Natural Antioxidants from Endemic Leaves in the Elaboration of Processed Meat Products: Current Status
Abstract
:1. Introduction
2. Leaves of Endemic Plants as Ingredients in Processed Meat Products
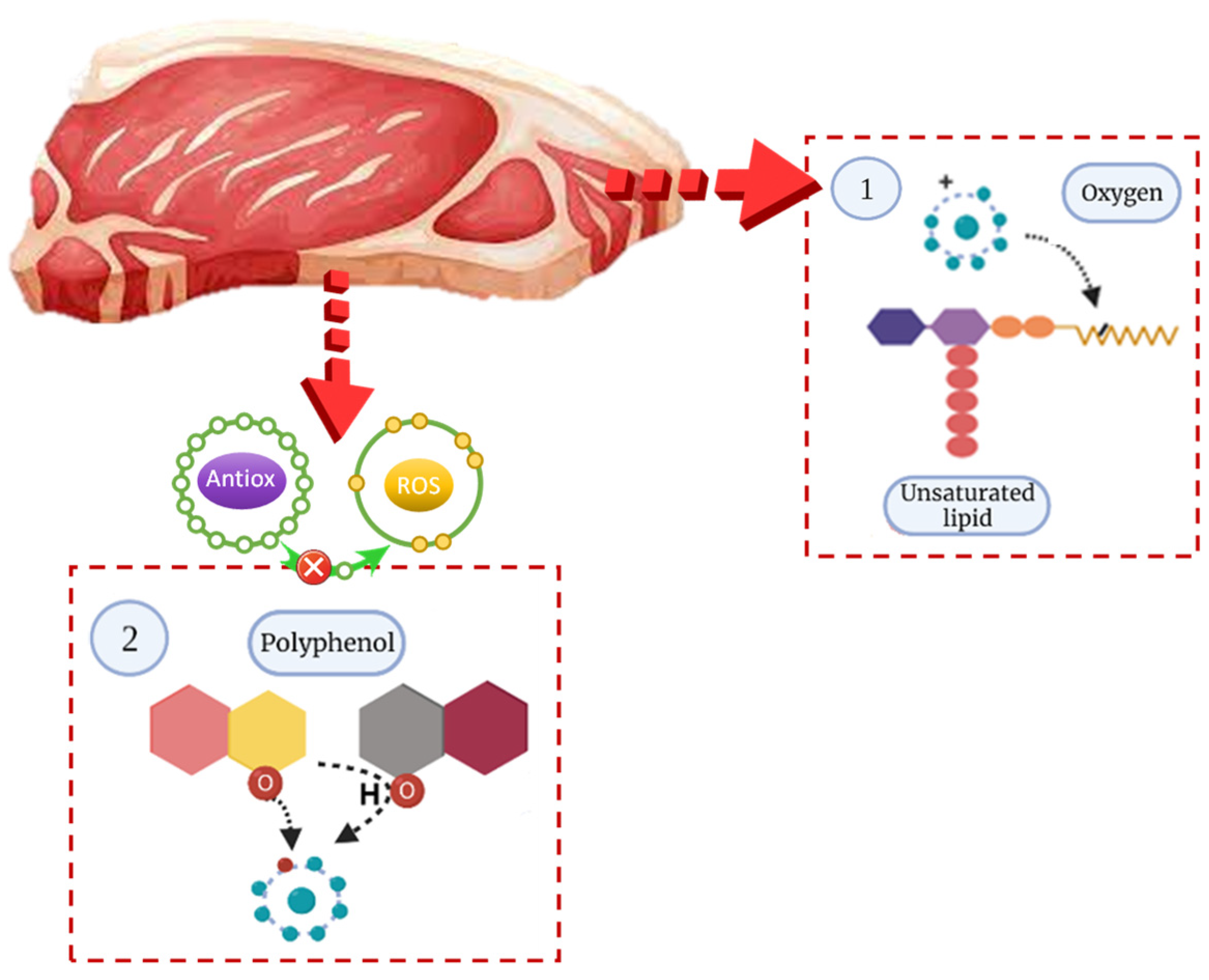
3. Effect of Natural Leaf Extracts on the Quality of Processed Meat Products
3.1. Physicochemical Parameters
3.2. Microbiological Changes
3.3. Organoleptic Attributes
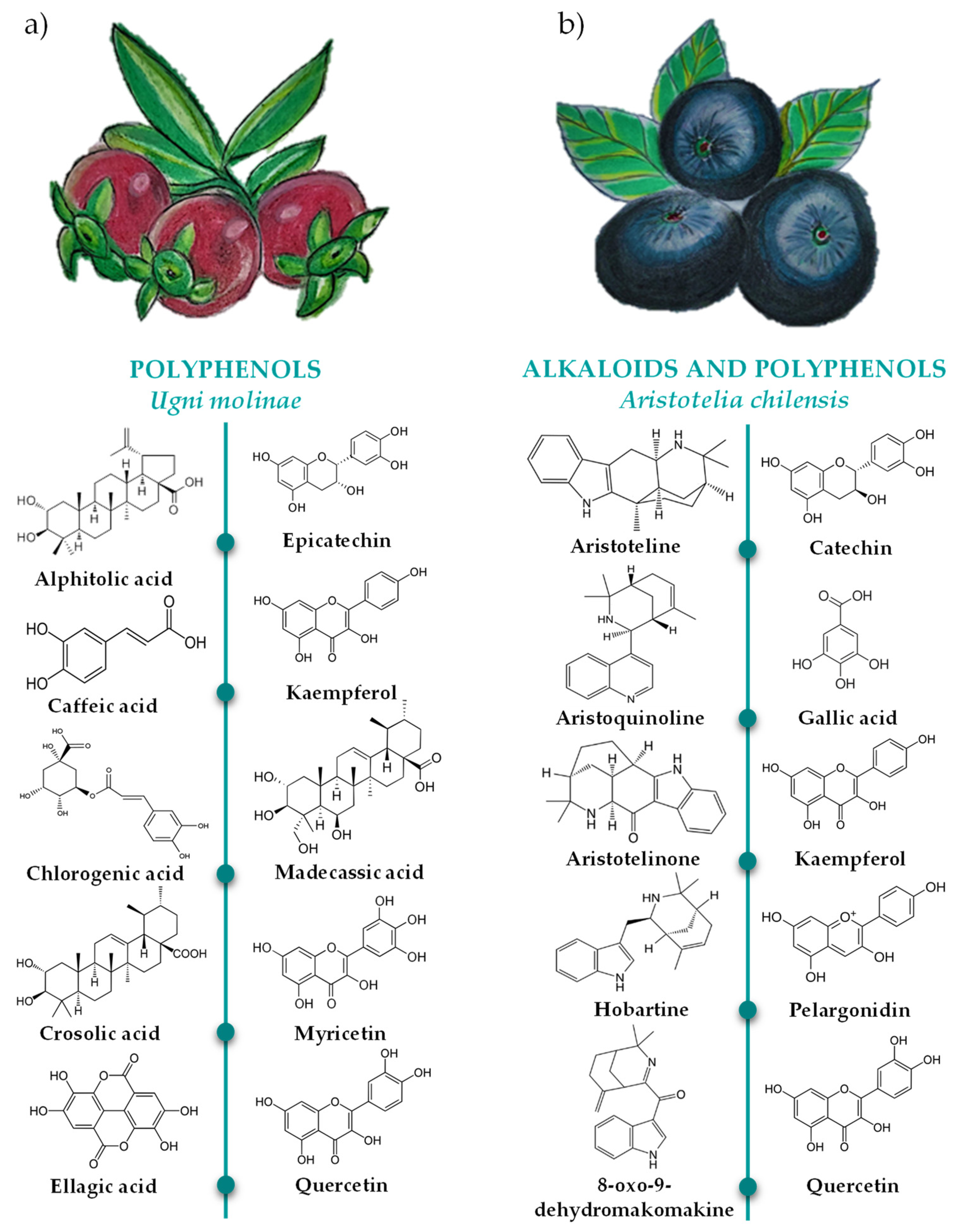
4. Aristotelia chilensis and Ugni molinae Leaves as Source of Natural Antioxidants
4.1. Ugni molinae Turcz
4.1.1. Bioactive Compound Profile of Ugni molinae Turcz Leaves
4.1.2. Antioxidant and Antimicrobial Capacity of Ugni molinae Turcz Leaves
4.2. Aristotelia chilensis (Mol.) Stuntz
4.2.1. Bioactive Compound Profile of Aristotelia chilensis Leaves
4.2.2. Antioxidant and Antimicrobial Capacity of Aristotelia chilensis Leaves
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horita, C.N.; Baptista, R.C.; Caturla, M.Y.R.; Lorenzo, J.M.; Barba, F.J.; Sant’Ana, A.S. Combining reformulation, active packaging and non-thermal post-packaging decontamination technologies to increase the microbiological quality and safety of cooked ready-to-eat meat products. Trends Food Sci. Technol. 2018, 72, 45–61. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, J.M.; Carballo, J. Changes in physico-chemical properties and volatile compounds throughout the manufacturing process of dry-cured foal loin. Meat Sci. 2015, 99, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of type of muscle on volatile compounds throughout the manufacture of Celta dry-cured ham. Food Sci. Technol. Int. 2015, 21, 581–592. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 61, 149–178. [Google Scholar] [CrossRef]
- Schnettler, B.; Sepúlveda, N.; Bravo, S.; Grunert, K.G.; Hueche, C. Consumer acceptance of a functional processed meat product made with different meat sources. Br. Food J. 2018, 120, 424–440. [Google Scholar] [CrossRef]
- Hung, Y.; de Kok, T.M.; Verbeke, W. Consumer attitude and purchase intention towards processed meat products with natural compounds and a reduced level of nitrite. Meat Sci. 2016, 121, 119–126. [Google Scholar] [CrossRef]
- Ouerfelli, M.; Villasante, J.; Ben Kaâb, L.B.; Almajano, M. Effect of neem (Azadirachta indica L.) on lipid oxidation in raw chilled beef patties. Antioxidants 2019, 8, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Carvalho, F.A.L.; Munekata, P.E.S.; Lopes de Oliveira, A.; Pateiro, M.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Turmeric (Curcuma longa L.) extract on oxidative stability, physicochemical and sensory properties of fresh lamb sausage with fat replacement by tiger nut (Cyperus esculentus L.) oil. Food Res. Int. 2020, 136, 109487. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Luo, A.; Feng, J.; Hu, B.; Lv, J.; Chen, C.-Y.O.; Xie, S. Polysaccharides in Spirulina platensis Improve Antioxidant Capacity of Chinese-Style Sausage. J. Food Sci. 2017, 82, 2591–2597. [Google Scholar] [CrossRef]
- Al-Hijazeen, M. Effect of direct adding oregano essential oil (Origanum syriacum L.) on quality and stability of chicken meat patties. Food Sci. Technol. 2018, 38, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Rojo, M.I.; Vargas-Sánchez, R.D.; del Mar Torres-Martínez, B.; Torrescano-Urrutia, G.R.; Sánchez-Escalante, A. Extractos de hojas de plantas para conservar la calidad de la carne y los productos cárnicos frescos. Revisión. Biotecnia 2018, 20, 155–164. [Google Scholar] [CrossRef]
- Alirezalu, K.; Hesari, J.; Eskandari, M.H.; Valizadeh, H.; Sirousazar, M. Effect of Green Tea, Stinging Nettle and Olive Leaves Extracts on the Quality and Shelf Life Stability of Frankfurter Type Sausage. J. Food Process. Preserv. 2017, 41, e13100. [Google Scholar] [CrossRef]
- Jayawardana, B.C.; Warnasooriya, V.B.; Thotawattage, G.H.; Dharmasena, V.A.K.I.; Liyanage, R. Black and green tea (Camellia sinensis L.) extracts as natural antioxidants in uncured pork sausages. J. Food Process. Preserv. 2019, 43, e13870. [Google Scholar] [CrossRef]
- Borella, T.G.; Peccin, M.M.; Mazon, J.M.; Roman, S.S.; Cansian, R.L.; Soares, M.B.A. Effect of rosemary (Rosmarinus officinalis) antioxidant in industrial processing of frozen-mixed hamburger during shelf life. J. Food Process. Preserv. 2019, 43, 43. [Google Scholar] [CrossRef]
- Nowak, A.; Czyzowska, A.; Efenberger, M.; Krala, L. Polyphenolic extracts of cherry (Prunus cerasus L.) and blackcurrant (Ribes nigrum L.) leaves as natural preservatives in meat products. Food Microbiol. 2016, 59, 142–149. [Google Scholar] [CrossRef]
- Burri, S.C.M.; Granheimer, K.; Rémy, M.; Ekholm, A.; Håkansson, Å.; Rumpunen, K.; Tornberg, E. Lipid Oxidation Inhibition Capacity of 11 Plant Materials and Extracts Evaluated in Highly Oxidised Cooked Meatballs. Foods 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Galvez, A.; Rodríguez, A.; Stucken, K. Antioxidant, functional properties and health-promoting potential of native South American berries: A review. J. Sci. Food Agric. 2021, 101, 364–378. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.I.; Suzuki, N.; Yamamoto, K.; Iio, S.I.; Yamada, T. Effects of MaquiBright® on improving eye dryness and fatigue in humans: A randomized, double-blind, placebo-controlled trial. J. Tradit. Complement. Med. 2019, 9, 172–178. [Google Scholar] [CrossRef]
- Salar, F.J.; Agulló, V.; García-Viguera, C.; Domínguez-Perles, R. Stevia vs. Sucrose: Influence on the Phytochemical Content of a Citrus–Maqui Beverage—A Shelf Life Study. Foods 2020, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, L.; Trostchansky, A.; Wood, I.; Mastrogiovanni, M.; Vogel, H.; González, B.; Maróstica Junior, M.; Fuentes, E.; Palomo, I. Antiplatelet activity and chemical analysis of leaf and fruit extracts from Aristotelia chilensis. PLoS ONE 2021, 16, e0250852. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Bustos, F.; Valenzuela, X.; López-Carballo, G.; Vilariño, J.M.; Galotto, M.J. Chilean berry Ugni molinae Turcz. fruit and leaves extracts with interesting antioxidant, antimicrobial and tyrosinase inhibitory properties. Food Res. Int. 2017, 102, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rojo, M.I.; Vargas-Sánchez, R.D.; del Mar Torres-Martínez, B.; Torrescano-Urrutia, G.R.; Lorenzo, J.M.; Sánchez-Escalante, A. Inclusion of ethanol extract of mesquite leaves to enhance the oxidative stability of pork patties. Foods 2019, 8, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gantner, M.; Guzek, D.; Najda, A.; Brodowska, M.; Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Godziszewska, J. Oxidative and microbial stability of poultry meatballs added with coriander extracts and packed in cold modified atmosphere. Int. J. Food Prop. 2017, 20, 2527–2537. [Google Scholar] [CrossRef] [Green Version]
- Van Hecke, T.; Ho, P.L.; Goethals, S.; De Smet, S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef]
- Mancuso, G.; Borgonovo, G.; Scaglioni, L.; Bassoli, A. Phytochemicals from Ruta graveolens Activate TAS2R Bitter Taste Receptors and TRP Channels Involved in Gustation and Nociception. Molecules 2015, 20, 18907–18922. [Google Scholar] [CrossRef] [Green Version]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef]
- Falowo, A.B.; Muchenje, V.; Hugo, A.; Aiyegoro, O.A.; Fayemi, P.O. Antioxidant activities of Moringa oleifera L. and Bidens pilosa L. leaf extracts and their effects on oxidative stability of ground raw beef during refrigeration storage. CyTA J. Food 2017, 15, 249–256. [Google Scholar] [CrossRef]
- Beal, P.; Faion, A.M.; Cichoski, A.J.; Cansian, R.L.; Valduga, A.T.; De Oliveira, D.; Valduga, E. Oxidative stability of fermented Italian-type sausages using mate leaves (Ilex paraguariensis St. Hil) extract as natural antioxidant. Int. J. Food Sci. Nutr. 2011, 62, 703–710. [Google Scholar] [CrossRef]
- Hać-Szymańczuk, E.; Cegiełka, A.; Chmiel, M.; Czaja, K. Antioxidant and Antimicrobial Effects of Oregano on Quality Characteristics of Model Pork Batters. J. Food Process. Preserv. 2017, 41, e12796. [Google Scholar] [CrossRef]
- Karpińska-Tymoszczyk, M. Effect of the addition of ground rosemary on the quality and shelf-life of turkey meatballs during refrigerated storage. Br. Poult. Sci. 2008, 49, 742–750. [Google Scholar] [CrossRef]
- Mahdavian Mehr, H.; Hosseini, Z.; Haddad Khodaparast, M.H.; Edalatian, M.R. Study on the antimicrobial effect of Salvia leriifolia (Nowroozak) leaf extract powder on the growth of staphylococcus aureus in hamburger. J. Food Saf. 2010, 30, 941–953. [Google Scholar] [CrossRef]
- Boruzi, A.I.; Nour, V. Antioxidant effects of walnut leaves and sweet cherry stems on color, lipid oxidation and sensory quality of cooked pork patties. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Campos-Requena, V. Antioxidant Compound Extraction from Maqui (Aristotelia chilensis [Mol] Stuntz) Berries: Optimization by Response Surface Methodology. Antioxidants 2017, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Khawli, F.A.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative green technologies of intensification for valorization of seafood and their by-products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastías-Montes, J.M.; Monterrosa, K.; Muñoz-Fariña, O.; García, O.; Acuña-Nelson, S.M.; Vidal-San Martín, C.; Quevedo-Leon, R.; Kubo, I.; Avila-Acevedo, J.G.; Domiguez-Lopez, M.; et al. Chemoprotective and antiobesity effects of tocols from seed oil of Maqui-berry: Their antioxidative and digestive enzyme inhibition potential. Food Chem. Toxicol. 2020, 136, 111036. [Google Scholar] [CrossRef]
- Gallego, M.; Gordon, M.; Segovia, F.; Almajano, M. Caesalpinia decapetala Extracts as Inhibitors of Lipid Oxidation in Beef Patties. Molecules 2015, 20, 13913–13926. [Google Scholar] [CrossRef] [Green Version]
- Alcántara, C.; Žugčić, T.; Abdelkebir, R.; García-Pérez, J.V.; Jambrak, A.R.; Lorenzo, J.M.; Collado, M.C.; Granato, D.; Barba, F.J. Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts. Molecules 2020, 25, 1718. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.M.; Kubota, E.H.; Prestes, R.C.; Mello, R.O.; Rosa, C.S.; Scapin, G.; Ferreira, S. Development and evaluation of pork burger with added natural antioxidant based on extract of banana inflorescence (Musa cavendishii). CyTA J. Food 2016, 14, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.K.; Ha, S.R.; Choi, J.S. Effect of Caesalpinia sappan L. extract on physico-chemical properties of emulsion-type pork sausage during cold storage. Meat Sci. 2015, 110, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Abdel-Atty, N.; Helmy, E. Improving the quality and extending the shelf life of chilled fresh sausages using natural additives and their extracts. J. Microbiol. Biotechnol. Food Sci. 2018, 7, 580–585. [Google Scholar] [CrossRef]
- Pateiro, M.; Franco, D.; Carril, J.A.; Lorenzo, J.M. Changes on physico-chemical properties, lipid oxidation and volatile compounds during the manufacture of celta dry-cured loin. J. Food Sci. Technol. 2015, 52, 4808–4818. [Google Scholar] [CrossRef] [Green Version]
- Alirezalu, K.; Hesari, J.; Nemati, Z.; Munekata, P.E.S.; Barba, F.J.; Lorenzo, J.M. Combined effect of natural antioxidants and antimicrobial compounds during refrigerated storage of nitrite-free frankfurter-type sausage. Food Res. Int. 2019, 120, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, H.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.B.; da Silva, M.V.; da Lannes, S.C.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; Torres-Martínez, B.D.M.; Pateiro, M.; Lorenzo, J.M.; Sánchez-Escalante, A. Propolis extract as antioxidant to improve oxidative stability of fresh patties during refrigerated storage. Foods 2019, 8, 614. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Holman, B.W.B.; Ponnampalam, E.N.; Kerr, M.G.; Bailes, K.L.; Kilgannon, A.K.; Collins, D.; Hopkins, D.L. Understanding beef flavour and overall liking traits using two different methods for determination of thiobarbituric acid reactive substance (TBARS). Meat Sci. 2019, 149, 114–119. [Google Scholar] [CrossRef]
- Georgantelis, D.; Blekas, G.; Katikou, P.; Ambrosiadis, I.; Fletouris, D.J. Effect of rosemary extract, chitosan and α-tocopherol on lipid oxidation and colour stability during frozen storage of beef burgers. Meat Sci. 2007, 75, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Agrimonti, C.; White, J.C.; Tonetti, S.; Marmiroli, N. Antimicrobial activity of cellulosic pads amended with emulsions of essential oils of oregano, thyme and cinnamon against microorganisms in minced beef meat. Int. J. Food Microbiol. 2019, 305, 108246. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef] [PubMed]
- Brodowska, A.J.; Nowak, A.; Śmigielski, K. Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 2176–2201. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Gómez, I.; Janardhanan, R.; Ibañez, F.C.; Beriain, M.J. The Effects of Processing and Preservation Technologies on Meat Quality: Sensory and Nutritional Aspects. Foods 2020, 9, 1416. [Google Scholar] [CrossRef]
- Duarte, A.; Luís, Â.; Oleastro, M.; Domingues, F.C. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Rocchetti, G.; Bernardo, L.; Pateiro, M.; Barba, F.J.; Munekata, P.E.S.; Trevisan, M.; Lorenzo, J.M.; Lucini, L. Impact of a Pitanga Leaf Extract to Prevent Lipid Oxidation Processes during Shelf Life of Packaged Pork Burgers: An Untargeted Metabolomic Approach. Foods 2020, 9, 1668. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Muchenje, V.; Hugo, C.J.; Charimba, G. In vitro antimicrobial activities of Bidens pilosa and Moringa oleifera leaf extracts and their effects on ground beef quality during cold storage. CyTA J. Food 2016, 14, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Angelini, P.; Matei, F.; Flores, G.A.; Pellegrino, R.M.; Vuguziga, L.; Venanzoni, R.; Tirillini, B.; Emiliani, C.; Orlando, G.; Menghini, L.; et al. Metabolomic Profiling, Antioxidant and Antimicrobial Activity of Bidens pilosa. Processes 2021, 9, 903. [Google Scholar] [CrossRef]
- Martínez, L.; Bastida, P.; Castillo, J.; Ros, G.; Nieto, G. Green alternatives to synthetic antioxidants, antimicrobials, nitrates, and nitrites in clean label Spanish chorizo. Antioxidants 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Biswas, B.; Rogers, K.; McLaughlin, F.; Daniels, D.; Yadav, A. Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two gram-negative and gram-positive bacteria. Int. J. Microbiol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Boyle, R.A. Sensory Evaluation of Oils/Fats and Oil/Fat–Based Foods. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Hu, M., Jacobsen, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 157–185. [Google Scholar]
- Shene, C.; Canquil, N.; Jorquera, M.; Pinelo, M.; Rubilar, M.; Acevedo, F.; Vergara, C.; von Baer, D.; Mardones, C. In vitro Activity on Human Gut Bacteria of Murta Leaf Extracts (Ugni molinae turcz.), a Native Plant from Southern Chile. J. Food Sci. 2012, 77, M323–M329. [Google Scholar] [CrossRef]
- Arancibia-Radich, J.; Peña-Cerda, M.; Jara, D.; Valenzuela-Bustamante, P.; Goity, L.; Valenzuela-Barra, G.; Silva, X.; Garrido, G.; Delporte, C.; Seguel, I. Comparative study of anti-inflammatory activity and qualitative-quantitative composition of triterpenoids from ten genotypes of Ugni molinae. Bol. Latinoam. Caribe Plantas Med. Aromat. 2016, 15, 274–287. [Google Scholar]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of maqui (Aristotelia chilensis) and murta (Ugni molinae Turcz.): Sources of antioxidant compounds and α-glucosidase/α-amylase inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef]
- Scheuermann, E.; Seguel, I.; Montenegro, A.; Bustos, R.O.; Hormazábal, E.; Quiroz, A. Evolution of aroma compounds of murtilla fruits (Ugni molinae Turcz) during storage. J. Sci. Food Agric. 2008, 88, 485–492. [Google Scholar] [CrossRef]
- Peña-Cerda, M.; Arancibia-Radich, J.; Valenzuela-Bustamante, P.; Pérez-Arancibia, R.; Barriga, A.; Seguel, I.; García, L.; Delporte, C. Phenolic composition and antioxidant capacity of Ugni molinae Turcz. leaves of different genotypes. Food Chem. 2017, 215, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.; Peñaloza, A.; Guarda, A.; Galotto, M.J.; Bruna, J.E.; Rodríguez, F.J. Development of an Active Packaging Film Based on a Methylcellulose Coating Containing Murta (Ugni molinae Turcz) Leaf Extract. Food Bioprocess Technol. 2016, 9, 298–307. [Google Scholar] [CrossRef]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin Characterization, Total Phenolic Quantification and Antioxidant Features of Some Chilean Edible Berry Extracts. Molecules 2014, 19, 936. [Google Scholar] [CrossRef] [Green Version]
- Cespedes, C.L.; Balbontin, C.; Avila, J.G.; Dominguez, M.; Alarcon, J.; Paz, C.; Burgos, V.; Ortiz, L.; Peñaloza-Castro, I.; Seigler, D.S.; et al. Inhibition on cholinesterase and tyrosinase by alkaloids and phenolics from Aristotelia chilensis leaves. Food Chem. Toxicol. 2017, 109, 984–995. [Google Scholar] [CrossRef]
- Jara-Moreno, D.; Castro-Torres, R.D.; Ettcheto, M.; Auladell, C.; Kogan, M.J.; Folch, J.; Verdaguer, E.; Cano, A.; Busquets, O.; Delporte, C.; et al. The Ethyl Acetate Extract of Leaves of Ugni molinae Turcz. Improves Neuropathological Hallmarks of Alzheimer’s Disease in Female APPswe/PS1dE9 Mice Fed with a High Fat Diet. J. Alzheimer’s Dis. 2018, 66, 1175–1191. [Google Scholar] [CrossRef]
- Fuentes, L.; Figueroa, C.R.; Valdenegro, M.; Vinet, R. Patagonian Berries: Healthy Potential and the Path to Becoming Functional Foods. Foods 2019, 8, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacón-Fuentes, M.; Mutis, A.; Bardehle, L.; Seguel, I.; Urzúa, A.; Quiroz, A. Decrease of flavonol synthase enzymatic activity in Ugni molinae turcz due to the domestication process. Int. J. Agric. Nat. Resour. 2019, 46, 30–39. [Google Scholar] [CrossRef]
- López, J.; Gálvez, A.V.; Rodríguez, A.; Uribe, E. Murta (Ugni molinae Turcz.): A review on chemical composition, functional components and biological activities of leaves and fruits. Chil. J. Agric. Anim. Sci. 2018, 34, 43–56. [Google Scholar] [CrossRef]
- Rubilar, M.; Pinelo, M.; Ihl, M.; Scheuermann, E.; Sineiro, J.; Nuñez, M.J. Murta leaves (Ugni molinae Turcz) as a source of antioxidant polyphenols. J. Agric. Food Chem. 2006, 54, 59–64. [Google Scholar] [CrossRef]
- Rubilar, M.; Gutiérrez, C.; Villarroel, M.; Shene, C. Influence of separation conditions on antimicrobial activity of polyphenolic fractions from murta leaves extract. CyTA J. Food 2010, 8, 139–149. [Google Scholar] [CrossRef]
- Hauser, C.; Peñaloza, A.; Rodríguez, F.; Guarda, A.; Galotto, M.J. Promising antimicrobial and antioxidant extracts of Murta leaves (Ugni molinae Turcz): Shelf-life extension and food safety. Food Packag. Shelf Life 2014, 1, 77–85. [Google Scholar] [CrossRef]
- Misle, E.; Garrido, E.; Contardo, H.; González, W. Maqui [Aristotelia chilensis (Mol.) Stuntz]-the Amazing Chilean Tree: A Review. J. Agric. Sci. Technol. B 2011, 1, 473–482. [Google Scholar]
- Ipinza Carmona, R.; Magni Díaz, C.; Gutiérrez Caro, B.; Torres Cuadros, J. La domesticación del maqui (Aristotelia chilensis): Un estudio de caso en Chile. INFOR 2019, Diciembre, 19–24. [Google Scholar]
- Muñoz, O.; Christen, P.; Cretton, S.; Backhouse, N.; Torres, V.; Correa, O.; Costa, E.; Miranda, H.; Delporte, C. Chemical study and anti-inflammatory, analgesic and antioxidant activities of the leaves of Aristotelia chilensis (Mol.) Stuntz, Elaeocarpaceae. J. Pharm. Pharmacol. 2011, 63, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Sobolev, A.P.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Di Giovanni, C.; Xiao, J.; Shirooie, S.; Tsetegho Sokeng, A.J.; et al. Antidepressive effects of a chemically characterized maqui berry extract (Aristotelia chilensis (molina) stuntz) in a mouse model of Post-stroke depression. Food Chem. Toxicol. 2019, 129, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, J.; Evelyn, J.A.R.A.; Molina, L.; Parada, F.; Burgos, R.A.; Hidalgo, M.A.; Hancke, J.L. Effects of Aristotelia chilensis berry juice on cyclooxygenase 2 expression, NF-κB, NFAT, ERK1/2 and PI3K/Akt activation in colon cancer. Bol. Latinoam. Caribe Plantas Med. Aromat. 2011, 10, 543–552. [Google Scholar]
- Cifuentes, F.; Palacios, J.; Paredes, A.; Nwokocha, C.; Paz, C. 8-Oxo-9-Dihydromakomakine Isolated from Aristotelia chilensis Induces Vasodilation in Rat Aorta: Role of the Extracellular Calcium Influx. Molecules 2018, 23, 3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, F.; Palacios, J.; Jofré, I.; Paz, C.; Nwokocha, C.R.; Paredes, A.; Cifuentes, F. Aristoteline, an Indole-Alkaloid, Induces Relaxation by Activating Potassium Channels and Blocking Calcium Channels in Isolated Rat Aorta. Molecules 2019, 24, 2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarado, J.; Schoenlau, F.; Leschot, A.; Salgad, A.M.; Portales, P.V. Delphinol® standardized maqui berry extract significantly lowers blood glucose and improves blood lipid profile in prediabetic individuals in three-month clinical. Panminerva Med. 2016, 58, 1–6. [Google Scholar]
- Zúñiga, G.E.; Tapia, A.; Arenas, A.; Contreras, R.A.; Zúñiga-Libano, G. Phytochemistry and biological properties of Aristotelia chilensis a Chilean blackberry: A review. Phytochem. Rev. 2017, 16, 1081–1094. [Google Scholar] [CrossRef]
- Schön, C.; Wacker, R.; Micka, A.; Steudle, J.; Lang, S.; Bonnländer, B. Bioavailability Study of Maqui Berry Extract in Healthy Subjects. Nutrients 2018, 10, 1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resolución 548 Exenta. In Listado de Medicamentos Herbarios Tradicionales; Available online: https://www.minsal.cl/mht/ (accessed on 3 July 2021).
- Cespedes, C.L.; Pavon, N.; Dominguez, M.; Alarcon, J.; Balbontin, C.; Kubo, I.; El-Hafidi, M.; Avila, J.G. The chilean superfruit black-berry Aristotelia chilensis (Elaeocarpaceae), Maqui as mediator in inflammation-associated disorders. Food Chem. Toxicol. 2017, 108, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, K.; Ah-Hen, K.S.; Vega-Gálvez, A.; Vásquez, V.; Quispe-Fuentes, I.; Rojas, P.; Lemus-Mondaca, R. Changes in bioactive components and antioxidant capacity of maqui, Aristotelia chilensis [Mol] Stuntz, berries during drying. LWT Food Sci. Technol. 2016, 65, 537–542. [Google Scholar] [CrossRef]
- Schreckinger, M.E.; Lotton, J.; Lila, M.A.; de Mejia, E.G. Berries from South America: A comprehensive review on chemistry, health potential, and commercialization. J. Med. Food 2010, 13, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.L.; Avello, L.M.; Loyola, C.C.; Campos, P.J.; Aqueveque, M.P.; Dungan, R.S.; Galotto, L.M.; Guarda, M.A. Microencapsulation of maqui (Aristotelia chilensis [Molina] Stuntz) leaf extracts to preserve and control antioxidant properties. Chil. J. Agric. Res. 2013, 73, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Fredes, C.; Yousef, G.G.; Robert, P.; Grace, M.H.; Lila, M.A.; Gómez, M.; Gebauer, M.; Montenegro, G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J. Sci. Food Agric. 2014, 94, 2639–2648. [Google Scholar] [CrossRef]
- González-Villagra, J.; Cohen, J.D.; Reyes-Díaz, M.M. Abscisic acid is involved in phenolic compounds biosynthesis, mainly anthocyanins, in leaves of Aristotelia chilensis plants (Mol.) subjected to drought stress. Physiol. Plant. 2019, 165, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Berardo, A.; Claeys, E.; Vossen, E.; Leroy, F.; De Smet, S. Protein oxidation affects proteolysis in a meat model system. Meat Sci. 2015, 106, 78–84. [Google Scholar] [CrossRef]
- Pateiro, M.; Domínguez, R.; Munekata, P.E.S.; Barba, F.J.; Lorenzo, J.M. Lipids and fatty acids. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Barba, F., Saraiva, J.A., Cravotto, G., Lorenzo, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 107–137. ISBN 9780128141748. [Google Scholar]
- Avello, M.; Valdivia, R.; Sanzana, R.; Mondaca, M.A.; Mennickent, S.; Aeschlimann, V.; Bittner, M.; Becerra, J. Extractos antioxidantes y antimicrobianos de Aristotelia chilensis y Ugni molinae y sus aplicaciones como preservantes en productos cosméticos. Bol. Latinoam. Caribe Plantas Med. Aromat. 2009, 8, 479–486. [Google Scholar]

| Plant Leaves | Meat Product | Extract Dose | Storage Conditions | Main Results | Ref. |
|---|---|---|---|---|---|
| Black and green tea (Camellia sinensis L.) | Uncured pork frankfurters | 0.05, 0.10, 0.20, and 0.30% | 5 days at 37 °C | Phenolic extracts inhibited the formation of hydroperoxides. Polyphenol extracts did not affect colour, odour, texture, juiciness, flavour, or general acceptability. | [15] |
| Black currant (Ribes nigrum L.), Cherry (Prunus cerasus L.) | Pork frankfurters | BCS 1.0 g/100 g CHS 0.5 g/100 g | 28 days at 4 °C | Samples treated with natural extracts showed significantly lower counts of Brochothrix thermosphacta, LAB, mesophiles, and psychrotrophs. Polyphenols from leaves of P. cerasus and R. nigrum inhibited MDA formation by 15.29% and 21.48%, respectively. | [17] |
| Black currant (Ribes nigrum L.), Blueberry (Vaccinium myrtillus L.), Garden savory (Satureja hortensis L.), Sea buckthorn (Hippophae rhamnoides L.) | Cooked meatballs | 100 and 200 ppm | 14 days at 4 °C | Aqueous extracts inhibited lipid oxidation by 13.8–21.8% during 14 days of cold storage. | [18] |
| Cadillo (Bidens pilosa), Moringa oleifera | Ground beef | 0.5 and 1 g/kg | 6 days at 4 °C | The extracts obtained from the leaves of M. oleifera and B. pilosa showed a polyphenol content of 77.5 and 75.9 mg GAE/g dw, respectively. Both plants displayed inhibitory effects (1 g/kg) against Staphylococcus aureus, Bacillus cereus, Enterococcus faecalis, and Escherichia coli, although the higher activity was observed against gram-negative bacteria. | [32] |
| Cilantro (Coriandrum sativum) | Turkey meatballs | 200 and 500 ppm | 9 days at 4 °C | The addition of C. sativum extract at 500 ppm delayed lipid oxidation by 58.33% during 6 days of storage and inhibited the growth of aerobic microorganisms for 9 days of storage. | [27] |
| Green tea (Camellia sinensis L.), Nettle (Urtica dioica L.), Olive (Olea europea L.) | Frankfurter sausages | 500 ppm | 45 days at 4 °C | The ethanolic extracts of C. sinensis, U. dioica, and O. europea leaves inhibited lipid oxidation by 40%, 20%, and 26%, respectively. | [14] |
| Indian nimbus (Azadirachta indica) | Raw, chilled beef patties | 0.7% (w/w) | 5 days at 4 °C | The hydroalcoholic extract limited colour loss and reduced metmyoglobin formation (36.70%). It prevented MDA formation by 67.30% after 11 days of storage, which was similar to the values found with the synthetic antioxidant (66.34%). Antimicrobial activity was observed against most of the bacterial strains tested, with the best observed activity against E. coli strains. | [8] |
| Mate (Ilex paraguariensis) | Fermented Italian-type sausages | 0.3–0.4% | 60 days at 18 °C | Aqueous extracts added at 0.4% reduced oxidation by 32.87% during 60 days of storage. Sensory characteristics (flavour, texture, and overall acceptability) were not affected by the addition of I. paraguariensis extract. | [33] |
| Oregano (Origanum vulgare) | Marinated pork | 0.5–2% | 10 days at 4 °C | Aqueous and ethanolic extracts of O. vulgare added at 2% inhibited lipid oxidation by 63.2% during 10 days of storage. The extracts showed inhibitory power against the growth of Enterobacteria and Enterococci. | [34] |
| Pitanga (Eugenia uniflora) | Pork burgers | 250, 500 and 1000 ppm | 18 days at 4 °C | Hydroalcoholic extracts showed to inhibit the growth of Salmonella spp., Bacillus cereus, Staphylococcus aureus, and Pseudomonas aeruginosa. | [29] |
| Rosemary (Salvia Rosmarinus) | Frozen pork, turkey and chicken patties | 0.03% and 0.06% | 120 days at −12 °C | The proximate composition of the different formulations did not show significant differences. The concentration of natural antioxidants was sufficient to maintain the oxidative stability of the products during frozen storage. | [16] |
| Duck meatballs | 1% | 15 days at 3 °C | Meatballs with extracts of S. rosmarinus inhibited hydroperoxide formation by 80.7%. The counts of psychrotrophic and coliform bacteria were lower in meatballs containing rosemary (≤10 cfu/g) compared to those found in control sample (1.3 × 104 and 8.7 × 102 cfu/g, respectively). | [35] | |
| Salvia (Salvia leriifolia) | Burgers | 5, 10, 15 and 20 mg/L | 45 days at −12 °C | The inclusion of powdered leaves of S. officinalis decreased the growth of S. aureus and TVC. This effect was significant at 15 and 30 days of storage, respectively. | [36] |
| Walnut (Juglans regia L.) | Pork meat | Extract (5.5%) or powder (0.5%) | 15 days at 4 °C | Aqueous extracts reduced MDA formation by 47.5% after 15 days of storage compared to controls, including samples without antioxidants and samples with 0.1% BHT. | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez, L.; Quiñones, J.; Díaz, R.; Pateiro, M.; Lorenzo, J.M.; Sepúlveda, N. Natural Antioxidants from Endemic Leaves in the Elaboration of Processed Meat Products: Current Status. Antioxidants 2021, 10, 1396. https://doi.org/10.3390/antiox10091396
Velázquez L, Quiñones J, Díaz R, Pateiro M, Lorenzo JM, Sepúlveda N. Natural Antioxidants from Endemic Leaves in the Elaboration of Processed Meat Products: Current Status. Antioxidants. 2021; 10(9):1396. https://doi.org/10.3390/antiox10091396
Chicago/Turabian StyleVelázquez, Lidiana, John Quiñones, Rommy Díaz, Mirian Pateiro, José Manuel Lorenzo, and Néstor Sepúlveda. 2021. "Natural Antioxidants from Endemic Leaves in the Elaboration of Processed Meat Products: Current Status" Antioxidants 10, no. 9: 1396. https://doi.org/10.3390/antiox10091396
APA StyleVelázquez, L., Quiñones, J., Díaz, R., Pateiro, M., Lorenzo, J. M., & Sepúlveda, N. (2021). Natural Antioxidants from Endemic Leaves in the Elaboration of Processed Meat Products: Current Status. Antioxidants, 10(9), 1396. https://doi.org/10.3390/antiox10091396









