta 2023, 251, 123765. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kozlova, E.; Morozova, E.; Budnikov, H. Chronocoulometric method for the evaluation of antioxidant capacity of medicinal plant tinctures. Anal. Methods 2018, 10, 4995–5003. [Google Scholar] [CrossRef]
- Capasso, R.; Evidente, A.; Avolio, S.; Solla, F. A highly convenient synthesis of hydroxytyrosol and its recovery from agricultural waste waters. J. Agric. Food Chem. 1999, 47, 1745–1748. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. In Antioxidants; InTech Open: London, UK, 2019; Volume 10, pp. 1–29. [Google Scholar] [CrossRef]
- Peyrat-Maillard, M.N.; Bonnely, S.; Berset, C. Determination of the antioxidant activity of phenolic compounds by coulometric detection. Talanta 2000, 51, 709–716. [Google Scholar] [CrossRef]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Bennett, B.; Chang, J.; Bard, A.J. Mechanism of the Br−/Br2 redox reaction on platinum and glassy carbon electrodes in nitrobenzene by cyclic voltammetry. Electrochim. Acta 2016, 219, 1–9. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Cong, P.N.; Budnikov, H. Ultrasound-assisted micellar extraction of phenolic antioxidants from spices and antioxidant properties of the extracts based on coulometric titration data. Anal. Methods 2016, 8, 7150–7157. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Chen, Y.; Sun, X.; Wang, L. AODB: A comprehensive database for antioxidants including small molecules, peptides and proteins. Food Chem. 2023, 418, 135992. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Radical chemistry of flavonoid antioxidants. In Antioxidants in Therapy and Preventive Medicine; Emerit, I., Packer, L., Auclair, C., Eds.; Springer: Boston, MA, USA, 1990; pp. 165–170. [Google Scholar]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Nenadis, N.; Pyrka, I.; Tsimidou, M.Z. The Contribution of Theoretical Prediction Studies to the Antioxidant Activity Assessment of the Bioactive Secoiridoids Encountered in Olive Tree Products and By-Products. Molecules 2023, 28, 2267. [Google Scholar] [CrossRef]
- Lins, P.G.; Marina Piccoli Pugine, S.; Scatolini, A.M.; de Melo, M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, e00805. [Google Scholar] [CrossRef]
- Zúñiga-Núñez, D.; Barrias, P.; Cárdenas-Jirón, G.; Ureta-Zanartu, M.S.; Lopez-Alarcón, C.; Vieyra, F.E.; Borsarelli, C.D.; Alarcon, E.I.; Aspée, A. Atypical antioxidant activity of non-phenolic amino-coumarins. RSC Adv. 2018, 8, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.P. Comprehensive Heterocyclic Chemistry; Boulton, A.J., Mc Killop, A., Eds.; Pergamon: Oxford, UK, 1984; Volume 3, p. 681. [Google Scholar]
- Petrucci, R.; Astolfi, P.; Greci, L.; Firuzi, O.; Saso, L.; Marrosu, G. A spectroelectrochemical and chemical study on oxidation of hydroxycinnamic acids in aprotic medium. Electrochim. Acta 2007, 52, 2461–2470. [Google Scholar] [CrossRef]
- David, I.G.; Popa, D.E.; Buleandră, M.; Cheregi, M.C. Electrochemical methods and (bio) sensors for rosmarinic acid investigation. Chemosensors 2020, 8, 74. [Google Scholar] [CrossRef]
- Malinda, K.; Sutanto, H.; Darmawan, A. Characterization and antioxidant activity of gallic acid derivative. AIP Conf. Proc. 2017, 1904, 020030. [Google Scholar]
- Kawabata, J.; Okamoto, Y.; Kodama, A.; Makimoto, T.; Kasai, T. Oxidative dimers produced from protocatechuic and gallic esters in the DPPH radical scavenging reaction. J. Agric. Food Chem. 2002, 50, 5468–5471. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Salikhova, I.; Budnikov, H. Coulometric titration with electrogenerated oxidants as a tool for evaluation of cognac and brandy antioxidant properties. Food Chem. 2014, 150, 80–86. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Fu, Z.M.; Deng, G.; Guo, R.; Chen, D.F. Free radical scavenging potency of ellagic acid and its derivatives in multiple H+/e− processes. Phytochemistry 2020, 180, 112517. [Google Scholar] [CrossRef]
- Konopko, A.; Litwinienko, G. Unexpected role of pH and microenvironment on the antioxidant and synergistic activity of resveratrol in model micellar and liposomal systems. J. Org. Chem. 2021, 87, 1698–1709. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, S.H.; Lee, K.A. A comparative study of hesperetin, hesperidin and hesperidin glucoside: Antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Majo, D.D.; Giammanco, M.; Guardia, M.L.; Tripoli, E.; Giammanco, S.; Finotti, E. Flavanones in Citrus fruit: Structure–antioxidant activity relationships. Food Res. Int. 2005, 38, 1161–1166. [Google Scholar] [CrossRef]
- Xiao, Z.; He, L.; Hou, X.; Wei, J.; Ma, X.; Ga, Z.; Yuan, Y.; Xiao, J.; Li, P.; Yue, T. Relationships between structure and antioxidant capacity and activity of glycosylated flavonols. Foods 2021, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chen, S.C.; Senthil Kumar, K.J.; Yu, K.N.; Lee Chao, P.D.; Tsai, S.Y.; Hou, Y.C.; Hseu, Y.C. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: An ex vivo approach. J. Agric. Food Chem. 2012, 60, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Jankun, J.; Wyganowska-Świątkowska, M.; Dettlaff, K.; Jelińska, A.; Surdacka, A.; Wątróbska-Świetlikowska, D.; Skrzypczak-Jankun, E. Determining whether curcumin degradation/condensation is actually bioactivation. Int. J. Mol. Med. 2016, 37, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Servillo, L.; D’Onofrio, N.; Casale, R.; Cautela, D.; Giovane, A.; Castaldo, D.; Balestrieri, M.L. Ergothioneine products derived by superoxide oxidation in endothelial cells exposed to high-glucose. Free Radic. Biol. Med. 2017, 108, 8–18. [Google Scholar] [CrossRef]
- Franzoni, F.; Colognato, R.; Galetta, F.; Laurenza, I.; Barsotti, M.; Di Stefano, R.; Bocchetti, R.; Regoli, F.; Carpi, A.; Balbarini, A.; et al. An in vitro study on the free radical scavenging capacity of ergothioneine: Comparison with reduced glutathione, uric acid and trolox. Biomed. Pharmacother. 2006, 60, 453–457. [Google Scholar] [CrossRef]
- Kitsanayanyong, L.; Ohshima, T. Ergothioneine: A potential antioxidative and antimelanosis agent for food quality preservation. FEBS Lett. 2022, 596, 1330–1347. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013, 57, 114–125. [Google Scholar] [CrossRef]
- Pompella, A.; Sies, H.; Wacker, R.; Brouns, F.; Grune, T.; Biesalski, H.K.; Frank, J. The use of total antioxidant capacity as surrogate marker for food quality and its effect on health is to be discouraged. Nutrition 2014, 30, 791–793. [Google Scholar] [CrossRef] [PubMed]

| Compound | Structural Formula | CDAC (mmol e− g−1) | MW (g mol−1) | CDACχ (mol e− mol−1) | ER | Major Food Source |
|---|---|---|---|---|---|---|
| Alkaloids | ||||||
| Caffeine | 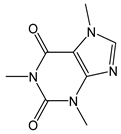 | - | 194.20 | - | - | Coffee, tea, cocoa, guarana |
| Amino acid and derivatives | ||||||
| Cysteine | 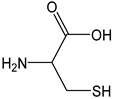 | 48 | 121.15 | 5.8 | 4.4 | Eggs, broccoli, garlic, wheat germ |
| Methionine | 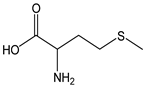 | 13 | 149.21 | 1.9 | 1.2 | Eggs, sesame seeds, cashew nuts, fish, meat |
| Istidine | 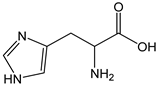 | <1 | 155.16 | - | <0.1 | Beans, peanuts |
| Hercynine | 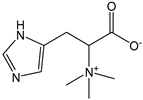 | <1 | 197.23 | - | <0.1 | Mushrooms |
| Ergothioneine | 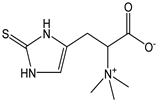 | 43 | 229.30 | 9.9 | 3.9 | Mushrooms |
| Glutathione | 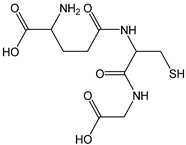 | 16 | 307.33 | 4.9 | 1.4 | Spinach, tomato, pumpkin, strawberries |
| Phenol compounds | ||||||
| 3,4-dihydroxy phenylacetic acid | 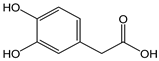 | 12 | 168.15 | 2.0 | 1.1 | Olives, olive oil |
| Vanillic acid | 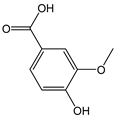 | 5 | 168.14 | 0.8 | 0.4 | Coriander, onion, sage |
| Gallic acid | 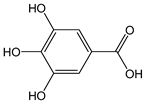 | 22 | 170.12 | 3.7 | 2 | Raspberries, blackberries, strawberries, mangoes |
| Syringic acid | 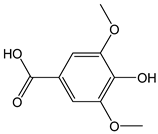 | 7 | 198.17 | 1.4 | 0.6 | Walnuts, chard, chickpeas, peanuts, cocoa |
| Ellagic acid | 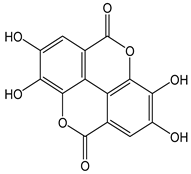 | 29 | 302.20 | 8.8 | 2.6 | Blackberries, pomegranates, raspberries, strawberries, walnuts, grapes, goji berries |
| Tannic acid | 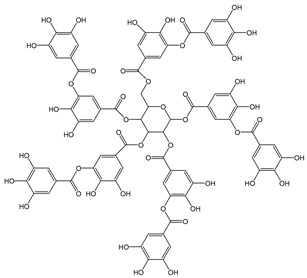 | 34 | 1701.19 | 57.8 | 3.1 | Walnuts, green tea |
| p-cumaric acid | 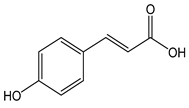 | 24 | 164.16 | 3.9 | 2.2 | Chili, basil, walnuts, spinach, pineapple, thyme, sunflower |
| Caffeic acid | 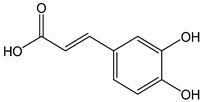 | 14 | 180.16 | 2.5 | 1.3 | Coffee, chicory, peas, artichokes, strawberries |
| Ferulic acid | 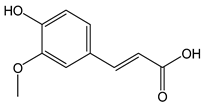 | 15 | 194.18 | 2.9 | 1.4 | Oats, wheat, rice, apples, oranges, pineapple, coffee |
| Curcumin | 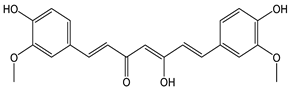 | 44 | 368.39 | 16.2 | 4.0 | Turmeric |
| Piceid | 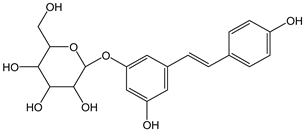 | 32 | 390.39 | 12.5 | 2.9 | Grape juice |
| Tyrosol | 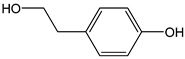 | 24 | 138.16 | 3.3 | 2.2 | Olives, olive oil |
| Hydroxytyrosol | 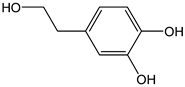 | 15 | 154.16 | 2.3 | 1.4 | Olives, olive oil |
| Oleuropein | 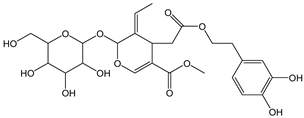 | 7 | 540.52 | 3.8 | 0.6 | Olives, olive oil |
| Chlorogenic acid | 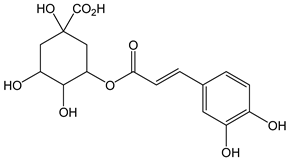 | 6 | 354.31 | 2.1 | 0.5 | Coffee, apples, tomato, aubergine, cherries, pears, blueberries |
| Cynarine | 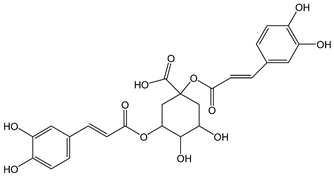 | 7 | 516.45 | 3.6 | 0.6 | Artichokes |
| Apigenin | 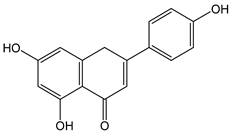 | 64 | 270.23 | 17.2 | 5.8 | Chamomile, celery, parsley |
| Kaempferol | 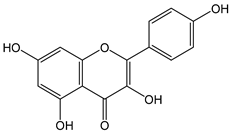 | 50 | 286.23 | 14.3 | 4.5 | Apples, grapes, tomatoes, onions, potatoes, spinach, cucumbers, lettuce, peaches, blackberries |
| Luteolin | 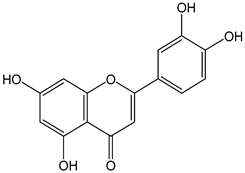 | 36 | 286.24 | 10.3 | 3.3 | Carrots, peppers, olive oil, thyme peppermint, rosemary, oregano, lettuce, pomegranates, chocolate, capers, cucumbers |
| Catechin | 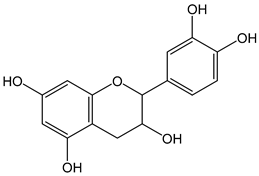 | 30 | 290.26 | 8.7 | 2.7 | Green tea, cocoa, wine |
| Quercetin | 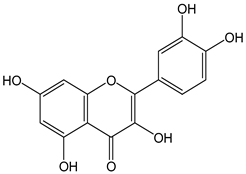 | 32 | 302.24 | 9.7 | 2.9 | Capers, grapes, apples, red onion, celery |
| Hesperetin | 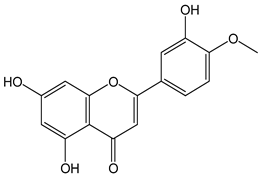 | 64 | 302.27 | 19.3 | 5.8 | Citrus fruit, grapes |
| Shaftoside | 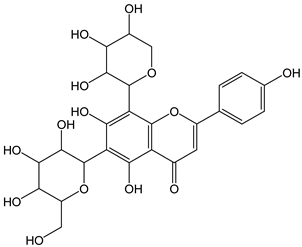 | 20 | 564.49 | 11.3 | 1.8 | Carob germ, wheat germ |
| Naringin | 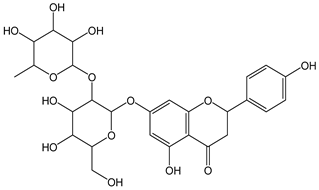 | 25 | 580.54 | 14.5 | 2.3 | Grapefruit |
| Rutin | 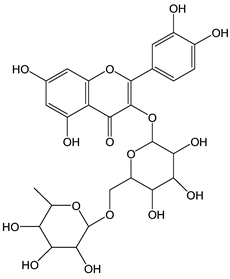 | 19 | 610.52 | 11.6 | 1.7 | Citrus fruit, red wine, buckwheat |
| Hesperidin | 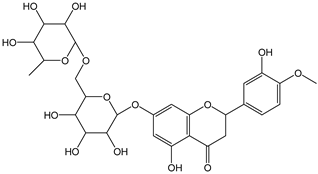 | 19 | 610.56 | 11.6 | 1.7 | Citrus fruit |
| Neohesperidin | 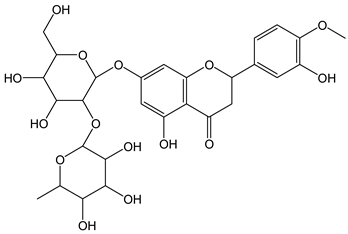 | 22 | 610.56 | 13.4 | 2.0 | Citrus fruit |
| Pelargonidin | 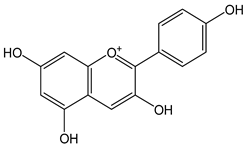 | 34 | 271.24 | 9.2 | 3.1 | Raspberries, strawberries, blueberries, blackberries, pomegranates, plums, red beans |
| Malvidin-3-O-galactosyde | 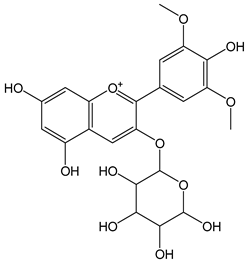 | 34 | 493.43 | 16.8 | 3.1 | Blueberries |
| Sterols | ||||||
| Ergosterol | 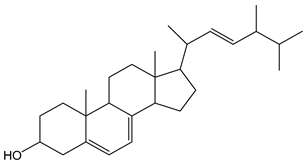 | 4 | 396.65 | 1.6 | 0.4 | Mushrooms |
| Terpenes | ||||||
| Limonene | 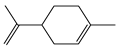 | 26 | 136.24 | 3.5 | 2.4 | Citrus essential olis |
| Linalool | 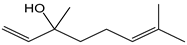 | 27 | 154.25 | 4.2 | 2.4 | Essential oils |
| Vitamins | ||||||
| Nicotinic acid | 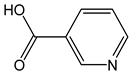 | <1 | 123.11 | - | <0.1 | Cereals, meat, brewer’s yeast |
| Ascorbic acid | 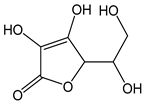 | 11 | 176.12 | 1.9 | 1 | Citrus fruit, kiwis, tomatoes, peppers, cabbage |
| Riboflavin |  | <1 | 376.36 | - | <0.1 | Milk, offal, kidney beans, egg whites |
| Other | ||||||
| Sulfite ion | 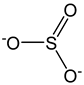 | 8 | 80.06 | 0.6 | 0.7 | Food additive |
| Furfural | 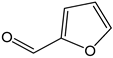 | <1 | 96.08 | - | <0.1 | Neoformation product from cooking food |
| Sorbate ion | 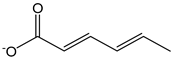 | 12 | 111.12 | 0.1 | 1.1 | Food additive |
| α-lipoic acid | 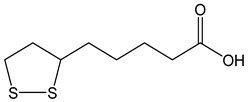 | 17 | 206.33 | 3.5 | 1.5 | Dietary supplement |
| Butylated hydroxytoluene (BHT) | 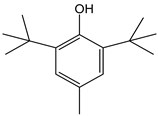 | 11 | 220.36 | 2.4 | 1 | Food additive |
| Trolox® | 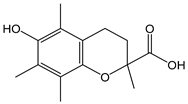 | 7 | 250.29 | 1.8 | 0.6 | Reference standard |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siano, F.; Sammarco, A.S.; Fierro, O.; Castaldo, D.; Caruso, T.; Picariello, G.; Vasca, E. Insights into the Structure–Capacity of Food Antioxidant Compounds Assessed Using Coulometry. Antioxidants 2023, 12, 1963. https://doi.org/10.3390/antiox12111963
Siano F, Sammarco AS, Fierro O, Castaldo D, Caruso T, Picariello G, Vasca E. Insights into the Structure–Capacity of Food Antioxidant Compounds Assessed Using Coulometry. Antioxidants. 2023; 12(11):1963. https://doi.org/10.3390/antiox12111963
Chicago/Turabian StyleSiano, Francesco, Anna Sofia Sammarco, Olga Fierro, Domenico Castaldo, Tonino Caruso, Gianluca Picariello, and Ermanno Vasca. 2023. "Insights into the Structure–Capacity of Food Antioxidant Compounds Assessed Using Coulometry" Antioxidants 12, no. 11: 1963. https://doi.org/10.3390/antiox12111963
APA StyleSiano, F., Sammarco, A. S., Fierro, O., Castaldo, D., Caruso, T., Picariello, G., & Vasca, E. (2023). Insights into the Structure–Capacity of Food Antioxidant Compounds Assessed Using Coulometry. Antioxidants, 12(11), 1963. https://doi.org/10.3390/antiox12111963









