Abstract
This study aimed to evaluate the effects of dietary supplementation of compound polysaccharides derived from Astragalus and Glycyrrhiza on growth performance, meat quality, antioxidant function, cecal microbiota and serum metabolomics of broilers. A total of 480 one-day-old male Arbor Acres (AA) broilers were randomly divided into four treatments with six replicates comprising 20 broilers each. Treatments: CON group was the basal diet; ANT group was supplemented with Terramycin calcium; LAG group was supplemented with 150 mg/kg Astragalus polysaccharides and 75 mg/kg Glycyrrhiza polysaccharides; HAG group was supplemented with 300 mg/kg Astragalus polysaccharides and 150 mg/kg Glycyrrhiza polysaccharides. The results showed that LAG and HAG supplementation increased growth performance, antioxidant function and meat quality compared with the CON group and ANT group and, especially, the effect of LAG treatment was better than HAG. Analysis of cecal microbiota showed that LAG and HAG supplementation altered cecal microbial diversity and composition in broilers. Serum metabolomics analysis showed that a total of 193 differential metabolites were identified in CON and LAG groups, which were mainly enriched in linoleic acid metabolism and glutathione metabolism pathways. Moreover, there was a close correlation between serum metabolites, cecal microbiota and phenotypic indicators. Conclusion: Dietary supplementation of 150 mg/kg Astragalus polysaccharides and 75 mg/kg Glycyrrhiza polysaccharides could improve the growth performance, antioxidant function and meat quality of broilers by changing the serum metabolites and cecal microbiota composition.
1. Introduction
The unreasonable use of antibiotics can easily lead to bacterial resistance, resulting in drug residues in meat, eggs and milk, causing food safety problems [1,2] and harming human health and environmental safety [3]. Considering food safety and human health, some countries, such as those of the European Union, the United States, China and India, have promulgated regulations prohibiting or restricting antibiotic growth promoters [4]. Thus, it is urgent to develop green feed additives to replace antibiotics. As feed additives, plant polysaccharides have safety characteristics, no resistance and minor toxic and side effects. Rational addition to the feed can improve the production performance, intestinal microecology, antioxidant function and livestock and poultry product quality [5,6]. Previous studies have shown that polysaccharides extracted from external pistachio hull could partly prevent enzymatic browning in potato slices [7], inhibit lipid peroxidation, have cytoprotective properties [8], thereby reducing lipid oxidation during refrigeration. and improve meat color stability [9]. Interestingly, supplementing green tea polysaccharides in broilers can also improve pH and a* value of chicken breast muscle, decrease the L* value and b* value, increase the abundance of Bacteroides and Lactobacillus and decrease the abundance of Proteobacteria [10]. Moreover, it has been reported that adding ginseng polysaccharides to the diet could display antioxidant, immunomodulatory properties and anti-tumor functions [11].
The primary component of the water-soluble heteropolysaccharide derived from the dried roots or stems of the traditional Chinese medicine herb Astragalus is Astragalus polysaccharides (APS), which has immunomodulatory, anti-inflammatory, antioxidant, antibacterial and antiviral properties [12]. Dietary supplementation with APS has been shown to boost juvenile broiler body weight gain, perhaps because of increased digestive enzyme activity and antioxidant ability [13]. Moreover, dietary supplementation of gamma-irradiated Astragalus polysaccharides in poultry diets can increase ADFI, reduce FCR [14] and alleviate growth decrease under cyclophosphamide and lipopolysaccharide stress in broilers [15,16]. In mice, APS effectively alleviated high-fat diet-induced metabolic disorders, altering gut microbiota composition and function [17]. Glycyrrhiza polysaccharides (GPS) are one of the primary active components of Glycyrrhiza and have antioxidant, antibacterial, antiviral, anticancer, anti-inflammatory, immunomodulatory, hypoglycemic, reconciling medicine and other biological activities [18]. Adding 1500 mg/kg Glycyrrhiza polysaccharide could increase the activity of serum T-SOD in broilers and inhibit the decrease of T-SOD activity and the increase of MDA content induced by lipopolysaccharide [19]. GPS has the potential to improve broiler growth performance by improving intestinal health and modulating gut microbiota [20]. The compatibility of Astragalus and Glycyrrhiza can promote the synergistic effect and improve efficacy [21]. However, there are few reports on broilers’ compatible use of Astragalus polysaccharides and Glycyrrhiza polysaccharides. The present study was carried out to investigate the effects of dietary supplementation of Astragalus-Glycyrrhiza polysaccharides on the production performance, nutrient apparent metabolic rate, meat quality and antioxidant function of broilers. Moreover, serum non-targeted metabolomics and gut microbiota were analyzed to provide insight into the beneficial effects of dietary Astragalus-Glycyrrhiza polysaccharides in broilers. The findings in this study will promote the application of Astragalus-Glycyrrhiza polysaccharides as antibiotic alternatives in poultry production.
2. Materials and Methods
2.1. Astragalus Polysaccharides and Glycyrrhiza Polysaccharides
Inner Mongolia Evergrand Pharmaceutical Co., Ltd. (Inner Mongolia, Tongliao, China) provided APS and GPS. The content of polysaccharides in APS and GPS is 70.23% and 61.36%, respectively.
2.2. Experimental Design and Animal Management
A total of 480 one-day-old male Arbor Acres (AA) broilers were randomly divided into four treatments with six replicates comprising 20 broilers each. Treatments: CON group was the basal diet; ANT group was supplemented with Terramycin calcium; LAG group was supplemented with 150 mg/kg Astragalus polysaccharides and 75 mg/kg Glycyrrhiza polysaccharides; HAG group was supplemented with 300 mg/kg Astragalus polysaccharides and 150 mg/kg Glycyrrhiza polysaccharides. The corn-soybean meal diet was fed to broilers in the experiment and the formula was divided into two stages, i.e., d1–d21 and d22–d42. The ingredients and nutrient levels of basal diets were formulated to meet the NRC (1994) nutrient requirements of broiler chickens (Supplementary Table S1). The broilers were vaccinated with the Newcastle disease vaccine and the infectious bursal vaccine on days 7 and 14 of the experiment. All broilers had free access to feed and clean water during the experiment. The temperature of the chicken coop was maintained at 33 °C at the age of 1 to 4 d and then reduced by 2 °C per week to a final temperature of around 24 °C.
2.3. Growth Performance
On the 21st and 42nd days of the experiment, broilers fasted with free drinking water at 8:00 p.m. Broilers were weighed in duplicate units at 8:00 a.m. on the 22nd and 43rd days and the body weight and feed consumption of the broilers were accurately recorded. The average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR) were calculated.
2.4. Serum and Breast Muscle Antioxidant Function
Each duplicate randomly selected one male broiler at 42 d. Blood samples were drawn from the wing vein and immediately transferred to coagulation tubes. After centrifugation (3000× g at 4 °C for 15 min), serum samples were collected and kept at −20 °C for analysis. The ipsilateral breast muscle was harvested after the broilers were euthanized. The activities of T-AOC, SOD, GSH-Px and MDA content in serum and breast muscles were determined using kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.5. Meat Quality
Ipsilateral breast muscle samples were collected and the following meat quality indicators were measured.
pH: pH was measured using the Testo 205 pH meter (Testo AG, Lenzkirch, Germany), which was inserted directly into the breast muscle within 45 min and 24 h after the euthanasia of broilers. pH meters were calibrated with standard buffers of pH 4.01 and pH 6.86 before use.
Color: About 45 min after the euthanasia of broilers, a colorimeter (CH-400, Konica Minolta Holdings, Inc., Tokyo, Japan) was used and three pieces of breast muscle samples (5 cm × 5 cm × 0.5 cm) were cut vertically. The samples’ L*, a* and b* values were measured thrice and the average value was taken as the final color value.
Drip loss: About 2 g of breast muscle (W1) from each broiler was weighed and placed in a sealed plastic bag. The plastic bag was inflated to prevent muscle mass from sticking to the wall and hung in a 4 °C refrigerator for 24 h. The filter paper was used to wipe off the water on the muscle surface. The resulting breast muscle (W2) was weighed. Drip loss was calculated using the equation:
Drip loss = (W1 − W2)/W1 × 100%
Shearing force: The breast muscle sample was packed in a plastic bag and placed in a constant-temperature water bath at 80 °C for heating. When the central meat temperature reached 70 °C, the meat was collected and cooled to room temperature. Then, the meat was trimmed into strips with length, width and height of 3, 1 and 1 cm, respectively, along the direction of muscle fibers and cut perpendicular to the direction of muscle fibers by using a digital meat tenderizer (model C-LM3B, Northeast Agricultural University).
Fatty acid: The fatty acid content of breast muscle was quantitatively determined by the gas chromatographic method (GB/5009.168-2016) [22]. Results of fatty acids were expressed as the percentage of the total fatty acids identified.
2.6. qRT-PCR of Intestinal Antioxidant Enzyme Genes
The expression levels of SOD1, SOD2 and GSH-Px mRNA in the intestinal mucosa of broilers (duodenum, jejunum and ileum) were detected by qRT-PCR. The extraction of total RNA from the samples was performed according to the instructions of the Trizol kit. The concentration of total RNA (OD260/280 = 1.8–2.0) was determined using a microspectrophotometer. The extracted RNA was transcribed into cDNA using the Takara reverse transcription kit. Fluorescence quantitative PCR detection was performed using the Takara fluorescence quantitative kit and the reaction volume was 20 μL. Primers were synthesized by Shanghai Sangon Biological Co., Ltd. (Shanghai, China). The specific information on primers is shown in Supplementary Table S2. GAPDH gene was used as an internal reference and the relative expression of the target gene was calculated using the 2−ΔΔCT method.
2.7. Cecal Microbiota
Using the QIAamp DNA Stool Mini Kit (QIAGEN, CA, Hamburg, Germany) by the manufacturer’s instructions, microbial DNA was extracted from the contents of the cecum. With primer pairs 338F (5′-ACTCCTACGGGAGGCACAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), the V3-V4 region of the 16S rRNA gene was amplified. By Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China), the 16S rRNA genes were sequenced using the Illumina MiSeq platform (Shanghai, China). The practical readings were obtained by demultiplexing the raw reads, quality-filtering them using Trimmomatic, then merging them using FLASH. Using UPARSE (version 7.11, http://www.drive5.com/uparse/ accessed on 10 July 2021), the acquired high-quality reads were assigned to operational taxonomic units (OTUs) with a 97% similarity and chimera sequences were eliminated by comparison with the Silva database using the UCHIME algorithm. The RDP Classifier examined the taxonomy of the representative sequences for each OTU. The bacterial communities’ diversity, composition and differences were examined on the I-Sanger Cloud Platform, which was made available by the Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
2.8. Metabolite Extraction and Analysis
The 400 µL methanol: water (4:1, v/v) solution was used to extract the metabolites from 100 µL of accurately weighed serum samples. The mixture was treated with a High throughput Tissue Crusher Wonbio-96c (Shanghai Wan Bo Biotechnology Co., Ltd., Shanghai, China) at 50 Hz for 6 min after being allowed to settle at −20 °C. It was followed by vortexing for 30 s and ultrasonic treatment at 40 kHz for 30 min at 5 °C. The samples were placed at −20 °C for 30 min to precipitate proteins. After centrifugation at 13,000× g at 4 °C for 15 min, the supernatant was carefully transferred to sample vials for LC-MS/MS analysis.
The mass spectrometric data were collected using a Thermo UHPLC-Q Exactive Mass Spectrometer equipped with an electrospray ionization (ESI) source operating in either positive or negative ion mode. Peak detection and alignment of raw data were performed in Progenesis QI 2.3 (Nonlinear Dynamics, Waters, Milfod, MA, USA). The final dataset was imported into the SIMCA16.0.2 software package (Sartorius Stedim Data Analytics AB, Umea, Sweden) for multivariate analysis. Orthogonal Partial Least Squares Discriminate Analysis (OPLS-DA) were performed using ropes (Version 1.6.2, http://bioconductor.org/packages/release/bioc/html/ropls.html, accessed on 25 July 2021) R package on Majorbio Cloud Platform (https://cloud.majorbio.com, accessed on 25 July 2021). Variable importance in the projection (VIP) was calculated in the OPLS-DA model. Differential metabolites were identified according to the standard of VIP > 1 and p < 0.05. Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/, accessed on 25 July 2021) was used for pathway enrichment analysis. The correlational heatmaps were generated according to the result of the Pearson correlation analysis. The process was conducted in the environment of a Python package named Scipy Stats (https://docs.scipy.org/doc/scipy/, accessed on 27 July 2021).
2.9. Statistical Analysis
Statistical analyses were performed using the SPSS Statistics Software (version 18.0, New York, NY, USA). One-way ANOVA evaluated data and the comparative analysis was conducted using Duncan’s test. Statistical results are shown in mean and standard error and p < 0.05 was considered statistically significant.
3. Results
3.1. Growth Performance
As shown in Table 1, compared with the CON group, the LAG and HAG groups had significantly increased 21 d body weight, 1–21 d ADG and 42 d body weight (p < 0.05) and the LAG group had significantly increased 22–42 d ADG (p < 0.05). For the whole feeding period (1–42 days), LAG and HAG groups had significantly increased ADG and significantly decreased FCR (p < 0.05). No significant difference was observed among the ANT, LAG and HAG groups (p > 0.05).

Table 1.
Effects of Astragalus-Glycyrrhiza polysaccharides on broiler performance.
3.2. Apparent Metabolic Rate
As shown in Table 2, compared with the CON group, the LAG and HAG groups had significantly increased apparent metabolic rate of energy (p < 0.05) and the LAG group had significantly increased apparent metabolic rate of the CP (p < 0.05), No significant difference was observed among the ANT, LAG and HAG groups (p > 0.05). No significant differences were observed in the apparent metabolic rates of EE, Ca and P among the four groups (p > 0.05).

Table 2.
Effects of Astragalus-Glycyrrhiza polysaccharides on apparent metabolic rate of nutrients in broilers (%).
3.3. Meat Quality
As shown in Table 3, compared with the CON group, the LAG group had significantly increased pH45min, pH24h and L* values (p < 0.05) and significantly decreased b* value, shear force and drip loss (p < 0.05), while the HAG group had significantly increased L* value (p < 0.05) and significantly decreased b* value (p < 0.05). Compared with the ANT group, the LAG group had significantly increased pH45min and L* values (p < 0.05) and significantly decreased b* value and shear force (p < 0.05) and the HAG group had significantly decreased b* value (p < 0.05). Compared with the HAG group, the LAG group had significantly increased pH45min and significantly decreased shear force (p < 0.05). No treatment difference was observed in the a* values (p > 0.05).

Table 3.
Effects of Astragalus-Glycyrrhiza polysaccharides on meat quality.
3.4. Breast Muscle Fatty Acid
As shown in Table 4, compared with the CON group, LAG and HAG groups had significantly decreased C22:0, total SFA and n-6/n-3 (p < 0.05) and significantly increased C16:1, C18:1n-9c, C24:1, C18:2n-6, C18:3n-3, C20:2, C22:6n-3, total MUFA, total PUFA and PUFA/SFA (p < 0.05). Compared with the ANT group, LAG and HAG groups had significantly increased C24:1, C18:3n-3, C20:2 and C22:6n-3 (p < 0.05) and significantly decreased n-6/n-3 (p < 0.05). Compared with the HAG group, the LAG group had significantly increased C16:1 and C18:1n-9c and significantly increased C18:3n-3 and C22:6n-3 (p < 0.05).

Table 4.
Effects of Astragalus-Glycyrrhiza polysaccharides on the fatty acid composition of breast muscle.
3.5. Serum and Breast Muscle Antioxidant Function
As shown in Table 5, compared with the CON group, LAG and HAG groups had significantly increased T-AOC activity in serum and breast muscle and SOD activity in serum (p < 0.05) and significantly decreased MDA content in serum and breast muscle (p < 0.05). Compared with the ANT group, LAG and HAG groups had significantly increased T-AOC activity in serum and breast muscle and significantly decreased MDA content (p < 0.05) and the LAG group had significantly increased serum GSH-Px and SOD activities (p < 0.05). The serum GSH-Px and SOD activities of the LAG group were significantly increased compared with those of the HAG group (p < 0.05). No treatment difference was observed in SOD and GSH-Px activities in the breast muscles (p > 0.05).

Table 5.
Effects of Astragalus-Glycyrrhiza polysaccharides on antioxidant function of broiler’s serum and breast muscle.
3.6. Intestine Antioxidant Enzyme mRNA Expression
As shown in Figure 1A–C, compared with the CON group, the expression levels of SOD1, SOD2 and GSH-Px mRNA in the duodenum of LAG and HAG groups were significantly increased (p < 0.05). Compared with the ANT group, the LAG and HAG groups had significantly increased expression levels of SOD2 and GSH-Px mRNA (p < 0.05) and the LAG group had significantly increased SOD1 mRNA expression (p < 0.05). No significant difference was observed between the LAG and HAG groups (p > 0.05).
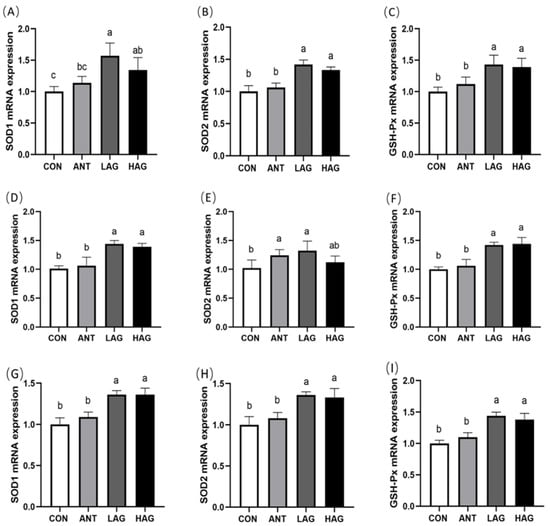
Figure 1.
(A–I) Effect of Astragalus-Glycyrrhiza polysaccharides on mRNA expression of antioxidant enzyme-related in the duodenum, jejunum and ileum of broilers. Data are means ± SEM, n = 6. Means with different letters indicate significant differences (p < 0.05).
As shown in Figure 1D–F, compared with the CON group, the LAG and HAG groups had significantly higher SOD1 and GSH-Px mRNA expression levels in the jejunum (p < 0.05) and the LAG group had significantly higher SOD2 mRNA expression (p < 0.05). Compared with the ANT group, the expression levels of SOD1 and GSH-Px mRNA in the LAG and HAG groups were significantly increased (p < 0.05). No significant difference was observed between the LAG and HAG groups (p > 0.05).
As shown in Figure 1G–I, compared with the CON and ANT groups, LAG and HAG groups had significantly higher SOD1, SOD2 and GSH-Px mRNA expression levels in the ileum (p < 0.05). No significant difference was observed between the LAG and HAG groups (p > 0.05).
3.7. Cecal Microbial Diversity
The Illumina Miseq high-throughput sequencing platform sequenced the V3-V4 region of the 16S rRNA gene. After removing incorrect chimeric sequences, 1,144,417 high-quality reads were generated. An average of 71,526 sequences was obtained per sample with an average length of 421 bp. As shown in Supplementary Figure S1A, the Shannon dilution curve reflected the sample’s microbial diversity index. In this experiment, the curves of each group tended to be flat, indicating that the amount of sequencing data was large enough and the sequencing results were reasonable. The Shannon index of the LAG group was the highest, indicating that the LAG group had the highest microbial diversity. Venn plots could count the number of common and unique operational taxonomic units (OTUs) in multiple samples. As shown in Supplementary Figure S1B, a total of 743 OTUs were shared by four groups and the unique OTUs of each group followed the order: LAG group (4208) > HAG group (4140) > CON group (2909) > ANT group (2785).
3.7.1. Alpha Diversity Analysis
The alpha diversity includes the Chao1 index, Shannon index, Simpson index and Observed species, which refers to the diversity within a specific area or ecosystem. The Chao index and Observed species are used to evaluate the richness of the microbiota. A higher Chao or Observed species indicates a higher richness of the microbiota. A higher Simpson index indicates a low microbiota diversity. The data of alpha diversity indexes are presented in Figure 2A–D. Compared with the CON group, the Shannon index and Observed species in the LAG group and the Observed species in the HAG group were significantly increased (p < 0.05). Compared with the ANT group, the Chao1 index, Shannon index and Observed species in the LAG group (p < 0.05) and the Chao1 index and Observed species in the HAG group (p < 0.05) were significantly increased. Compared with the HAG group, the Shannon index in the LAG group was significantly increased (p < 0.05). No significant difference in the Simpson index was observed among the groups (p > 0.05).
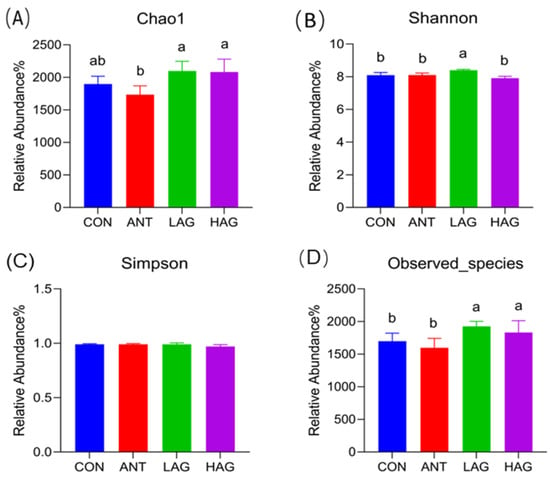
Figure 2.
(A–D). Effects of Astragalus-Glycyrrhiza polysaccharides on alpha diversity index of cecal microbiota in broilers. Means with different small letters indicate significant differences (p < 0.05).
3.7.2. Beta Diversity Analysis
Beta diversity was used to analyze the similarity of cecal microbiota among different groups. The differences or similarities of cecal microbial diversity among groups were comprehensively analyzed by the principal coordinates analysis (PCoA) based on the Weighted UniFrac distance. As shown in Figure 3, the distances of the samples in the CON group were scattered, indicating that the samples in this group had poor uniformity. LAG and HAG groups were wholly separated from the CON and ANT groups, indicating that LAG and HAG groups changed the bacterial community structure. A crossover was observed between LAG and HAG groups, suggesting a similarity in the structures of their cecal microbiota.
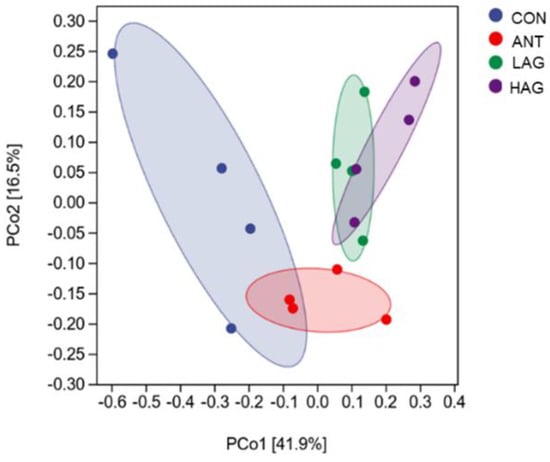
Figure 3.
The cecal microbiota’s principal coordinate analysis (PCoA) was based on the Weighted UniFrac distance (n = 4).
3.7.3. Microflora Structure
At the phylum level, the effects of Astragalus- Glycyrrhiza polysaccharides on the cecal microbial composition of broilers are shown in Figure 4A,B. The dominant phyla of the four groups were Bacteroidetes and Firmicutes. CON, ANT, LAG and HAG had relative abundance values of Bacteroidetes of 49.12%, 54.15%, 40.87% and 40.91%, respectively and relative abundance values of Firmicutes of 37.67%, 32.42%, 43.97%, 42.49%, respectively. Compared with the CON and ANT groups, LAG and HAG groups had increased the relative abundance of Firmicutes and the ratio of Firmicutes and Bacteroidetes(F/B) decreased the relative abundance of Bacteroidetes. At the genus level, the effect of Astragalus-Glycyrrhiza polysaccharides on the cecal microbial composition of broilers was shown in Figure 4C,D. The dominant genera of the four groups were Bacteroides, Oscillospira, Phascolarctobacterium and Faecalibacterium. Figure 4D showed the significant difference in the relative abundance of 9 of the top 15 genera among the four treatment groups.
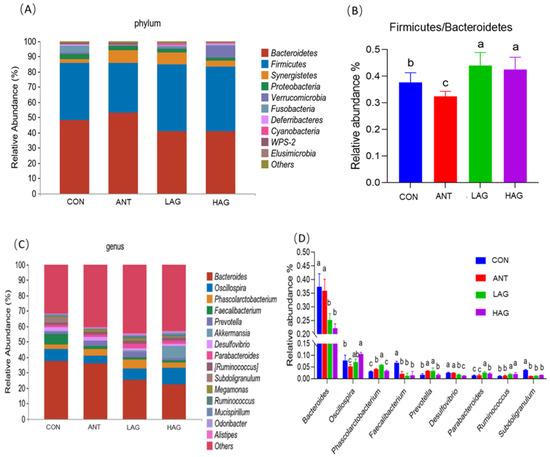
Figure 4.
Effect of Astragalus-Glycyrrhiza polysaccharides on the abundance of cecal microflora at phylum and genus level in broilers (A) Relative abundance of cecal microbiota at the phylum level; (B) Comparison of Firmicutes and Bacteroidetes ratio. (p < 0.05). (C) Relative abundance of cecal microbiota at the genus level; (D) Relative abundance difference analysis of cecal bacterial species at the genus level (p < 0.05). Means with different small letters indicate significant differences (p < 0.05).
Compared with the CON and ANT groups, LAG and HAG groups increased the relative abundance of Oscillospira, Parabacteroides and Ruminococcus while decreased the abundance of Bacteroides, Faecalibacterium, Desulfovibrio and Subdoligranulum. In addition, the LAG group increased the relative abundance of Phascolarctobacterium.
3.8. Serum Metabolomic Analysis
Through the analysis of apparent performance and gut microbiota, we found that the production performance of the LAG group was better than that of HAG. Furthermore, CON and LAG were used to analyze serum metabolites. Orthogonal partial least squares discriminant analysis (OPLS-DA) was used to distinguish the difference between groups in positive and negative ion mode. Response permutation testing (RPT) was used to evaluate the accuracy of the OPLS-DA model. R2Ywas closer to 1, the more stable and reliable the model; Q2Y was below 0.05 and represented a credible predictive ability. In this experiment, R2Y was above 0.7 and Q2Ywas below 0.05, indicating a good model prediction ability. (Supplementary Figure S2). Further OPLS-DA analysis of the data was performed in positive and negative ion modes; the samples in the same group were clustered closely, indicating that their metabolites were highly similar and stable and the samples in different groups were wholly separated, indicating that there were significant differences in serum metabolites between the CON and LAG group.
As shown in Figure 5A differential volcanic plot, a total of 193 differential metabolites were identified in the two treatments, of which 113 differential metabolites were significantly up-regulated and 80 differential metabolites were significantly down-regulated (VIP ≥ 1.0, p-values < 0.05). Moreover, VIP in the OPLS-DA model was calculated to evaluate the changes in serum metabolites. Metabolites with VIP values > 1.0 and p-values < 0.05 (t-test) were considered significantly influenced by LAG supplementation and the top 40 metabolites with the highest VIP values were listed in Figure 5B, which revealed that serum metabolites among the two groups formed distinct clusters. Figure 5C shows that the identified metabolites were functionally annotated through the KEGG database. The annotated metabolites were mainly focused on the processes of amino acid metabolism, lipid metabolism, nucleotide metabolism, membrane transport, etc. Furthermore, as shown in Figure 5D, pathway enrichment and topology analysis were performed.
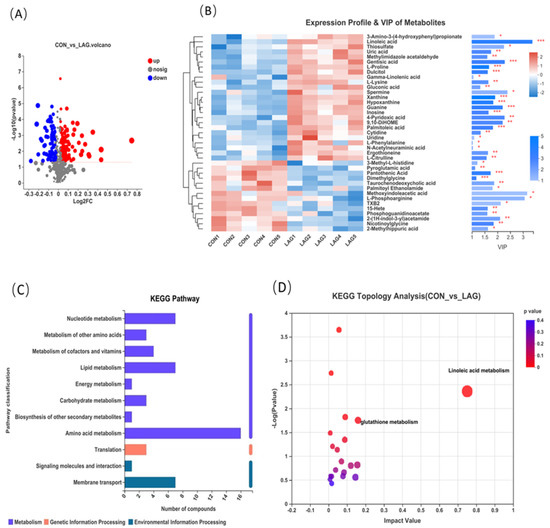
Figure 5.
(A) Differential volcano plot of CON and LAG groups, each point in the graph represents a specific metabolite and larger points indicate higher VIP values. Blue indicates significant downregulation and red indicates significant upregulation. (B) Heatmap combined with hierarchical clustering of the most significantly influenced metabolites in CON and LAG groups. Only the metabolites with the highest variable importance in projection (VIP) value in the OPLS-DA model (top 40 named metabolites) were listed. The t-test was jointly applied with the OPLS-DA to identify the discrepant metabolites and the p value in the t-test was shown. The heatmap colors represent the relative expression of metabolites in the sample, with VIP bar graphs of metabolites on the right. The bars’ length represents the metabolites’ contribution value to the difference between the two groups. The larger the value, the more significant the difference between the two groups. The bar’s color indicates the significance of the metabolite difference between the two groups (* 0.01 < p ≤ 0.05, ** 0.001 < p ≤ 0.01, *** p ≤ 0.001). (C) KEGG pathway, the ordinate, is the second classification of the KEGG metabolic pathway and the abscissa is the number of metabolites annotated to the pathway. (D) KEGG topology analysis. Each bubble in the figure represents a KEGG Pathway; the horizontal axis represents the relative importance of the metabolites in the pathway and the size of the Impact Value; the vertical axis represents the enrichment significance of metabolites involved in the pathway-log10 (p-value); bubbles of the size represents the Impact Value; the larger the bubble, the greater the importance of the pathway.
The identified metabolites with an impact value higher than 0.1 and p-values below 0.05 were mainly enriched in two pathways, namely the linoleic acid metabolism pathway (Impact Value = 0.75, p = 0.004) and glutathione metabolism pathway (Impact Value = 0.16, p = 0.018). Differential metabolites from the linoleic acid metabolism pathway, including linoleic acid and gamma-linolenic acid and the glutathione metabolism pathway includes two differential metabolites, spermine and pyroglutamic acid.
3.9. Correlation Analysis
In order to predict the correlation between the top nine differential bacterial genera and 21 differential phenotypic indicators, a Pearson correlation analysis was conducted. As shown in Figure 6, Bacteroides was positively correlated with n-6/n-3, MDA and FCR but negatively correlated with ADG, L*, T-AOC, GSH-Px, SOD1 mRNA expression, GSH-Px mRNA expression, MUFA and PUFA (p < 0.05).
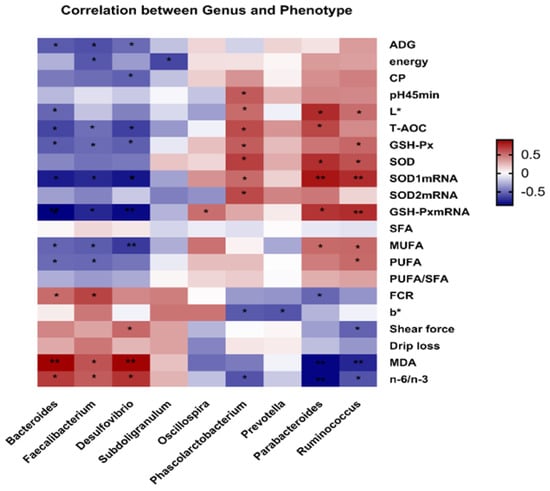
Figure 6.
Correlation between the differential cecal microbial community of genera and the phenotype of broilers by Pearson correlation analysis. Red indicates positive correlation and blue indicates negative correlation. (* 0.01 < p ≤ 0.05, ** 0.001 < p ≤ 0.01).
Faecalibacterium was positively correlated with n-6/n-3, MDA and FCR but negatively correlated with ADG, energy, T-AOC, GSH-Px, SOD1 mRNA expression, GSH-Px mRNA expression, MUFA and PUFA (p < 0.05). Desulfovibrio was positively correlated with n-6/n-3, MDA and shear force but negatively correlated with ADG, CP, T-AOC, GSH-Px, SOD1 mRNA expression, GSH-Px mRNA expression and MUFA (p < 0.05). Subdoligranulum is only negatively correlated with energy. Similarly, Oscillospir was only positively correlated with GSH-Px mRNA expression (p < 0.05). Phascolarctobacterium was positively correlated with pH 45 min, L*, T-AOC, GSH-Px, SOD, SOD1 mRNA expression and SOD2 mRNA expression, but negatively correlated with n-6/n-3 and b* (p < 0.05). Prevotella was only negatively correlated with b* (p < 0.05). Parabacteroides was positively correlated with L*, T-AOC, SOD, SOD1 mRNA expression, GSH-Px mRNA expression and MUFA but negatively correlated with FCR, MDA and n-6/n-3 (p < 0.05). Ruminococcus was positively correlated with L*, GSH-Px, SOD, SOD1 mRNA expression, GSH-Px mRNA expression, MUFA and PUFA, but negatively correlated with shear force, MDA and n-6/n-3 (p < 0.05). These results indicated a specific correlation between the bacterial genera and phenotypic indicators.
Since two critical metabolic pathways, linoleic acid metabolism and glutathione metabolism, were found in metabolites pathway enrichment analysis, we investigated the correlation of 9 differential bacterial genera with four differential metabolites enriched in these two pathways by Pearson’s correlation analysis. The heatmap revealed that nine species were correlated with the top 40 metabolites (Figure 7).
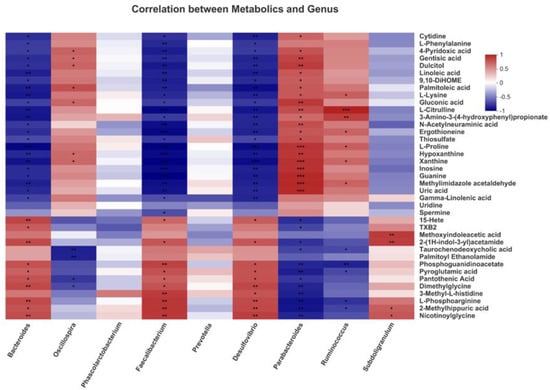
Figure 7.
Correlation between the differential cecal microbial community of genera and the serum metabolites of broilers by Pearson correlation analysis. Red indicates positive correlation and blue indicates negative correlation. (* 0.01 < p ≤ 0.05, ** 0.001 < p ≤ 0.01, *** p ≤ 0.001).
Differential metabolites from the linoleic acid metabolism pathway, including linoleic acid and gamma-linoleic acid, negatively correlated with Bacteroides, Faecalibacterium and Desulfovibrio; moreover, linoleic acid was positively correlated with Parabacteroides (p < 0.05). Differential metabolites from the glutathione metabolism pathway, including pyroglutamic acid and spermine. Pyroglutamic acid was positively correlated with Bacteroides, Faecalibacterium and Desulfovibrio but negatively correlated with Parabacteroides and Ruminococcus; moreover, spermine was positively correlated with Faecalibacterium (p < 0.05). In addition, many differential metabolites such as gluconic acid, L-proline and nicotinylglycine were significantly correlated with certain bacteria such as Bacteroides, Faecalibacterium and Desulfovibrio (p < 0.05). These results suggested that LAG-mediated change of certain bacteria were correlated with metabolites in linoleic acid metabolism and glutathione metabolism, indicating the critical roles of these bacteria in LAG-associated beneficial effects.
Correlation analysis of the top 40 differential serum metabolites and 21 differential phenotypic indicators showed that in Figure 8.
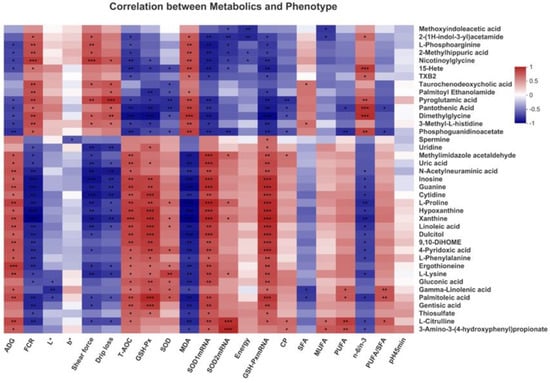
Figure 8.
Correlation between the differential serum metabolites and the phenotype of broilers by Pearson correlation analysis. Red indicates positive correlation and blue indicates negative correlation. (* 0.01 < p ≤ 0.05, ** 0.001 < p ≤ 0.01, *** p ≤ 0.001).
Linoleic acid and gamma-linoleic acid were positively correlated with ADG, T-AOC, GSH-Px, SOD1 mRNA and GSH-Px mRNA but negatively correlated with FCR and MDA (p < 0.05). Gamma-linoleic acid was positively correlated with SOD, PUFA and PUFA/SFA but negatively correlated with SFA (p < 0.05). Conversely, Pyroglutamic acid was positively correlated with FCR, shear force, drip loss, MDA and n-6/n-3 but negatively correlated with ADG, T-AOC, GSH-Px, SOD, SOD1mRNA and GSH-Px mRNA (p < 0.05). Spermine was positively correlated with GSH-Px mRNA but negatively correlated with b*. In addition, many differential metabolites such as inosine, guanine and cytidine were significantly correlated with phenotypic indicators such as T-AOC, GSH-Px, SOD, SOD1mRNA and GSH-Px mRNA (p < 0.05). These results suggested that specific phenotypic indicators that LAG changed were correlated with metabolites in linoleic acid metabolism and glutathione metabolism, indicating the critical roles of these indicators in LAG-associated beneficial effects.
4. Discussion
Under the comprehensive “ban on antibiotics”, exploring “new, efficient, safe and green” antibiotic substitutes has gradually become a research hotspot in the feed industry. Several studies showed that adding plant extracts to poultry diets can improve poultry immunity, prevent disease and promote poultry growth [6,23]. Adding APS to poultry diets can increase ADFI, reduce FCR [14] and alleviate growth decrease under cyclophosphamide and lipopolysaccharide stress in broilers [15,16]. Adding 10 g/kg of Astragalus powder to the diet can increase the ADG and reduce the FCR of broilers [24]. Drinking water containing Glycyrrhiza extract could improve body weight, ADFI and ADG and reduce FCR in broilers under heat stress [25]. Adding 500 mg/kg of Glycyrrhiza extract to high-density broilers diets effectively improves weight gain in late growth and throughout the growth period [26]. Our experiment showed that LAG and HAG supplementation increased body weight and ADG and decreased FCR. In addition, linoleic acid and gamma-linoleic acid were positively correlated with ADG but negatively correlated with FCR; conversely, pyroglutamic acid was positively correlated with FCR, indicating that the addition of Astragalus-Glycyrrhiza polysaccharide could improve the growth performance of broilers by altering the linoleic acid metabolism and glutamate metabolism pathways.
The nutrient metabolic rate is an important indicator to measure the digestion and absorption of nutrients by animals. Its level directly affects the growth performance of animals and reflects the diet’s nutritional value. Several studies have shown that dietary supplementation with APS can improve the metabolism of energy and CP [16]. Ibrahim et al. [27] showed that adding 3% Glycyrrhiza glabra extract residue to the broiler diet can increase CP’s apparent metabolic rate and decrease fat’s apparent metabolic rate. Our experiment showed that LAG and HAG groups increased broiler diets’ apparent metabolic energy rate. Moreover, the LAG group achieved a higher apparent metabolic rate of CP than antibiotics. It speculated that the improvement of nutrient apparent metabolic rate might be related to the improvement of production performance, further indicating that the Astragalus-Glycyrrhiza polysaccharides have a specific promotion effect on the growth and feed utilization of broilers.
Muscle pH, shear force and drip loss are indicators for evaluating the physicochemical properties of meat quality. The decrease in pH after animal slaughter is related to the glycolysis of muscle glycogen. Stress accelerates glycogenolysis in the body and causes the rapid decrease of muscle’s pH, seriously affecting muscle’s color and taste [28]. Galli et al. [29] showed that adding microencapsulated organic acids to broiler diets can reduce the rate of glycolysis and pH decline and improve meat quality. A high pH24h results in a low shear force, high water-holding capacity and improved meat quality [30]. Studies showed that the fermented Astragalus-Glycyrrhiza water extract as a feed additive could reduce drip loss of breast and leg muscles [31]. Our experiment found that the LAG group increases broilers’ pH45min and pH24h and reduces drip loss and shear force, indicating that the moderate addition of Astragalus-Glycyrrhiza polysaccharides could effectively alleviate the glycolysis post-slaughter and maintain the juiciness of the meat. This finding shows that the Astragalus-Glycyrrhiza polysaccharides are better at improving meat quality than antibiotics.
The color of meat, the most intuitive external expression for consumers to evaluate the freshness of meat, is usually expressed by L*, a* and b* values. The L* value indicates the muscle’s oxidized myoglobin content and the normal range is 46–53. Within this range, a higher value represents improved gloss. The a* value represents the content of deoxy-myoglobin in the muscle and a higher value indicates improved meat color and fresh meat. The b* value reflects the content of oxidized methemoglobin and a lower value indicates fresh meat [26,32,33]. Alagawany et al. [21] showed that drinking fermented Astragalus-Glycyrrhiza water extract improved the a* value and decreased the b* value and drip loss in broilers. Our experiment showed that the L* value was within the normal range and LAG and HAG supplementation increased the L* value and decreased the b* value, indicating that the addition of Astragalus-Glycyrrhiza polysaccharides helps in improving the freshness of broiler meat.
Chicken has become a popular functional food for consumers because of its high protein, low cholesterol and low saturated fatty acids [34]. The fatty acid content in chicken primarily consists of unsaturated fatty acids, such as linoleic acid (C18:2n-6), linolenic acid (C18:3n-3) and arachidonic acid (C20:4) is an essential precursor of chicken flavor [35]. Our results showed that LAG and HAG groups increased the contents of C18:2n-6 and C18:3n-3 in chicken and the content of total PUFA, thereby improving the flavor of the meat. Studies showed that imbalances in the ratios of PUFA to SFA and PUFA n-6 to PUFA n-3 are associated with various diseases, such as cardiovascular disease, inflammatory disease, diabetes and autoimmune diseases [36,37]. Hu et al. [38] showed that high PUFA n-3 and low SFA contents can improve meat quality and nutritional value, thereby reducing the risk of cardiovascular disease. PUFA n-3 has excellent anti-aging, anti-inflammatory, antioxidant, anti-cancer, anti-arthritis, anti-depressant, anti-hypertensive and insulin-sensitizing effects and cardiovascular health benefits [39]. The present study showed that LAG and HAG groups increased the ratio of PUFA to SFA and decreased the ratio of PUFA n-6 to PUFAn-3. In addition, gamma-linoleic acid was positively correlated with PUFA and PUFA/SFA but negatively correlated with SFA, indicating that the addition of Astragalus-Glycyrrhiza polysaccharide could improve meat quality by changing the linoleic acid metabolic pathway.
GSH-Px, SOD, T-AOC and MDA are indicators used to measure the body’s antioxidant capacity. Studies showed that APS could scavenge free radicals in time by activating various enzyme activities in the body, reducing oxidative stress in animals and enhancing animal immune responses [40]. The present experiment showed that LAG and HAG groups increased the expression levels of SOD1 mRNA, SOD2 mRNA and GSH-Px mRNA in the small intestine (duodenum, jejunum and ileum) of broilers. Moreover, the LAG supplementation increased serum T-AOC, SOD, GSH-Px and breast muscle’s T-AOC activity. At the same time, it decreased the MDA content in serum and breast muscle. Similar results were reported by Wu et al. [13] who found that adding the diet with 0.5–1.0 g/kg APS could improve the growth performance and serum SOD, GSH-Px, IgG, IgM and IgA and reduce the MDA content in broilers. Adding Astragalus root powder can enhance broilers’ growth performance, antioxidant status and serum metabolites and improve liver and kidney functions by improving the antioxidant status [41,42]. Adding the Glycyrrhiza extract to chicken patties reduces the production of MDA and increases the pH and a* values in the patties, thereby improving the oxidative stability of chicken patties and prolonging the shelf life [43].
The change of gut microbiome disrupts gut function when broilers are immunosuppressed by environmental stress or viral infection [44]. Studies showed that some plant polysaccharides reaching the distal gastrointestinal tract could be fermented by the gut microbiota and further regulate the gut microenvironment [45]. Broilers fed with γ-irradiated Astragalus polysaccharides show higher bacterial OTUs and the Shannon index but decrease the Simpson index than control and cyclophosphamide-treated groups [46]. Consistent with these findings, our study showed that LAG and HAG had more bacterial OTUs than the CON and ANT groups. Moreover, the result of Alpha diversity revealed that LAG and HAG groups increased the Chao1 index and Observed species. Beta diversity results showed that LAG and HAG groups altered the gut microbiota structure and composition, indicating that dietary supplementation with Astragalus-Glycyrrhiza polysaccharide increased the richness and diversity of cecal microbiota of broilers.
The dominant phyla in this experiment are Bacteroidetes and Firmicutes. Many bacteria belonging to Firmicutes are involved in energy metabolism and maintenance of intestinal health [47]. An increase in F/B favors nutrient absorption and is closely related to gut microbiota composition and the ability of the host to acquire energy [48,49]. The present experimental study showed that adding Astragalus-Glycyrrhiza polysaccharides increased F/B, which was beneficial to the absorption of nutrients by intestinal microorganisms in broilers.
At the genus level, the dominant genera with significant differences in this experiment are Bacteroides, Oscillospira, Phascolarctobacterium and Faecalibacterium. Bacteroides is one of the predominant genera of anaerobic bacteria in the chicken cecum [50]. Previous studies have shown that Bacteroides was positively related to serum inflammatory cytokines TNF-a, IL-1b and IL-6 and dietary supplementation with APS and GPS could inhibit the proliferation of Bacteroides [20]. Liu et al. [46] showed that the abundance of Bacteroides in broilers fed with γ-irradiated Astragalus polysaccharides was lower than those in the cyclophosphamide-treated groups. These findings are consistent with the results of the present experiment that LAG and HAG supplementation reduce the abundance of Bacteroides and Bacteroides were positively correlated with MDA but negatively correlated with T-AOC GSH-Px, SOD1 mRNA expression and GSH-Px mRNA expression. It has been reported that Oscillospira could produce butyrate by gluconate and human health is positively correlated with Oscillospira [51,52]. The supplementation of probiotic preparations and Bacillus subtilis in laying hen diets increases the abundance of Oscillospira, decreases the abundance of pathogenic E. coli and improves the performance and intestinal function in laying hens [53]. Our experiment showed that Oscillospira was positively correlated with GSH-Px mRNA expression and LAG and HAG supplementation increased the abundance of Oscillospira. Hou et al. [54] found that the abundance of Phascolarctobacterium in the gut microbiota in patients with ulcerative colitis was remarkably reduced compared with that in healthy individuals. Plantain is a widely used remedy for constipation. Jalanka et al. [55] found that plantain could increase the abundance of Phascolarctobacterium in the fecal microorganisms of constipated patients and reduce constipation. In this experiment, LAG supplementation increased the relative abundance of Phascolarctobacterium while Phascolarctobacterium was positively correlated with T-AOC, GSH-Px, SOD, SOD1 mRNA expression and SOD2 mRNA expression. Maier et al. [56] reported that enhancing the activity of Faecalibacterium does not enhance the integrity of the intestinal barrier. Liu et al. [46] found that APS supplementation significantly decreased the abundance of Faecalibacterium in broilers. In our experiment, LAG and HAG supplementation decrease the relative abundance of Faecalibacterium. Faecalibacterium was positively correlated with MDA but negatively correlated with T-AOC, GSH-Px, SOD1 mRNA expression and GSH-Px mRNA expression, indicating that the Astragalus-Glycyrrhiza polysaccharides could improve antioxidant function by modulating gut microbiota in broilers.
Metabolomics is widely utilized to investigate the impact of changes in animal diet, environment, genes and gut microbial communities on the response pathways of metabolic systems [57]. In this study, untargeted metabolomics of broiler serum were analyzed by LC-MS. Through the OPLS-DA model analysis, it was found that there was a significant difference between LAG and CON groups, indicating that the addition of Astragalus-Glycyrrhiza polysaccharides could significantly affect the metabolism of broiler serum. A total of 193 significant differential metabolites were identified for both groups, of which 113 differential ions were significantly up-regulated and 80 differential ions were significantly down-regulated. Through the KEGG database, the identified metabolites were functionally annotated. Amino acid metabolism, lipid metabolism, nucleotide metabolism and membrane transport are these compounds’ primary biological metabolic and signal transduction processes. Furthermore, the pathway of linoleic acid metabolism and glutathione metabolism pathway were identified as the most significant pathway through KEGG topology analysis.
Linoleic acid belongs to omega-6 PUFA and is essential for normal growth and development, cell function and signal transduction and immune response [58]. Linoleic acid can be elongated and desaturated to other bioactive omega-6 PUFAs, such as gamma-linolenic acid (18:3n6) and arachidonic acid (20:4n6). Subsequently, arachidonic acid can be converted to bioactive compounds, such as prostaglandins and leukotrienes. These eicosanoids are important in the normal metabolic function of tissues and cells [59]. Studies have shown that supplementing 1% linoleic acid in parental pigeon diets could improve the health of pigeons by increasing antioxidant capacity and lipid metabolism [60]. Linoleic acid could also improve antioxidative capacity in laying hens [61] and lipid metabolism in broilers and ducks [62,63]. The high intake of linoleic acid is associated with reduced risk for heart diseases and type 2 diabetes [64]. Linoleic acid induced autophagy and increased antioxidant ability through the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway and the AMPK-target of rapamycin (TOR) signaling pathway in hepatocytes in vitro and could greatly aid in the prevention and treatment of multiple pathologies [65]. In our study, LAG supplementation elevated linoleic acid levels and gamma-linolenic acid levels. T-AOC, GSH-Px, SOD and GSH-Px mRNA were positively correlated with these two metabolites, indicating that LAG could improve antioxidant capacity by modulating linoleic acid metabolism. In addition, Parabacteroides was positively correlated with linoleic acid. Conversely, Bacteroides, Faecalibacterium and Desulfovibrio were negatively correlated with linoleic acid and gamma-linolenic acid, suggesting that these species might participate in the modulation of linoleic acid metabolism by LAG.
The glutathione metabolism pathway includes two differential metabolites, spermine and pyroglutamic acid, which were changed by LAG. Spermine and pyroglutamic acid are intermediates in glutathione metabolism. Glutathione plays a critical role in protecting cells from oxidative damage and the toxicity of xenobiotic electrophiles and maintaining redox homeostasis [66]. Studies have shown that elevated levels of pyroglutamic acid are associated with impaired glutathione metabolism [67,68]. In our study, LAG supplementation reduced the levels of pyroglutamic acid and T-AOC, GSH-Px, SOD, SOD1mRNA and GSH-Px mRNA were negatively correlated with pyroglutamic acid, indicating the protective effect of LAG supplementation on glutathione metabolism. In addition, Bacteroides, Faecalibacterium and Desulfovibrio were positively correlated with pyroglutamic acid. Conversely, Parabacteroides and Ruminococcus were negatively correlated with pyroglutamic acid. Faecalibacterium was positively correlated with spermine, suggesting that these species might participate in the modulation of glutathione metabolism by LAG.
5. Conclusions
This study showed that dietary supplementation with 150 mg/kg Astragalus polysaccharides and 75 mg/kg Glycyrrhiza polysaccharides or 300 mg/kg Astragalus polysaccharides and 150 mg/kg Glycyrrhiza polysaccharides could improve production performance, antioxidant function, meat quality and modulate the diversity, richness and composition of cecal microbiota of broilers, which achieved the effects of Terramycin calcium. In addition, the metabolomics analysis showed that supplementation with 150 mg/kg Astragalus polysaccharides and 75 mg/kg Glycyrrhiza polysaccharides screened some differential metabolites, mainly concentrated in the linoleic acid metabolism and glutathione metabolism pathway. Correlation analysis showed that production performance, antioxidant function and meat quality significantly correlated with cecal microbiota and serum metabolites. Overall, these findings indicate that dietary supplementation with 150 mg/kg Astragalus polysaccharides and 75 mg/kg Glycyrrhiza polysaccharides could improve production performance, antioxidant function and meat quality by changing cecal microbiota and serum metabolites, which would further provide helpful information for developing effective and safe antibiotic alternatives in the poultry industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11101872/s1, Figure S1: (A) Shannon dilution curve analysis (B) Venn diagram analysis; Figure S2: (A) Orthogonal partial least squares discriminant analysis (OPLS-DA) and (C) response permutation testing (RPT) of serum metabolites between CON and LAG groups in positive ion mode. (B) Orthogonal partial least squares discriminant analysis (OPLS-DA) and (D) response permutation testing (RPT) of serum metabolites between CON and LAG groups in negative ion mode. R2X and R2Y represent the interpretation rate of the built model to the X and Y matrix, R2X (cum) and R2Y (cum) represent the cumulative interpretation rate; Q2 indicates the predictive power of the model.; Table S1: Composition and nutrient levels of basal diet (air-dry basis,%); Table S2: Primer sequences used for the quantitative real-time PCR.
Author Contributions
Z.W. and C.L. designed the research. Y.Q. conducted the research and wrote the manuscript. W.Z., W.G. and Y.G. analyzed the data. K.O. and N.B. provided advice in experiment design. Z.W. and C.L. are responsible for the final content of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Zhongyuan high level talents special support plan (Grant No. 204200510010).
Institutional Review Board Statement
All the animals were managed according to the guidelines for the care and use of experimental animals approved by The Ethics Committee of Henan Agricultural University (No. HNND2021031503).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw reads of sequencing data in this study have been uploaded to NCBI under the accession number of PRJNA793377.
Acknowledgments
Special thanks to the Inner Mongolia Evergrand Pharmaceutical Co., Ltd., Chifeng, China for providing experiment material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350. [Google Scholar] [CrossRef]
- Suresh, G.; Das, R.K.; Kaur Brar, S.; Rouissi, T.; Avalos Ramirez, A.; Chorfi, Y.; Godbout, S. Alternatives to antibiotics in poultry feed: Molecular perspectives. Crit. Rev. Microbiol. 2018, 44, 318. [Google Scholar] [CrossRef]
- Haque, M.; Sarker, S.; Islam, M.; Karim, M.; Kayesh, M.E.H.; Shiddiky, M.J.; Anwer, M.S. Sustainable Antibiotic-Free Broiler Meat Production: Current Trends, Challenges, and Possibilities in a Developing Country Perspective. Biology 2020, 9, 411. [Google Scholar] [CrossRef]
- Salim, H.M.; Huque, K.S.; Kamaruddin, K.M.; Haque Beg, A. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci. Prog. 2018, 101, 52. [Google Scholar] [CrossRef]
- Zhang, X.H.; Sun, Z.Y.; Cao, F.L.; Ahmad, H.; Yang, X.H.; Zhao, L.G.; Wang, T. Effects of dietary supplementation with fermented ginkgo leaves on antioxidant capacity, intestinal morphology and microbial ecology in broiler chicks. Br. Poult. Sci. 2015, 56, 370. [Google Scholar] [CrossRef]
- Diaz-Sanchez, S.; D’Souza, D.; Biswas, D.; Hanning, I. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 2015, 94, 1419. [Google Scholar] [CrossRef]
- Abolhasani, A.; Barzegar, M.; Sahari, M.A. Effect of gamma irradiation on the extraction yield, antioxidant, and antityrosinase activities of pistachio green hull extract. Radiat. Phys. Chem. 2018, 144, 373. [Google Scholar] [CrossRef]
- Hamed, M.; Bougatef, H.; Karoud, W.; Krichen, F.; Haddar, A.; Bougatef, A.; Sila, A. Polysaccharides extracted from pistachio external hull: Characterization, antioxidant activity and potential application on meat as preservative. Ind. Crops Prod. 2020, 148, 112315. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493. [Google Scholar] [CrossRef]
- Zhao, Z.T.; Ye, X.M.; Ouyang, K.H.; Wang, W.J. Effects of polysaccharides from Yingshan Yunwu tea on meat quality, immune status and intestinal microflora in chickens. Int. J. Biol. Macromol. 2020, 155, 61. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019, 125, 906. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A review of the pharmacological action of Astragalus polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Wu, S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018, 97, 3489. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.F.; Xing, T.; Li, J.L.; Zhu, X.D.; Zhang, L.; Gao, F. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult. Sci. 2021, 100, 100909. [Google Scholar] [CrossRef]
- Shan, L.; Ren, L.; Zhu, X.; Li, J.; Zhou, G. Immunomodulatory effect of γ-irradiated astragalus polysaccharides on immunosuppressed broilers. Anim. Sci. J. 2018, 90, 13133. [Google Scholar] [CrossRef]
- Liu, L.; Shen, J.; Zhao, C.; Wang, X.; Yao, J.; Gong, Y.; Yang, X. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide. Int. J. Biol. Macromol. 2015, 72, 624. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, B.; Zheng, N.; Wu, G.; Ma, J.; Tao, X.; Chen, L.; Zhong, J.; Sheng, L.; Li, H. Integrated metagenomic and metabolomic analyses of the effect of astragalus polysaccharides on alleviating high-fat diet–induced metabolic disorders. Front. Pharmacol. 2020, 11, 833. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.X.; Shao, Q.; Chen, W.B.; Ma, L.; Xu, W.H.; Ma, Y.B. Effects of Glycyrrhiza polysaccharide in diet on growth performance, serum antioxidant capacity, and biochemistry of broilers. Poult. Sci. 2021, 100, 25. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, C.; Guo, Y.; Zhang, W.; Guo, W.; Oleksandr, K.; Wang, Z. Polysaccharides derived from Astragalus membranaceus and Glycyrrhiza uralensis improve growth performance of broilers by enhancing intestinal health and modulating gut microbiota. Poult. Sci. 2022, 101, 101905. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R. Use of liquorice (Glycyrrhiza glabra) in poultry nutrition: Global impacts on performance, carcass and meat quality. World’s Poult. Sci. J. 2019, 75, 293. [Google Scholar] [CrossRef]
- GB 5009.168-2016.2016-12-23; National Food Safety Standard Determination of Fatty Acids in Food. Standards Press of China: Beijing, China, 2016. (In Chinese)
- Wallace, R.J.; Oleszek, W.; Franz, C.; Hahn, I.; Baser, K.H.C.; Mathe, A.; Teichmann, K. Dietary plant bioactives for poultry health and productivity. Br. Poult. Sci. 2010, 51, 461. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Yang, W.R.; Yang, H.W.; Wang, Y.; Yang, Z.B.; Jiang, S.Z.; Zhang, G.G. Effects of Astragalus membranaceus on growth performance, carcass characteristics, and antioxidant status of broiler chickens. Acta Agric. Scand. Sect. A 2010, 60, 151. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Abdelnour, S.A.; Taha, A.E.; Khafaga, A.F.; Arif, M.; Ayasan, T.; Abdel-Daim, M.M. Herbs as thermoregulatory agents in poultry: An overview. Sci. Total Environ. 2020, 703, 134399. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, N.; Ghorbani, M.R.; Tatar, A.; Salari, S. Response of broiler chickens reared at high density to dietary supplementation with licorice extract and probiotic. J. Anim. Physiol. Anim. Nutr. 2019, 103, 100. [Google Scholar] [CrossRef]
- Ibrahim, D.; Sewid, A.H.; Arisha, A.H.; Abd El-Fattah, A.H.; Abdelaziz, A.M.; Al-Jabr, O.A.; Kishawy, A.T. Influence of Glycyrrhiza glabra extract on growth, gene expression of gut integrity, and Campylobacter jejuni colonization in broiler chickens. Front. Vet. Sci. 2020, 22, 612063. [Google Scholar] [CrossRef]
- Ali, M.; Kang, G.H.; Joo, S.T. A review: Influences of pre-slaughter stress on poultry meat quality. Asian-Australas. J. Anim. Sci. 2008, 21, 912. [Google Scholar] [CrossRef]
- Galli, G.M.; Aniecevski, E.; Petrolli, T.G.; da Rosa, G.; Boiago, M.M.; Simões, C.A.; Da Silva, A.S. Growth performance and meat quality of broilers fed with microencapsulated organic acids. Anim. Feed Sci. Technol. 2021, 271, 114706. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, C.W.; Kwon, S.G.; Hwang, J.H.; Park, D.H.; Kang, D.G.; Kim, I.S. pH as analytical indicator for managing pork meat quality. Sains Malays. 2016, 45, 1097–1103. [Google Scholar]
- Zhang, X.; Shi, H.; Niu, X.; Song, Y.; Tang, F.; Bian, C.; Qiao, H. Effect of aqueous extract of fermented Astragalia and Glycyrrhizaon on growth performance, immune organ indexes and meat quality of 817 broilers. China Anim. Husb. Vet. Med. 2018, 145, 933–939. (In Chinese) [Google Scholar] [CrossRef]
- Brewer, S. Irradiation effects on meat color–a review. Meat Sci. 2004, 68, 1. [Google Scholar] [CrossRef]
- Mingyuan, W.; Xiping, Y.; Xi, Z.; Bizhi, H.; Jianyong, J.; Zhe, W. Study of banana stems and leaves silage on beef quality and growth performance of Yunnan yellow cattle. Feed Ind. 2015, 36, 12. (In Chinese) [Google Scholar] [CrossRef]
- Oh, H.J.; Song, M.H.; Yun, W.; Lee, J.H.; An, J.S.; Kim, Y.J.; Cho, J.H. Effects of replacing soybean meal with perilla seed meal on growth performance, and meat quality of broilers. J. Anim. Sci. Technol. 2020, 62, 495. [Google Scholar] [CrossRef]
- Manilla, H.; Husvéth, F. N-3 fatty acid enrichment and oxidative stability of broiler chicken (A review). Acta Aliment. 1999, 28, 235. [Google Scholar] [CrossRef]
- Kouba, M.; Enser, M.; Whittington, F.M.; Nute, G.R.; Wood, J.D. Effect of a high-linolenic acid diet on lipogenic enzyme activities, fatty acid composition, and meat quality in the growing pig. J. Anim. Sci. 2003, 81, 1967. [Google Scholar] [CrossRef]
- Corino, C.; Rossi, R.; Cannata, S.; Ratti, S. Effect of dietary linseed on the nutritional value and quality of pork and pork products: Systematic review and meta-analysis. Meat Sci. 2014, 98, 679–688. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.E.; Willett, W.C. Types of dietary fat and risk of coronary heart disease: A critical review. J. Am. Coll. Nutr. 2001, 20, 5. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012, 65, 211. [Google Scholar] [CrossRef]
- Alagawany, M.; Ashour, E.A.; El-Fakhrany, H.H.H.; Ismail, T.A.; Nasr, M. Early nutrition programming with Astragalus membranaceus polysaccharide: Its effect on growth, carcasses, immunity, antioxidants, lipid profile and liver and kidney functions in broiler chickens. Anim. Biotechnol. 2022, 33, 362–368. [Google Scholar] [CrossRef]
- Zhang, G.G.; Yang, Z.B.; Wang, Y.; Yang, W.R. Effects of Astragalus membranaceus root processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poult. Sci. 2013, 92, 178. [Google Scholar] [CrossRef] [PubMed]
- El-Shafei, A.A.; Al-Gamal, M.A.; Abdelrahman, A.S.; Arafa, M.M. Influence of different levels of astragalus root powder in broiler chick diets on the physiological and biochemical changes. J. Appl. Sci. Res. 2013, 9, 2104–2118. [Google Scholar]
- Aslam, M.N.; Sohaib, M.; Khan, A.U.; Ali, S.; Amjad, A.; Ahmed, S. Lipids Oxidative Stability and Microbial Shelf Life Quality of Licorice (Glycyrrhiza glabra L.) Extract Supplemented Chicken Patties. Braz. J. Poult. Sci. 2020, 22, 1316. [Google Scholar] [CrossRef]
- Hoerr, F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010, 54, 2–15. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef]
- Liu, Y.S.; Li, S.; Wang, X.F.; Xing, T.; Li, J.L.; Zhu, X.D.; Gao, F. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing γ-irradiated Astragalus polysaccharides. Poult. Sci. 2021, 100, 273. [Google Scholar] [CrossRef]
- Downs, I.A.; Aroniadis, O.C.; Kelly, L.; Brandt, L.J. Postinfection irritable bowel syndrome. J. Clin. Gastroenterol. 2017, 51, 869. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Kwok, L.Y.; Zheng, Y.; Wang, L.; Guo, Z.; Zhang, J.; Zhang, H. Differential fecal microbiota are retained in broiler chicken lines divergently selected for fatness traits. Sci. Rep. 2016, 6, 1. [Google Scholar] [CrossRef]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Takase, K. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015, 15, 1. [Google Scholar] [CrossRef]
- Houston, S.; Blakely, G.W.; McDowell, A.; Martin, L.; Patrick, S. Binding and degradation of fibrinogen by Bacteroides fragilis and characterization of a 54 kDa fibrinogen-binding protein. Microbiology 2010, 156, 2516. [Google Scholar] [CrossRef]
- Gophna, U.; Konikoff, T.; Nielsen, H.B. Oscillospira and related bacteria–From metagenomic species to metabolic features. Environ. Microbiol. 2017, 19, 835. [Google Scholar] [CrossRef]
- Konikoff, T.; Gophna, U. Oscillospira: A central, enigmatic component of the human gut microbiota. Trends Microbiol. 2016, 24, 523. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yan, T.; Li, X.Y.; Duan, Y.L.; Yang, X.; Yang, X.J. Effects of Bacillus subtilis and antibiotic growth promoters on the growth performance, intestinal function and gut microbiota of pullets from 0 to 6 weeks. Animal 2020, 14, 1619. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Huang, Z.; Zeng, S.; Liu, J.; Wei, D.; Deng, X.; He, J. Intestinal bacterial signatures of white feces syndrome in shrimp. Appl. Microbiol. Biotechnol. 2018, 102, 3701. [Google Scholar] [CrossRef] [PubMed]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Spiller, R. The effect of psyllium husk on intestinal microbiota in constipated patients and healthy controls. Int. J. Mol. Sci. 2019, 20, 433. [Google Scholar] [CrossRef] [PubMed]
- Maier, E.; Anderson, R.C.; Roy, N.C. Live Faecalibacterium prausnitzii does not enhance epithelial barrier integrity in an apical anaerobic co-culture model of the large intestine. Nutrients 2017, 9, 1349. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Ren, D.; Yan, W.; Wang, Y.; Liu, H.; Shen, M. Linoleic acid inhibits Lactobacillus activity by destroying cell membrane and affecting normal metabolism. J. Sci. Food Agric. 2020, 100, 2057. [Google Scholar] [CrossRef]
- Whelan, J.; Fritsche, K. Linoleic acid. Adv. Nutr. 2013, 4, 311. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Q.; Ma, X.W.; Dong, X.Y.; Tao, Z.R.; Lu, L.Z.; Zou, X.T. Effects of parental dietary linoleic acid on growth performance, antioxidant capacity, and lipid metabolism in domestic pigeons (Columba livia). Poult. Sci. 2020, 99, 1471. [Google Scholar] [CrossRef]
- Qi, X.; Wu, S.; Zhang, H.; Yue, H.; Xu, S.; Ji, F.; Qi, G. Effects of dietary conjugated linoleic acids on lipid metabolism and antioxidant capacity in laying hens. Arch. Anim. Nutr. 2011, 65, 354. [Google Scholar] [CrossRef]
- Du, M.; Ahn, D.U. Dietary CLA affects lipid metabolism in broiler chicks. Lipids 2003, 38, 505. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, W.; Dong, R.; Wang, S.; Lin, Y.; Zheng, C. Effects of dietary linoleic acid level on laying performance, egg quality and lipids metabolism of ducks during the early laying period. Chin. J. Anim. Nutr. 2015, 27, 731. [Google Scholar] [CrossRef]
- Belury, M.A.; Cole, R.M.; Snoke, D.B.; Banh, T.; Angelotti, A. Linoleic acid, glycemic control and Type 2 diabetes. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 30. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhou, Y.; Wu, M.; Li, X.; Mai, K.; Ai, Q. ω-6 Polyunsaturated fatty acids (linoleic acid) activate both autophagy and antioxidation in a synergistic feedback loop via TOR-dependent and TOR-independent signaling pathways. Cell Death Dis. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489. [Google Scholar] [CrossRef] [PubMed]
- Emmett, M. Acetaminophen toxicity and 5-oxoproline (pyroglutamic acid): A tale of two cycles, one an ATP-depleting futile cycle and the other a useful cycle. Clin. J. Am. Soc. Nephrol. 2014, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hyde, A.S.; Simpson, M.A.; Barycki, J.J. Emerging regulatory paradigms in glutathione metabolism. Adv. Cancer Res. 2014, 122, 69. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).