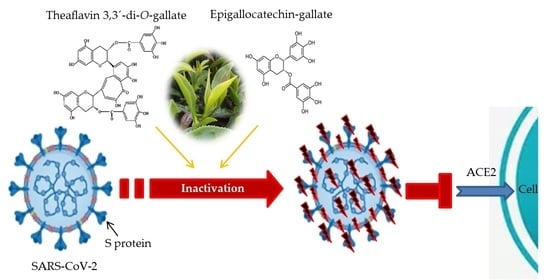
Investigation of the Azorean Camellia sinensis Processing Conditions to Maximize the Theaflavin 3,3′-di-O-Gallate Content as a Potential Antiviral Compound
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Sample Origin, Withering, and Drying Methodologies
2.3. Sample Preparation and Extraction Methodology for Crude Catechins and CAF Content
2.4. Sample Preparation and Extraction Methodology for Theaflavin Content
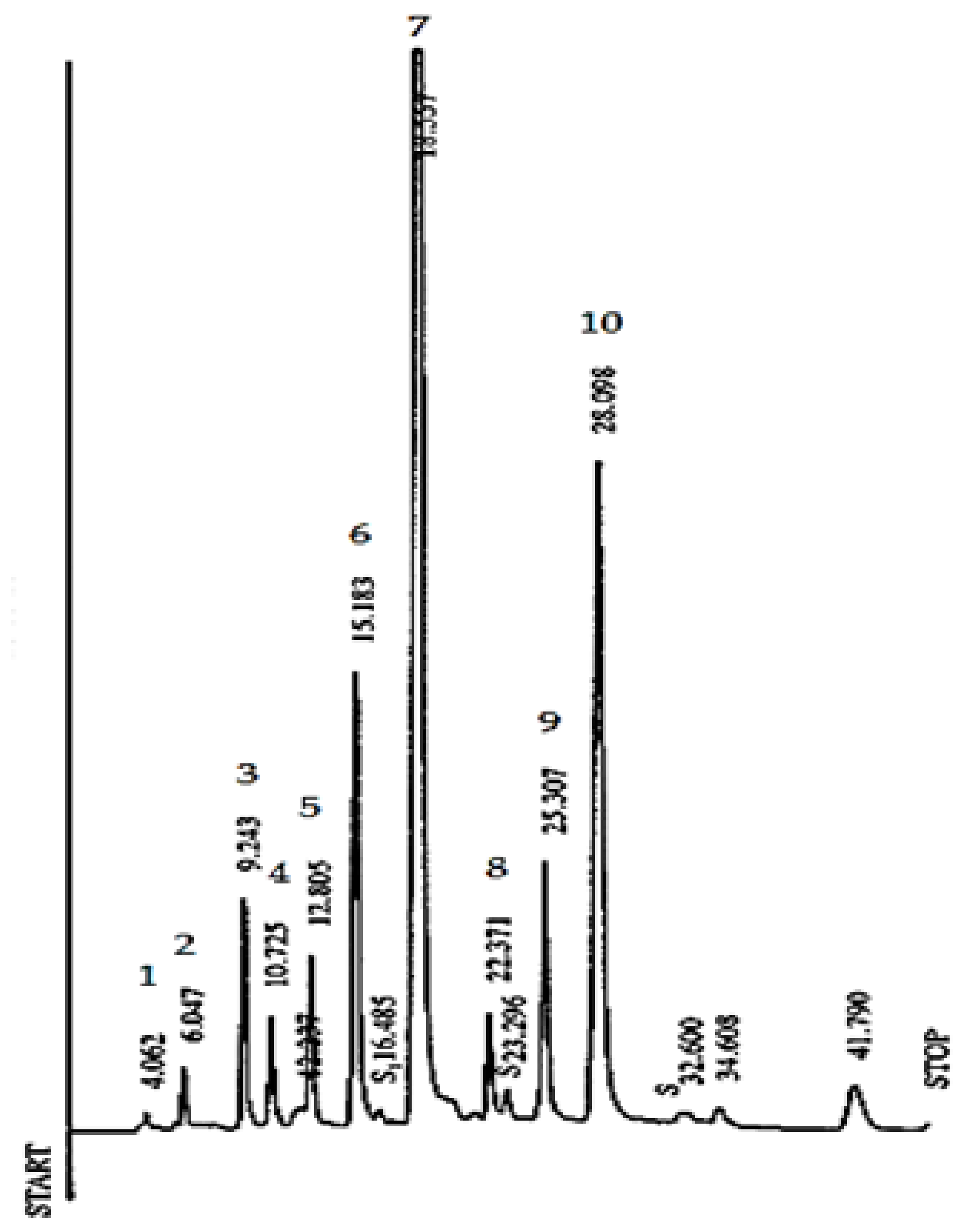
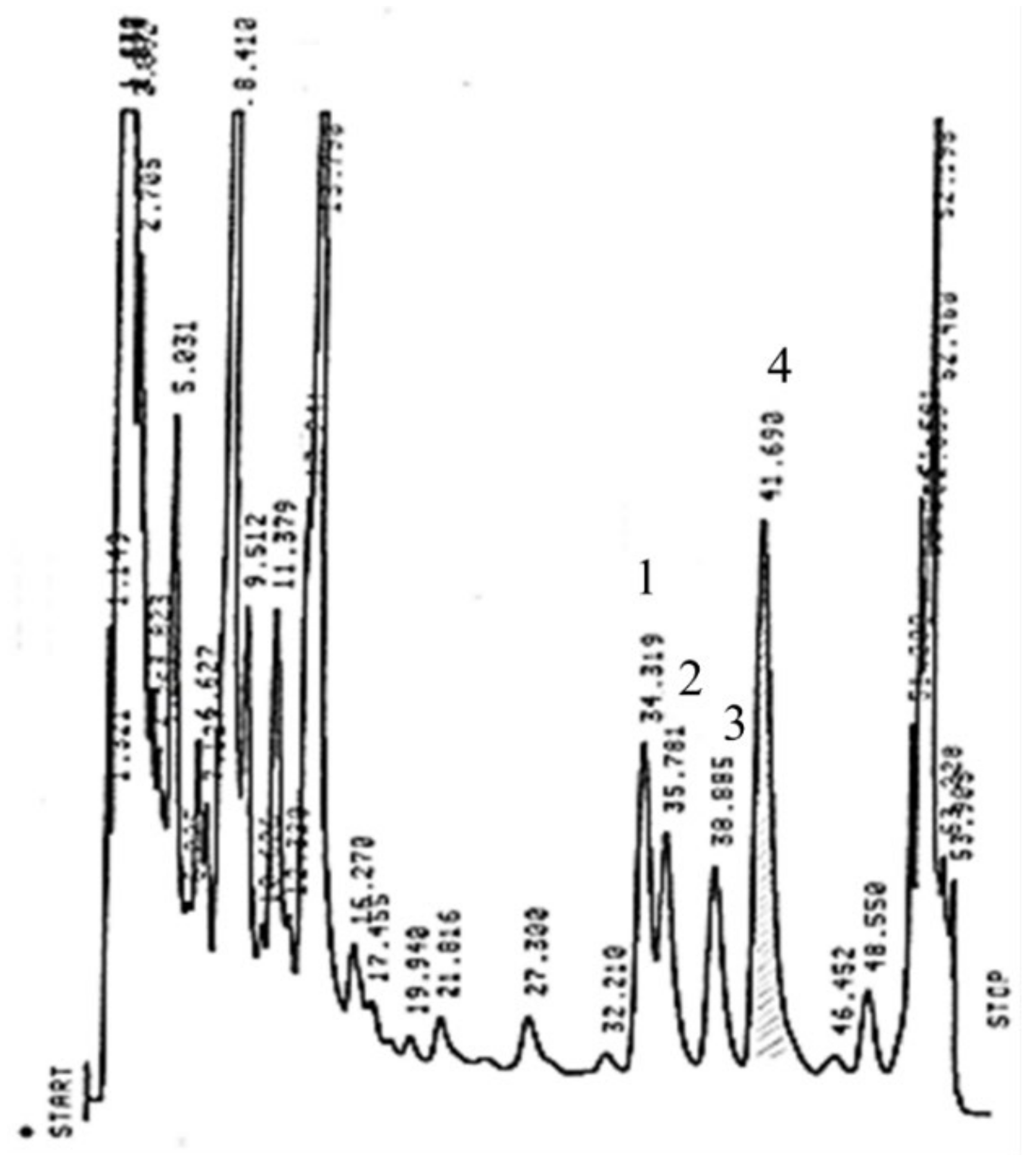
2.5. RP-HPLC Analysis of Catechins, CAF and Theaflavins
2.6. Statistical Analysis
3. Results and Discussion
3.1. Collection and Processing the Different Azorean C. sinensis Samples and Determination of their Catechin Profiles and CAF Content
3.2. Determination of Theaflavin Content Profiles and their Variability in Different Zones of Tea Plantation and in Different Seasons
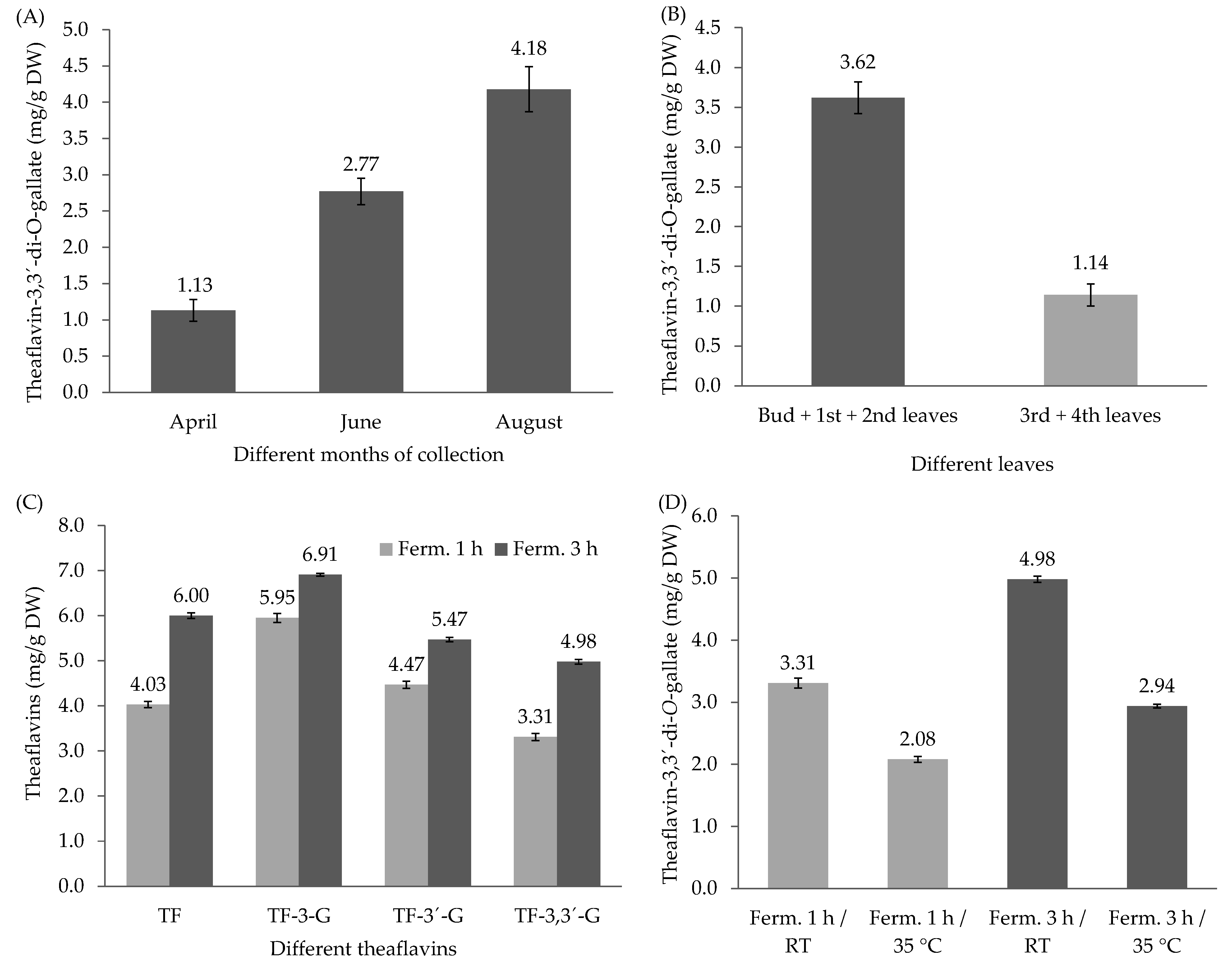
3.3. Variability of TF-3,3′-DG in Different Seasons, in Different Parts of the Tea Plant, and in Different Fermentation Times and the Effect of Fermentation Temperature on TF-3,3′-DG Content from Different Zones of Tea Plantation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, W.O.; Chun, O.K. Tea is the major source of flavan-3-ol and flavonol in the U.S. diet. J. Nutr. 2008, 138, 1543S–1547S. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.A.B.; Tavares, J.F.P.; Carvalho, R.C.B. Comparison of catechins and aromas among different green teas, using HPLC/SPME-GC. Food Res. Int. 1999, 31, 729–736. [Google Scholar] [CrossRef]
- Chen, M.-L. Tea and health—An overview. In Tea Bioactivity and Therapeutic Potential; Zhen, Y., Chen, Z., Cheng, S., Chen, M., Eds.; Taylor and Francis: London, UK, 2002; pp. 1–16. [Google Scholar]
- Ohgitani, E.; Shin-Ya, M.; Ichitani, M.; Kobayashi, M.; Takihara, T.; Kawamoto, M.; Kinugasa, H.; Mazda, O. Significant inactivation of SARS-CoV-2 in vitro by a green tea catechin, a catechin-derivative, and black tea galloylated theaflavins. Molecules 2021, 26, 3572. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, D.-Y. Docking characterization and in vitro inhibitory activity of flavan-3-ols and dimeric proanthocyanidins against the main protease activity of SARS-Cov-2. Front. Plant Sci. 2020, 11, 601316. [Google Scholar] [CrossRef]
- He, H.-F. Research progress on theaflavins: Efficacy, formation, and preparation. Food Nutr. Res. 2017, 61, 1344521. [Google Scholar] [CrossRef]
- Collings, E.R.; Alamar, M.C.; Redfern, S.; Cools, K.; Terry, L.A. Spatial changes in leaf biochemical profile of two tea cultivars following cold storage under two different vapour pressure deficit (VPD) conditions. Food Chem. 2019, 277, 179–185. [Google Scholar] [CrossRef]
- Satish, S.; Rathinavel, A.K.; Lutz, W.E.; Struble, L.R.; Khurana, S.; Schnaubelt, A.T.; Mishra, N.K.; Guda, C.; Broadhurst, M.J.; Reid, S.P.M.; et al. Bromelain inhibits SARS-CoV-2 infection in veroE6 cells. bioRxiv 2020, 20200916297366. [Google Scholar]
- Mhatre, S.; Srivastava, T.; Naik, S.; Patravale, V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine 2021, 85, 153286. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, I.; Tverdal, A.; Solvoll, K.; Foss, O.P. Tea consumption. Relationship to cholesterol, blood pressure, and coronary and total mortality. Prev. Med. 1992, 21, 546–553. [Google Scholar] [CrossRef]
- Loke, W.M.; Proudfoot, J.M.; Hodgson, J.M.; McKinley, A.J.; Hime, N.; Magat, M.; Stocker, R.; Croft, K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jin, F.; Wang, Y.; Li, F.; Wang, L.; Wang, Q.; Ren, Z.; Wang, Y. In vitro and in vivo anti-inflammatory effects of theaflavin-3,3´-digallate on lipopolysaccharide-induced inflammation. Eur. J. Pharmacol. 2017, 794, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Rankin, G.O.; Tu, Y.; Chen, Y.C. Inhibitory effects of the four main theaflavin derivatives found in black tea on ovarian cancer cells. Anticancer Res. 2016, 36, 643–651. [Google Scholar]
- Muthumani, T.; Kumar, R.S.S. Influence of fermentation time on the development of compounds responsible for quality in black tea. Food Chem. 2007, 101, 98–102. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Nakanishi, T.; Nakae, H.; Matsuo, T. Tea polyphenols inhibit IL-6 production in tumor necrosis factor superfamily 14-stimulated human gingival fibroblasts. Mol. Nutr. Food Res. 2010, 54 (Suppl. S2), S151–S158. [Google Scholar] [CrossRef]
- Ohba, M.; Oka, T.; Ando, T.; Arahata, S.; Ikegaya, A.; Takagi, H.; Ogo, N.; Zhu, C.; Owada, K.; Kawamori, F.; et al. Antiviral effect of theaflavins against caliciviruses. J. Antibiot. 2017, 70, 443–447. [Google Scholar] [CrossRef]
- Yang, Z.F.; Bai, L.P.; Huang, W.B.; Li, X.Z.; Zhao, S.S.; Zhong, N.S.; Jiang, Z.H. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure-activity relationship analysis. Fitoterapia 2014, 93, 47–53. [Google Scholar] [CrossRef]
- Baptista, J.; Lima, E.; Paiva, L.; Castro, A.R. Value of off-season fresh Camellia sinensis leaves. Antiradical activity, total phenolics content and catechin profiles. LWT Food Sci. Technol. 2014, 59, 1152–1158. [Google Scholar] [CrossRef]
- Matsubara, S.; Rodriguez-Amaya, D.B. Catechin and theaflavin levels of teas commercialized in Brazil. Food Sci. Technol. 2006, 26, 401–407. [Google Scholar] [CrossRef][Green Version]
- Koch, W.; Kukula-Koch, W.; Komsta, Ł.; Marzec, Z.; Szwerc, W.; Glowniak, K. Green tea quality evaluation based on its catechins and metals composition in combination with chemometric analysis. Molecules 2018, 23, 1689. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukula-Koch, W.; Komsta, L. Black tea samples origin discrimination using analytical investigations of secondary metabolites, antiradical scavenging activity and chemometric approach. Molecules 2018, 23, 513. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Jin, J.; Chen, H.; Du, Y.Y.; Ye, J.H.; Lu, J.L.; Lin, C.; Dong, J.J.; Sun, Q.L.; Wu, L.Y.; et al. Effect of ultraviolet B irradiation on accumulation of catechins in tea (Camellia sinensis (L.) O. Kuntze). Afr. J. Biotechnol. 2008, 7, 3283–3287. [Google Scholar]
- Sharma, V.; Joshi, R.; Gulati, A. Seasonal clonal variations and effects of stresses on quality chemicals and prephenate dehydratase enzyme activity in tea (Camellia sinensis). Eur. Food Res. Technol. 2011, 232, 307–317. [Google Scholar] [CrossRef]
- Tounekti, T.; Joubert, E.; Hernández, I.; Munné-Bosch, S. Improving the polyphenol content of tea. Crit. Rev. Plant Sci. 2013, 32, 192–215. [Google Scholar] [CrossRef]
- Ahmed, S.; Griffin, T.S.; Kraner, D.; Schaffner, M.K.; Sharma, D.; Hazel, M.; Leitch, A.R.; Orians, C.M.; Han, W.; Stepp, J.R.; et al. Environmental factors variably impact tea secondary metabolites in the context of climate change. Front. Plant Sci. 2019, 10, 939. [Google Scholar] [CrossRef]
- Jiang, Y.; Hua, J.; Wang, B.; Yuan, H.; Ma, H. Effects of variety, season, and region on theaflavins content of fermented Chinese Congou black tea. J. Food Qual. 2018, 2018, 5427302. [Google Scholar] [CrossRef]
- Takemoto, M.; Takemoto, H. Synthesis of theaflavins and their functions. Molecules 2018, 23, 918. [Google Scholar] [CrossRef]
- German, D.P.; Marcelo, K.R.B.; Stone, M.M.; Allison, S.D. The Michaelis–Menten kinetics of soil extracellular enzymes in response to temperature: A cross-latitudinal study. Glob. Chang. Biol. 2012, 18, 1468–1479. [Google Scholar] [CrossRef]
- Storozhuk, M. COVID-19: Could green tea catechins reduce the risks? medRxiv 2020, 2020102320218479. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Menegazzi, M.; Campagnari, R.; Bertoldi, M.; Crupi, R.; Di Paola, R.; Cuzzocrea, S. Protective effect of epigallocatechin-3-gallate (EGCG) in diseases with uncontrolled immune activation: Could such a scenario be helpful to counteract COVID-19? Int. J. Mol. Sci. 2020, 21, 5171. [Google Scholar] [CrossRef]
- Johns Hopkins Coronavirus Resource Center: Home Page. Available online: https://coronavirus.jhu.edu (accessed on 24 December 2021).


| Parameter | Compound | Zone 1 | Zone 2 | Zone 3 | |||
|---|---|---|---|---|---|---|---|
| Spring | Summer | Spring | Summer | Spring | Summer | ||
| Catechins | EGC | 8.14 ± 0.41 b | tr b | 10.25 ± 0.51 a | 6.25 ± 0.31 a | tr c | tr b |
| C | 5.76 ± 0.10 a | 6.07 ± 0.09 a | 3.94 ± 0.06 c | 6.69 ± 0.09 a | 4.38 ± 0.08 b | 4.77 ± 0.11 b | |
| EC | 20.36 ± 1.12 a | 17.80 ± 0.95 c | 18.37 ± 1.06 b | 26.55 ± 1.42 a | 14.62 ± 0.66 c | 24.07 ± 1.01 b | |
| EGCG | 67.01 ± 1.17 c | 54.03 ± 1.12 b | 94.71 ± 1.01 b | 54.23 ± 1.39 b | 133.64 ± 1.80 a | 121.30 ± 3.44 a | |
| ECG | 73.47 ± 1.15 a | 105.69 ± 2.28 a | 73.63 ± 1.27 a | 97.64 ± 1.52 ab | 59.11 ± 0.73 b | 64.95 ± 1.83 c | |
| TOTAL | 174.72 c | 183.59 c | 200.90 ab | 191.36 b | 211.75 a | 215.09 a | |
| Caffeine | 16.12 ± 0.54 a | 16.18 ± 1.03 a | 13.67 ± 0.48 b | 12.27 ± 0.29 c | 6.40 ± 0.07 c | 14.15 ± 0.38 b | |
| Theaflavins | TF | 3.29 ± 0.12 b | 3.41 ± 0.21 b | 1.75 ± 0.06 c | 1.90 ± 0.12 c | 4.04 ± 0.13 a | 6.43 ± 0.40 a |
| TF-3-G | 3.99 ± 0.18 a | 4.22 ± 0.19 b | 2.10 ± 0.06 b | 1.99 ± 0.08 c | 4.16 ± 0.18 a | 5.39 ± 0.16 a | |
| TF-3′-G | 2.45 ± 0.09 b | 3.45 ± 0.15 b | 1.56 ± 0.11 c | 2.10 ± 0.16 c | 3.78 ± 0.11 a | 7.25 ± 0.25 a | |
| TF-3,3′-DG | 2.68 ± 0.11 b | 3.78 ± 0.07 b | 1.13 ± 0.15 c | 2.06 ± 0.25 c | 3.72 ± 0.09 a | 8.91 ± 0.40 a | |
| TOTAL | 12.41 b | 14.86 B | 6.54 c | 8.05 C | 15.7 a | 27.98 A | |
| Analyte | Linear Range (mg/L) | R2 | LOD (mg/L) | LOQ (mg/L) | Recovery (%) | Inter-Day/Intra-Day (% RSD) |
|---|---|---|---|---|---|---|
| TF-3,3′-DG | 0.18–94.0 | 0.9998 | 0.16–0.20 | 0.40–1.08 | 95.8–104.8 | 0.57/0.06 |
| Country | Population in Millions | Total COVID-19 Cases 1 | Total COVID-19 Mortality 1 | COVID-19 Mortality (per Million Population) 1 | % COVID-19 Mortality per Total Cases 2 | % COVID-19 Cases per Million Population 1 |
|---|---|---|---|---|---|---|
| Low Tea Consumption Countries | ||||||
| Germany | 83.13 | 7,279,037 | 112,707 | 1356 | 1.55 | 0.87 |
| France | 67.51 | 10,694,804 | 125,551 | 1860 | 1.17 | 15.84 |
| Italy | 59.09 | 6,566,947 | 138,045 | 2336 | 2.10 | 11.11 |
| Spain | 47.39 | 6,785,286 | 89,689 | 1893 | 1.32 | 14.32 |
| Portugal | 10.51 | 1,460,406 | 19,015 | 1809 | 1.30 | 13.89 |
| High Tea Consumption Countries | ||||||
| China | 1439.32 | 115,611 | 4849 | 3.37 | 4.19 | 0.08 |
| Japan | 126.93 | 1,735,050 | 18,393 | 144.91 | 1.06 | 1.37 |
| Senegal | 16.74 | 77,935 | 1895 | 113.20 | 2.43 | 0.47 |
| Korea | 51.67 | 645,226 | 5781 | 111.88 | 0.89 | 1.25 |
| Kenya | 47.56 | 302,134 | 5401 | 113.56 | 1.79 | 0.64 |
| Mozambique | 31.26 | 196,346 | 2042 | 65.32 | 1.04 | 0.63 |
| Taiwan | 23.83 | 17,129 | 849 | 35.63 | 4.96 | 0.07 |
| Uzbekistan | 33.47 | 199,758 | 1488 | 44.46 | 0.74 | 0.60 |
| Mali | 20.25 | 23,600 | 670 | 33.09 | 2.84 | 0.12 |
| Other Tea Consumption Countries | ||||||
| Canada | 38.51 | 2,323,772 | 30,475 | 791.14 | 1.31 | 6.03 |
| USA | 332.90 | 56,803,703 | 829,351 | 2491 | 1.46 | 1.71 |
| Brazil | 212.56 | 22,309,081 | 619,473 | 2914 | 2.78 | 10.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, L.; Lima, E.; Motta, M.; Marcone, M.; Baptista, J. Investigation of the Azorean Camellia sinensis Processing Conditions to Maximize the Theaflavin 3,3′-di-O-Gallate Content as a Potential Antiviral Compound. Antioxidants 2022, 11, 1066. https://doi.org/10.3390/antiox11061066
Paiva L, Lima E, Motta M, Marcone M, Baptista J. Investigation of the Azorean Camellia sinensis Processing Conditions to Maximize the Theaflavin 3,3′-di-O-Gallate Content as a Potential Antiviral Compound. Antioxidants. 2022; 11(6):1066. https://doi.org/10.3390/antiox11061066
Chicago/Turabian StylePaiva, Lisete, Elisabete Lima, Madalena Motta, Massimo Marcone, and José Baptista. 2022. "Investigation of the Azorean Camellia sinensis Processing Conditions to Maximize the Theaflavin 3,3′-di-O-Gallate Content as a Potential Antiviral Compound" Antioxidants 11, no. 6: 1066. https://doi.org/10.3390/antiox11061066
APA StylePaiva, L., Lima, E., Motta, M., Marcone, M., & Baptista, J. (2022). Investigation of the Azorean Camellia sinensis Processing Conditions to Maximize the Theaflavin 3,3′-di-O-Gallate Content as a Potential Antiviral Compound. Antioxidants, 11(6), 1066. https://doi.org/10.3390/antiox11061066