1. Introduction
Fish emulsions, such as salmon pâté, represent a valuable dietary source of polyunsaturated fatty acids (PUFA), in particular, of some essential long-chain n-3 PUFA, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [
1]. The intake of these two PUFA has been correlated with a lesser risk of cardiovascular diseases [
2], neurodegenerative diseases and inflammatory disorders [
3]. However, PUFA are particularly prone to oxidation and can be degraded during manufacturing and storage of fish pâté due to oxidising factors such as oxygen, iron, salt, heating and mechanical treatments [
4,
5]. Lipid oxidation can generate secondary compounds, such as malondialdehyde (MDA) and cholesterol oxidation products (COP), together with volatile organic compounds (VOC), including hydrocarbons, aldehydes, ketones and alcohols, among others, responsible for flavour and rancidity [
6]. An excessive intake of oxidised lipids may increase the risk of some pathologies such as metabolic and neurodegenerative diseases and some cancers [
7,
8]. Oxidation reactions involving other pâté components may also lead to some changes in colour and pH. Therefore, it is justified that fish pâté, a product rich in unsaturated fat, is stabilised with antioxidants. Synthetic antioxidants, including tertiary butylated hydroquinone, butylated hydroxy anisole, propyl gallate and butylated hydroxy toluene (BHT), are used in the food industry to stabilise food lipids due to their low cost and high efficiency [
9]. Nevertheless, some of these antioxidants are being questioned due to their technological limitations (low dosage, thermal sensibility and high volatility) [
10], and, in particular, to their possible role as toxicological and carcinogenic agents in humans [
11,
12].
Nowadays, “green label” formulations for fish products often include “generally recognised-as-safe” (GRAS) natural preservatives. Among these, phenolic compounds present in fruits, vegetables, beverages (tea, wine, juices), plants, seaweed and some herbs show antioxidant and antimicrobial activities, considered as emerging natural additives for fish products [
13]. Phenolic compounds would mainly have an antioxidant role in fish products where microbiological quality is ensured by heating treatments. In recent studies, oxidative stability could be improved in different fish products (burgers, cooked and frozen cuts) using different phenolic extracts (rosemary, sage, lavender, moringa, pomegranate and olive) [
14,
15,
16,
17,
18]. These studies reveal the importance of using active ingredients with suitable properties (e.g., solubility and antioxidant power) to obtain good results in fish matrixes.
Rosemary (
Rosmarinus officinalis L.) is considered a source of phenolic antioxidants, being particularly rich in some hydrophobic diterpenes such as carnosic acid and carnosol, which can act as free radical scavengers in lipid matrixes [
19]. The rosemary plant is subjected to successive distillation and extraction processes: (i) essential oil containing volatile phenols (e.g., carvacrol and eucalyptol) is removed with water steam, (ii) oil-free by-product is extracted with water to obtain the rosemary water extract (RWE), which is rich in hydrophilic polyphenols (e.g., rosmarinic acid) and (iii) the resulting by-product is extracted again with an acetone and water mix to obtain the rosemary lipophilic extract (RLE) [
11,
20,
21]. RLE has been authorised by the European Union (E392) as an antioxidant for fish products at maximum doses of 150 mg/kg (carnosic acid + carnosol) (European Union Regulation 1129/2011/EU) [
22]. The antioxidant effectiveness of different rosemary extracts has been checked in food products such as chicken and beef burgers [
11,
23], pork and chicken liver pâté [
5,
24,
25], frozen salmon [
18], fish patties [
16], sea bream [
17] and sardines [
26]. In general, rosemary extracts showed good antioxidant properties in muscle-based products, although their use has some technological limitations. Rosemary extracts may not reach a suitable balance between dosage, polyphenols stability, preservative effects and sensory impact [
27]. This might be due to various reasons: (i) polyphenol content of rosemary extract may vary depending on the raw material, which is related to genetic and agronomical variations, and the extraction procedure used; (ii) once the extract is added, rosemary polyphenols may degrade to a greater or lesser degree during food processing, particularly during thermal treatments and further storage; and (iii) rosemary extracts provide an intense herbal flavour that may negatively affect the acceptance of food products. Therefore, it is recommended to use typified rosemary extracts obtained from selected plants to attempt to standardise their preservative, sensory and nutritional properties [
19].
Consumers increasingly demand healthier food products. Salmon pâté, a fatty product with a high caloric value, is often prepared with vegetable oils (e.g., sunflower oil) that provides a spreadable texture and a good flavour to the product. In less-caloric formulations, vegetable oils can be partially replaced by linseed [
28], an ingredient with a neutral flavour and good thickening properties that can be used as a fat substitute and emulsion stabiliser, while also being a good source of n-6 PUFA and dietary fibre [
29,
30]. The working hypothesis of the present study was that RLE, applied at authorised doses, can stabilise lipids against oxidation in a PUFA-enriched fish pâté. The objective was therefore to test RLE (rich in carnosic acid and carnosol and obtained from selected plants) as a lipid antioxidant in a pasteurised-chilled salmon pâté made with linseed.
3. Results
Twenty-two polyphenols were determined in RLE (
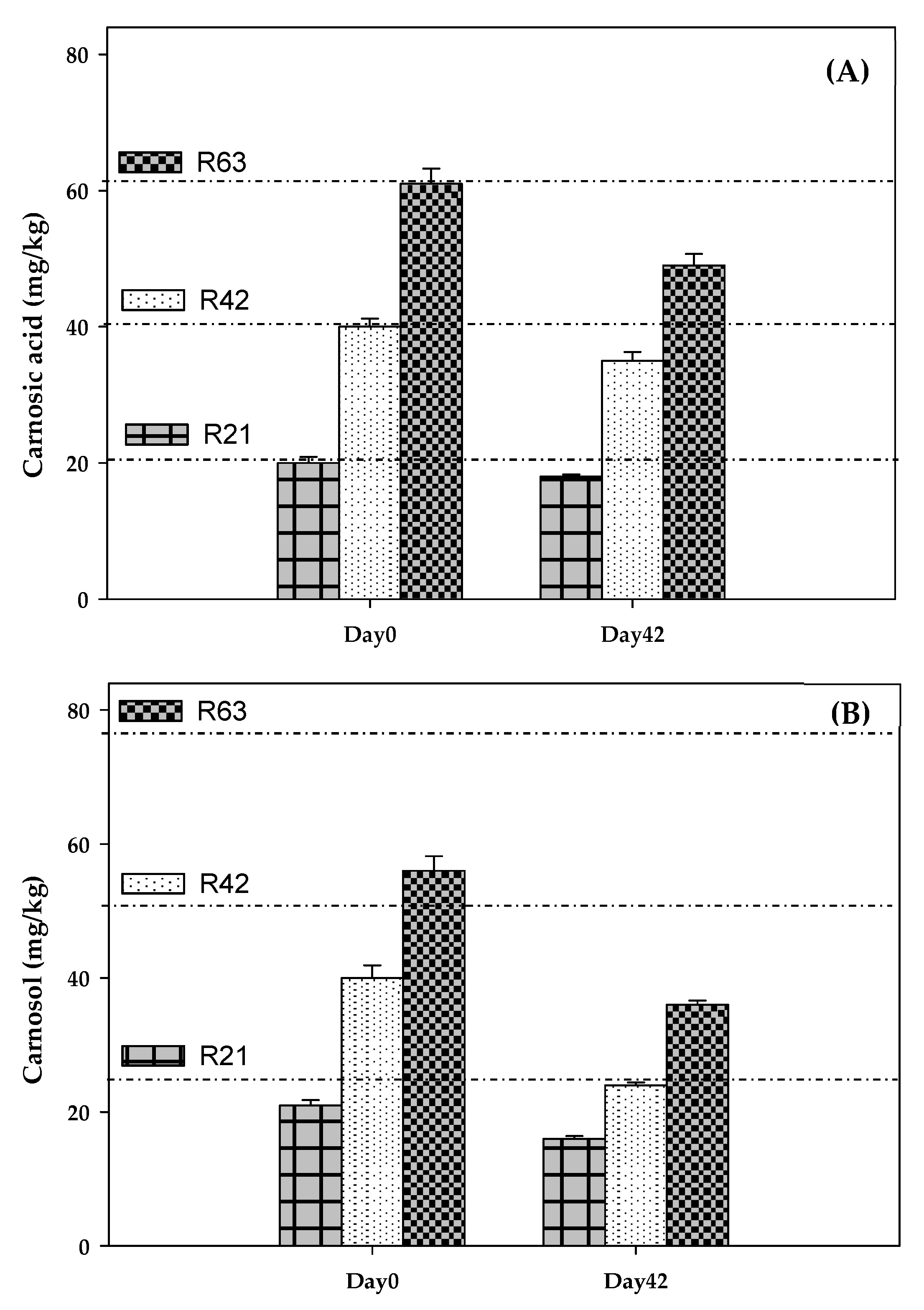
Table 2), including phenolic acids (salvianic, caffeic, isoferulic, rosmarinic, salvianolic), flavonoids (lithospermic acid, luteolin-4-glucoside, cirsimaritin, genkwanin, hesperidin, salvigenin and protocateic acid) and diterpenes (carnosol, carnosic acid, 7-methyl-rosmanol, 12-methyl-carnosic acid, rosmanol and different derivatives from carnosol and methyl-rosmanol). Carnosol and carnosic acid were the most abundant compounds (125 and 98 mg/g, respectively), representing 67% of total polyphenols in RLE (334 mg/g). Degradation of carnosic acid and carnosol was studied in salmon pâté (
Figure 1). According to the polyphenol content of RLE, the initial quantities (mg/kg) of carnosic acid + carnosol added to raw pâté were: 21 + 26 (R21), 41 + 53 (R42) and 62 + 79 (R63). RLE also contained relevant levels of methyl-carnosic acid, carnosol derivatives and other diterpenes. On day 0, the concentrations (mg/kg) of carnosic acid + carnosol determined in pâté were: 20 + 21 (R21), 40 + 39 (R42) and 61 + 56 (R63), while, on day 42, these concentrations were: 18 + 16 (R21), 35 + 24 (R42) and 49 + 35 (R63). Thus, both diterpenes were retained without degrading in the pasteurised salmon pâté, even though a part (10–18% of carnosic acid and 24–38% of carnosol) degraded during chill storage.
Salmon pâté contained 12 g/100 g total protein, 19 g/100 g total lipids and 61 g/100 g moisture, contributing with 270.6 kcal/100 g (
Table 3). Data regarding general lipid oxidation, CIELab colour and pH are shown in
Table 4. RLE addition did not affect PV on day 0, but R63 pâté presented the highest PV on day 42, while not impacting CD and CT values on day 0 and 42. Thus, PV discriminated the effects of RLE on primary lipid oxidation better than CD and CT, which provide similar information. Chill storage did not affect PV but actually decreased CD and CT values. The antioxidant effects of RLE addition on lipids were confirmed by TBARS. MDA levels were higher in the untreated pâté on day 0 and 42 than in the pâté with RLE (at any dose). No differences were found in the MDA levels among R21, R42 and R63 pâtés. Similarly, adding RLE did not affect colour on day 0, while pâtés with more RLE (R42 and R63) had lower values of L* and a* and higher values of b* and h* than the untreated pâté on day 42. Therefore, salmon pâté underwent some discolouration (slight browning) during chill storage that might be inhibited using RLE. Regardless of the dose used, RLE slightly decreased the pâté pH on days 0 and 42. Overall, the pH values hardly decreased by around 0.2 after chill storage.
Twenty-two FA were quantified (g FA/100 g fat) (
Table 5). The most abundant class was MUFA (22.7–26.2 g/100 g fat) with C18:1
t n-9 as main FA (18.3–22.2 g/100 g fat), followed by PUFA (17.3–20.2 g/100 g fat), with C18:2
c n-6 (11.8–13.8 g/100 g fat) and C18:3α n-3 (2.1–2.7 g/100 g fat) as major FA, and by SFA (9.2–10.6 g/100 g fat), with C16:0 as predominant FA (3.6–4.6 g/100 g fat). Levels of EPA and DHA were around 1 and 1.4 g/100 g fat, respectively. RLE addition did not affect the FA levels on day 0 and 42, while chill storage only led to some changes in minor FA. TFA content was similar for the untreated and the RLE pâtés on day 0 and 42. Consequently, nutritional FA ratios (n-6/n-3 and P/S) were unaffected by RLE addition or chill storage.
The most abundant sterol was cholesterol (33.7–41.7 mg/100 g pâté), followed by β-sitosterol (12.9–17.8 mg/100 g pâté), campesterol (2.3–3.9 mg/100 g pâté) and stigmasterol (1.6–2.2 mg/100 g pâté) (
Table 6). Campesterol content was higher in the untreated than in the R21 and R63 pâtés on day 0, whereas on day 42 a reduction was observed for all sterols in all R21 pâtés with respect to untreated ones. Chill storage generally led to a decrease in sterol content, except for stigmasterol. Among the sterol oxidation products, only COP were determined, as cholesterol was the most abundant sterol in salmon pâté. The main COP was 7-ketocholesterol (7-KC), followed by 7α-hydroxycholesterol (7α-HC), 7β-hydroxycholesterol (7β-HC), cholestane-3β,5α,6β-triol (triol), 5β,6β-epoxycholesterol (β-EC) and 5α,6α-epoxycholesterol (α-EC). RLE addition had an antioxidant effect on cholesterol, since the untreated pâté presented the highest COP contents on day 0 and 42. Consequently, the OR were clearly higher in untreated (0.49–0.70%) than in pâtés added with RLE (0.22–0.32%). As observed for single and total COP, RLE addition, storage time and its interaction influenced this ratio. OR increased with chill storage in untreated and R21, while remaining stable in R42 and R63 pâtés.
A total of twenty VOC were identified in the headspace of salmon pâté including several hydrocarbons (3-methoxy-1-propene, toluene, butylated-hydroxytoluene, 1-ethyl-4-methyl-benzene, and 1H-trindene, 2,3,4,5,6,7,8,9-octoctahydro-1,1,4,4,9,9-hexamethyl), alcohols (1-pentanol, 1-hexanol, 1-penten-3-ol, (Z)-2-penten-1-ol, 1-octen-3-ol, eugenol, and benzyl alcohol), aldehydes (hexanal, 2-methyl-propanal, 2-(phenylmethylene)-octanal and 2,5-bis(trimethyl)-benzaldehyde), ketones (2’,6’-dihydroxyacetophenone), organic acids (2-amino-4-methyl-benzoic acids) and terpenes (D-limonene and α-pinene). The relative abundance of most of these VOC was unaffected by the addition of RLE or chill storage, with some exceptions (
Table 7). Of these, 1-pentanol was less abundant in the untreated than in the pâté with RLE (at any dose) on day 42. Overall, the abundance of 1-pentanol and 1-hexanol increased with chill storage, while, in contrast, the abundance of hexanal and 2-methyl-propanal decreased on day 42. The above four VOC were studied as possible lipid oxidation markers in salmon pâté.
4. Discussion
Recovery of rosemary polyphenols depends on the raw materials, solvents and operating conditions applied to obtain the extracts [
19,
40]. Different studies agree that carnosic acid and carnosol are the most abundant polyphenols present in RLE [
19,
21,
40]. The reported concentration ranges are 47–179 mg/g for carnosic acid, 5–28 mg/g for carnosol and 50–200 mg/g for total polyphenols [
19,
41]. Thus, the RLE tested in the present study was particularly rich in polyphenols (333 mg/g), which was one of the main objectives of the plant’s selection. As a purity criterion, European Union Directive 2010/67/UE [
42] establishes that the RLE for food application must contain at least 10% (
w:
w) of carnosic acid plus carnosol, a percentage widely exceeded by the RLE used in this experiment. Assessment of the stability of carnosic acid and carnosol provides an idea of how rosemary antioxidants behave in food matrices. Under oxidising conditions, carnosic acid is transformed into carnosol [
21,
43], which can be regenerated or not by other antioxidants acting in the food matrix. As observed in the experimental data, both diterpenes were quite resistant to the pasteurisation conditions applied, as has also been reported for carnosic acid in pork liver pâté [
24]. In general, rosemary diterpenes are quite resistant to the cooking procedures applied in food [
24,
41]. In the present study, the degradation of carnosic acid and carnosol mainly occurred during chill storage. This was expected, since salmon pâté was aerobically homogenised, which favours the presence of occluded oxygen, and jars were kept under refrigeration and fluorescent lighting (600 lux) for 42 days. Doolaege et al. (2012) [
24] found that a part (6–32 mg/kg) of the added carnosic acid (250–750 mg/kg) degraded in liver pâté kept at 4 °C for 48 h. There were no available data on the stability of rosemary polyphenols in other studies on the antioxidant properties of RE in meat and fish products [
11,
23].
The formulation used for salmon pâté in the present study enabled the reduction of fat content and caloric intake compared to commercial fish pâtés (30% fat and 330 kcal/100 g pâté) [
44,
45,
46]. Salmon pâté reflected the FA and sterol profiles of the different fat sources used as ingredients (salmon muscle, sunflower oil and linseed). The FA profile was similar to those reported for other fish pâtés [
45,
46,
47]. The relevance of the different FA classes (MUFA > PUFA > SFA) is coherent with the above-mentioned fat sources, since salmon flesh and linseed are rich in MUFA and PUFA [
30,
48], while sunflower oil is rich in PUFA [
49]. Salmon muscle is rich in cholesterol, while β-sitosterol is the main phytosterol in sunflower oil and linseed [
50,
51]. Sterol content tended to decrease in all pâtés following chilled storage, likely due to the oxidation reactions involving lipids. The cholesterol content of the salmon pâté coincides with that reported by Echarte et al. (2004) [
47]. From a nutritional standpoint, salmon pâté showed a n-6/n-3 ratio (2.3–2.6) below the recommended level of 4 [
52], resulting in a balanced dietary source of n-3 FA. An adequate n-6/n-3 ratio in the diet contributes to optimising bioavailability, metabolism and incorporation of FA into membrane phospholipids [
30]. Likewise, the P/S ratio (1.79–1.97) was above the minimum recommended (0.5–0.7) [
52], which is considered a good nutritional trait. An increase in the P/S ratio can lead to reduced plasma total cholesterol [
52], while, at the technological level, it is a useful indicator of fat oxidation susceptibility, which mainly affects PUFA. In the present study, pâté lipids, integrated by a high proportion of PUFA, were quite stable during chill storage, perhaps partly due to linseed, a thickening agent containing hydrocolloids that might help stabilising the emulsion [
53].
Rosemary polyphenols, containing benzene rings able to act as free radical scavengers, fulfilled their antioxidant role delaying lipid oxidation in the pâté; however, in quantitative terms, the addition of up to 210 mg/kg of rosemary polyphenols does not seem enough to protect a fatty fish emulsion (19 g fat/100 g pâté, of which, 4.7 g MUFA and 3.4 g PUFA) against oxidation. Lipid oxidation reactions involve three stages: initiation, propagation and termination. Some techniques measure the loss of initial reactants (such as oxygen, lipid and antioxidants), others the formation of primary oxidation products (such as hydroperoxides and conjugated dienes) and others the formation of secondary oxidation products (such as alcohols, aldehydes, hydrocarbons and ketones) [
54]. Thermal exposure in the presence of oxygen induces lipid oxidation, which forms hydroperoxides and causes double bond displacement or isomerisation, resulting in an increased production of CD and CT from unsaturated FA [
55]. As oxidation advances in pâté during the display period, secondary oxidised lipids are formed to the detriment of the early oxidised lipids, even though they may still be generated. This may explain why primary oxidation stabilised or decreased after chill storage, as indicated by the reduction observed in the CD and CT values. The reactive chemical species that gave rise to CT evolved towards other secondary compounds such as TBARS [
56]. PV was the sole index that discriminated the antioxidant activity of rosemary polyphenols in the early stages. As observed in
Table 4, R63 pâté clearly had more hydroperoxides than the other samples on day 42, suggesting that the formation of secondary oxidised lipids such as TBARS and VOC was inhibited. Rosemary polyphenols may inhibit the formation of oxidised lipids through hydrogen donation or preventing the cleavage of lipid hydroperoxides [
55].
TBARS is a widely used index with technological (flavour and rancidity) and nutritional (toxicity) implications that measures the levels of aldehydes and other secondary oxidised lipids in the pâté. Lipid oxidation is enhanced by the thermal treatment and favoured by fat unsaturation and the presence of oxygen. Salmon pâté presented an incipient lipid oxidation just after pasteurisation. The antioxidant role of RLE during pâté preparation was modest, since it only slightly inhibited the formation of MDA and was not dose-dependent. Salmon was cooked before adding RLE, which would explain why there were no marked differences regarding MDA levels among the different freshly pasteurised pâtés. TBARS formation continued during further retail display. On day 42, MDA level practically doubled in the pâté formulated without RLE, reaching TBARS values near 2 mg MDA/kg, the threshold value from which rancidity is sensorially detected in cooked meat [
57]. In contrast, RLE clearly inhibited MDA formation in the chill-stored pâté. However, increasing RLE dose did not improve results. In other studies, lipid oxidation was inhibited when using different RLE in pork liver pâté [
24,
58], chicken pâté [
5] or RWE in frozen salmon [
18] and refrigerated sardines [
26], confirming the present findings.
The antioxidant effect of RLE was also confirmed for cholesterol. The COP profile found in this study is similar to that reported by Echarte et al. (2004) [
47] for salmon pâté, where the most abundant COP originated from the monomolecular oxidation reaction pathway involving B ring (i.e., 7-oxysterols), while epoxy and triol derivatives, generated from a bimolecular reaction pathway, were found in smaller quantities (β-EC and triol) or even undetected (α-EC) in the pâtés with RLE. The amounts of total COP formed do not correspond to the observed decrease in cholesterol, which might be due to the reaction of COP with amino groups from amino acids, peptides and proteins leading to the formation of Schiff bases [
59] and/or the formation of mid-polarity sterol oxidation products [
60] that cannot be determined under the analytical conditions used. Total COP and OR showed the same trend as TBARS, with values in untreated samples that were about twice as much as those found in pâtés with RLE at 0 and 42 days of chilled storage. Cholesterol oxidation is known to be favoured in the presence of oxygen and during chilled display [
61], but RLE addition was able to greatly hinder COP formation, without a dose-dependent effect.
RLE addition did not initially affect pâté colour, as also reported in pork liver pâté with RLE [
25]. It is unlikely that a small amount of RE can alter the colour of an orange salmon pâté that was also coloured with capsanthin. Other studies on pork liver pâté made with tea and grape seed extracts [
62] and with seaweed extracts [
56] reported some changes in CIELab colour due to the contribution of pigments such as chlorophylls [
63] or by the action of polyphenol oxidases able to condense quinones, forming dark compounds [
62,
64]. As these enzymes are inactivated by heating, it is unlikely that enzymes from a rosemary by-product (distilled with hot water steam) remain active in a thermally treated pâté. The untreated pâté showed some discolouration on day 42, as indicated by the increased Hue angle, a browning index, thus evidencing a general oxidation of product. Discolouration processes affecting the cooked–chilled muscle products may be ascribable to metmyoglobin formation, the denaturation of myofibrillar proteins producing colour changes when interacting with myoglobin [
18,
65] and lipid oxidation [
25]. Similarly, colour could be stabilised in pork liver pâtés with RLE [
24,
25] or with beer residue, chestnut leaves and peanut skin [
66], as well as in frozen salmon with rosemary whole extract [
18].
The pH of pâté was slightly lower when RLE was incorporated into its formulation. Similar results were found in pork liver pâté made with RE [
24] and with other vegetable extracts [
62,
66]. The natural acidity of plant extracts may decrease the pH of treated pâtés [
62]. In the present study, this effect was not dose-dependent, which suggests that some components of RLE were implicated in chemical reactions involving small changes in pH. The pH dropped slightly more (by 0.2) after storage in all pâtés, formulated with and without RLE, likely due to oxidation phenomena, since microbial events often result in more pronounced changes in pH and, in addition, the efficacy of pasteurisation treatment had been checked previously [
28]. Moreover, carnosol and carnosic acid may present bacteriostatic activities, as these can alter the lipidic order of phospholipidic membranes [
67].
A relevant part of the VOC identified in headspace corresponded to C5–C8 aliphatic aldehydes and alcohols. Hexanal has been identified as the most abundant VOC in sunflower and linseed oil blends, together with other VOC (propanal, pentanal, 1-penten-3-one, 1-pentanol, octanal, 1-octen-3-one, 1-octen-3-ol and (E,Z)-2,4-heptadienal) that contribute to oil aroma [
68]. In addition, saturated and unsaturated aliphatic VOC can be generated from the thermal degradation and oxidation of C18–C20 MUFA and C18–C22 PUFA present in fish lipids. In fact, propanal and hexanal are the most abundant VOC detected in cooked salmon aroma [
69,
70]. Benzene derivatives at different oxidation stages (e.g., toluene; benzaldehyde and benzoic acid) can also be formed in cooked salmon from aromatic precursors [
69,
70]. BHT can be used to stabilise carotenoid dyes, which might explain why BHT appeared in pâté headspace. α-Pinene and eugenol are volatile terpenes that can proceed from rosemary by-products [
71] or can be directly formed in cooked fish [
69,
70]. Unlike the TBARS trend, the VOC levels, except for 1-pentanol, did not allow discrimination of the antioxidant effect of RLE on salmon pâté lipids. For instance, inhibition by RLE of hexanal, a well-known VOC generated by cooking, could not be proven. The VOC profile of salmon pâté suggests that vegetable VOC from sunflower and linseed predominated over VOC from fish in headspace under the conditions used for HS-SPME. The number of VOC identified in salmon pâté was actually modest compared to those reported for cooked salmon [
69,
70]. Moreover, many characteristic C9–C11 aliphatic aldehydes (e. g. nonanal and nonenal) of flesh origin were not detected. In general, the VOC detected in salmon pâté seems to provide little information about lipid oxidation and would thus be of little interest as lipid oxidation markers. In any case, sampling of different manufacturing batches might have increased intra-group variability in parameters (FA, sterols and others) related to the composition of pâté, making treatment effects less evident.