Quantification of Amino Acids, Phenolic Compounds Profiling from Nine Rice Varieties and Their Antioxidant Potential
Abstract
:1. Introduction
2. Experimental Research Materials and Methods
2.1. Research Samples
2.2. Chemicals and Cultures
2.3. Sample Preparation
Preparation of Rice Samples Ethanolic Extracts
2.4. Measurement of Total Anthocyanin Content (TAC)
2.5. Measurement of Total Phenolic Content (TPC)
2.6. Measurement of Total Flavonoid Content (TFC)
2.7. Estimation of Antioxidant Potential of Various Rice Varieties
2.7.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.7.2. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Radical Scavenging Activity
2.7.3. The Ferric Reducing Antioxidant Power (FRAP) Activity for Rice Varieties
2.8. HPLC-FLD-MS/MS and UHPLC Q-TOF-MS/MS for Identification and Quantification of Amino Acid and Phenolic Compounds in Rice Samples
2.8.1. Amino Acid Detection
2.8.2. Phenolic Compounds Detection in Nine Rice Varieties
2.9. Statistical Analysis
3. Results and Discussion
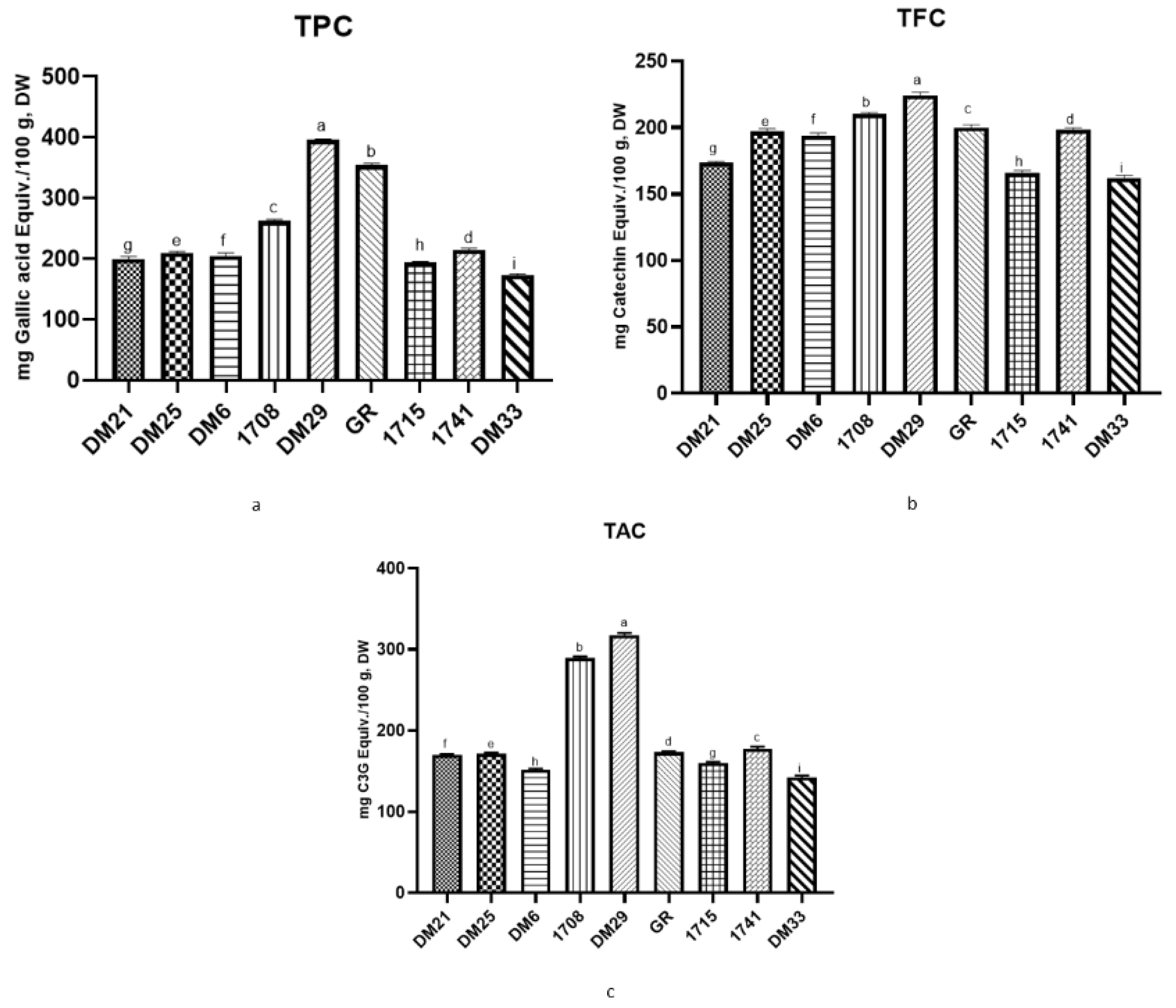
3.1. TPC, TFC, and TAC of Different Nine Rice Varieties
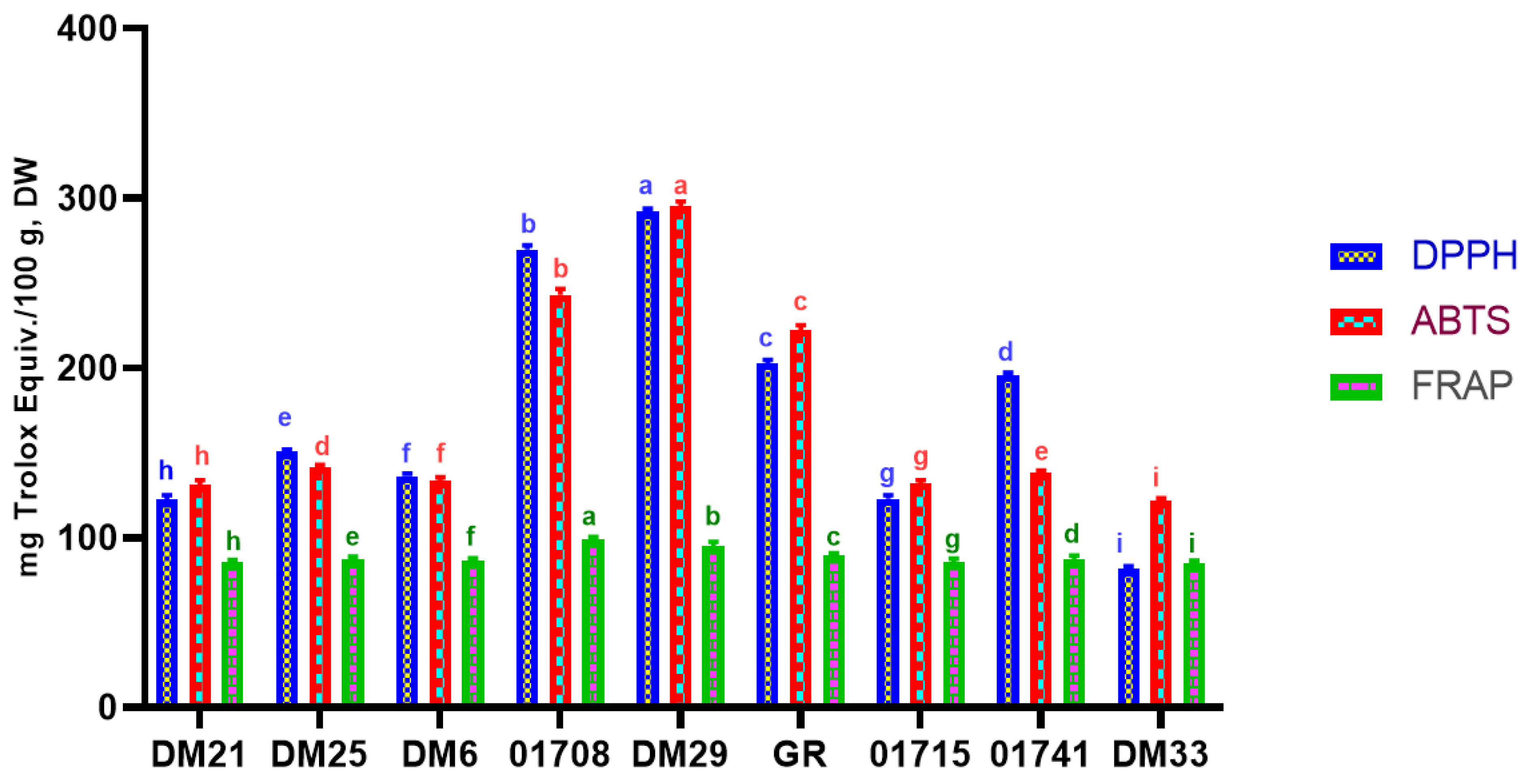
3.2. In-Vitro Antioxidant Analysis (DPPH, ABTS, & FRAP)
3.3. HPLC-FLD-MS/MS and UHPLC Q-TOF-MS/MS Identification and Quantification of Amino Acid and Phenolic Compounds in Different Rice Samples
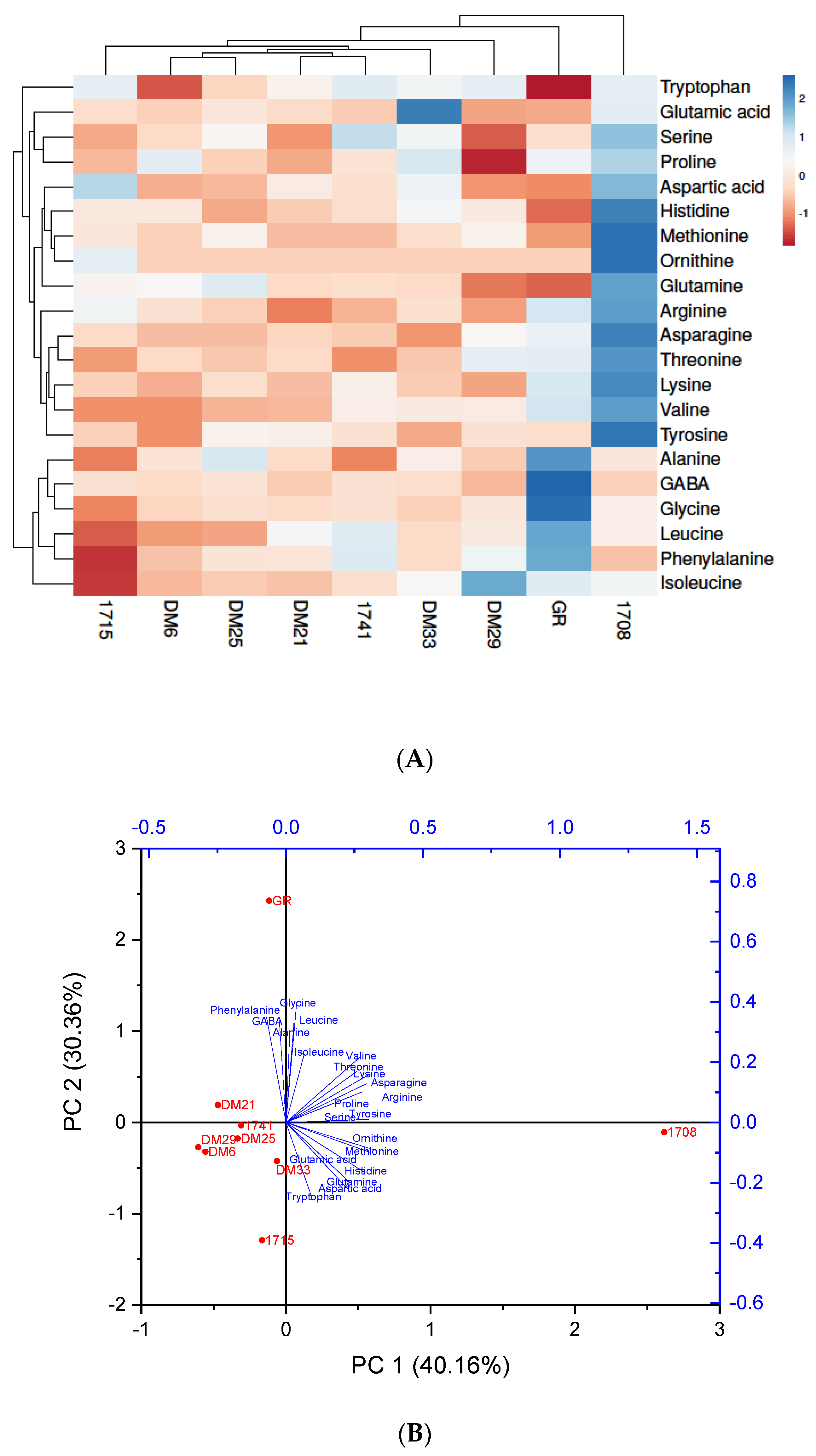
3.3.1. Amino Acid Detection in Different Colored Rice
3.3.2. Phenolic Compounds Identification in Nine Different Colored Rice Varieties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ni, S.; Li, Z.; Ying, J.; Zhang, J.; Chen, H. Decreased Spikelets 4 encoding a novel tetratricopeptide repeat domain-containing protein is involved in DNA repair and spikelet number determination in rice. Genes 2019, 10, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samyor, D.; Das, A.B.; Deka, S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Technol. 2017, 52, 1073–1081. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The Genetic Basis and Nutritional Benefits of Pigmented Rice Grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.-F.; Xu, X.-R.; Zhang, Y.; Li, D.; Gan, R.-Y.; Li, H.-B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Yeon, S.-J.; Daliri, E.B.-M.; Chen, X.; Chelliah, R.; Oh, D.-H. Untargeted Metabolomics of Korean Fermented Brown Rice Using UHPLC Q-TOF MS/MS Reveal an Abundance of Potential Dietary Antioxidative and Stress-Reducing Compounds. Antioxidants 2021, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Shabbir, U.; Chelliah, R.; Daliri, E.B.-M.; Chen, X.; Oh, D.-H. Limosilactobacillus reuteri Fermented Brown Rice: A Product with Enhanced Bioactive Compounds and Antioxidant Potential. Antioxidants 2021, 10, 1077. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Trindade, H. Factors influencing antioxidant compounds in rice. Crit. Rev. Food Sci. Nutr. 2017, 57, 893–922. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The role of oxidative stress in physiopathology and pharmacological treatment with pro-and antioxidant properties in chronic diseases. Oxidative Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Dordevic, D.; Kushkevych, I.; Jancikova, S.; Zeljkovic, S.C.; Zdarsky, M.; Hodulova, L. Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations. Open Life Sci. 2020, 15, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Chen, X.; Shabbir, U.; Chelliah, R.; Oh, D.H. Effect of slightly acidic electrolyzed water on amino acid and phenolic profiling of germinated brown rice sprouts and their antioxidant potential. LWT 2022, 157, 113119. [Google Scholar] [CrossRef]
- Rao, S.; Callcott, E.T.; Santhakumar, A.B.; Chinkwo, K.A.; Vanniasinkam, T.; Luo, J.; Blanchard, C.L. Profiling polyphenol composition and antioxidant activity in Australian-grown rice using UHPLC Online-ABTS system. J. Cereal Sci. 2018, 80, 174–179. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Q.; Dong, L.; Jia, X.; Liu, L.; Huang, F.; Liu, G.; Sun, Z.; Chi, J.; Zhang, M. Phenolic Profiles and Bioactivities of Different Milling Fractions of Rice Bran from Black Rice. Food Chem. 2022, 378, 132035. [Google Scholar] [CrossRef]
- Nayeem, S.; Sundararajan, S.; Ashok, A.K.; Abusaliya, A.; Ramalingam, S. Effects of cooking on phytochemical and antioxidant properties of pigmented and non-pigmented rare Indian rice landraces. Biocatal. Agric. Biotechnol. 2021, 32, 101928. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Karbalaii, M.T.; Jaafar, H.Z.; Rahmat, A. Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem. Cent. J. 2018, 12, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hung, P. Phenolic compounds of cereals and their antioxidant capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Min, B.; Gu, L.; McClung, A.M.; Bergman, C.J.; Chen, M.-H. Free and bound total phenolic concentrations, antioxidant capacities, and profiles of proanthocyanidins and anthocyanins in whole grain rice (Oryza sativa L.) of different bran colours. Food Chem. 2012, 133, 715–722. [Google Scholar] [CrossRef]
- Chatthongpisut, R.; Schwartz, S.J.; Yongsawatdigul, J. Antioxidant activities and antiproliferative activity of Thai purple rice cooked by various methods on human colon cancer cells. Food Chem. 2015, 188, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.S.; Liu, C.; Li, B.; Zhou, W.; Chen, H.; Li, T.; Wu, J.; Zeng, Z.; Wang, Y.; Si, X.; et al. Phytochemical profiles of rice and their cellular antioxidant activity against ABAP induced oxidative stress in human hepatocellular carcinoma HepG2 cells. Food Chem. 2020, 318, 126484. [Google Scholar] [CrossRef]
- Ito, V.C.; Lacerda, L.G. Black rice (Oryza sativa L.): A review of its historical aspects, chemical composition, nutritional and functional properties, and applications and processing technologies. Food Chem. 2019, 301, 125304. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.; Juraimi, A.S.; Tayebi-Meigooni, A. Comparative evaluation of different extraction techniques and solvents for the assay of phytochemicals and antioxidant activity of hashemi rice bran. Molecules 2015, 20, 10822–10838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Phytochemical constituents and biological activities of different extracts of Strobilanthes crispus (L.) Bremek leaves grown in different locations of Malaysia. BMC Complementary Altern. Med. 2015, 15, 422. [Google Scholar] [CrossRef] [Green Version]
- Devraj, L.; Panoth, A.; Kashampur, K.; Kumar, A.; Natarajan, V. Study on physicochemical, phytochemical, and antioxidant properties of selected traditional and white rice varieties. J. Food Process Eng. 2020, 43, e13330. [Google Scholar] [CrossRef]
- Chinprahast, N.; Tungsomboon, T.; Nagao, P. Antioxidant activities of T hai pigmented rice cultivars and application in sunflower oil. Int. J. Food Sci. Technol. 2016, 51, 46–53. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. The composition, extraction, functionality and applications of rice proteins: A review. Trends Food Sci. Technol. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Saleh, A.S.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okarter, N.; Liu, R.H. Health benefits of whole grain phytochemicals. Crit. Rev. Food Sci. Nutr. 2010, 50, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Daroi, P.A.; Dhage, S.N.; Juvekar, A.R. p-Coumaric acid mitigates lipopolysaccharide induced brain damage via alleviating oxidative stress, inflammation and apoptosis. J. Pharm. Pharmacol. 2021, 74, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; de Camargo, A.C.; Atala, E.; Gotteland, M.; Olea-Azar, C.; Speisky, H. Quercetin Oxidation Metabolite Present in Onion Peel Protects Caco-2 Cells against the Oxidative Stress, NF-kB Activation, and Loss of Epithelial Barrier Function Induced by NSAIDs. J. Agric. Food Chem. 2021, 69, 2157–2167. [Google Scholar] [CrossRef]
- Hathout, H.M.; Sobhy, H.M.; Abou-Ghanima, S.; El-Garawani, I.M. Ameliorative role of ascorbic acid on the oxidative stress and genotoxicity induced by acetamiprid in Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2021, 28, 55089–55101. [Google Scholar] [CrossRef] [PubMed]
- Rahman Mazumder, M.A.; Hongsprabhas, P. Genistein as antioxidant and antibrowning agents in in vivo and in vitro: A review. Biomed. Pharmacother. 2016, 82, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Goffman, F.; Bergman, C. Rice kernel phenolic content and its relationship with antiradical efficiency. J. Sci. Food Agric. 2004, 84, 1235–1240. [Google Scholar] [CrossRef]
- Sumczynski, D.; Kotásková, E.; Družbíková, H.; Mlček, J. Determination of contents and antioxidant activity of free and bound phenolics compounds and in vitro digestibility of commercial black and red rice (Oryza sativa L.) varieties. Food Chem. 2016, 211, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; McClung, A.M.; Chen, M.H. Phytochemicals and antioxidant capacities in rice brans of different color. J. Food Sci. 2011, 76, C117–C126. [Google Scholar] [CrossRef]


| Amino Acid | Sample Name | Retention Time (min) | Area LU * min | Detected Concentration (µg/g) | Relative Area % | Height LU |
|---|---|---|---|---|---|---|
| Aspartic acid | 01708 | 2.03 | 0.56 | 4.20 | 12.17 | 5.07 |
| 01715 | 2.04 | 0.53 | 4.00 | 17.31 | 4.90 | |
| 01741 | 2.05 | 0.41 | 3.11 | 140.02 | 3.68 | |
| DM6 | 2.04 | 0.37 | 2.80 | 12.86 | 3.41 | |
| DM29 | 2.04 | 0.36 | 2.68 | 12.10 | 3.29 | |
| DM25 | 2.05 | 0.38 | 2.85 | 12.30 | 3.46 | |
| DM21 | 2.04 | 0.44 | 3.31 | 15.21 | 4.09 | |
| DM33 | 2.04 | 0.48 | 3.57 | 14.51 | 4.35 | |
| GR | 2.05 | 0.35 | 2.64 | 9.49 | 3.26 | |
| Glutamic acid | 01708 | 3.54 | 0.76 | 6.71 | 16.49 | 5.05 |
| 01715 | 3.54 | 0.66 | 5.81 | 21.28 | 4.28 | |
| 01741 | 3.54 | 0.64 | 5.62 | 21.50 | 4.18 | |
| DM6 | 3.53 | 0.64 | 5.66 | 22.02 | 4.29 | |
| DM29 | 3.54 | 0.60 | 5.34 | 20.44 | 4.02 | |
| DM25 | 3.54 | 0.68 | 6.00 | 21.93 | 4.55 | |
| DM21 | 3.54 | 0.65 | 5.77 | 22.47 | 4.35 | |
| DM33 | 3.54 | 0.91 | 8.05 | 27.67 | 6.06 | |
| GR | 3.54 | 0.61 | 5.36 | 16.34 | 4.06 | |
| Asparagine | 01708 | 7.07 | 1.27 | 8.93 | 27.62 | 9.64 |
| 01715 | 7.08 | 0.49 | 3.42 | 15.76 | 3.64 | |
| 01741 | 7.08 | 0.42 | 2.96 | 14.26 | 3.18 | |
| DM6 | 7.07 | 0.39 | 2.75 | 13.46 | 3.01 | |
| DM29 | 7.07 | 0.69 | 4.84 | 23.36 | 5.21 | |
| DM25 | 7.07 | 0.39 | 2.72 | 12.52 | 2.97 | |
| DM21 | 7.07 | 0.45 | 3.13 | 15.37 | 3.39 | |
| DM33 | 7.07 | 0.28 | 1.95 | 8.42 | 2.11 | |
| GR | 7.07 | 0.77 | 5.40 | 20.73 | 5.87 | |
| Serine | 01708 | 7.67 | 0.25 | 1.31 | 5.46 | 1.81 |
| 01715 | 7.68 | 0.16 | 0.82 | 5.09 | 1.13 | |
| 01741 | 7.68 | 0.24 | 1.24 | 8.00 | 1.72 | |
| DM6 | 7.67 | 0.18 | 0.92 | 6.03 | 1.28 | |
| DM29 | 7.67 | 0.13 | 0.70 | 4.55 | 0.98 | |
| DM25 | 7.67 | 0.20 | 1.05 | 6.51 | 1.46 | |
| DM21 | 7.68 | 0.15 | 0.79 | 5.17 | 1.09 | |
| DM33 | 7.67 | 0.21 | 1.09 | 6.36 | 1.53 | |
| GR | 7.67 | 0.18 | 0.94 | 4.86 | 1.32 | |
| Glutamine | 01708 | 8.70 | 0.21 | 1.60 | 4.51 | 1.61 |
| 01715 | 8.70 | 0.13 | 1.00 | 4.21 | 1.00 | |
| 01741 | 8.71 | 0.11 | 0.82 | 3.59 | 0.81 | |
| DM6 | 8.70 | 0.13 | 1.03 | 4.60 | 1.03 | |
| DM29 | 8.70 | 0.06 | 0.47 | 2.08 | 0.49 | |
| DM25 | 8.70 | 0.16 | 1.21 | 5.09 | 1.24 | |
| DM21 | 8.70 | 0.10 | 0.78 | 3.50 | 0.81 | |
| DM33 | 8.69 | 0.10 | 0.79 | 3.13 | 0.80 | |
| GR | 8.69 | 0.05 | 0.41 | 1.45 | 0.42 | |
| Histidine | 01708 | 9.25 | 0.05 | 0.74 | 1.09 | 0.37 |
| 01715 | 9.25 | 0.03 | 0.43 | 0.96 | 0.20 | |
| 01741 | 9.26 | 0.03 | 0.39 | 0.91 | 0.19 | |
| DM6 | 9.25 | 0.03 | 0.43 | 1.02 | 0.21 | |
| DM29 | 9.25 | 0.03 | 0.44 | 1.01 | 0.19 | |
| DM25 | 9.24 | 0.02 | 0.32 | 0.71 | 0.15 | |
| DM21 | 9.25 | 0.02 | 0.37 | 0.86 | 0.18 | |
| DM33 | 9.24 | 0.03 | 0.49 | 1.02 | 0.25 | |
| GR | 9.24 | 0.02 | 0.26 | 0.47 | 0.13 | |
| Glycine | 01708 | 9.72 | 0.11 | 0.39 | 2.43 | 0.81 |
| 01715 | 9.71 | 0.08 | 0.29 | 2.64 | 0.59 | |
| 01741 | 9.72 | 0.10 | 0.36 | 3.50 | 0.76 | |
| DM6 | 9.72 | 0.10 | 0.34 | 3.38 | 0.71 | |
| DM29 | 9.71 | 0.11 | 0.38 | 3.64 | 0.79 | |
| DM25 | 9.72 | 0.10 | 0.36 | 3.33 | 0.75 | |
| DM21 | 9.72 | 0.10 | 0.35 | 3.47 | 0.73 | |
| DM33 | 9.71 | 0.10 | 0.34 | 2.97 | 0.73 | |
| GR | 9.71 | 0.17 | 0.59 | 4.51 | 1.22 | |
| Threonine | 01708 | 9.97 | 0.09 | 0.51 | 1.91 | 0.59 |
| 01715 | 9.97 | 0.05 | 0.31 | 1.73 | 0.37 | |
| 01741 | 9.97 | 0.05 | 0.30 | 1.77 | 0.37 | |
| DM6 | 9.97 | 0.06 | 0.35 | 2.07 | 0.39 | |
| DM29 | 9.97 | 0.07 | 0.42 | 2.45 | 0.47 | |
| DM25 | 9.97 | 0.06 | 0.34 | 1.87 | 0.39 | |
| DM21 | 9.97 | 0.06 | 0.35 | 2.09 | 0.40 | |
| DM33 | 9.96 | 0.06 | 0.34 | 1.77 | 0.41 | |
| GR | 9.96 | 0.07 | 0.42 | 1.96 | 0.48 | |
| Arginine | 01708 | 10.74 | 0.23 | 1.69 | 4.98 | 1.78 |
| 01715 | 10.74 | 0.15 | 1.10 | 4.85 | 1.17 | |
| 01741 | 10.75 | 0.09 | 0.63 | 2.88 | 0.63 | |
| DM6 | 10.75 | 0.11 | 0.84 | 3.95 | 0.91 | |
| DM21 | 10.74 | 0.08 | 0.56 | 2.56 | 0.60 | |
| DM25 | 10.74 | 0.10 | 0.74 | 3.26 | 0.77 | |
| DM29 | 10.74 | 0.06 | 0.45 | 2.08 | 0.48 | |
| DM33 | 10.74 | 0.11 | 0.82 | 3.40 | 0.88 | |
| GR | 10.74 | 0.18 | 1.32 | 4.84 | 1.35 | |
| Alanine | 01708 | 11.85 | 0.26 | 1.10 | 5.73 | 1.96 |
| 01715 | 11.84 | 0.17 | 0.71 | 5.52 | 1.26 | |
| 01741 | 11.86 | 0.18 | 0.74 | 5.95 | 1.31 | |
| DM6 | 11.86 | 0.26 | 1.08 | 8.86 | 1.92 | |
| DM29 | 11.84 | 0.22 | 0.94 | 7.61 | 1.68 | |
| DM25 | 11.86 | 0.35 | 1.45 | 11.22 | 2.56 | |
| DM21 | 11.85 | 0.24 | 1.01 | 8.32 | 1.82 | |
| DM33 | 11.84 | 0.28 | 1.16 | 8.46 | 2.08 | |
| GR | 11.84 | 0.44 | 1.82 | 11.77 | 3.30 | |
| GABA | 01708 | 12.46 | 0.04 | 0.19 | 0.86 | 0.30 |
| 01715 | 12.45 | 0.06 | 0.28 | 1.89 | 0.43 | |
| 01741 | 12.47 | 0.06 | 0.27 | 1.92 | 0.43 | |
| DM6 | 12.47 | 0.05 | 0.23 | 1.62 | 0.35 | |
| DM29 | 12.45 | 0.03 | 0.13 | 0.90 | 0.21 | |
| DM25 | 12.47 | 0.06 | 0.30 | 2.00 | 0.45 | |
| DM21 | 12.46 | 0.04 | 0.18 | 1.27 | 0.28 | |
| DM33 | 12.45 | 0.05 | 0.26 | 1.63 | 0.40 | |
| GR | 12.46 | 0.23 | 1.13 | 6.32 | 1.75 | |
| Tyrosine | 01708 | 13.51 | 0.04 | 0.35 | 0.94 | 0.33 |
| 01715 | 13.51 | 0.03 | 0.21 | 0.86 | 0.19 | |
| 01741 | 13.52 | 0.03 | 0.22 | 0.95 | 0.21 | |
| DM6 | 13.52 | 0.02 | 0.18 | 0.80 | 0.18 | |
| DM29 | 13.51 | 0.03 | 0.22 | 0.95 | 0.21 | |
| DM25 | 13.52 | 0.03 | 0.24 | 0.99 | 0.22 | |
| DM21 | 13.51 | 0.03 | 0.24 | 1.04 | 0.22 | |
| DM33 | 13.50 | 0.02 | 0.20 | 0.75 | 0.18 | |
| GR | 13.51 | 0.03 | 0.22 | 0.74 | 0.21 | |
| Valine | 01708 | 16.41 | 0.09 | 0.44 | 2.01 | 0.64 |
| 01715 | 16.41 | 0.06 | 0.27 | 1.87 | 0.40 | |
| 01741 | 16.41 | 0.07 | 0.33 | 2.40 | 0.46 | |
| DM6 | 16.42 | 0.06 | 0.27 | 1.99 | 0.40 | |
| DM29 | 16.42 | 0.07 | 0.33 | 2.40 | 0.48 | |
| DM25 | 16.42 | 0.06 | 0.29 | 1.98 | 0.43 | |
| DM21 | 16.41 | 0.06 | 0.29 | 2.12 | 0.43 | |
| DM33 | 16.41 | 0.07 | 0.33 | 2.13 | 0.49 | |
| GR | 16.42 | 0.08 | 0.39 | 2.23 | 0.58 | |
| Methionine | 01708 | 16.71 | 0.03 | 0.15 | 0.56 | 0.16 |
| 01715 | 16.72 | 0.01 | 0.08 | 0.42 | 0.07 | |
| 01741 | 16.71 | 0.01 | 0.06 | 0.34 | 0.06 | |
| DM6 | 16.71 | 0.01 | 0.06 | 0.38 | 0.06 | |
| DM29 | 16.71 | 0.01 | 0.08 | 0.49 | 0.08 | |
| DM25 | 16.71 | 0.01 | 0.08 | 0.46 | 0.08 | |
| DM21 | 16.71 | 0.01 | 0.06 | 0.34 | 0.06 | |
| DM33 | 16.71 | 0.01 | 0.07 | 0.37 | 0.07 | |
| GR | 16.72 | 0.01 | 0.05 | 0.23 | 0.05 | |
| Tryptophan | 01708 | 17.86 | 0.28 | 0.88 | 6.18 | 1.40 |
| 01715 | 17.87 | 0.28 | 0.87 | 9.18 | 1.26 | |
| 01741 | 17.86 | 0.29 | 0.90 | 9.69 | 1.30 | |
| DM6 | 17.88 | 0.24 | 0.39 | 8.15 | 1.14 | |
| DM29 | 17.87 | 0.28 | 0.86 | 9.58 | 1.27 | |
| DM25 | 17.87 | 0.26 | 0.63 | 8.41 | 1.15 | |
| DM21 | 17.86 | 0.27 | 0.77 | 9.42 | 1.31 | |
| DM33 | 17.86 | 0.28 | 0.83 | 8.49 | 1.32 | |
| GR | 17.87 | 0.23 | 0.30 | 6.16 | 1.04 | |
| Phenylalanine | 01708 | 18.51 | 0.01 | 0.10 | 0.32 | 0.10 |
| 01715 | 18.51 | 0.01 | 0.07 | 0.34 | 0.08 | |
| 01741 | 18.50 | 0.02 | 0.14 | 0.70 | 0.14 | |
| DM6 | 18.52 | 0.01 | 0.10 | 0.51 | 0.10 | |
| DM29 | 18.51 | 0.02 | 0.13 | 0.65 | 0.13 | |
| DM25 | 18.51 | 0.02 | 0.11 | 0.53 | 0.11 | |
| DM21 | 18.51 | 0.02 | 0.11 | 0.58 | 0.12 | |
| DM33 | 18.50 | 0.02 | 0.10 | 0.48 | 0.11 | |
| GR | 18.51 | 0.02 | 0.16 | 0.66 | 0.17 | |
| Isoleucine | 01708 | 18.83 | 0.03 | 0.16 | 0.70 | 0.21 |
| 01715 | 18.83 | 0.02 | 0.09 | 0.59 | 0.12 | |
| 01741 | 18.83 | 0.03 | 0.14 | 0.92 | 0.18 | |
| DM6 | 18.84 | 0.02 | 0.12 | 0.84 | 0.15 | |
| DM29 | 18.83 | 0.04 | 0.21 | 1.41 | 0.27 | |
| DM25 | 18.84 | 0.03 | 0.13 | 0.83 | 0.15 | |
| DM21 | 18.83 | 0.03 | 0.13 | 0.87 | 0.16 | |
| DM33 | 18.83 | 0.03 | 0.16 | 0.95 | 0.20 | |
| GR | 18.83 | 0.03 | 0.17 | 0.93 | 0.23 | |
| Ornitnine | 01708 | 19.40 | 0.01 | 0.29 | 0.28 | 0.08 |
| 01715 | 19.39 | 0.01 | 0.12 | 0.17 | 0.04 | |
| 01741 | ND | ND | ND | ND | ND | |
| DM6 | ND | ND | ND | ND | ND | |
| DM29 | ND | ND | ND | ND | ND | |
| DM25 | ND | ND | ND | ND | ND | |
| DM21 | ND | ND | ND | ND | ND | |
| DM33 | ND | ND | ND | ND | ND | |
| GR | ND | ND | ND | ND | ND | |
| Leucine | 01708 | 19.75 | 0.03 | 0.17 | 0.73 | 0.22 |
| 01715 | 19.75 | 0.02 | 0.11 | 0.74 | 0.15 | |
| 01741 | 19.74 | 0.04 | 0.19 | 1.29 | 0.25 | |
| DM6 | 19.76 | 0.03 | 0.13 | 0.89 | 0.17 | |
| DM29 | 19.75 | 0.03 | 0.16 | 1.11 | 0.22 | |
| DM25 | 19.76 | 0.03 | 0.13 | 0.86 | 0.18 | |
| DM21 | 19.75 | 0.04 | 0.18 | 1.23 | 0.24 | |
| DM33 | 19.74 | 0.03 | 0.15 | 0.92 | 0.21 | |
| GR | 19.75 | 0.05 | 0.23 | 1.23 | 0.30 | |
| Lysine | 01708 | 20.42 | 0.03 | 0.36 | 0.54 | 0.15 |
| 01715 | 20.42 | 0.01 | 0.22 | 0.49 | 0.10 | |
| 01741 | 20.42 | 0.02 | 0.25 | 0.59 | 0.10 | |
| DM6 | 20.43 | 0.01 | 0.20 | 0.47 | 0.09 | |
| DM29 | 20.42 | 0.01 | 0.19 | 0.45 | 0.09 | |
| DM25 | 20.43 | 0.02 | 0.23 | 0.51 | 0.10 | |
| DM21 | 20.42 | 0.01 | 0.21 | 0.49 | 0.08 | |
| DM33 | 20.42 | 0.01 | 0.21 | 0.45 | 0.09 | |
| GR | 20.42 | 0.02 | 0.29 | 0.55 | 0.13 | |
| Proline | 01708 | 24.61 | 0.21 | 1.30 | 4.48 | 0.90 |
| 01715 | 24.61 | 0.10 | 0.65 | 3.36 | 0.45 | |
| 01741 | 24.61 | 0.13 | 0.81 | 4.38 | 0.57 | |
| DM6 | 24.61 | 0.18 | 1.12 | 6.11 | 0.78 | |
| DM29 | 24.62 | 0.05 | 0.29 | 1.59 | 0.21 | |
| DM25 | 24.62 | 0.11 | 0.72 | 3.70 | 0.49 | |
| DM21 | 24.61 | 0.10 | 0.60 | 3.3.0 | 0.42 | |
| DM33 | 24.61 | 0.19 | 1.18 | 5.69 | 0.82 | |
| GR | 24.61 | 0.17 | 1.06 | 4.54 | 0.74 |
| Phenolic Compound | Sample Name | Retention Time (min) | Precursor Mass | Concentration (µg/g) | Formula |
|---|---|---|---|---|---|
| Ascorbic acid | 01741 | 0.81 | 175.025 | 101.381 | C6H8O6 |
| 01715 | 0.80 | 175.025 | 118.182 | ||
| 01708 | 0.78 | 175.025 | 137.831 | ||
| DM6 | 0.80 | 175.025 | 134.043 | ||
| DM29 | 0.82 | 175.025 | 125.843 | ||
| DM25 | 0.74 | 175.025 | 134.974 | ||
| DM21 | 0.81 | 175.025 | 117.102 | ||
| DM33 | 0.72 | 175.025 | 110.336 | ||
| GR | 0.78 | 175.025 | 180.642 | ||
| p-coumaric acid | 01741 | 10.68 | 163.040 | 0.627 | C9H8O3 |
| 01715 | 10.66 | 163.040 | 1.401 | ||
| 01708 | 10.66 | 163.040 | 0.713 | ||
| DM6 | 10.68 | 163.040 | 1.058 | ||
| DM29 | 10.68 | 163.040 | 11.67 | ||
| DM25 | 10.69 | 163.040 | 0.401 | ||
| DM21 | 10.67 | 163.040 | 0.870 | ||
| DM33 | 10.65 | 163.040 | 0.212 | ||
| GR | 10.65 | 163.040 | 1.181 | ||
| Ferulic acid | 01741 | 12.94 | 193.051 | 0.174 | C10H10O4 |
| 01715 | 12.93 | 193.051 | 0.857 | ||
| 01708 | 12.93 | 193.051 | 1.621 | ||
| DM6 | 12.94 | 193.051 | 2.816 | ||
| DM29 | 12.95 | 193.051 | 71.539 | ||
| DM25 | 12.94 | 193.051 | 0.064 | ||
| DM21 | 12.92 | 193.051 | 0.541 | ||
| DM33 | 12.94 | 193.051 | 0.804 | ||
| GR | 12.92 | 193.051 | 19.527 | ||
| Catechin | 01741 | 4.97 | 289.072 | 0.253 | C15H14O6 |
| 01715 | ND | ND | ND | ||
| 01708 | 5.25 | 289.072 | 1.071 | ||
| DM6 | ND | ND | ND | ||
| DM29 | 5.60 | 289.072 | 0.023 | ||
| DM25 | ND | ND | ND | ||
| DM21 | 5.27 | 289.072 | 0.197 | ||
| DM33 | 5.65 | 289.072 | 0.626 | ||
| GR | ND | ND | ND | ||
| Quercetin | 01741 | 17.71 | 301.035 | 1.721 | C15H10O7 |
| 01715 | 17.73 | 301.035 | 0.784 | ||
| 01708 | 17.72 | 301.035 | 0.290 | ||
| DM6 | 17.71 | 301.035 | 1.010 | ||
| DM29 | 17.71 | 301.035 | 1025.277 | ||
| DM25 | 17.73 | 301.035 | 0.978 | ||
| DM21 | 17.73 | 301.035 | 0.613 | ||
| DM33 | 17.73 | 301.035 | 0.163 | ||
| GR | 17.72 | 301.035 | 84.211 | ||
| Caffeic acid | 01741 | 6.92 | 179.035 | 0.615 | C9H8O4 |
| 01715 | 6.87 | 179.035 | 1.024 | ||
| 01708 | 6.89 | 179.035 | 0.12 | ||
| DM6 | 6.96 | 179.035 | 1.174 | ||
| DM29 | 6.88 | 179.035 | 1.785 | ||
| DM25 | ND | ND | ND | ||
| DM21 | 6.90 | 179.035 | 0.384 | ||
| DM33 | 6.88 | 179.035 | 0.911 | ||
| GR | 6.86 | 179.035 | 1.015 | ||
| Gallic acid | 01741 | ND | ND | ND | C7H6O5 |
| 01715 | 1.34 | 169.014 | 0.576 | ||
| 01708 | 1.28 | 169.014 | 0.811 | ||
| DM6 | 1.48 | 169.014 | 0.679 | ||
| DM21 | ND | ND | ND | ||
| DM25 | ND | ND | ND | ||
| DM29 | ND | ND | ND | ||
| DM33 | ND | ND | ND | ||
| GR | ND | ND | ND | ||
| Genistein | 01741 | 17.97 | 269.046 | 0.294 | C15H10O5 |
| 01715 | 1.34 | 269.046 | 0.576 | ||
| 01708 | 17.97 | 269.046 | 0.292 | ||
| DM6 | 17.97 | 269.046 | 0.70 | ||
| DM29 | 17.97 | 269.046 | 0.248 | ||
| DM25 | 17.99 | 269.046 | 0.845 | ||
| DM21 | 17.98 | 269.046 | 0.128 | ||
| DM33 | 17.98 | 269.046 | 0.10 | ||
| GR | 17.97 | 269.046 | 0.257 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyagi, A.; Lim, M.-J.; Kim, N.-H.; Barathikannan, K.; Vijayalakshmi, S.; Elahi, F.; Ham, H.-J.; Oh, D.-H. Quantification of Amino Acids, Phenolic Compounds Profiling from Nine Rice Varieties and Their Antioxidant Potential. Antioxidants 2022, 11, 839. https://doi.org/10.3390/antiox11050839
Tyagi A, Lim M-J, Kim N-H, Barathikannan K, Vijayalakshmi S, Elahi F, Ham H-J, Oh D-H. Quantification of Amino Acids, Phenolic Compounds Profiling from Nine Rice Varieties and Their Antioxidant Potential. Antioxidants. 2022; 11(5):839. https://doi.org/10.3390/antiox11050839
Chicago/Turabian StyleTyagi, Akanksha, Min-Jin Lim, Nam-Hyeon Kim, Kaliyan Barathikannan, Selvakumar Vijayalakshmi, Fazle Elahi, Hun-Ju Ham, and Deog-Hwan Oh. 2022. "Quantification of Amino Acids, Phenolic Compounds Profiling from Nine Rice Varieties and Their Antioxidant Potential" Antioxidants 11, no. 5: 839. https://doi.org/10.3390/antiox11050839
APA StyleTyagi, A., Lim, M.-J., Kim, N.-H., Barathikannan, K., Vijayalakshmi, S., Elahi, F., Ham, H.-J., & Oh, D.-H. (2022). Quantification of Amino Acids, Phenolic Compounds Profiling from Nine Rice Varieties and Their Antioxidant Potential. Antioxidants, 11(5), 839. https://doi.org/10.3390/antiox11050839








