Water Caltrop (Trapa quadrispinosa Roxb.) Husk Improves Oxidative Stress and Postprandial Blood Glucose in Diabetes: Phenolic Profiles, Antioxidant Activities and α-Glycosidase Inhibition of Different Fractions with In Vitro and In Silico Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Sample Preparation
2.3. Determination of Total Phenolic Content (TPC)
2.4. Determination of Total Flavonoid Content (TFC)
2.5. Characterization of Phenolics by UHPLC-ESI-HRMS/MS
2.6. ABTS Radical Scavenging Activity
2.7. DPPH Radical Scavenging Activity
2.8. Inhibition of α-Glycosidase
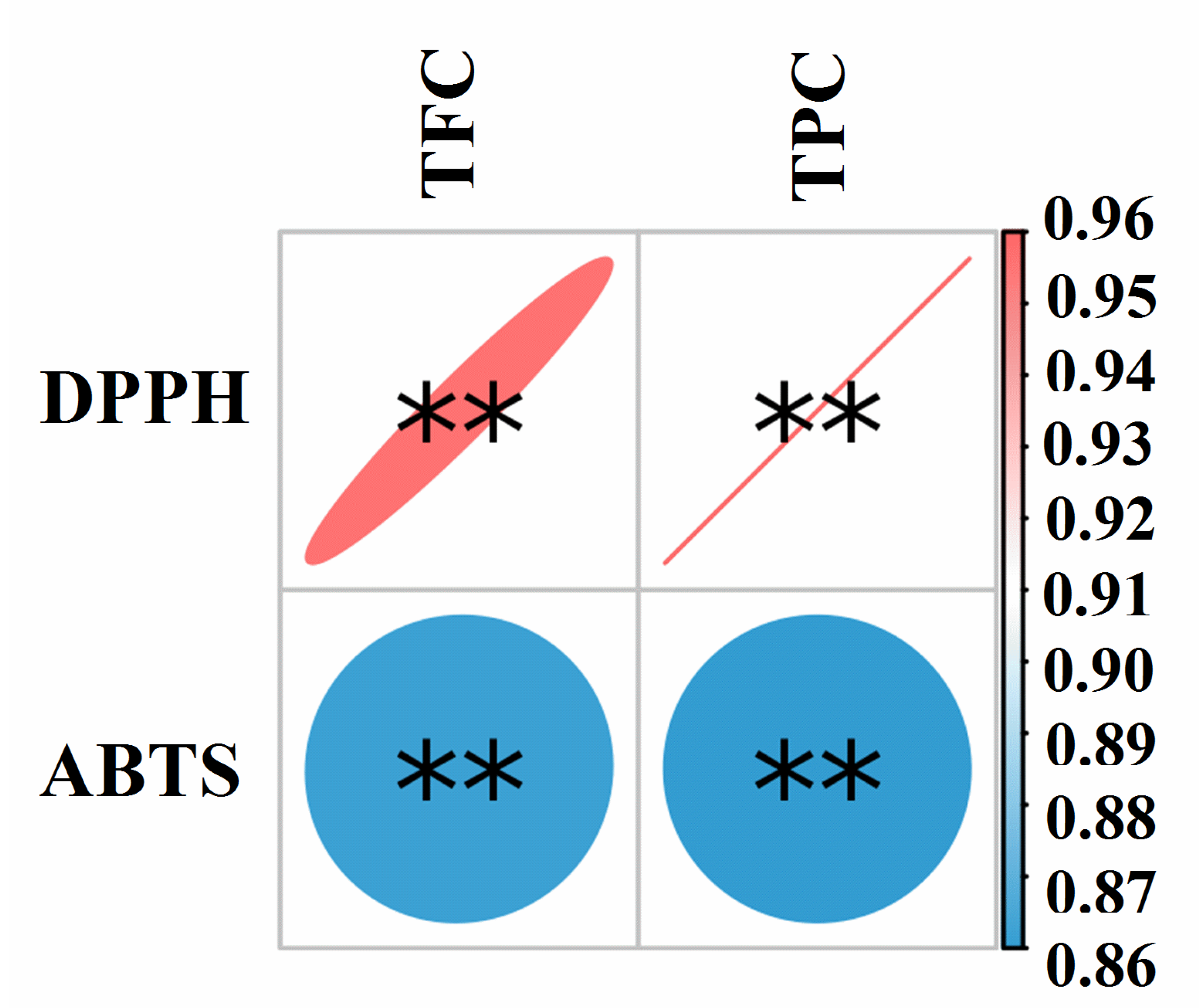
2.9. Correlation Analysis
2.10. Molecular Docking and Molecular Dynamics (MD) Simulation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Identification and Quantification of Phenolic Compounds and TFC and TPC
3.2. Antioxidant Activity
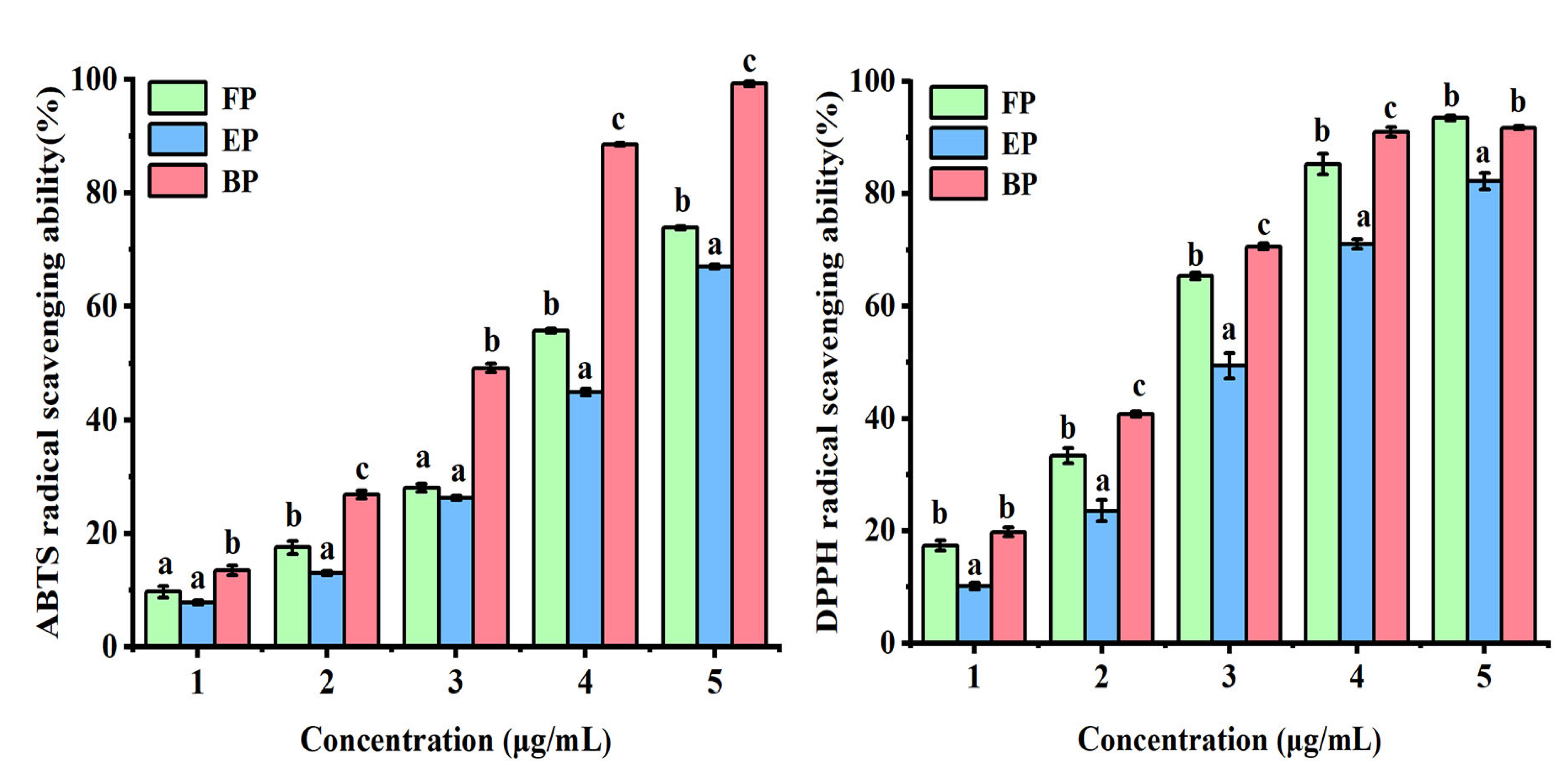
3.2.1. ABTS Radical Scavenging Activity
3.2.2. DPPH Radical Scavenging Activity
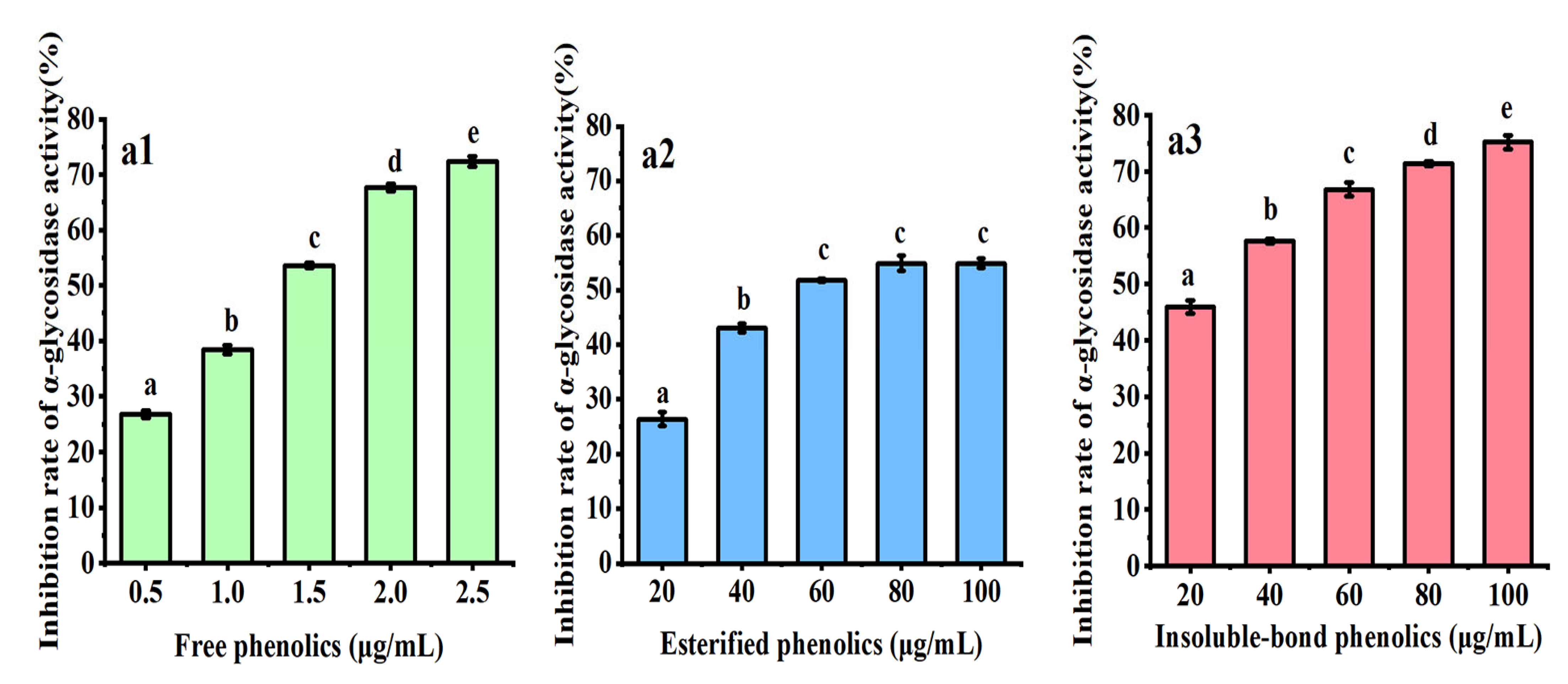
3.3. Inhibition of α-Glycosidase
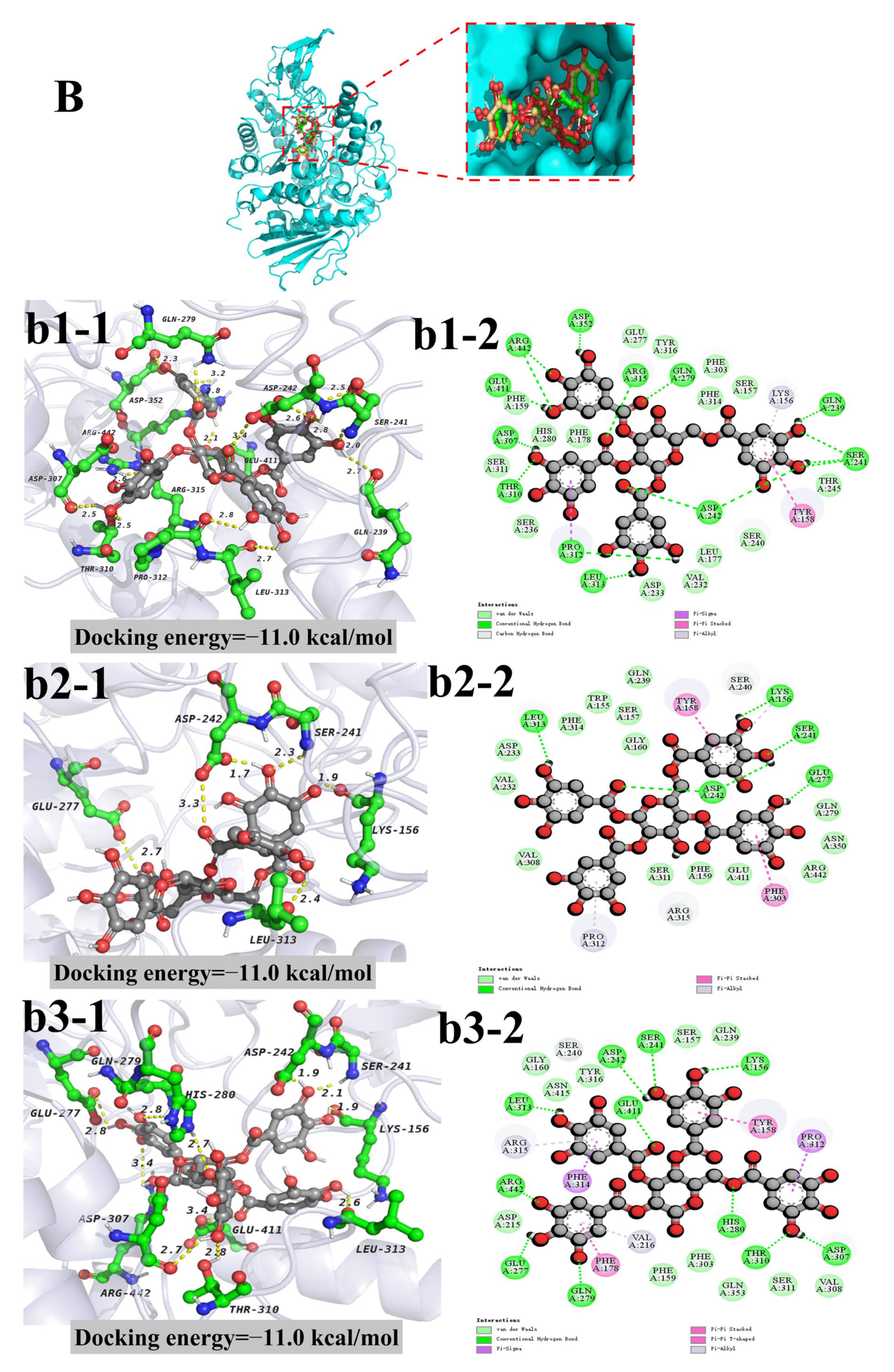
3.4. Molecular Docking
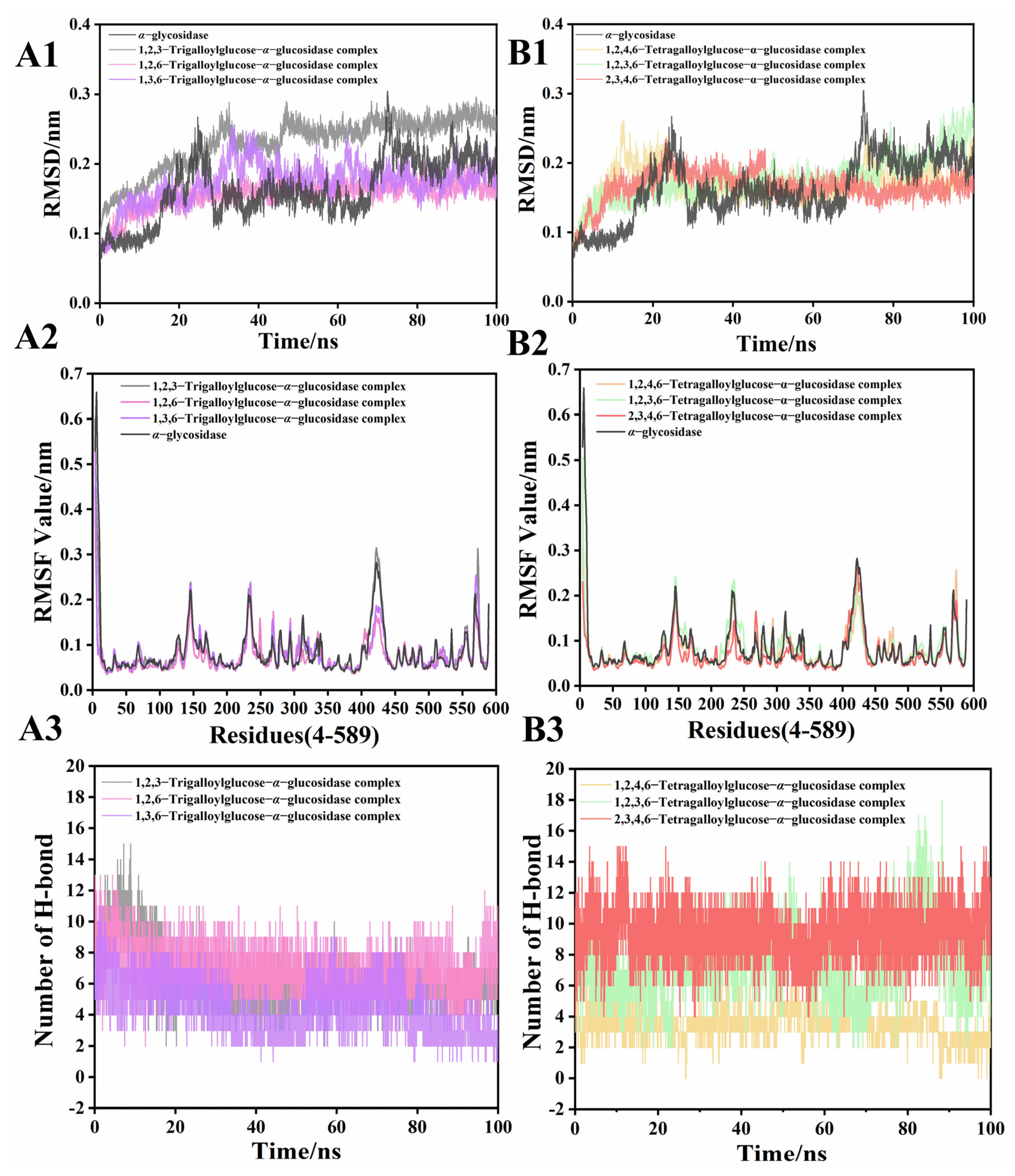
3.5. Molecular Dynamic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-C.; Zhang, X.-H.; Chen, J.; Shao, J.-H.; Zhao, Z.-Y.; Tang, Y.-Y. Lignans with α-glucosidase, protein tyrosine phosphatase 1B, and aldose reductase inhibitory activities from the fruits of Viburnum cylindricum. Ind. Crop. Prod. 2022, 178, 114601. [Google Scholar] [CrossRef]
- Laube, H. Acarbose. Clin. Drug Investig. 2002, 22, 141–156. [Google Scholar] [CrossRef]
- Takewaki, F.; Nakajima, H.; Takewaki, D.; Hashimoto, Y.; Majima, S.; Okada, H.; Senmaru, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; et al. Habitual Dietary Intake Affects the Altered Pattern of Gut Microbiome by Acarbose in Patients with Type 2 Diabetes. Nutrients 2021, 13, 2107. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Zommara, M.; Eweedah, N.M.; Helal, A.I. Synergistic Effects of Selenium Nanoparticles and Vitamin E on Growth, Immune-Related Gene Expression, and Regulation of Antioxidant Status of Nile Tilapia (Oreochromis niloticus). Biol. Trace Element Res. 2019, 195, 624–635. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P. UPLC/ESI-Q-TOF-MS analysis of (poly)phenols, tocols and amino acids in Chaenomeles leaves versus in vitro anti-enzyme activities. Ind. Crop. Prod. 2022, 181, 114829. [Google Scholar] [CrossRef]
- Yang, M.; Ma, Y.; Wang, Z.; Khan, A.; Zhou, W.; Zhao, T.; Cao, J.; Cheng, G.; Cai, S. Phenolic constituents, antioxidant and cytoprotective activities of crude extract and fractions from cultivated artichoke inflorescence. Ind. Crop. Prod. 2019, 143, 111433. [Google Scholar] [CrossRef]
- Yue, H.; Jiang, S.; Wang, L.; Banma, C.; Zhou, G.; Shao, Y.; Tao, Y.; Zhao, X. Hypoglycemic ingredients identification of Rheum tanguticum Maxim. ex Balf. by UHPLC-triple-TOF-MS/MS and interrelationships between ingredients content and glycosidase inhibitory activities. Ind. Crop. Prod. 2022, 178, 114595. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, X.; Ma, Q.; Yi, J.; Cai, S. Phytochemical bioaccessibility and in vitro antidiabetic effects of Chinese sumac (Rhus chinensis Mill.) fruits after a simulated digestion: Insights into the mechanisms with molecular docking analysis. Int. J. Food Sci. Technol. 2021, 57, 2656–2669. [Google Scholar] [CrossRef]
- Liu, J.-L.; He, Y.-L.; Wang, S.; He, Y.; Wang, W.-Y.; Li, Q.-J.; Cao, X.-Y. Ferulic acid inhibits advanced glycation end products (AGEs) formation and mitigates the AGEs-induced inflammatory response in HUVEC cells. J. Funct. Foods 2018, 48, 19–26. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, Y.; Jia, Y.; Pang, M.; Cheng, G.; Cai, S. Phenolic profiles, antioxidant activities and cytoprotective effects of different phenolic fractions from oil palm (Elaeis guineensis Jacq.) fruits treated by ultra-high pressure. Food Chem. 2019, 288, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, Y.; Ma, Q.; Yi, J.; Cai, S. Anti-Diabetic Effects of Different Phenolic-Rich Fractions from Rhus Chinensis Mill. Fruits in vitro. eFood 2021, 2, 37–46. [Google Scholar] [CrossRef]
- Adkar, P.; Dongare, A.; Ambavade, S.; Bhaskar, V.H. Trapa bispinosa Roxb.: A Review on Nutritional and Pharmacological Aspects. Adv. Pharmacol. Sci. 2014, 2014, 959830. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mao, Y.-D.; Wang, Y.-F.; Raza, A.; Qiu, L.-P.; Xu, X.-Q. Optimization of Ultrasonic-Assisted Enzymatic Extraction Conditions for Improving Total Phenolic Content, Antioxidant and Antitumor Activities In Vitro from Trapa quadrispinosa Roxb. Residues. Molecules 2017, 22, 396. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Jiang, Y.; Guo, Z.; Fang, S. Separation, UPLC-QTOF-MS/MS analysis, and antioxidant activity of hydrolyzable tannins from water caltrop (Trapa quadrispinosa) pericarps. LWT 2020, 133, 110010. [Google Scholar] [CrossRef]
- Takeshita, S.; Ishioka, Y.; Yagi, M.; Uemura, T.; Yamada, M.; Yonei, Y. The effects of water chestnut (Trapa bispinosa Roxb.) on the inhibition of glycometabolism and the improvement in postprandial blood glucose levels in humans. Glycative Stress Res. 2016, 3, 124–132. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Gao, F.; Zhao, Y.; Cai, S.; Pang, M. The free, esterified, and insoluble-bound phenolic profiles of Rhus chinensis Mill. fruits and their pancreatic lipase inhibitory activities with molecular docking analysis. J. Funct. Foods 2018, 40, 729–735. [Google Scholar] [CrossRef]
- Ma, Q.; Cai, S.; Liu, X.; Shi, J.; Yi, J. Characterization of phytochemical components and identification of main antioxidants in Crateva unilocalaris Buch. shoots by UHPLC-Q-Orbitrap-MS2 analysis. Food Res. Int. 2021, 143, 110264. [Google Scholar] [CrossRef]
- Jia, Y.; Ma, Y.; Cheng, G.-G.; Zhang, Y.; Cai, S. Comparative Study of Dietary Flavonoids with Different Structures as α-Glucosidase Inhibitors and Insulin Sensitizers. J. Agric. Food Chem. 2019, 67, 10521–10533. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Yi, J.; Zhang, X.; Ma, Q.; Cai, S. The effect of in vitro simulated gastrointestinal digestion on phenolic bioaccessibility and bioactivities of Prinsepia utilis Royle fruits. LWT 2020, 138, 110782. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, W.; Xu, H.; Liu, J.; Ren, L.; Yang, Y.; Li, S.; Wang, J.; Ji, T.; Du, G. Sinomenine ester derivative inhibits glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway. Acta Pharm. Sin. B 2021, 11, 3465–3480. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Jian, P.; Zhang, Q.; Liang, W.Y.; Zhou, K.; Hu, Q.; Liu, Y.; Liu, R.; Zhang, L.-Z. Tannins in Terminalia bellirica inhibit hepatocellular carcinoma growth by regulating EGFR-signaling and tumor immunity. Food Funct. 2021, 12, 3720–3739. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Sun, Y.; Yi, J.; Zhou, L.; Cai, S. Chinese sumac (Rhus chinensis Mill.) fruits alleviate indomethacin-induced gastric ulcer in mice by improving oxidative stress, inflammation and apoptosis. J. Ethnopharmacol. 2021, 284, 114752. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ma, Y.; Gong, X.; Zhang, Y.; Zhao, L.; Cheng, G.; Cai, S. Rhus chinensis Mill. fruits prevent high-fat/ethanol diet-induced alcoholic fatty liver in rats via AMPK/SREBP-1/FAS signaling pathway. J. Funct. Foods 2019, 61, 103498. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, Y.; Zhao, L.; Cai, S.; Cheng, G. Acute and subchronic toxicities of the ethanol and hot-water extracts from Chinese sumac (Rhus chinensis Mill.) fruits by oral administration in rats. Food Chem. Toxicol. 2018, 119, 14–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, O.; Ma, N.; Yi, J.; Mi, H.; Cai, S. The preventive effect and underlying mechanism of Rhus chinensis Mill. fruits on dextran sulphate sodium-induced ulcerative colitis in mice. Food Funct. 2021, 12, 9965–9978. [Google Scholar] [CrossRef]
- Zengin, G.; Paksoy, M.Y.; Aumeeruddy, M.Z.; Glamocilja, J.; Sokovic, M.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J.; Custodio, L.; et al. New insights into the chemical profiling, cytotoxicity and bioactivity of four Bunium species. Food Res. Int. 2019, 123, 414–424. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhang, A.; Zhou, X.; Sun, H. Serum Pharmacochemistry of TCM Screening the Bioactive Components from Moutan Cortex. Serum Pharmacochem. Tradit. Chin. Med. 2017, 21, 287–302. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, N.; Huang, S.; Fan, X.; Zhang, Y.; Ren, D.; Yi, L. Chemical profiling and discrimination of green tea and Pu-erh raw tea based on UPLC–Q–Orbitrap–MS/MS and chemometrics. Food Chem. 2020, 326, 126760. [Google Scholar] [CrossRef]
- Kumar, S.; Chandra, P.; Bajpai, V.; Singh, A.; Srivastava, M.; Mishra, D.; Kumar, B. Rapid qualitative and quantitative analysis of bioactive compounds from Phyllanthus amarus using LC/MS/MS techniques. Ind. Crop. Prod. 2015, 69, 143–152. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Li, Y.; Huang, Y.; Zhang, J. Antioxidant activity of free and hydrolyzed phenolic compounds in soluble and insoluble dietary fibres derived from hulless barley. LWT. 2019, 111, 534–540. [Google Scholar] [CrossRef]
- Yao, J.; Chen, J.; Yang, J.; Hao, Y.; Fan, Y.; Wang, C.; Li, N. Free, soluble-bound and insoluble-bound phenolics and their bioactivity in raspberry pomace. LWT 2020, 135, 109995. [Google Scholar] [CrossRef]
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Mo, R.; Zheng, Y.; Zhou, Y.; Liu, Y. Phenolic profiles and antioxidant activities of free, esterified and bound phenolic compounds in walnut kernel. Food Chem. 2021, 350, 129217. [Google Scholar] [CrossRef] [PubMed]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays—A Practical Approach. Molecules 2021, 27, 50. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liang, L.; Yang, T.; Feng, L.; Mao, X.; Wang, Y. In-vitro bioactivity evaluation and non-targeted metabolomic analysis of green tea processed from different tea shoot maturity. LWT 2021, 152, 112234. [Google Scholar] [CrossRef]
- Huang, S.; Ma, Y.; Zhang, C.; Cai, S.; Pang, M. Bioaccessibility and antioxidant activity of phenolics in native and fermented Prinsepia utilis Royle seed during a simulated gastrointestinal digestion in vitro. J. Funct. Foods 2017, 37, 354–362. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, H.; Zhang, P.; Gao, L.; Yan, N.; Li, P.; Liu, X.; Du, Y.; Shen, G. Chemical Composition, Antioxidant Activity and α-Glucosidase Inhibitory Activity of Chaenomeles Speciosa from Four Production Areas in China. Molecules 2018, 23, 2518. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, G.; Pan, J.; Wang, Y. α-Glucosidase inhibition by luteolin: Kinetics, interaction and molecular docking. Int. J. Biol. Macromol. 2014, 64, 213–223. [Google Scholar] [CrossRef]
- Limanto, A.; Simamora, A.; Santoso, A.W.; Timotius, K.H. Antioxidant, α-Glucosidase Inhibitory Activity and Molecular Docking Study of Gallic Acid, Quercetin and Rutin: A Comparative Study. Mol. Cell. Biomed. Sci. 2019, 3, 67–74. [Google Scholar] [CrossRef]
- Maurady, A.; M’Guil, M.; Sadoq, B.-E.; Eslami, G.; Britel, M.R. Molecular Docking Study of Selected Molecules as Novel Targets for ATPase of Mycobacterium Tuberculosis Inhibitors. In International Conference on Advanced Intelligent Systems for Sustainable Developmen, 21–26 December 2020, Tangier, Morocco; Springer: Berlin/Heidelberg, Germany, 2022; pp. 157–170. [Google Scholar] [CrossRef]
- Murugesu, S.; Ibrahim, Z.; Ahmed, Q.-U.; Yusoff, N.-I.N.; Uzir, B.-F.; Perumal, V.; Abas, F.; Saari, K.; El-Seedi, H.; Khatib, A. Characterization of α-Glucosidase Inhibitors from Clinacanthus nutans Lindau Leaves by Gas Chromatography-Mass Spectrometry-Based Metabolomics and Molecular Docking Simulation. Molecules 2018, 23, 2402. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rana, H.K.; Singh, V.; Yadav, T.C.; Varadwaj, P.; Pandey, A.K. Evaluation of antidiabetic activity of dietary phenolic compound chlorogenic acid in streptozotocin induced diabetic rats: Molecular docking, molecular dynamics, in silico toxicity, in vitro and in vivo studies. Comput. Biol. Med. 2021, 134, 104462. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Song, Y.; Chen, Y.; Ma, Y.; Fu, M.; Liu, X. The galloyl moiety enhances the inhibitory activity of catechins and theaflavins against α-glucosidase by increasing the polyphenol–enzyme binding interactions. Food Funct. 2020, 12, 215–229. [Google Scholar] [CrossRef] [PubMed]


| Peak | Compounds | TR * (min.) | [M-H]− (m/z) | Molecular Formula | MS/MS Fragment Ions | Reference | Extracts | ||
|---|---|---|---|---|---|---|---|---|---|
| FP | EP | BP | |||||||
| 1 | Gallic acid | 2.51 | 169.0136 | C7H5O5 | 125.0234 (100.00), 69.0332 (98.97) | [15] | 3364.65 ± 13.67 c | 30,001.66 ± 175.78 b | 38,947.59 ± 56.68 a |
| 2 | Di-galloylglucose isomer I | 4.00 | 483.0793 | C20H19O14 | 169.0134 (100.00), 125.0235 (15.81) | [15,30] | ND | ND | 4.73 ± 0.24 |
| 3 | Di-galloylglucose isomer II | 7.51 | 483.0794 | C20H19O14 | 169.0134 (100.00), 211.0244 (28.64) | [15,30] | ND | ND | 8.39 ± 0.42 |
| 4 | Digallic acid isomer I | 7.81 | 321.0259 | C14H9O9 | 125.0232 (100.00), 169.0134 (71.97) | [15,30] | 20.37 ± 1.02 c | 605.37 ± 30.27 a | 395.43 ± 19.77 b |
| 5 | 4-O-Methylgallic acid | 8.21 | 183.0293 | C8H7O5 | 140.0140 (100.00) 111.0076 (64.41), 139.0027 (58.57) | [15,28] | 5589.97 ± 55.89 b | 70.15 ± 3.51 c | 8050.32 ± 17.62 a |
| 6 | DiG-HHDP-glucose isomer I | 8.74 | 785.0866 | C34H25O22 | 300.9990 (82.14) | [15] | 274.57 ± 8.24 | ND | ND |
| 7 | Di-galloylglucose isomer III | 8.85 | 483.0792 | C20H19O14 | 271.0461 (100.00) 169.0134 (85.86), 211.0244 (24.05) | [15,30] | 1044.93 ± 10.45 | ND | ND |
| 8 | Digallic acid isomer II | 9.04 | 321.0259 | C14H9O9 | 125.0233 (100.00), 169.0134 (71.99) | [15,30] | 111.76 ± 5.59 c | 5366.87 ± 162.18 a | 2816.03 ± 140.80 b |
| 9 | Trigalloylglucose isomer I | 9.35 | 635.0909 | C27H23O18 | 169.0134 (100.00), 313.0568 (62.85) | [15,29] | 919.29 ± 45.96 | ND | ND |
| 10 | Trigalloylglucose isomer II | 10.12 | 635.0906 | C27H23O18 | 169.0134 (59.59), 313.0566 (42.80) | [15,29] | 617.04 ± 8.02 | ND | ND |
| 11 | DiG-HHDP-glucose isomer II | 10.45 | 785.0863 | C34H25O22 | 300.9991 (100.00) | [15] | 664.41 ± 13.29 a | ND | 7.82 ± 0.39 b |
| 12 | Trigalloylglucose isomer III | 11.11 | 635.0903 | C27H23O18 | 313.0568 (23.98) 169.0134 (21.22), | [15,29] | 10,460.24 ± 102.58 a | 20.85 ± 1.04 b | ND |
| 13 | Syringic acid isomer I | 12.14 | 197.0453 | C9H9O5 | 123.0074 (5.67) | [28] | 383.80 ± 10.48 b | ND | 590.59 ± 3.51 a |
| 14 | p-Coumaric acid isomer I | 12.34 | 163.0394 | C9H7O3 | 119.0490 (100.00), 93.0368 (25.31) | [30] | 51.09 ± 2.04 b | 364.83 ± 18.24 a | ND |
| 15 | Tri-O-galloyl-HHDP-D-glucose | 13.05 | 937.0975 | C41H29O26 | 169.0130 (1.90), 767.0746 (4.85) | [15] | 2886.32 ± 13.54 | ND | ND |
| 16 | Tetragalloylglucose isomer I | 13.17 | 787.1012 | C34H27O22 | 465.0674 (100.00), 635.0897 (47.64) | [15,17,29,30] | 3898.93 ± 129.39 a | ND | 3.19 ± 0.16 b |
| 17 | Isolariciresinol 9-O-glucoside isomer I | 13.41 | 521.2041 | C26H33O11 | 101.0231 (24.47), 89.0231 (21.59) | [20] | ND | trace | trace |
| 18 | Tetragalloyl glucose isomer II | 13.56 | 787.1014 | C34H27O22 | 617.0793 (100.00) 465.0676 (44.87) | [15,17,29,30] | 20,762.27 ± 510.50 a | 126.28 ± 6.31 b | 62.61 ± 3.76 c |
| 19 | Myricitrin | 13.90 | 463.0894 | C21H19O12 | 316.0225 (100.00), 317.0282 (33.49) | Standard | 15.67 ± 0.78 | ND | ND |
| 20 | Ellagic acid | 13.95 | 300.9994 | C14H5O8 | 283.9969 (20.92), 201.0186 (14.63) | Standard | 963.22 ± 31.75 b | 931.76 ± 37.93 c | 1442.99 ± 104.08 a |
| 21 | Tri-O-galloyl-brevifolincarboxyl-D-glucose | 14.37 | 909.1033 | C40H29O25 | 435.0587 (15.78), 291.0146 (13.30) | [15] | 78.99 ± 3.95 | ND | ND |
| 22 | Digallic acid isomer III | 15.29 | 321.0253 | C14H9O9 | 125.0232 (100.00), 169.0134 (57.66) | [15,30] | 14.19 ± 0.71 | ND | ND |
| 23 | Pentagalloylglucose | 15.59 | 939.1132 | C41H31O26 | 787.1010 (100.00), 617.0793 (12.54) | [15,29,30] | 8868.45 ± 86.43 a | 41.45 ± 2.07 b | 18.69 ± 0.46 b |
| 24 | Quercitrin | 15.82 | 447.0941 | C21H19O11 | 301.0345 (74.98), 300.0277 (100.00) | [31] | 38.55 ± 1.93 a | 19.62 ± 0.98 b | 18.06 ± 0.66 b |
| 25 | Isolariciresinol 9-O-glucoside isomer II | 15.87 | 521.2043 | C26H33O11 | 89.0231 (100.00), 101.0231 (37.42) | [20] | ND | 12.40 ± 0.03 b | 27.97 ± 0.39 a |
| 26 | Syringic acid isomer II | 16.43 | 197.0452 | C9H9O5 | 123.0075 (1.97) | [28] | 142.24 ± 7.34 | ND | ND |
| 27 | p-Coumaric acid isomer II | 16.54 | 163.0393 | C9H7O3 | 93.0332 (18.45), 119.0492 (3.94) | [30] | 18.56 ± 0.93 | ND | ND |
| 28 | Hexagallyol glucose | 16.95 | 1091.1245 | C48H35O30 | 617.0792 (1.89), 939.1115 (80.16) | [15] | 236.72 ± 11.84 | ND | ND |
| 29 | p-Coumaric acid isomer III | 18.38 | 163.0394 | C9H7O3 | 93.0333 (19.65), 119.0490 (52.81) | [30] | 11.85 ± 1.06 | ND | ND |
| TPC (mgGAE/g) | 406.06 ± 3.94 a | 275.96 ± 2.34 b | 360.06 ± 3.94 c | ||||||
| TFC (mgRE/g) | 82.85 ± 0.70 b | 83.20 ± 0.35 b | 99.90 ± 0.35 a | ||||||
| Compounds | Pubchem ID | Docking Energy (kcal/mol) | Compounds | Pubchem ID | Docking Energy (kcal/mol) |
|---|---|---|---|---|---|
| 2,3,4,6-Tetragalloylglucose | 49,777,225 | −11.0 | 2,3,6-Trigalloylglucose | 129,819,625 | −8.9 |
| 1,2,4,6-Tetragalloylglucose | 11,297,287 | −11.0 | Quercitin | 5,280,459 | −8.8 |
| 1,2,3,6-Tetragalloylglucose | 73,178 | −11.0 | Myricitin | 5,281,673 | −8.7 |
| 1,3,6-Trigalloylglucose | 452,707 | −10.6 | 1,2,3,4-Tetragalloylglucose | 133,556,532 | −8.5 |
| 1,2,6-Trigalloylglucose | 440,308 | −10.5 | Digallic acid | 341 | −8.5 |
| 1,2,3-Trigalloylglucose | 13,270,010 | −10.1 | Ellagic acid | 5,281,855 | −8.3 |
| Pentagalloylglucose | 65,238 | −10.0 | p-Coumaric acid | 637,542 | −6.7 |
| 1,2,6-Trigalloylglucose | 124,156,722 | −9.8 | Methylgallate | 7428 | −6.5 |
| (-)-Isolariciresinol 9-O-glucoside | 74,191,750 | −9.7 | Gallic acid | 370 | −6.4 |
| (+)-Isolariciresinol 9-O-glucoside | 93,473,218 | −9.6 | 4-O-Methylgallic acid | 78,016 | −6.5 |
| DiG-HHDP-glucose | 471,120 | −9.2 | Syringic acid | 10,742 | −5.7 |
| Compounds | Pubchem ID | Docking Energy (kcal/mol) | The Number of Amino Acid Residues Forming Van der Waals Force | Amino Acid Residues Forming Hydrogen Bonds (Bond Length) |
|---|---|---|---|---|
| 1,2,3-Trigalloylglucose | 132,707 | −10.1 | 21 | His-280 (2.1 Å), Asp-307 (2.8 Å), Gln-353 (2.0 Å) Asp-352 (2.1 Å), Ser-311 (2.3 Å), Asn-415 (2.1 Å), Ser-240 (2.6 Å) |
| 1,2,6-Trigalloylglucose | 440,308 | −10.5 | 10 | Asp-242 (2.6 Å), Asp-215 (3.1 Å), Glu-277 (2.2 Å), Gln-279 (2.5 Å), His-280 (2.4 Å), Asp-307 (2.4 Å) |
| 1,3,6-Trigalloylglucose | 452,707 | −10.6 | 11 | Lys-156 (2.6 Å), Thr-310 (2.6 Å), Gln-279 (2.4 Å), Tyr-158 (2.3 Å), Glu-411 (2.1 Å), Glu-277 (1.9 Å), Arg-442 (2.9 Å,3.0 Å) |
| 1,2,3,6-Tetragalloylglucose | 73,178 | −11.0 | 15 | Asp-352 (2.3 Å), Arg-442 (2.6 Å,3.2 Å), Glu-411 (3.4 Å), Asp-307 (2.5 Å), Thr-310 (2.5 Å), Pro-312 (2.8 Å), Leu-313 (2.7 Å), Asp-242 (2.6 Å), Arg-315 (2.8 Å) Ser-241 (2.5 Å, 2.8 Å, 2.0 Å), Gln-239 (2.7 Å), Gln-279 (2.8 Å) |
| 1,2,4,6-Tetragalloylglucose | 11,297,287 | −11.0 | 14 | Glu-277 (2.7 Å), Asp-242 (3.3 Å, 1.7 Å), Ser-241 (2.3 Å), Lys-156 (1.9 Å), Leu-313 (2.4 Å) |
| 2,3,4,6-Tetragalloylglucose | 49,777,225 | −11.0 | 11 | Glu-277 (2.8 Å), Gln-279 (2.8 Å), His-280 (2.7 Å), Thr-310 (2.8 Å), Asp-307 (2.7 Å), Lys-156 (1.9 Å), Ser-241 (2.1 Å), Asp-242 (1.9 Å), Glu-411(3.4 Å), Leu-313 (2.6 Å), Arg-442 (3.4 Å) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cai, S.; Ma, S.; Zhao, S.; Yi, J.; Zhou, L. Water Caltrop (Trapa quadrispinosa Roxb.) Husk Improves Oxidative Stress and Postprandial Blood Glucose in Diabetes: Phenolic Profiles, Antioxidant Activities and α-Glycosidase Inhibition of Different Fractions with In Vitro and In Silico Analyses. Antioxidants 2022, 11, 1873. https://doi.org/10.3390/antiox11101873
Zhang Y, Cai S, Ma S, Zhao S, Yi J, Zhou L. Water Caltrop (Trapa quadrispinosa Roxb.) Husk Improves Oxidative Stress and Postprandial Blood Glucose in Diabetes: Phenolic Profiles, Antioxidant Activities and α-Glycosidase Inhibition of Different Fractions with In Vitro and In Silico Analyses. Antioxidants. 2022; 11(10):1873. https://doi.org/10.3390/antiox11101873
Chicago/Turabian StyleZhang, Yuanyue, Shengbao Cai, Shuang Ma, Shuai Zhao, Junjie Yi, and Linyan Zhou. 2022. "Water Caltrop (Trapa quadrispinosa Roxb.) Husk Improves Oxidative Stress and Postprandial Blood Glucose in Diabetes: Phenolic Profiles, Antioxidant Activities and α-Glycosidase Inhibition of Different Fractions with In Vitro and In Silico Analyses" Antioxidants 11, no. 10: 1873. https://doi.org/10.3390/antiox11101873
APA StyleZhang, Y., Cai, S., Ma, S., Zhao, S., Yi, J., & Zhou, L. (2022). Water Caltrop (Trapa quadrispinosa Roxb.) Husk Improves Oxidative Stress and Postprandial Blood Glucose in Diabetes: Phenolic Profiles, Antioxidant Activities and α-Glycosidase Inhibition of Different Fractions with In Vitro and In Silico Analyses. Antioxidants, 11(10), 1873. https://doi.org/10.3390/antiox11101873







