Curcumin Electrochemistry—Antioxidant Activity Assessment, Voltammetric Behavior and Quantitative Determination, Applications as Electrode Modifier
Abstract
1. Introduction
1.1. Curcumin—History and Occurrence
1.2. Curcumin—Chemical Structure and Properties
1.3. Curcumin—Uses
2. CU Electrochemical Behavior
2.1. Curcumin Electro-Reduction
2.2. Curcumin Electro-Oxidation
3. CU Electrochemical Analysis
3.1. Electrochemical Sensors and Methods for CU Quantification
3.2. CU Voltammetric Quantification in the Presence of Other Electroactive Species
3.3. Application of Voltammetric Methods in CU Release Kinetics Studies
4. Electrochemical Investigation of CU Biological Activities and Its Interactions with Various Chemical Species
4.1. Antioxidant Activity
4.2. Antitumoral Activity
4.3. Antibacterial Activity
4.4. CU Interaction with Metal Ions
4.5. CU Interaction with DNA
4.6. CU Interaction with Other Molecules
5. CU Applications in the Development of Electrochemical Sensors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | adenine; |
| ABS | acetate buffer solution; |
| ACV | alternating current voltammetry; |
| AdSCV | adsorptive stripping cyclic voltammetry; |
| AFB1 | aflatoxin B1; |
| Al3+-Pd_NPs | aluminum ions/palladium nanoparticles; |
| Amp | amperometry; |
| AOA | antioxidant activity; |
| AOC | antioxidant capacity; |
| Au_NPs-FMWCNTs-IL-Chit | gold nanoparticles-amino functionalized multi-wall carbon nanotubes-ionic liquid-chitosan nanocomposite |
| Az-rGO@MWCNTs | azobenzene-modified reduced graphene oxide@multi-walled carbon nanotubes; |
| β-CD | beta-cyclodextrin; |
| β-CD–rGO | beta-cyclodextrin–reduced graphene oxide; |
| BDDE | boron doped diamond electrode; |
| BDMCU | bis-demethoxycurcumin; |
| BPPGE | basal plane pyrolytic graphite electrode; |
| BRB | Britton Robinson buffer; |
| C | cytosine; |
| CAB | citric acid buffer; |
| CB | carbon black; |
| CdO-IL | cadmium oxide-ionic liquid (1,3-dipropylimidazolium bromide); |
| Ce-BDC-MOF_NPs | Ce-1,4-benzenedicarboxylic metalorganic framework nanoparticles; |
| CNTs-CMC/Au-PET | carbon nanotubes-carboxymethylcellulose/Au on polyethylenetherephthalate; |
| CNTsPE | carbon nanotubes paste electrode; |
| CPE | carbon paste electrode; |
| CQDs | carbon quantum dots; |
| CTAB | cetyltrimethylammonium bromide; |
| ct-dsDNA | calf thymus double stranded DNA; |
| CU@ZIF-8-PDA | curcumin@zeolitic imidazolate framework-8-polydopamine; |
| CV | cyclic voltammetry; |
| DFT | density functional theory; |
| DMCU | demethoxycurcumin; |
| DME | dropping mercury electrode; |
| DMF | dimethylformamide; |
| DPP/DPV | differential pulse polarography/differential pulse voltammetry; |
| DPAd(C)SV | differential pulse adsorptive (cathodic) stripping voltammetry; |
| DPASV | differential pulse anodic stripping voltammetry; |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl; |
| Dy_NWs | dysprosium nanowires; |
| Eacc | accumulation potential; |
| Eappl | applied potential; |
| ECE | electrochemical-chemical-electrochemical; |
| EIS | electrochemical impedance spectroscopy; |
| Ep (Epa and Epc) | peak potential (anodic peak potential and cathodic peak potential); |
| EPPGE | edge plane pyrolytic graphite electrode; |
| EQCM | electrochemical quartz crystal microbalance; |
| erGO | electrochemically reduced graphene oxide; |
| ET | electron transfer; |
| FCM | ferrocenemethanol; |
| FDA | US Food and Drug Administration |
| FFTSWV | fast Fourier transform square wave voltammetry; |
| FIA | flow injection analysis; |
| FRAP | ferric reducing antioxidant power; |
| GCE | glassy carbon electrode; |
| GE | graphite electrode; |
| GO | graphene oxide; |
| GPE | graphite paste electrode; |
| GQDs | graphene quantum dots; |
| Gr | graphene; |
| GRAS | Generally Recognized As Safe |
| HaP-IL | hydroxyapatite nanoparticles and ionic liquid; |
| HAT | H-atom transfer; |
| HER-2 | human epidermal growth factor receptor 2 |
| hIL-2 | human interleukin-2; |
| HIV | human immunodeficiency virus; |
| HMDE | hanging mercury drop electrode; |
| HPSAM | H-point standard addition method; |
| HPV | human papilloma virus; |
| ITO | indium tin oxide; |
| LC-MS | Liquid chromatography-mass spectrometry |
| LOD | limit of detection; |
| LSV | linear sweep voltammetry; |
| MBA_pAAM | N, N’-methylenebisacrylamide cross-linked polyacrylamide; |
| MBMIP_NPs | magnetic biocompatibility molecularly imprinted nanoparticles; |
| MIP | molecularly imprinted polymer; |
| MIpAA@CU_NPs/μPAD | molecularly imprinted polyacrylamide coated curcumin nanoparticles micro-paper-based analytical device; |
| MnO2-c-MWCNTs | MnO2 nanoparticles functionalized carboxylated multi-walled carbon nanotubes; |
| MS | mass spectrometry; |
| MWCNTs | multi-walled carbon nanotubes; |
| NADH | β-nicotinamide adenine dinucleotide; |
| NOS | nitric oxide species; |
| NPs | nanoparticles; |
| NSs | nanospheres; |
| NSrGO/Ru@Au_NPs | Ru@Au nanoparticles decorated nitrogen and sulfur functionalized reduced graphene oxide; |
| oCNTs | oxidized carbon nanotubes; |
| pAA-MIP | polyacrylic-acid-based molecularly imprinted polymer; |
| pACBK | poly(acid chrome blue K); |
| PACO_MIP | 4-pentenoyl-alanyl-chitosan oligosaccharide-based molecularly imprinted polymer; |
| PBS | phosphate buffer solution; |
| PC3 | polyaniline–curcumin–copper–cobalt hybrid composite |
| PCR | polymerase chain reaction; |
| pCys_MIP-CuCo2O4-N-CNTs-P-GO | poly(L-cysteine)-based molecularly imprinted polymer-CuCo2O4-nitrogen-doped carbon nanotubes -phosphorus-doped graphene oxide; |
| Pd_NPs-pPr | palladium nanoparticles on polyproline film; |
| PEC | photoelectrochemistry; |
| pGA | poly(glutamine); |
| PGE | pencil graphite electrode; |
| pMAA_MIP | poly(methacrylic acid)-based molecularly imprinted polymer; |
| poly A | 5′-AAA AAA AAA AAA AAA AAA-3′ where A: adenine; |
| poly C | 5′-CCC CCC CCC CCC CCC CCC-3′ where C: cytosine; |
| poly G | 5′-GGG GGG GGG GGG GGG GGG-3′ where G: guanine; |
| poly T | 5′-TTT TTT TTT TTT TTT TTT-3′ where T: thymine; |
| potentiom | potentiometry; |
| PT | proton transfer; |
| pTY | poly(Titan yellow); |
| pTMS_MIP | poly(trimethoxysilane)-based molecularly imprinted polymer; |
| p(Van-co-Caf) | poly(vanillin-co-caffeic acid); |
| PWIGE | paraffin wax impregnated graphite electrode modified; |
| QDs | quantum dots; |
| Rct | charge transfer resistance at the electrode/solution interface; |
| rGO | reduced graphene oxide; |
| ROS | reactive oxygen species; |
| SASPM | surface active superparamagnetic maghemite (γ-Fe2O3); |
| SCE | saturated calomel electrode; |
| SDLSV | second-order derivative linear sweep voltammetry; |
| SDS | sodium dodecyl sulfate; |
| SPCE | screen printed carbon electrode; |
| SPE | screen printed electrode; |
| SWASV | square wave anodic stripping voltammetry; |
| SWV | square wave voltammetry; |
| T | thymine; |
| tacc | accumulation time; |
| TGACdTe@NiTAPc-Gr | thioglycolic acid-capped CdTe nanoparticles and nickel tetra-amined phthalocyanine-linked graphene oxide; |
| tinc | incubation time; |
| ZnO_NPs-PVP_NFs-FC | zinc oxide nanoparticles-poly vinyl pyrrolidone-nanofibers-ferrocene. |
References
- Lis, K.; Bartuzi, Z. Plant Food Dyes with Antioxidant Properties and Allergies—Friend or Enemy? Antioxidants 2023, 12, 1357. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of Curcumin, a Component of Golden Spice, and its Miraculous Biological Activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Zokhtareh, R.; Moghadamnia, A.A.; Asghary, M. An Electrochemical Sensor Based on Reduced Graphene Oxide Modified Carbon Paste Electrode for Curcumin Determination in Human Blood Serum. Port. Electrochim. Acta 2020, 38, 29–42. [Google Scholar] [CrossRef]
- Sun, X.; Follett, P.A.; Wall, M.M.; Duff, K.S.; Wu, X.; Shu, C.; Plotto, A.; Liang, P.; Stockton, D.G. Physical, Chemical, and Sensory Properties of a Turmeric-Fortified Pineapple Juice Beverage. Foods 2023, 12, 2323. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.P.; Swetanshu; Singh, P.; Yadav, S.; Nigam, M.; Seidel, V.; Rodrigues, C.F. Role of the Dietary Phytochemical Curcumin in Targeting Cancer Cell Signalling Pathways. Plants 2023, 12, 1782. [Google Scholar] [CrossRef]
- Burç, M.; Gungor, O.; Duran, S.T. Voltammetric Determination of Curcumin in Spices using Platinum Electrode Electrochemically Modified with Poly (Vanillin-co-Caffeic Acid). Anal. Bioanal. Chem. 2020, 12, 625–643. [Google Scholar]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Curcumin and its Uses in Active and Smart Food Packaging Applications—A Comprehensive Review. Food Chem. 2022, 375, 131885. [Google Scholar] [CrossRef] [PubMed]
- Chaisiwamongkhol, K.; Ngamchuea, K.; Batchelor-McAuley, C.; Compton, R.G. Multiwalled Carbon Nanotube Modified Electrodes for the Adsorptive Stripping Voltammetric Determination and Quantification of Curcumin in Turmeric. Electroanalysis 2017, 29, 1049–1055. [Google Scholar] [CrossRef]
- Stanić, Z. Curcumin, a Compound from Natural Sources, a True Scientific Challenge—A Review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef]
- Smirnova, E.; Moniruzzaman, M.; Chin, S.; Sureshbabu, A.; Karthikeyan, A.; Do, K.; Min, T. A Review of the Role of Curcumin in Metal Induced Toxicity. Antioxidants 2023, 12, 243. [Google Scholar] [CrossRef]
- Serpi, C.; Stanić, Z.; Girousi, S. Electroanalytical Study of the Interaction Between dsDNA and Curcumin in the Presence of Copper(II). Talanta 2010, 81, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Guerra, J.; Palomar-Pardavé, M.; Romero-Romo, M.; Corona-Avendaño, S.; Rojas-Hernández, A.; Ramírez-Silva, M.T. New Insights on the Chemical Stability of Curcumin in Aqueous Media at Different pH: Influence of the Experimental Conditions. Int. J. Electrochem. Sci. 2019, 14, 5373–5385. [Google Scholar] [CrossRef]
- Jain, R.; Haque, A.; Verma, A. Voltammetric Quantification of Surfactant Stabilized Curcumin at MWCNT/GCE Sensor. J. Mol. Liq. 2017, 230, 600–607. [Google Scholar] [CrossRef]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Basmaz, G.; Öztürk, N. Determination of Curcumin in Turmeric Sample Using Edge Plane Pyrolytic Graphite Electrode. Celal Bayar Univ. J. Sci. 2017, 13, 689–694. [Google Scholar] [CrossRef]
- Daneshgar, P.; Norouzi, P.; Moosavi-Movahedi, A.A.; Ganjali, M.R.; Haghshenas, E.; Dousty, F.; Farhadi, M. Fabrication of Carbon Nanotube and Dysprosium Nanowire Modified Electrodes as a Sensor for Determination of Curcumin. J. Appl. Electrochem. 2009, 39, 1983–1992. [Google Scholar] [CrossRef]
- Raril, C.; Manjunatha, J.G.; Tigari, G. Low-Cost Voltammetric Sensor Based on an Anionic Surfactant Modified Carbon Nanocomposite Material for the Rapid Determination of Curcumin in Natural Food Supplement. Instrumen. Sci. Technol. 2020, 48, 561–582. [Google Scholar] [CrossRef]
- Deng, P.; Wei, Y.; Li, W.; Shi, S.; Zhou, C.; Li, J.; Yao, L.; Ding, J.; He, Q. A Novel Platform Based on MnO2 Nanoparticles and Carboxylated Multi-walled Carbon Nanotubes Composite for Accurate and Rapid Determination of Curcumin in Commercial Food Products. J. Food Compos. Anal. 2023, 115, 104940. [Google Scholar] [CrossRef]
- Stanić, Z.; Voulgaropoulos, A.; Girousi, S. Electroanalytical Study of the Antioxidant and Antitumor Agent Curcumin. Electroanalysis 2008, 20, 1263–1266. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhai, H.Y.; Pan, Y.F.; Li, K. A Simple and Sensitive Sensor Based on a Molecularly Imprinted Polymer-Modified Carbon Paste Electrode for the Determination of Curcumin in Foods. RSC Adv. 2017, 7, 22913–22918. [Google Scholar] [CrossRef]
- Suhito, I.R.; Lee, W.; Baek, S.; Lee, D.; Min, J.; Kim, T.-H. Rapid and Sensitive Electrochemical Detection of Anticancer Effects of Curcumin on Human Glioblastoma Cells. Sens. Actuators B Chem. 2019, 288, 527–534. [Google Scholar] [CrossRef]
- Ahmed, A.H.M.T.; Naskar, H.; Banerjee, S.; Ghatak, B.; Das, N.; Tudu, B.; Bandyopadhyay, R. Electrochemical Sensor Based on Molecularly Imprinted Polymer Embedded Graphite Electrode for Detecting Curcumin. Sens. Actuator A Phys. 2022, 344, 113748. [Google Scholar] [CrossRef]
- Monticelli, M.; Hay Mele, B.; Allocca, M.; Liguori, L.; Lukas, J.; Monti, M.C.; Morretta, E.; Cubellis, M.V.; Andreotti, G. Curcumin Has Beneficial Effects on Lysosomal Alpha-Galactosidase: Potential Implications for the Cure of Fabry Disease. Int. J. Mol. Sci. 2023, 24, 1095. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Devasena, T.; Verma, R.S. Validation of Copper Decorated Graphene Oxide Material for Assaying Curcumin. Front. Nanosci. Nanotech. 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Stura, I.; Munir, Z.; Cavallo, L.; Torri, L.; Mandras, N.; Banche, G.; Spagnolo, R.; Pertusio, R.; Cavalli, R.; Guiot, C. Combining Blue Light and Yellow Curcumin to Obtain a “Green” Tool for Berry Preservation against Bacterial Contamination: A Preliminary Investigation. Foods 2023, 12, 2038. [Google Scholar] [CrossRef]
- Salama, A.M.; Ramadan, A.M.; Alakhdar, H.H.; Khan, T.K.; El-Garhy, H.A.S.; Shoala, T. Influence of Spraying Nano-Curcumin and Nano-Glycyrrhizic Acid on Resistance Enhancement and Some Growth Parameters of Soybean (Glycine max) in Response to Tetranychus urticae Infestation and Drought Stress. Plants 2023, 12, 114. [Google Scholar] [CrossRef]
- Han, T.; Chen, W.; Zhong, Q.; Chen, W.; Xu, Y.; Wu, J.; Chen, H. Development and Characterization of an Edible Zein/Shellac Composite Film Loaded with Curcumin. Foods 2023, 12, 1577. [Google Scholar] [CrossRef]
- Bondar, A.; Horodincu, L.; Solcan, G.; Solcan, C. Use of Spirulina platensis and Curcuma longa as Nutraceuticals in Poultry. Agriculture 2023, 13, 1553. [Google Scholar] [CrossRef]
- Qin, J.; Park, J.S.; Jo, D.G.; Cho, M.; Lee, Y. Curcumin-Based Electrochemical Sensor of Amyloid-β Oligomer for the Early Detection of Alzheimer’s Disease. Sens. Actuators B Chem. 2018, 273, 1593–1599. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.; Kumar, A.; Kumar, R.; Pal, R.; Sachan, A.K.; Dixit, R.K.; Nath, R. Effect of Curcumin and Coenzyme Q10 Alone and in Combination on Learning and Memory in an Animal Model of Alzheimer’s Disease. Biomedicines 2023, 11, 1422. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sureda, A.; Devkota, H.P.; Pittala, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the Golden Spice in Treating Cardiovascular Diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef]
- D’andurain, J.; López, V.; Arazo-Rusindo, M.; Tiscornia, C.; Aicardi, V.; Simón, L.; Mariotti-Celis, M.S. Effect of Curcumin Consumption on Inflammation and Oxidative Stress in Patients on Hemodialysis: A Literature Review. Nutrients 2023, 15, 2239. [Google Scholar] [CrossRef]
- Fessler, S.N.; Chang, Y.; Liu, L.; Johnston, C.S. Curcumin Confers Anti-Inflammatory Effects in Adults Who Recovered from COVID-19 and Were Subsequently Vaccinated: A Randomized Controlled Trial. Nutrients 2023, 15, 1548. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q.; Zhang, X.; Yang, X.; Shi, Y.; Li, Y.; Song, M. Curcumin Alleviates Aflatoxin B1-Induced Liver Pyroptosis and Fibrosis by Regulating the JAK2/NLRP3 Signaling Pathway in Ducks. Foods 2023, 12, 1006. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Lee, S.-J.; Chandrasekaran, P.; Lamichhane, G.; O’Connell, J.F.; Egan, J.M.; Kim, Y. Dietary Curcumin Attenuates Hepatic Cellular Senescence by Suppressing the MAPK/NF-κB Signaling Pathway in Aged Mice. Antioxidants 2023, 12, 1165. [Google Scholar] [CrossRef]
- Chang, G.-R.; Hsieh, W.-T.; Chou, L.-S.; Lin, C.-S.; Wu, C.-F.; Lin, J.-W.; Lin, W.-L.; Lin, T.-C.; Liao, H.-J.; Kao, C.-Y.; et al. Curcumin Improved Glucose Intolerance, Renal Injury, and Nonalcoholic Fatty Liver Disease and Decreased Chromium Loss through Urine in Obese Mice. Processes 2021, 9, 1132. [Google Scholar] [CrossRef]
- Zeng, Y.; Luo, Y.; Wang, L.; Zhang, K.; Peng, J.; Fan, G. Therapeutic Effect of Curcumin on Metabolic Diseases: Evidence from Clinical Studies. Int. J. Mol. Sci. 2023, 24, 3323. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Mielgo-Ayuso, J.; Martínez-Guardado, I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371. [Google Scholar] [CrossRef]
- Gorza, L.; Germinario, E.; Vitadello, M.; Guerra, I.; De Majo, F.; Gasparella, F.; Caliceti, P.; Vitiello, L.; Danieli-Betto, D. Curcumin Administration Improves Force of mdx Dystrophic Diaphragm by Acting on Fiber-Type Composition, Myosin Nitrotyrosination and SERCA1 Protein Levels. Antioxidants 2023, 12, 1181. [Google Scholar] [CrossRef]
- Saud Gany, S.L.; Chin, K.-Y.; Tan, J.K.; Aminuddin, A.; Makpol, S. Curcumin as a Therapeutic Agent for Sarcopenia. Nutrients 2023, 15, 2526. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, H.D.; Lee, S.H.; Lee, J.H. Curcumin Ameliorates Particulate Matter-Induced Pulmonary Injury through Bimodal Regulation of Macrophage Inflammation via NF-κB and Nrf2. Int. J. Mol. Sci. 2023, 24, 1858. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-J.; Kim, K.; Kim, C.; Lee, S.-E. Antimelanogenic Effects of Curcumin and Its Dimethoxy Derivatives: Mechanistic Investigation Using B16F10 Melanoma Cells and Zebrafish (Danio rerio) Embryos. Foods 2023, 12, 926. [Google Scholar] [CrossRef]
- Farhat, F.; Sohail, S.S.; Siddiqui, F.; Irshad, R.R.; Madsen, D.Ø. Curcumin in Wound Healing—A Bibliometric Analysis. Life 2023, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Scomoroscenco, C.; Teodorescu, M.; Nistor, C.L.; Gifu, I.C.; Petcu, C.; Banciu, D.D.; Banciu, A.; Cinteza, L.O. Preparation and In Vitro Characterization of Alkyl Polyglucoside-Based Microemulsion for Topical Administration of Curcumin. Pharmaceutics 2023, 15, 1420. [Google Scholar] [CrossRef]
- Mirzaei, B.; Zarrabi, A.; Noorbakhsh, A.; Amini, A.; Makvandi, P. A reduced graphene oxide-b-cyclodextrin nanocomposite-based electrode for electrochemical detection of curcumin. RSC Adv. 2021, 11, 7862–7872. [Google Scholar] [CrossRef] [PubMed]
- Nasery, M.M.; Varzandeh, M.; Pahlavanneshan, S.; Mohamadi, N.; Sarhadi, S.; Fekri, H.S.; Mohammadinejad, R.; Ahn, K.S. Curcumin: A Potential Therapeutic Natural Product for Adenocarcinomas. Phytochem. Lett. 2022, 49, 45–55. [Google Scholar] [CrossRef]
- Barcelos, K.A.; Mendonça, C.R.; Noll, M.; Botelho, A.F.; Francischini, C.R.D.; Silva, M.A.M. Antitumor Properties of Curcumin in Breast Cancer Based on Preclinical Studies: A Systematic Review. Cancers 2022, 14, 2165. [Google Scholar] [CrossRef]
- Nocito, M.C.; Avena, P.; Zavaglia, L.; De Luca, A.; Chimento, A.; Hamad, T.; La Padula, D.; Stancati, D.; Hantel, C.; Sirianni, R.; et al. Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment. Cancers 2023, 15, 1050. [Google Scholar] [CrossRef]
- Boccellino, M.; Ambrosio, P.; Ballini, A.; De Vito, D.; Scacco, S.; Cantore, S.; Feola, A.; Di Donato, M.; Quagliuolo, L.; Sciarra, A.; et al. The Role of Curcumin in Prostate Cancer Cells and Derived Spheroids. Cancers 2022, 14, 3348. [Google Scholar] [CrossRef]
- Salucci, S.; Bavelloni, A.; Stella, A.B.; Fabbri, F.; Vannini, I.; Piazzi, M.; Volkava, K.; Scotlandi, K.; Martinelli, G.; Faenza, I.; et al. The Cytotoxic Effect of Curcumin in Rhabdomyosarcoma is Associated with the Modulation of AMPK, AKT/mTOR, STAT, and p53 Signaling. Nutrients 2023, 15, 740. [Google Scholar] [CrossRef]
- Güllü, N.; Smith, J.; Herrmann, P.; Stein, U. MACC1-Dependent Antitumor Effect of Curcumin in Colorectal Cancer. Nutrients 2022, 14, 4792. [Google Scholar] [CrossRef] [PubMed]
- Shih, K.-C.; Chan, H.-W.; Wu, C.-Y.; Chuang, H.-Y. Curcumin Enhances the Abscopal Effect in Mice with Colorectal Cancer by Acting as an Immunomodulator. Pharmaceutics 2023, 15, 1519. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, L.; Stawny, M.; Mlynarczyk, D.T.; Muszalska-Kolos, I.; Goslinski, T.; Jelińska, A. Role of Curcumin and (−)-Epigallocatechin-3-O-Gallate in Bladder Cancer Treatment: A Review. Cancers 2020, 12, 1801. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-F.; Huang, Y.-H.; Chiu, L.-Y.; Cherng, S.-H.; Sheu, G.-T.; Yang, T.-Y. Curcumin Induces Apoptosis of Chemoresistant Lung Cancer Cells via ROS-Regulated p38 MAPK Phosphorylation. Int. J. Mol. Sci. 2022, 23, 8248. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zang, Y.; Zhang, Y.; Xie, J.; Li, J.; Gao, J.; Xue, H. Composite Polymerized Molecular Imprinting Membrane-Based Electrochemical Sensor for Sensitive Determination of Curcumin by Using 4-Pentenoyl-Aminoacyl-Chitosan Oligosaccharide as Functional Monomer Oligomer. J. Electroanal. Chem. 2020, 879, 114793. [Google Scholar] [CrossRef]
- Sravani, A.B.; Mathew, E.M.; Ghate, V.; Levis, S.A. A Sensitive Spectrofluorimetric Method for Curcumin Analysis. J. Fluoresc. 2022, 32, 1517–1527. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—From Molecule to Biological Function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476. [Google Scholar] [CrossRef]
- Deljoo, S.; Rabiee, N.; Rabiee, M. Curcumin-hybrid Nanoparticles in Drug Delivery System. Asian J. Nano. Mat. 2019, 2, 66–91. [Google Scholar] [CrossRef]
- Mandal, D.; Sarkar, T.; Chakraborty, R. Critical Review on Nutritional, Bioactive, and Medicinal Potential of Spices and Herbs and Their Application in Food Fortification and Nanotechnology. Appl. Biochem. Biotechnol. 2023, 195, 1319–1513. [Google Scholar] [CrossRef] [PubMed]
- Pan-On, S.; Dilokthornsakul, P.; Tiyaboonchai, W. Trends in Advanced Oral Drug Delivery System for Curcumin: A Systematic Review. J. Control. Release 2022, 348, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Flores-Frias, E.A.; Barba, V.; Lucio-Garcia, M.A.; Lopez-Cecenes, R.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G. Use of Curcuma and Curcumin as a Green Corrosion Inhibitors for Carbon Steel in Sulfuric Acid. Int. J. Electrochem. Sci. 2019, 14, 5026–5041. [Google Scholar] [CrossRef]
- Ashwini, N.; Dileep, R.; Ranganatha, S. Curcumin and Curcumin Derivatives as Green Corrosion Inhibitor-A Review. Phys. Chem. Res. 2023, 11, 825–835. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Kaul, V.; Gorantla, S.; Waghule, T.; Dubey, S.K.; Pandey, M.M.; Singhvi, G. UV Spectrophotometric Method for Characterization of Curcumin Loaded Nanostructured Lipid Nanocarriers in Simulated Conditions: Method Development, in-vitro and ex-vivo Applications in Topical Delivery. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 224, 117392. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Hesampour, S. Spectrophotometric Determination of Curcumin after Preconcentration by Ultrasonic Assisted Supramolecular Dispersive Liquid-liquid Microextraction based on Solidification of Floating Organic Drops using Taguchi Design Method. Adv. Mater. Lett. 2021, 12, 21111680. [Google Scholar] [CrossRef]
- Suryawanshi, B.; Nehete, J.Y. Qualitative Analysis of Curcumin in Marketed Dosage Form by Using UV Spectroscopy. Int. J. Pharm. Res. Appl. 2021, 6, 845–850. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H. Qualitative and Quantitative Analysis of Curcumin in Dried Ginger by the Resonance Rayleigh Scattering Technique and Absorption Spectroscopy. J. Food Comp. Anal. 2023, 115, 104923. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Li, L.; Yin, J.; Yu, X.; Wu, X.; Xu, L. Turn on Fluorescence Detection of Curcumin in Food Matrices by the Novel Fluorescence Sensitizer. Anal. Chim. Acta 2023, 1254, 341094. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, S.; Chen, C.; Wang, X.; Luo, C.; Wei, Q. A Fluorescent Nanoprobe Based on N/S co-Doped Carbon Dots Coupled with Molecularly Imprinted Polymers for the Detection of Curcumin. Opt. Mater. 2023, 139, 113800. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Y.; Guo, G.; Li, T.; Xing, H.; Hu, H.; Leng, X.; Gu, C.; Chen, D. Activated Cascade Effect for Dual-Mode Ratiometric and Smartphone-Assisted Visual Detection of Curcumin and F− Based on Nitrogen-Doped Carbon Dots. Sci. Total Environ. 2023, 872, 162277. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Shukla, B.; Dwivedi, J.; Saxena, S. Validated high-performance thin-layer chromatographic analysis of curcumin in the methanolic fraction of Curcuma longa L. rhizomes. Futur. J. Pharm. Sci. 2021, 7, 178. [Google Scholar] [CrossRef]
- Alvarado, H.-L.; Limón, D.; Calpena-Campmany, A.-C.; Mallandrich, M.; Rodríguez-Cid, L.; Aliaga-Alcalde, N.; González-Campo, A.; Pérez-García, L. Intrinsic Permeation and Anti-Inflammatory Evaluation of Curcumin, Bisdemethoxycurcumin and Bisdemethylcurcumin by a Validated HPLC-UV Method. Int. J. Mol. Sci. 2023, 24, 6640. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Alsahabi, D.S.; Hegazy, A.M.; Khan, R.A.; Ahmed, A.M. Analytical Purity Determinations of Universal Food-Spice Curcuma longa through a QbD Validated HPLC Approach with Critical Parametric Predictors and Operable-Design’s Monte Carlo Simulations: Analysis of Extracts, Forced-Degradants, and Capsules and Tablets-Based Pharmaceutical Dosage Forms. Foods 2023, 12, 1010. [Google Scholar] [CrossRef]
- Kroon, M.A.G.M.; van Laarhoven, H.W.M.; Swart, E.L.; Kemper, E.M.; van Tellingen, O. A Validated HPLC-MS/MS Method for Simultaneously Analyzing Curcumin, Demethoxycurcumin, Bisdemethoxycurcumin, Tetra-hydrocurcumin and Piperine in Human Plasma, Urine or Feces. Heliyon 2023, 9, e15540. [Google Scholar] [CrossRef]
- Mohammadinejad, A.; Abouzari-Lotf, E.; Aleyaghoob, G.; Rezayi, M.; Oskuee, R.K. Application of a Transition Metal Oxide/Carbon-Based Nanocomposite for Designing a Molecularly Imprinted Poly (l-Cysteine) Electrochemical Sensor for Curcumin. Food Chem. 2022, 386, 132845. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Guerra, J.; Palomar-Pardavé, M.; Romero-Romo, M.; Corona-Avendaño, S.; Guzmán-Hernández, D.-S.; Rojas-Hernández, A.; Ramírez-Silva, M.T. On the Curcumin and β-Cyclodextrin Interaction in Aqueous Media. Spectrophotometric and Electrochemical Study. ChemElectroChem 2022, 9, 202101534. [Google Scholar] [CrossRef]
- Modarresi, M.; Harfbol, M.R.; Eshraghi, A.; Ahmadi, F. Development of Ternary H-Point Standard Addition Method for Simultaneous Analysis of Curcuminoids by Differential Pulse Voltammetry. Food Res. Int. 2022, 161, 111802. [Google Scholar] [CrossRef]
- Kotra, V.S.R.; Satyabanta, L.; Goswami, T.K. A Critical Review of Analytical Methods for Determination of Curcuminoids in Turmeric. J. Food Sci. Technol. 2019, 56, 5153–5166. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C. Spice Antioxidants as Objects of Analytical Chemistry. J. Anal. Chem. 2018, 73, 946–965. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Tasbandi, A.; Jamialahmadi, T.; Sahebkar, A. Carbon-based Nanomaterials and Curcumin: A Review of Biosensing Applications. In Studies on Biomarkers and New Targets in Aging Research in Iran; Advances in Experimental Medicine and Biology; Guest, P.C., Ed.; Springer: Cham, Switzerland, 2021; Volume 1291. [Google Scholar] [CrossRef]
- Modi, G.; Pitre, K. Electrochemical Analysis of Natural Chemopreventive Agent (Curcumin) in Extracted Sample and Pharmaceutical Formulation (Short Communication). Def. Sci. J. 2010, 60, 255–258. [Google Scholar] [CrossRef][Green Version]
- Gholivdan, M.B.; Ahmadi, F.; Pourhossein, A. Adsorptive Cathodic Stripping Voltammetric Determination of Curcumin in Turmeric and Human Serum. Collect. Czech. Chem. Commun. 2011, 76, 143–157. [Google Scholar] [CrossRef]
- Shereema, R.M.; Rao, T.P.; Kumar, V.B.S.; Sruthi, T.V.; Vishnu, R.; Prabhu, G.R.D.; Shankar, S.S. Individual and Simultaneous Electrochemical Determination of Metanil Yellow and Curcumin on Carbon Quantum Dots Based Glassy Carbon Electrode. Mat. Sci. Eng. C. 2018, 93, 21–27. [Google Scholar] [CrossRef]
- Zokhtareh, R.; Rahimnejad, M. A Novel Sensitive Electrochemical Sensor Based on Nickel Chloride Solution Modified Glassy Carbon Electrode for Curcumin Determination. Electroanalysis 2018, 30, 921–927. [Google Scholar] [CrossRef]
- Kotan, G.; Kardas, F.; Yokus, O.A.; Akyıldırım, O.; Saral, H.; Eren, T.; Yola, M.L.; Atar, N. A Novel Determination of Curcumin via Ru@Au Nanoparticle Decorated Nitrogen and Sulfur Functionalized Reduced Graphene Oxide Nanomaterials. Anal. Methods 2016, 8, 401–408. [Google Scholar] [CrossRef]
- Peng, J.; Nong, K.; Cen, L. Electropolymerization of Acid Chrome Blue K on Glassy Carbon Electrode for the Determination of Curcumin. J. Chin. Chem. Soc. 2012, 59, 1415–1420. [Google Scholar] [CrossRef]
- Jha, N.S.; Mishra, S.; Jha, S.K.; Surolia, A. Antioxidant Activity and Electrochemical Elucidation of the Enigmatic Redox Behavior of Curcumin and its Structurally Sodified Analogues. Electrochim. Acta 2015, 151, 574–583. [Google Scholar] [CrossRef]
- Tigari, G.; Manjunatha, J.G. Poly(glutamine) Film-Coated Carbon Nanotube Paste Electrode for the Determination of Curcumin with Vanillin: An Electroanalytical Approach. Monatsh. Chem. 2020, 151, 1681–1688. [Google Scholar] [CrossRef]
- D’Souza, E.S.; Manjunatha, J.G.; Raril, C.; Tigari, G.; Arpitha, H.J.; Shenoy, S. Electro-Polymerized Titan Yellow Modified Carbon Paste Electrode for the Analysis of Curcumin. Surfaces 2021, 4, 191–204. [Google Scholar] [CrossRef]
- Elfiky, M.; Beltagi, A.M.; Abuzalat, O. Selective Modified Stripping Voltammetric Sensor Based on Ce-1,4-Benzenedicarboxylic Metal–Organic Frameworks Porous Nanoparticles for Picomolar Detection of Curcumin. J. Electroanal. Chem. 2021, 898, 115606. [Google Scholar] [CrossRef]
- Lungu, A.; Sandu, I.; Boscornea, C.; Tomas, S.; Mihailciuc, C. Electrochemical Study of Curcumin and bisDemethoxycurcumin on Activated Glassy Carbon Electrode. Rev. Roum. Chim. 2010, 55, 109–115. [Google Scholar]
- Masek, A.; Chrzescijanska, E.; Zaborski, M. Characteristics of Curcumin Using Cyclic Voltammetry, UV–Vis, Fluorescence and Thermogravimetric Analysis. Electrochim. Acta 2013, 107, 441–447. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Nizamova, A.M.; Budnikov, H.C. Voltammetric Determination of Curcumin in Spices. J. Anal. Chem. 2012, 67, 591–594. [Google Scholar] [CrossRef]
- Manaia, M.A.N.; Diculescu, V.C.; de Souza Gil, E.; Oliveira-Brett, A.M. Guaicolic Spices Curcumin and Capsaicin Electrochemical Oxidation Behaviour at a Glassy Carbon Electrode. J. Electroanal. Chem. 2012, 682, 83–89. [Google Scholar] [CrossRef]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H. Fabrication of Fast and Sensitive Nanostructure Voltammetric Sensor for Determination of Curcumin in the Presence of Vitamin B9 in Food Samples. Electroanalysis 2016, 28, 2590–2597. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Yang, L.; Wang, L.; Ye, B. The Electrochemical Characterization of Curcumin and its Selective Detection in Curcuma Using a Graphene-Modified Electrode. Anal. Methods 2014, 6, 7801–7808. [Google Scholar] [CrossRef]
- Zhang, D.; Ouyang, X.; Ma, J.; Li, L.; Zhang, Y. Electrochemical Behavior and Voltammetric Determination of Curcumin at Electrochemically Reduced Graphene Oxide Modified Glassy Carbon Electrode. Electroanalysis 2016, 28, 749–756. [Google Scholar] [CrossRef]
- Çakır, S.; Biçer, E.; Arslan, E.Y. A Newly Developed Electrocatalytic Oxidation and Voltammetric Determination of Curcumin at the Surface of PdNp-graphite Electrode by an Aqueous Solution Process with Al3+. Croat. Chem. Acta 2015, 88, 105–112. [Google Scholar] [CrossRef]
- Wada, R.; Takahashi, S.; Muguruma, H.; Osakabe, N. Electrochemical Detection of Curcumin in Food with a Carbon Nanotube-Carboxymethylcellulose Electrode. Anal. Sci. 2020, 36, 1113–1118. [Google Scholar] [CrossRef]
- Arslan, E.; Çakır, S. A Novel Palladium Nanoparticles-Polyproline-Modified Graphite Electrode and its Application for Determination of Curcumin. J. Solid State Electrochem. 2014, 18, 1611–1620. [Google Scholar] [CrossRef]
- Cittan, M.; Altuntaş, E.; Çelik, A. Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode as Curcumin Sensor. Monatsh. Chem. 2020, 151, 881–888. [Google Scholar] [CrossRef]
- Wang, H.; Hao, L.; Wang, P.; Chen, M.; Jiang, S.; Jiang, S. Release Kinetics and Antibacterial Activity of Curcumin Loaded Zein Fibers. Food Hydrocoll. 2017, 63, 437–446. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Zokhtare, R.; Moghadamnia, A.A.; Asghary, M. Fabrication of Electrochemical Curcumin Sensor Based on Carbon Paste Electrode. J. Appl. Chem. 2018, 13, 91–104. [Google Scholar] [CrossRef]
- Mousaabadi, K.Z.; Ensafi, A.A.; Hadadzadeh, H.; Rezaei, B. Reduced Graphene Oxide and Carbon Nanotubes Composite Functionalized by Azobenzene, Characterization and its Potential as a Curcumin Electrochemical Sensor. J. Electroanal. Chem. 2020, 873, 114418. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Z.-F.; Tan, L.; Wang, S.-X.; Wang, C.-Z.; Zhang, J.-W.; Zhou, L.-D.; Zhang, Q.-H.; Yuan, C.-S. Rapid Measurements of Curcumin from Complex Samples Coupled with Magnetic Biocompatibility Molecularly Imprinted Polymer Using Electrochemical Detection. J. Sep. Sci. 2020, 43, 1173–1182. [Google Scholar] [CrossRef]
- Li, S.; Ferrag, C.; Mikhaylichenko, S.; Kerman, K. Electrochemical Sensor for the Controlled-Release of Curcumin from a Novel Polyacrylamide Hydrogel. In Proceedings of the 17th International Meeting on Chemical Sensors—IMCS 2018, Vienna, Austria, 15–19 July 2018. [Google Scholar] [CrossRef]
- Dey, N.; Devasena, T.; Sivalingam, T. A Comparative Evaluation of Graphene Oxide Based Materials for Electrochemical Non-Enzymatic Sensing of Curcumin. Mater. Res. Express. 2018, 5, 025406. [Google Scholar] [CrossRef]
- Uca, M.; Eksin, E.; Erac, Y.; Erdem, A. Electrochemical Investigation of Curcumin–DNA Interaction by Using Hydroxyapatite Nanoparticles–Ionic Liquids Based Composite Electrodes. Materials 2021, 14, 4344. [Google Scholar] [CrossRef]
- Wray, D.M.; Batchelor-McAuley, C.; Compton, R.G. Selective Curcuminoid Separation and Detection via Nickel Complexation and Adsorptive Stripping Voltammetry. Electroanalysis 2012, 24, 2244–2248. [Google Scholar] [CrossRef]
- Kar, S.; Naskar, H.; Tudu, B.; Bandyopadhyay, R. Application of a Polytrimethoxysilane Based Molecularly Imprinted Polymer (MIP) Electrode Towards Discrimination of Different Types of Turmeric Powder. Carbon Sci. Tech. 2018, 10, 8–16. [Google Scholar]
- Afzali, M.; Mostafavi, A.; Shamspur, T. Electrospun Composite Nanofibers of Poly Vinyl Pyrrolidone and Zinc Oxide Nanoparticles Modified Carbon Paste Electrode for Electrochemical Detection of Curcumin. Mat. Sci. Eng. C 2016, 68, 789–797. [Google Scholar] [CrossRef]
- Peng, J.; Huang, Q.; Liu, Y.; Liu, P.; Zhang, C. Photoelectrochemical Sensor Based on Composite of CdTe and Nickel Tetra-Amined Phthalocyanine Covalently Linked with Graphene Oxide for Ultrasensitive Detection of Curcumin. Sens. Actuators B Chem. 2019, 294, 157–165. [Google Scholar] [CrossRef]
- Wahyuni, W.T.; Darusman, L.K.; Diksy, Y. Deteksi Kurkumin dan Bisdemetoksikurkumin dengan Teknik Voltammetri Menggunakan Elektrode Boron-Doped Diamond. ALCHEMY J. Pen. Kim. 2018, 14, 253–266. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, W.; Wang, F.; Chen, Z. Simultaneous Determination of Three Curcuminoids in Curcuma longa L. by High Performance Liquid Chromatography Coupled with Electrochemical Detection. J. Pharm. Anal. 2014, 4, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Doménech-Carbó, A.; Doménech-Carbó, T.; Saurí-Peris, C.; Gimeno-Adelantado, J.V.; Bosch-Reig, F. Identification of Curcuma and Safflower Dyes by Voltammetry of Microparticles Using Paraffin-Impregnated Graphite Electrodes. Microchim. Acta 2005, 152, 75–84. [Google Scholar] [CrossRef]
- Kupaeva, N.V.; Kotenkova, E.A. Current View on the Assessment of Antioxidant and Antiradical Activities: A Mini Review. In Proceedings of the IOP Conference Series: Earth and Environmental Science, 854, 012048, 61st International Meat Industry Conference, Zlatibor, Serbia, 26−29 September 2021. [Google Scholar] [CrossRef]
- Alam, M.W.; Najeeb, J.; Naeem, S.; Usman, S.M.; Nahvi, I.; Alismail, F.; Abuzir, A.; Farhan, M.; Nawaz, A. Electrochemical Methodologies for Investigating the Antioxidant Potential of Plant and Fruit Extracts: A Review. Antioxidants 2022, 11, 1205. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, M.A.; Chevion, M. The Use of Cyclic Voltammetry for the Evaluation of Antioxidant Capacity. Free Radic. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Blasco, A.J.; Rogerio, M.; González, M.; Escarpa, A. “Electrochemical Index” as a screening method to determine “total polyphenolics” in foods: A proposal. Anal. Chim. Acta 2005, 539, 237–244. [Google Scholar] [CrossRef]
- David, I.G.; Litescu, S.C.; Moraru, R.; Albu, C.; Buleandra, M.; Popa, D.E.; Riga, S.; Ciobanu, A.M.; Noor, H. Electroanalysis of Naringin at Electroactivated Pencil Graphite Electrode for the Assessment of Polyphenolics with Intermediate Antioxidant Power. Antioxidants 2022, 11, 2306. [Google Scholar] [CrossRef]
- Chen, C.; Xue, H.; Mu, S. pH Dependence of Reactive Sites of Curcumin Possessing Antioxidant Activity and Free Radical Scavenging Ability Studied Using the Electrochemical and ESR Techniques: Polyaniline Used as a Source of the Free Radical. J. Electroanal. Chem. 2014, 713, 22–27. [Google Scholar] [CrossRef]
- Barzegar, A. The Role of Electron-Transfer and H-Atom Donation on the Superb Antioxidant Activity and Free Radical Reaction of Curcumin. Food Chem. 2012, 135, 1369–1376. [Google Scholar] [CrossRef]
- Belmont-Bernal, F.; Aguilar, J.C.; Ramos, E.; Guadarrama, P. Systematic Derivatization of Curcumin and its Effect on Antioxidant Capacity and Action Mechanism. Cyclic Voltammetry and DFT as Tools of Analysis. ChemistrySelect 2016, 1, 5091–5098. [Google Scholar] [CrossRef]
- Borra, S.K.; Gurumurthy, P.; Mahendra, J.; Jayamathi, K.M.; Cherian, C.N.; Chand, R. Antioxidant and Free Radical Scavenging Activity of Curcumin Determined by Using Different in vitro and ex vivo Models. J. Med. Plants Res. 2013, 7, 2680–2690. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Cong, F.N.; Budnikov, H.C. Assessment of the Antioxidant Properties of Micellar Spice Extracts by Galvanostatic Coulometry with Electrogenerated Hexacyanoferrate(III) ions. J. Anal. Chem. 2015, 70, 974–982. [Google Scholar] [CrossRef]
- Toniolo, R.; Di Narda, F.; Susmel, S.; Martelli, M.; Martelli, L.; Bontempelli, G. Quenching of Superoxide Ions by Curcumin. A Mechanistic Study in Acetonitrile. Ann. Chim. 2002, 92, 281–288. [Google Scholar]
- Ziyatdinova, G.K.; Ziganshina, E.R.; Nguyen Cong, P.; Budnikov, H.C. Determination of the Antioxidant Capacity of the Micellar Extracts of Spices in Brij® 35 Medium by Differential Pulse Voltammetry. J. Anal. Chem. 2016, 71, 573–580. [Google Scholar] [CrossRef]
- Barbosa, G.B.; Gomez, E.C.; Inutan, E.D.V. Cyclic Voltammetry and Spectrophotometric Determination of Antioxidant Activities of Selected Ginger Species. Asian J. Biol. Life Sci. 2018, 7, 98–104. [Google Scholar] [CrossRef]
- Chen, M.; Li, L.; Xia, L.; Jiang, S.; Kong, Y.; Chen, X.; Wang, H. The Kinetics and Release Behaviour of Curcumin Loaded pH-Responsive PLGA/Chitosan Fibers with Antitumor Activity against HT-29 Cells. Carbohydr. Polym. 2021, 265, 118077. [Google Scholar] [CrossRef]
- Yagati, A.K.; Chavan, S.G.; Baek, C.; Lee, D.; Lee, M.-H.; Min, J. RGO-PANI Composite Au Microelectrodes for Sensitive ECIS Analysis of Human Gastric (MKN-1) Cancer Cells. Bioelectrochemistry 2023, 150, 108347. [Google Scholar] [CrossRef]
- Suhito, I.R.; Angeline, N.; Lee, K.-H.; Kim, H.; Kim, T.-H.; Park, C.G.; Luo, Z.; Kim, T.-H. A Spheroid-Forming Hybrid Gold Nanostructure Platform That Electrochemically Detects Anticancer Effects of Curcumin in a Multicellular Brain Cancer Model. Small 2021, 17, 2002436. [Google Scholar] [CrossRef]
- Angeline, N.; Choo, S.S.; Kim, C.H.; Bhang, S.H.; Kim, T.-H. Precise Electrical Detection of Curcumin Cytotoxicity in Human Liver Cancer Cells. BioChip J. 2020, 15, 52–60. [Google Scholar] [CrossRef]
- Liu, L.; Cao, X.; Ma, W.; Chen, L.; Li, S.; Hu, B.; Xu, Y. In-situ and Continuous Monitoring of Pyocyanin in the Formation Process of Pseudomonas aeruginosa Biofilms by an Electrochemical Biosensor Chip. Sens. Actuators B Chem. 2021, 327, 128945. [Google Scholar] [CrossRef]
- Samayamanthula, D.R.; Alhalaili, B.; Yapati, H.; Akber, A.; Sabarathinam, C. Innovative Bacterial Removal Technique Using Green Synthetic Nano Curcumin Zinc (II) Complex for Sustainable Water Resource Management. Sustainability 2022, 14, 4289. [Google Scholar] [CrossRef]
- Maghool, F.; Emami, M.H.; Alipour, R.; Mohammadzadeh, S.; Sereshki, N.; Dehkordi, S.A.E.; Fahim, A.; Tayarani-Najaran, Z.; Sheikh, A.; Kesharwani, P.; et al. Rescue Effect of Curcumin Against Copper Toxicity. J. Trace Elem. Med. Biol. 2023, 78, 127153. [Google Scholar] [CrossRef]
- Ciszewski, A.; Milczarek, G.; Lewandowska, B.; Krutowski, K. Electrocatalytic Properties of Electropolymerized Ni(II)curcumin Complex. Electroanalysis 2003, 15, 518–523. [Google Scholar] [CrossRef]
- Majdi, S.; Jabbari, A.; Heli, H.; Moosavi-Movahedi, A.A. Electrocatalytic Oxidation of Some Amino Acids on a Nickel–Curcumin Complex Modified Glassy Carbon Electrode. Electrochim. Acta 2007, 52, 4622–4629. [Google Scholar] [CrossRef]
- Yousef Elahi, M.; Heli, H.; Bathaie, S.Z.; Mousavi, M.F. Electrocatalytic Oxidation of Glucose at a Ni-Curcumin Modified Glassy Carbon Electrode. J. Solid State Electrochem. 2007, 11, 273–282. [Google Scholar] [CrossRef]
- Yousef Elahi, M.; Mousavi, M.F.; Ghasemi, S. Nano-structured Ni(II)–Curcumin Modified Glassy Carbon Electrode for Electrocatalytic Oxidation of Fructose. Electrochim. Acta 2008, 54, 490–498. [Google Scholar] [CrossRef]
- Heli, H.; Jabbari, A.; Majdi, S.; Mahjoub, M.; Moosavi-Movahedi, A.A.; Sheibani, S. Electrooxidation and Determination of Some Non-Steroidal Anti-Inflammatory Drugs on Nanoparticles of Ni–Curcumin-Complex-Modified Electrode. J. Solid State Electrochem. 2009, 13, 1951–1958. [Google Scholar] [CrossRef]
- Ojani, R.; Raoof, J.-B.; Zamani, S. A Novel Voltammetric Sensor for Amoxicillin Based on Nickel–Curcumin Complex Modified Carbon Paste Electrode. Bioelectrochemistry 2012, 85, 44–49. [Google Scholar] [CrossRef]
- Nayak, S.P.; Ventrapragada, L.K.; Ramamurthy, S.S.; Kumar, J.K.K.; Rao, A.M. Green Synthesis of a Novel Porous Gold-Curcumin Nanocomposite for Super-Efficient Alcohol Oxidation. Nano Energy 2022, 94, 106966. [Google Scholar] [CrossRef]
- Bernabé-Pineda, M.; Ramírez-Silva, M.T.; Romero-Romo, M.A.; González-Vergara, E.; Rojas-Hernández, A. Spectrophotometric and Electrochemical Determination of the Formation Constants of the Complexes Curcumin–Fe(III)–Water and Curcumin–Fe(II)–Water. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Özbolat, G.; Yegani, A.A.; Tuli, A. Synthesis, Characterization and Electrochemistry Studies of Iron(III) Complex with Curcumin Ligand. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1221–1226. [Google Scholar] [CrossRef]
- Özbolat, G.; Yegani, A.A. Synthesis, Characterization, Biological Activity and Electrochemistry Studies of Iron(III) Complex with Curcumin-Oxime Ligand. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Campos, R.; Baratella, D.; Lima, G.; Hola, K.; Divoky, C.; Stollberger, R.; Malina, O.; Aparicio, C.; Zoppellaro, G.; et al. A Magnetically Drivable Nanovehicle for Curcumin with Antioxidant Capacity and MRI Relaxation Properties. Chem. Eur. J. 2014, 20, 11913–11920. [Google Scholar] [CrossRef]
- Iwunze, M.O. Characterization of Cr-Curcumin Complex by Differential Pulse Voltammetry and UV-Vis Spectrophotometry. ISRN Anal. Chem. 2014, 2014, 372576. [Google Scholar] [CrossRef]
- Ahmadi, F.; Alizadeh, A.A.; Shahabadi, N.; Rahimi-Nasrabadi, M. Study Binding of Al–Curcumin Complex to ds-DNA, Monitoring by Multispectroscopic and Voltammetric Techniques. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1466–1474. [Google Scholar] [CrossRef]
- Dinesh, B.; Saraswathi, R. Electrochemical Synthesis of Nanostructured Copper-Curcumin Complex and its Electrocatalytic Application Towards Reduction of 4-Nitrophenol. Sens. Actuators B Chem. 2017, 253, 502–512. [Google Scholar] [CrossRef]
- Xi, W.; Zhai, J.; Tian, L.; Zhou, S.; Zhang, Z. Curcumin-Cu2+ Complex Generated on Carbon Nanotubes for Electrocatalytic Application Toward Electrooxidation of Hydroxylamine. Microchem. J. 2021, 161, 105792. [Google Scholar] [CrossRef]
- Merzouk, L.A.; Adkhis, A. Synthesis, Characterization, Electrochemical of Ligational Behavior of Curcumin Drug Towards Some Transition Metal Ions. In Proceedings of the Third International Symposium on Materials and Sustainable Development; SMSD 2017; Abdelbaki, B., Safi, B., Saidi, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 337–344. [Google Scholar] [CrossRef]
- Serpi, C.; Stanić, Z.; Girousi, S. Electroanalytical Study of the Interaction Between Double Stranded DNA and Antitumor Agent Curcumin. Anal. Lett. 2010, 43, 1491–1506. [Google Scholar] [CrossRef]
- Alipour, E.; Shahabi, H.; Mahmoudi-Badiki, T. Introducing Curcumin as an Electrochemical DNA Hybridization Indicator and Its Application for Detection of Human Interleukin-2 Gene. J. Solid State Electrochem. 2016, 20, 1645–1653. [Google Scholar] [CrossRef]
- Rajesh, J.; Rajasekaran, M.; Rajagopal, G.; Athappan, P. Analytical Methods to Determine the Comparative DNA Binding Studies of Curcumin–Cu(II) Complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 223–230. [Google Scholar] [CrossRef]
- Shahabadi, N.; Falsafi, M.; Moghadam, N.H. DNA Interaction Studies of a Novel Cu(II) Complex as an Intercalator Containing Curcumin and Bathophenanthroline Ligands. J. Photochem. Photobiol. B Biol. 2013, 122, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.Y.; Langari, H.; Sany, S.B.T.; Rezayi, M.; Sahebkar, A. The Role of Curcumin and its Derivatives in Sensory Applications. Mat. Sci. Eng. C 2019, 103, 109792. [Google Scholar] [CrossRef] [PubMed]
- Devasena, T.; Balasubramanian, N.; Muninathan, N.; Baskaran, K.; John, S.T. Curcumin Is an Iconic Ligand for Detecting Environmental Pollutants. Bioinorg. Chem. Appl. 2022, 2022, 9248988. [Google Scholar] [CrossRef] [PubMed]
- Devadas, B.; Rajkumar, M.; Chen, S.-M. Electropolymerization of Curcumin on Glassy Carbon Electrode and its Electrocatalytic Application for the Voltammetric Determination of Epinephrine and p-Acetoaminophenol. Colloids Surf. B 2014, 116, 674–680. [Google Scholar] [CrossRef]
- Zheng, L.; Song, J.-F. Curcumin Multi-Wall Carbon Nanotubes Modified Glassy Carbon Electrode and its Electrocatalytic Activity Towards Oxidation of Hydrazine. Sens. Actuators B Chem. 2009, 135, 650–655. [Google Scholar] [CrossRef]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Curcumin Graphite Pencil Electrode Modified with Molybdenum Disulfide Nanosheets Decorated Gold Foams for Simultaneous Quantification of Nitrite and Hydrazine in Water Samples. Anal. Chim. Acta 2020, 1137, 19–27. [Google Scholar] [CrossRef]
- Güneş, M.; Şap, A.; Karakaya, S.; Yaman, M.; Dilgin, Y. Sensitive Amperometric Detection of Hydroxylamine at Electropolymerized Curcumin Film Coated Pencil Graphite Electrode. ChemistrySelect 2023, 8, e202204104. [Google Scholar] [CrossRef]
- Nayak, S.P.; Prathyusha, V.; Kumar, J.K.K. Eco-Friendly Surface Modification of Oxidized Carbon Nanotubes with Curcumin for Simultaneous Electrochemical Detection of Dopamine and Serotonin. Mat. Chem. Phys. 2022, 287, 126293. [Google Scholar] [CrossRef]
- Dinesh, B.; Devi, K.S.S.; Kumar, A.S. Curcumin-Quinone Immobilised Carbon Black Modified Electrode Prepared by in-situ Electrochemical Oxidation of Curcumin-Phytonutrient for Mediated Oxidation and Flow Injection Analysis of Sulfide. J. Electroanal. Chem. 2017, 804, 116–127. [Google Scholar] [CrossRef]
- Kumara, K.K.; Devendirana, M.; Jyothithamizhanban, N.S.S. Curcumin/MWCNT Modified Graphite Electrode for Electrochemical Determination Of BHA. Intern. J. Innov. Res. Sci. Eng. 2014, 2, 654–659. [Google Scholar]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Reduced Graphene Oxide Nanosheets Modified with Nickel Disulfide and Curcumin Nanoparticles for Non-Enzymatic Electrochemical Sensing of Methyl Parathion and 4-Nitrophenol. Microchim. Acta 2019, 186, 704. [Google Scholar] [CrossRef]
- Haghnegahdar, N.; Abbasi Tarighat, M.; Dastan, D. Curcumin-Functionalized nanocomposite AgNPs/SDS/MWCNTs for Electrocatalytic Simultaneous Determination of Dopamine, Uric Acid, and Guanine in Co-existence of Ascorbic Acid by Glassy Carbon Electrode. J. Mater. Sci. Mater. Electron. 2021, 32, 5602–5613. [Google Scholar] [CrossRef]
- Ragu, S.; Chen, S.-M.; Ranganathan, P.; Rwei, S.-P. Fabrication of a Novel Nickel-Curcumin/Graphene Oxide Nanocomposites for Superior Electrocatalytic Activity Toward the Detection of Toxic p-Nitrophenol. Int. J. Electrochem. Sci. 2016, 11, 9133–9144. [Google Scholar] [CrossRef]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Voltammetric Simultaneous Quantification of p-Nitrophenol and Hydrazine by Using Magnetic Spinel FeCo2O4 Nanosheets on Reduced Graphene Oxide Layers Modified with Curcumin-Stabilized Silver Nanoparticles. Microchim. Acta 2019, 186, 561. [Google Scholar] [CrossRef]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Graphene Nanosheets Modified with Curcumin-Decorated Manganese Dioxide for Ultrasensitive Potentiometric Sensing of Mercury(II), Fluoride and Cyanide. Microchim. Acta 2018, 185, 529. [Google Scholar] [CrossRef]
- Duan, S.; Wu, X.; Shu, Z.; Xiao, A.; Chai, B.; Pi, F.; Wang, J.; Dai, H.; Liu, X. Curcumin-Enhanced MOF Electrochemical Sensor for Sensitive Detection of Methyl parathion in Vegetables and Fruits. Microchem. J. 2023, 184, 108182. [Google Scholar] [CrossRef]
- Dai, H.; Wu, X.; Duan, S.; Li, Z.; Zhang, Q.; Shen, Y.; Bi, J.; Shu, Z.; Xiao, A.; Pi, F.; et al. An Electrochemical Sensor Based on Curcumin-Encapsulated Zeolitic Imidazolate Framework-8 for the Sensitive Determination of Aflatoxin B1 in Grain Products. Microchem. J. 2023, 191, 108852. [Google Scholar] [CrossRef]
- Mars, A.; Mejri, A.; Hamzaoui, A.H.; Elfi, H. Molecularly Imprinted Curcumin Nanoparticles Decorated Paper for Electrochemical and Fluorescence Dual-Mode Sensing of Bisphenol A. Microchim. Acta 2021, 188, 94. [Google Scholar] [CrossRef]
- Mars, A.; Hamami, M.; Bechnak, L.; Patra, D.; Raouafi, N. Curcumin-Graphene Quantum Dots for Dual Mode Sensing Platform: Electrochemical and Fluorescence Detection of APOe4, Responsible of Alzheimer’s Disease. Anal. Chim. Acta 2018, 1036, 141–146. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Raoof, J.B.; Ojani, R. Preparation of Epirubicin Aptasensor Using Curcumin as Hybridization Indicator: Competitive Binding Assay between Complementary Strand of Aptamer and Epirubicin. Electroanalysis 2018, 30, 378–385. [Google Scholar] [CrossRef]
- Tugce Yaman, Y.; Vural, O.A.; Bolat, G.; Abaci, S. Fabrication of Trastuzumab Conjugated Curcumin Nanoparticles Based Impedimetric Cytosensor for the Cancer Cell Detection. Microchem. J. 2023, 191, 108773. [Google Scholar] [CrossRef]
- Ashraf, P.M.; Lalitha, K.V.; Edwin, L. Synthesis of Polyaniline Hybrid Composite: A New and Efficient Sensor for the Detection of Total Volatile Basic Nitrogen Molecules. Sens. Actuators B Chem. 2015, 208, 369–378. [Google Scholar] [CrossRef]
- Kumar, K.K.; Devendiran, M.; Kumar, P.S.; Babu, R.S.; Narayanan, S.S. Green Synthesis of Curcumin-Silver Nanoparticle and its Modified Electrode Assisted Amperometric Sensor for the Determination of Paracetamol. Chemosphere 2022, 303, 134994. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.K.; Devendiran, M.; Narayanan, S.S. Curcumin Functionalized Cadmium Selenide Quantum Dots Modified Electrode for Voltammetric Determination of Ascorbic Acids. Int. J. Res. Anal. Rev. 2019, 6, 308–314. [Google Scholar]
- Sivalingam, T.; Devasena, T.; Dey, N.; Maheswari, U. Curcumin-Loaded Chitosan Sensing System for Electrochemical Detection of Bilirubin. Sens. Lett. 2019, 17, 228–236. [Google Scholar] [CrossRef]
- Iffath, B.; Renjithkumar, R.; Devasena, T. Novel One Pot Synthesis of Curcumin Quantum Dots for Non-Enzymatic Highly Sensitive and Selective Detection of Dopamine. Dig. J. Nanomater. Biostructures 2023, 18, 183–193. [Google Scholar] [CrossRef]
- Ouyang, X.; Ding, Y.; Ba, X.; Gu, S. Amperometric Determination of NADH Based on a poly-Ni(II)–Curcumin Composite Film Modified Glassy Carbon Electrode. Anal. Methods 2014, 6, 7496–7501. [Google Scholar] [CrossRef]
- Kumar, K.K.; Devendiran, M.; Kalaivani, R.A.; Narayanan, S.S. Polycurcumin Nanospheres Modified Electrode for Nanoscale Detection of Mercury Ions in Seawater. Chem. Phys. Lett. 2021, 781, 138974. [Google Scholar] [CrossRef]
- Frag, E.Y.; Mohamed, G.G.; Saad, M. Chemically Modified Copper Potentiometric Sensors Based on Curcumin and Amino Acid. J. Iran. Chem. Soc. 2021, 18, 651–660. [Google Scholar] [CrossRef]
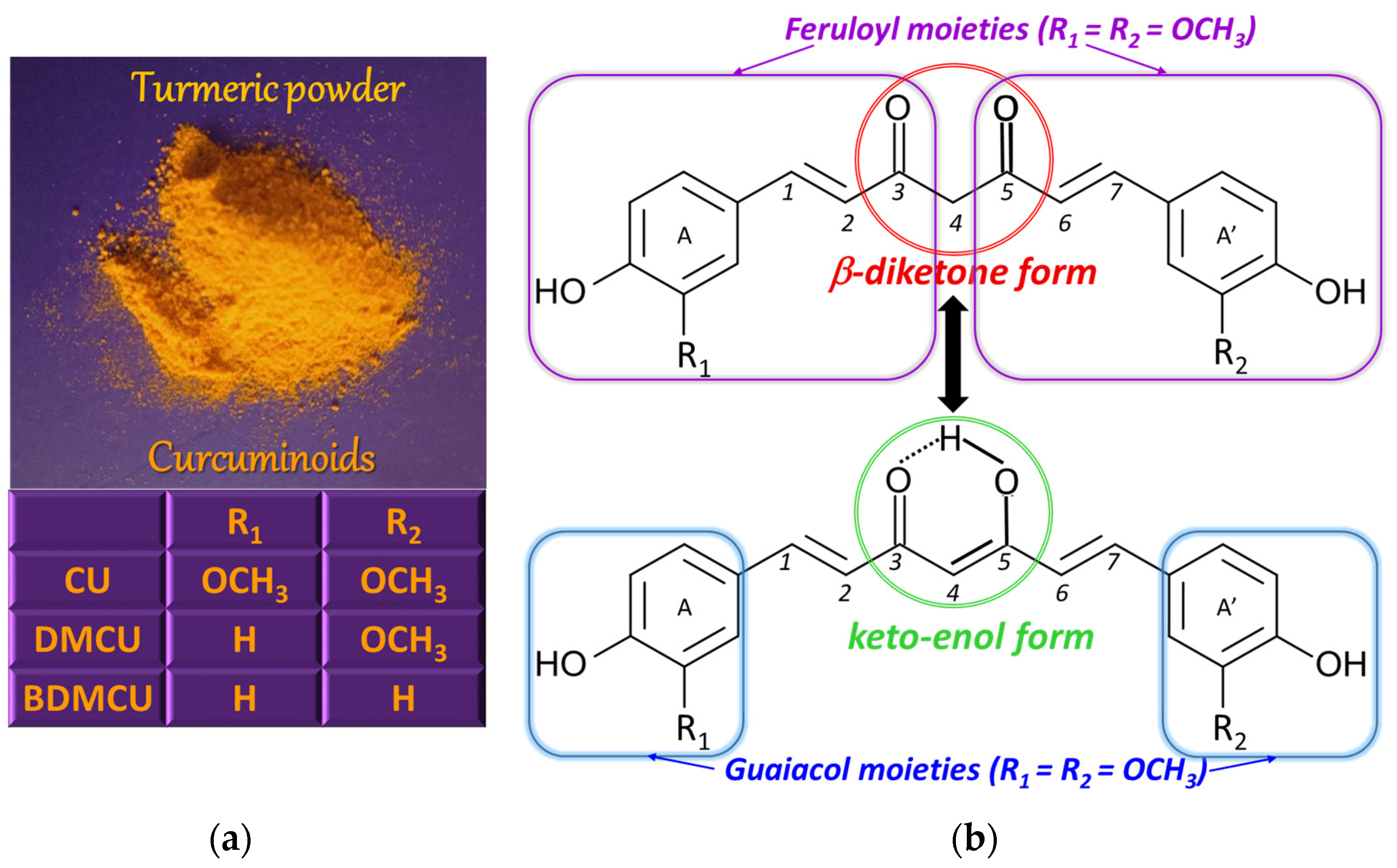

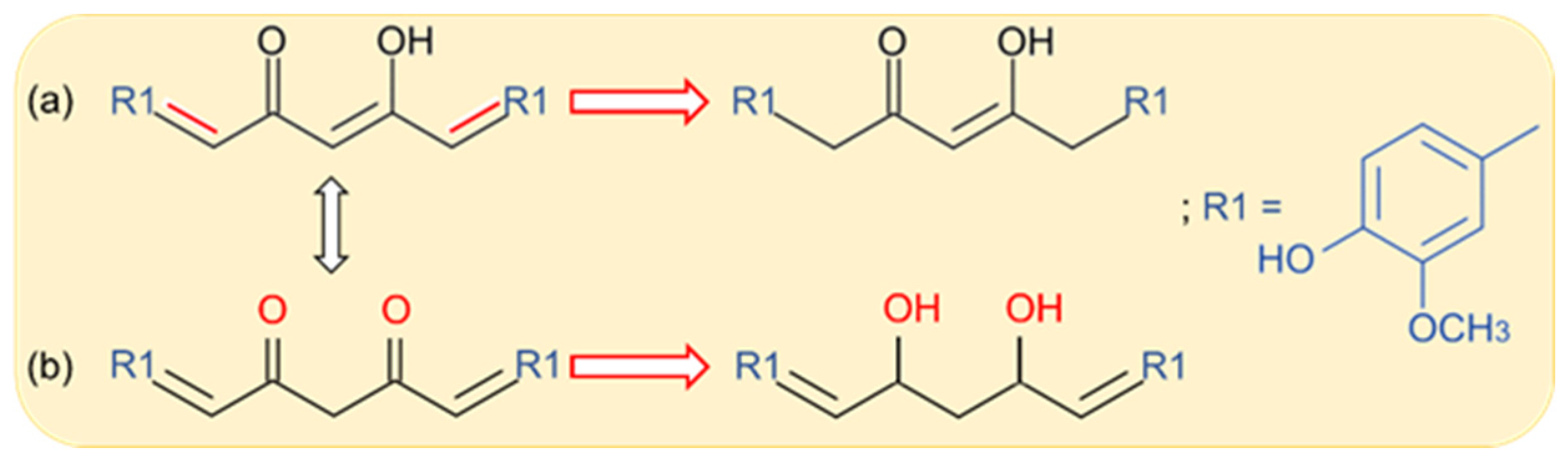
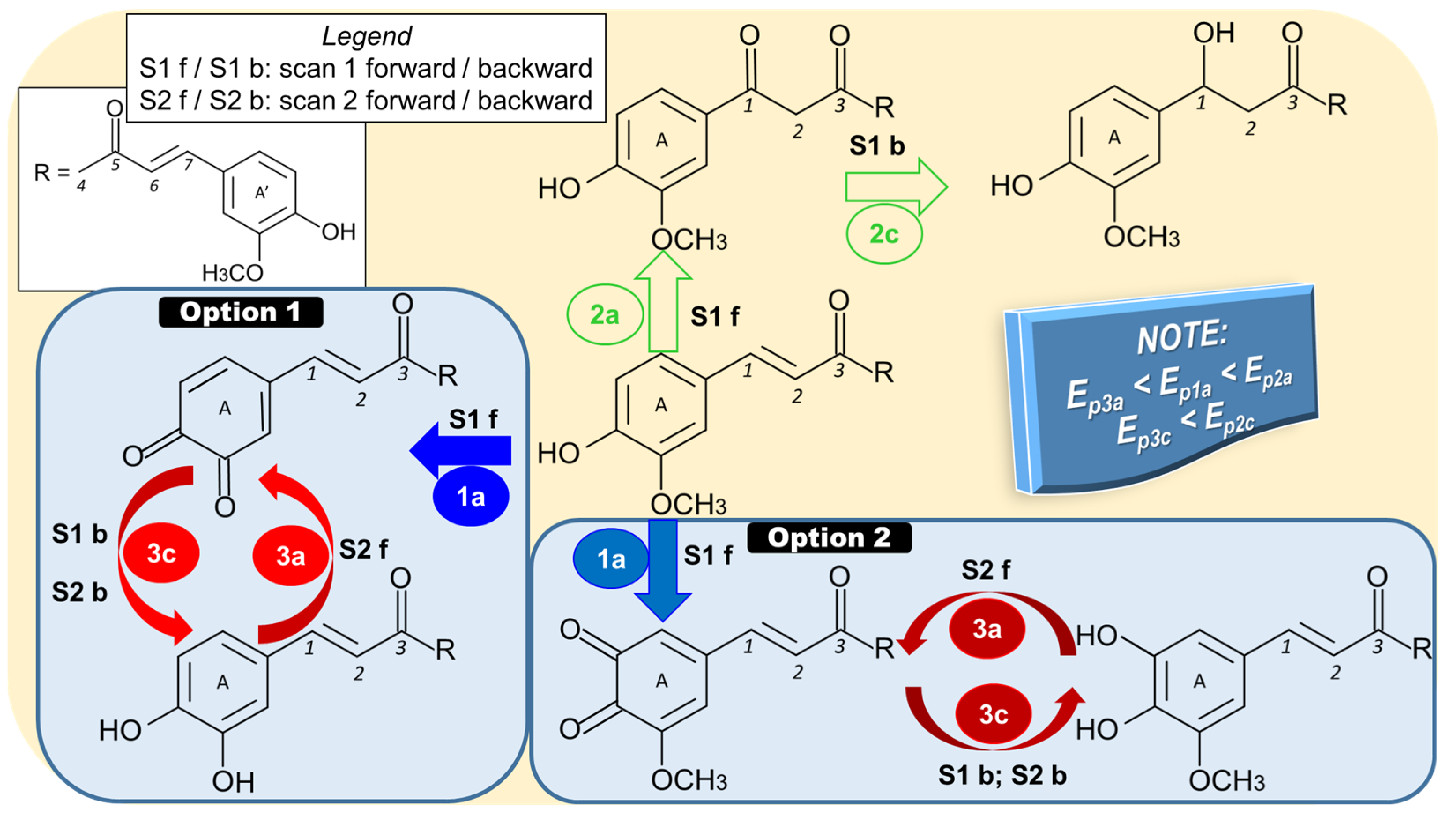

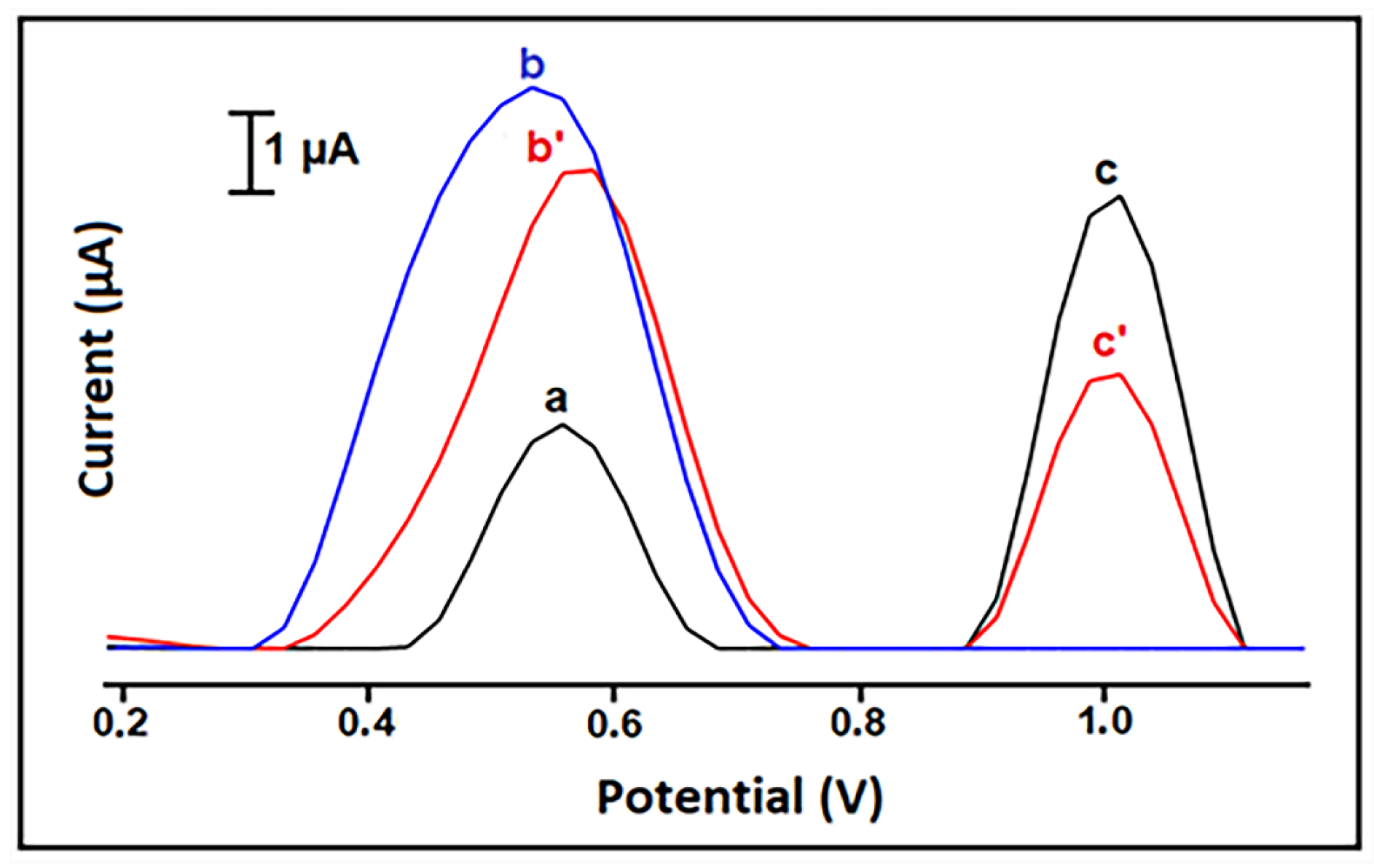
| Technique | Electrode | Peak, Ep (V); Conditions | Linear Range (mol/L) | LOD (mol/L) | Sample | Ref. |
|---|---|---|---|---|---|---|
| Reduction | ||||||
| DPAdCSV | HMDE | −1.100 BRB pH 9.50 Eacc = −0.300 V; tacc = 50 s | 5.00 × 10−9–2.80 × 10−7 | 1.50 × 10−9 | Human serum, turmeric | [83] |
| DPAdSV DPV | CPE HMDE | 0.300 ABS pH 3.50 + 0.02 mol/L NaCl Eacc = 0.300 V; tacc = 120 s −1.100 PBS pH 8.50 + 0.10 mol/L NaCl Eacc = −0.800 V; tacc = 60 s −1.600 PBS pH 8.50 + 0.10 mol/L NaCl | 5.76 × 10−8–2.74 × 10−6 4.95 × 10−7–2.76 × 10−5 9.60 × 10−7–4.84 × 10−5 | [20] | ||
| Oxidation | ||||||
| CV | GCE | 0.740 0.10 mol/L LiClO4 in ethanol | 9.90 × 10−6–1.07 × 10−4 | 4.10 × 10−6 | Spices | [94] |
| DPV | GCE | 0.090 PBS pH 7.40 + 0.10 mol/L KCl | 1.00 × 10−7–3.50 × 10−6 | 1.00 × 10−7 | CU release from MBA_pAAM hydrogel | [107] |
| DPV | NiCl2/GCE | ~0.350 PBS pH 4.00 | 1.00 × 10−5–6.00 × 10−4 | 1.09 × 10−7 | Human blood serum | [85] |
| CV DPV | CQDs/GCE | 0.288 PBS pH 4.50 | 8.00 × 10−7–1.00 × 10−4 4.00 × 10−7–2.00 × 10−4 | 1.00 × 10−7 | Turmeric powder | [84] |
| SWV | MWCNTs /GCE | ~0.700 PBS pH 2.50 + CTAB | 1.09 × 10−6–5.43 × 10−6 | 8.97 × 10−7 | Curcuma longa | [14] |
| SWV | MWCNTs /GCE | ~0.550 0.10 mol/L HCl tacc = 10 min | 1.00 × 10−8–5.00 × 10−3 | 5.00 × 10−9 | Turmeric extract | [102] |
| SWV FFTSWV | MWCNTs /GCE Dy_NWs /CPE | ~0.450 PBS pH 4.00 Eacc = −0.700 V; tacc,SWV = 110 s; tacc,FFTSWV = 0.4 s | 1.00 × 10−8–1.00 × 10−6 2.00 × 10−9–1.00 × 10−6 | 5.00 × 10−9 5.00 × 10−10 | Milk | [17] |
| AdSCV | MWCNTs /BPPGE | 0.740 BRB pH 1.81 tinc = 1 min | 2.00 × 10−6–1.00 × 10−4 | 4.50 × 10−7 | Turmeric powder | [8] |
| LSV | Gr/GCE | ~0.750 0.10 mol/L H2SO4 tacc = 80 sec | 5.00 × 10−8–3.00 × 10−6 | 3.00 × 10−8 | Curcuma longa L. | [97] |
| DPV | erGO/GCE | ~0.150 PBS pH 7.40 + 0.10 mol/L KCl | 2.00 × 10−7–6.00 × 10−5 | 1.00 × 10−7 | Turmeric capsules | [98] |
| CV | GO/GCE rGO/GCE | PBS pH 7.40 | 1.00 × 10−12–1.00 × 10−9 1.00 × 10−9–1.00 × 10−7 1.00 × 10−12–1.00 × 10−10 1.00 × 10−10–1.00 × 10−8 | 9.00 × 10−13 9.00 × 10−13 | [108] | |
| CV | Cu-GO/GCE Cu-rGO /GCE | PBS pH 7.40 | - | 4.70 × 10−9 2.00 × 10−11 | Plasma | [25] |
| DPV | pACBK/GCE | ~0.175 PBS pH 6.40 tacc = 70 sec | 1.00 × 10−7–7.00 × 10−5 | 4.10 × 10−8 | Human urine | [87] |
| SWV | Az-rGO@ MWCNTs /GCE | ~0.450 PBS pH 3.50 | 8.00 × 10−9–2.00 × 10−6 2.00 × 10−6–1.00 × 10−5 | 3.00 × 10−9 | Curcuma tablets, human plasma, urine, | [105] |
| SDLSV | MnO2- c-MWCNTs /GCE | 0.758 0.10 mol/L H2SO4 Eacc = −0.300 V; tacc = 90 s | 1.00 × 10−8–1.00 × 10−6 1.00 × 10−6–8.00 × 10−5 | 6.00 × 10−9 | Turmeric powder, curry, mustard, instant noodle seasoning, ginger powder | [19] |
| DPV | β-CD–rGO /GCE | ~0.500 PBS pH 7.00 tinc = 45 min | 5.00 × 10−8–1.00 × 10−5 | 3.30 × 10−8 | - | [46] |
| SWV | NSrGO/Ru@Au_NPs /GCE | ~0.600 PBS pH 5.00 | 1.00 × 10−12–1.00 × 10−10 | 2.00 × 10−13 | Plasma | [86] |
| CV | MBMIP_NPs /GCE | ~0.150 PBS pH 3.06 | 1.00 × 10−7–1.00 × 10−4 | 1.00 × 10−8 | Potato chips | [106] |
| DPV | pCys_MIP-CuCo2O4-N-CNTs-P-GO /GCE | ~0.450 PBS pH 3.06 | 1.00 × 10−7–1.00 × 10−6 1.00 × 10−6–3.00 × 10−5 | 3.00 × 10−8 | Human blood serum | [76] |
| SWV | Al3+-Pd_NPs /PGE | 0.560 PBS pH 2.00 | 3.00 × 10−8–6.00 × 10−7 | 2.20 × 10−8 | Turmeric powder | [99] |
| SWV | Pd_NPs-pPr /PGE | 0.504 PBS pH 2.00 | 5.00 × 10−9–1.00 × 10−7 | 1.20 × 10−9 | [101] | |
| DPV | HaP-IL/PGE | 0.560 ABS pH 4.80 tacc = 5 min | 5.43 × 10−6–2.71 × 10−5 | 5.04 × 10−6 | - | [109] |
| DPV | EPPGE | 0.10 mol/L KCl pH 2.00 | 3.25 × 10−7–1.95 × 10−6 | 2.96 × 10−7 | Turmeric powder | [16] |
| AdSCV | SPCE | 0.700 0.10 mol/L HCl (40% ethanol); tacc = 420 s | 2.20 × 10−6–7.00 × 10−5 | 4.90 × 10−6 | - | [110] |
| DPV | CPE | ~0.650 PBS pH 3.00 | 3.00 × 10−6–3.00 × 10−4 | 5.03 × 10−6 | Human blood serum | [104] |
| DPAdSV | CPE | 0.300 ABS pH 3.50 Eacc = 0.800 V; tacc = 120 s 0.600 ABS pH 3.50 | 5.76 × 10−8–4.83 × 10−6 9.60 × 10−7–1.08 × 10−5 | [20] | ||
| CV | CPE in 0.01 mol/L β-CD | 0.800 ABS pH 3.57 | 2.50 × 10−6–2.70 × 10−5 | 9.30 × 10−7 | Turmeric spice | [77] |
| DPV | rGO/CPE | ~0.650 PBS pH 3.00 | 1.00 × 10−5–6.00 × 10−3 | 3.18 × 10−6 | Human blood serum | [3] |
| CV | pTY/CPE | 0.239 PBS pH 6.50 | 2.00 × 10−6–1.00 × 10−5 1.00 × 10−5–4.00 × 10−5 | 1.09 × 10−6 | Natural food supplement | [90] |
| DPV | pAA_MIP /GE | ~0.700 ABS pH 5.50 | 1.00 × 10−6–1.00 × 10−5 1.00 × 10−5–1.80 × 10−4 | 4.00 × 10−8 | Raw turmeric, turmeric powder, capsule | [23] |
| CV | pMAA_MIP /CPE | 0.434 PBS pH 3.06 tacc = 20 s | 1.00 × 10−7–5.00 × 10−5 | 1.01 × 10−8 | Curcuma powder, cookies | [21] |
| CV | pTMS_MIP /CPE | 0.400 and 0.700 PBS pH 6.00 tacc = 20 s | 1.00 × 10−6–1.00 × 10−4 | Turmeric powder | [111] | |
| SWV | CdO-IL/CPE | 0.420 PBS pH 7.00 | 2.00 × 10−7–3.20 × 10−4 | 8.00 × 10−8 | Spices | [96] |
| SWV | ZnO_NPs-PVP_NFs-FC /CPE | ~0.250 PBS pH 8.00 | 1.00 × 10−7–7.00 × 10−6 7.00 × 10−6–5.00 × 10−4 | 2.40 × 10−8 | Urine; turmeric powder | [112] |
| DPV | SDS /CNTsPE | ~0.150 PBS pH 6.00 | 2.00 × 10−7–1.00 × 10−6 1.50 × 10−6–4.50 × 10−6 | 2.70 × 10−8 | Natural food supplement | [18] |
| DPV | pGA /CNTsPE | 0.116 PBS pH 7.50 | 4.00 × 10−7–6.00 × 10−6 6.00 × 10−6–1.00 × 10−5 | 2.79 × 10−8 | Food supplement | [89] |
| SWASV | Ce-BDC-MOF_NPs /GPE | ~0.550 BRB pH 3.00 Eacc = 0.100 V; tacc = 40 s | 2.00 × 10−11–2.00 × 10−9 2.00 × 10−9–9.00 × 10−9 5.00 × 10−11–7.00 × 10−9 3.00 × 10−11–6.00 × 10−9 | 6.00 × 10−12 1.50 × 10−11 9.00 × 10−12 | Bulk Human plasma Human urine | [91] |
| DPV | p(Van-co-Caf)/Pt | ~0.350 PBS pH 7.25 | 1.00 × 10−5–7.00 × 10−5 1.00 × 10−4–1.00 × 10−3 | 5.00 × 10−6 | Turmeric powder, curry powder | [6] |
| CV | CNTs-CMC /Au-PET | 0.300 CAB pH 6.00 | 1.00 × 10−6–4.80 × 10−6 | 8.40 × 10−8 | Turmeric powder | [100] |
| DPV | PACO_MIP /GCE | Indirect ~0.170 V 1.00 × 10−3 mol/L FCM ABS pH 6.50 | 1.00 × 10−8–2.00 × 10−6 | 5.00 × 10−9 | Turmeric extract | [56] |
| PEC | TGACdTe@NiTAPc-Gr /ITO | PBS pH 8.00 Eappl = −0.250 V | 2.50 × 10−7–1.00 × 10−4 | 1.25 × 10−8 | [113] |
| Technique | Electrode | Analyte | Linear Range (mol/L) | LOD (mol/L) | Sample | Ref. |
|---|---|---|---|---|---|---|
| LSV | polyCU/GCE | Epinephrine Paracetamol | 4.90 × 10−6–2.31 × 10−4 9.90 × 10−7–2.31 × 10−4 | 5.40 × 10−8 1.10 × 10−7 | Injections Tablets | [159] |
| Amp | CU-Ag_NPs/PWIGE | Paracetamol | 5.90 × 10−7–3.42 × 10−4 | 2.90 × 10−7 | − | [178] |
| CV | CU-CdSe_QDs/PWIGE | Ascorbic acid | 1.86 × 10−7–7.00 × 10−6 | − | − | [179] |
| CV | CU-chitosan/GCE | Bilirubin | 1.00 × 10−8–1.00 × 10−7 | 3.30 × 10−9 | Blood serum | [180] |
| DPV | ||||||
| Amp | poly-GQDS/ITO | APOe4 DNA | 20.00–400.00 pg/mL | 0.48 pg/mL | Human blood plasma | [174] |
| LSV | CU_QDs/GCE | Dopamine | 5.00 × 10−11–1.00 × 10−9 | 6.00 × 10−12 | − | [181] |
| LSV | polyCU-oCNTs/GCE | Dopamine Serotonin | 1.00 × 10−5–1.70 × 10−4 1.00 × 10−5–1.30 × 10−4 | 1.00 × 10−5 1.10 × 10−5 | − | [163] |
| DPV | CU-Ag_NPs-SDS-MWCNTs/GCE | Dopamine Guanine Uric acid | 1.20 × 10−5–2.00 × 10−4 1.60 × 10−5–4.00 × 10−4 1.80 × 10−5–6.50 × 10−4 | 1.40 × 10−7 1.90 × 10−7 3.80 × 10−7 | Blood, serum, urine, pharmaceuticals | [167] |
| EIS | poly(Ni2+-CU)/ Ni foam | Amyloid β oligomer | 1.00 × 10−12–5.00 × 10−9 | 1.00 × 10−12 | Artificial cerebrospinal fluid | [30] |
| Amp | poly(Ni2+-CU)/CPE | Amoxicillin | 8.00 × 10−6–1.00 × 10−4 | 5.00 × 10−6 | Urine, capsules | [142] |
| Amp | poly(Ni2+-CU)/GCE | NADH | 3.00 × 10−7–3.00 × 10−4 | 1.80 × 10−7 | Human serum | [182] |
| Amp | polyCU/PGE | Hydroxylamine | 5.00 × 10−7–5.00 × 10−4 | 1.50 × 10−7 | Pharmaceuticals water | [162] |
| Amp | Cu(II)-CU-SWCNTs/GCE | Hydroxylamine | 1.00 × 10−6–1.00 × 10−4 1.00 × 10−4–1.00 × 10−3 | 1.90 × 10−8 | Pharmaceuticals | [151] |
| Amp | polyCU-MWCNTs/GCE | Hydrazine | 2.00 × 10−6–4.40 × 10−5 | 1.40 × 10−6 | − | [160] |
| DPV | MIpAA@CU/μPAD | Bisphenol A | 4.38 × 10−9–8.76 × 10−6 | 2.06 × 10−9 | Cans, plastic bottles | [173] |
| Amp FIA | polyCU-quinone-CB/GCE | Sulfide | 1.00 × 10−5–1.00 × 10−4 1.00 × 10−5–1.20 × 10−3 | 2.40 × 10−6 7.12 × 10−6 | [164] | |
| CV | polyCU-MWCNTs/PWIGE | Butylated hydroxyanisole | 3.37 × 10−6–3.32 × 10−4 | 2.25 × 10−7 | − | [165] |
| DPV | CU-UiO-66/GCE | Methyl parathion | 6.88 × 10−8–6.88 × 10−5 | 3.36 × 10−9 | Vegetables, fruits | [171] |
| DPV | poly(Cu2+-CU)/GCE | 4-Nitrophenol | 1.00 × 10−7–1.03 × 10−3 | 6.82 × 10−8 | − | [150] |
| LSV | Ni(CU)2-GO/GCE | 4-Nitrophenol | 4.90 × 10−7–7.60 × 10−4 | 1.60 × 10−8 | − | [168] |
| DPV | CU_NPs-NiS2-rGO/SPCE | Methyl parathion 4-Nitrophenol | 2.50 × 10−6–5.00 × 10−6 5.00 × 10−6–8.00 × 10−5 2.50 × 10−6–5.00 × 10−6 5.00 × 10−6–8.00 × 10−5 | 8.70 × 10−8 6.90 × 10−8 | Tomato and apple juices; spiked river water | [166] |
| Amp | CU-Ag_NPs-rGO-FeCo2O4/SPCE | Hydrazine 4-Nitrophenol | 2.00 × 10−6–3.00 × 10−4 3.00 × 10−4–1.20 × 10−3 2.00 × 10−6–3.00 × 10−4 3.00 × 10−4–1.20 × 10−3 | 2.37 × 10−8 1.84 × 10−8 | Industrial wastewater, river water | [169] |
| DPV | MoS2-Au-polyCU/PGE | Hydrazine Nitrite | 2.00 × 10−5–3.50 × 10−4 3.50 × 10−4–1.20 × 10−3 2.00 × 10−5–3.50 × 10−4 3.50 × 10−4–1.20 × 10−3 | 1.83 × 10−8 2.17 × 10−8 | Industrial wastewater, river water | [161] |
| DPASV | polyCU_NSs/PWIGE | Hg2+ | 1.05 × 10−9–1.05 × 10−7 | 3.51 × 10−10 | Seawater | [183] |
| DPV | polyCU-MnO2-Gr/GCE | Hg2+ CN− F− | 5.00 × 10−8–1.20 × 10−6 5.00 × 10−8–1.20 × 10−6 5.00 × 10−8–1.20 × 10−6 | 1.92 × 10−8 2.83 × 10−8 1.72 × 10−8 | Spiked river water, tap water, petrochemical refinery wastewater | [170] |
| Potentiom | CU-CPE | Cu2+ | 1.00 × 10−6–1.00 × 10−2 | 1.00 × 10−6 | Water | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, I.G.; Iorgulescu, E.E.; Popa, D.E.; Buleandra, M.; Cheregi, M.C.; Noor, H. Curcumin Electrochemistry—Antioxidant Activity Assessment, Voltammetric Behavior and Quantitative Determination, Applications as Electrode Modifier. Antioxidants 2023, 12, 1908. https://doi.org/10.3390/antiox12111908
David IG, Iorgulescu EE, Popa DE, Buleandra M, Cheregi MC, Noor H. Curcumin Electrochemistry—Antioxidant Activity Assessment, Voltammetric Behavior and Quantitative Determination, Applications as Electrode Modifier. Antioxidants. 2023; 12(11):1908. https://doi.org/10.3390/antiox12111908
Chicago/Turabian StyleDavid, Iulia Gabriela, Emilia Elena Iorgulescu, Dana Elena Popa, Mihaela Buleandra, Mihaela Carmen Cheregi, and Hassan Noor. 2023. "Curcumin Electrochemistry—Antioxidant Activity Assessment, Voltammetric Behavior and Quantitative Determination, Applications as Electrode Modifier" Antioxidants 12, no. 11: 1908. https://doi.org/10.3390/antiox12111908
APA StyleDavid, I. G., Iorgulescu, E. E., Popa, D. E., Buleandra, M., Cheregi, M. C., & Noor, H. (2023). Curcumin Electrochemistry—Antioxidant Activity Assessment, Voltammetric Behavior and Quantitative Determination, Applications as Electrode Modifier. Antioxidants, 12(11), 1908. https://doi.org/10.3390/antiox12111908









