A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat
Abstract
:1. Introduction
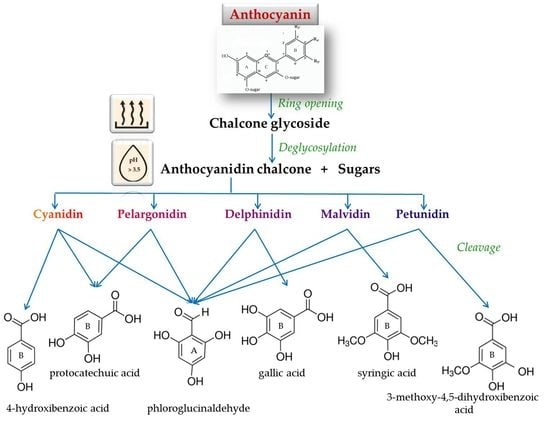
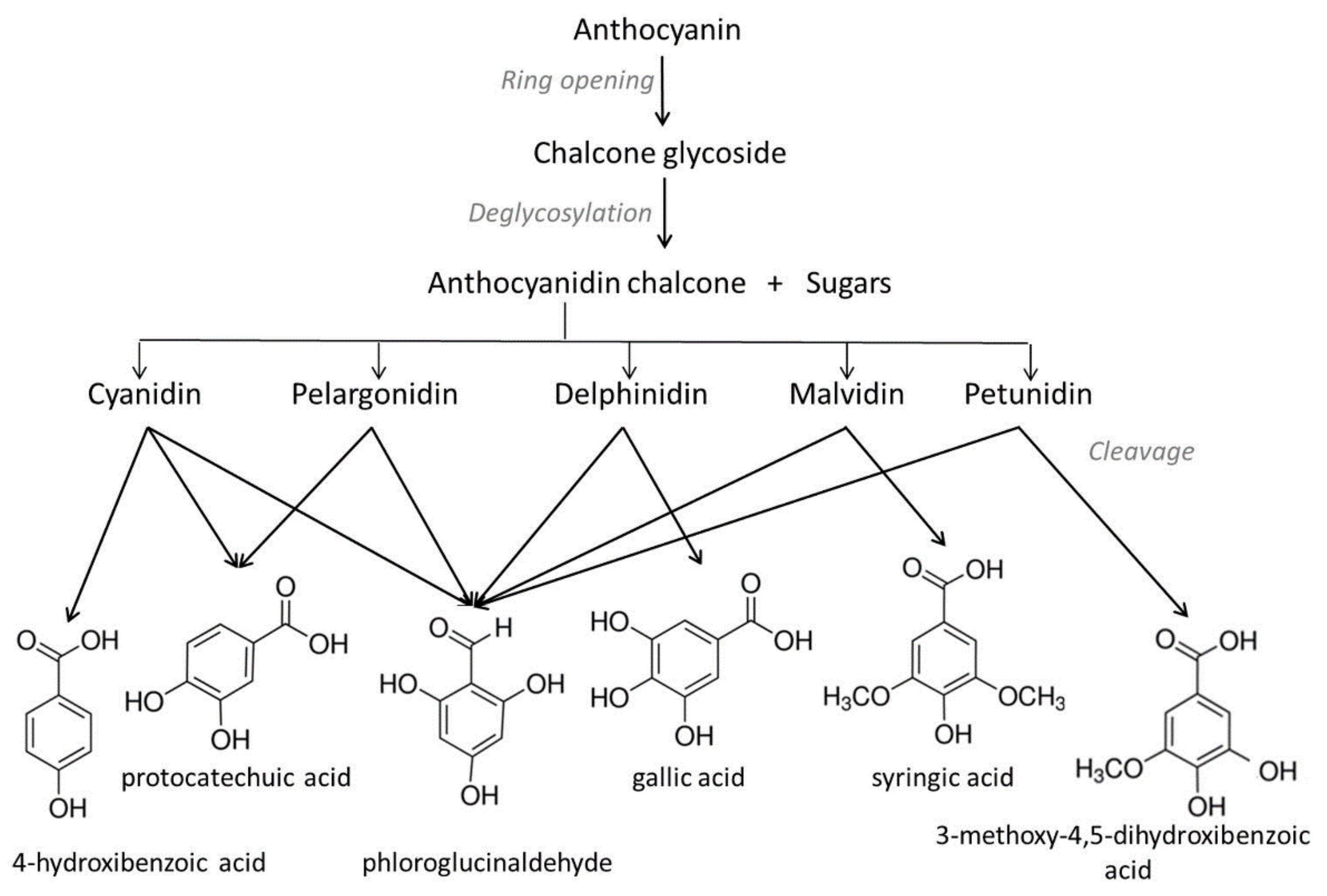
2. Chemical Degradation of Anthocyanins during Heating: Molecular Mechanism and Kinetics
2.1. Chemical Reversible Transformations of Anthocyanins
2.2. Kinetics and Thermal Degradation Route of Anthocyanins
3. Behavior of Anthocyanins within Extracts and Real Foods, as Main Influence of Temperature and Time
3.1. Anthocyanin Thermal Degradation in Crude and Purified Extracts
3.2. Behavior of Anthocyanins during Processing Involving Thermal Treatment of Food Rich or Enriched with Anthocyanins
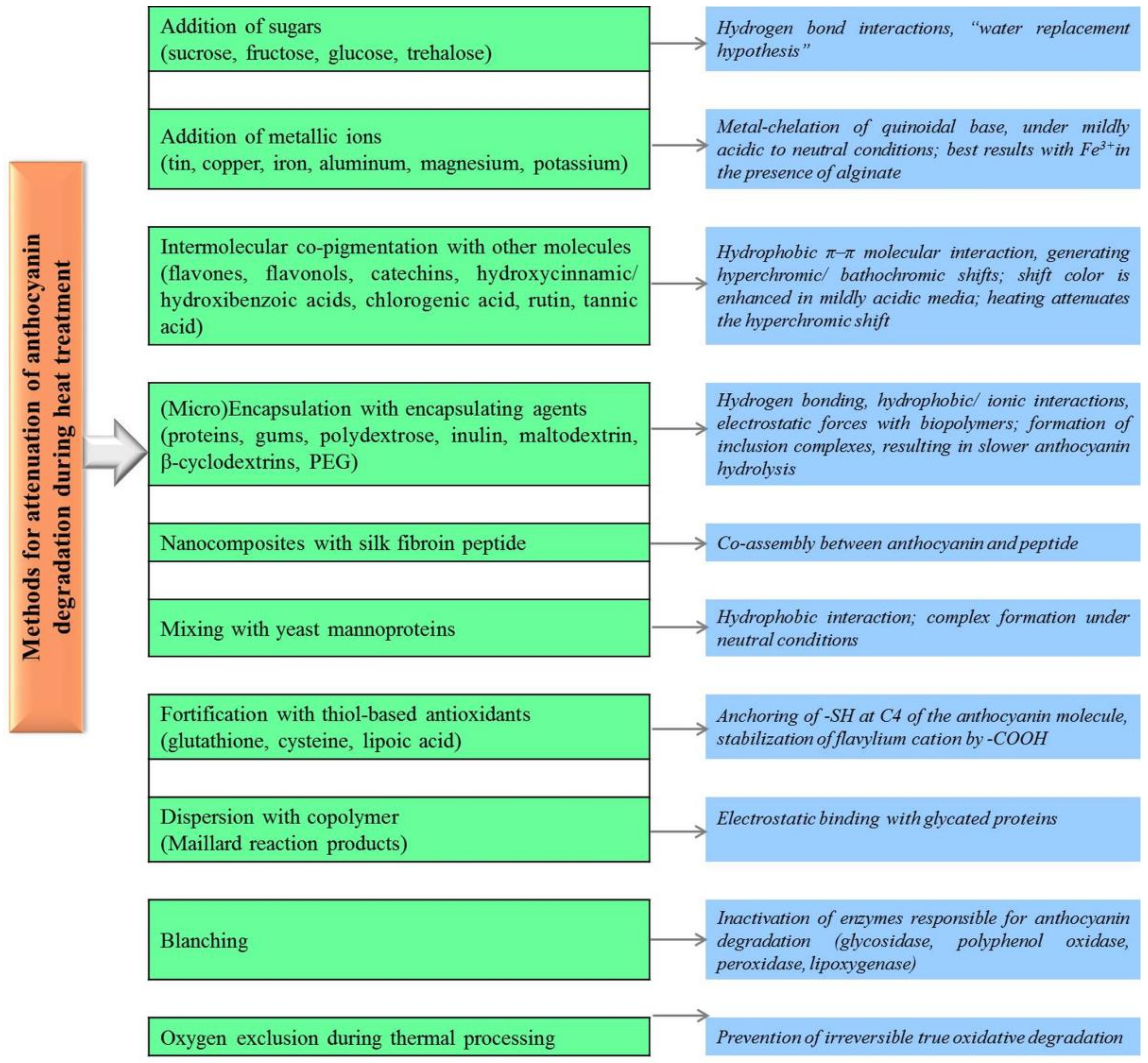
4. Exploring Methods Designed to Enhance the Stability of Anthocyanins to Heat
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Durazzo, A.; Lucarini, M. Editorial: The State of Science and Innovation of Bioactive Research and Applications, Health, and Diseases. Front. Nutr. 2019, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Cicero, N. Development of Food Chemistry, Natural Products, and Nutrition Research: Targeting New Frontiers. Foods 2020, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Stoia, M.; Oancea, S. (Eds.) Overview on plant bioactive compounds. In Phytopharmaceuticals Market Research; Lambert Academic Publishing: Sunnyvale, CA, USA, 2014; pp. 11–12. [Google Scholar]
- Oancea, S.; Linn, Z.M. Anthocyanins: Powerful natural antioxidant pigments with significant biomedical and technological applications. Oxid. Commun. 2018, 41, 92–106. [Google Scholar]
- Albuquerque, B.R.; Oliveira, M.B.P.P.L.; Barros, L.; Ferreira, I.C.F.R. Could fruits be a reliable source of food colorants? Pros and cons of these natural additives. Crit. Rev. Food Sci. Nutr. 2021, 61, 805–835. [Google Scholar] [CrossRef] [PubMed]
- Oancea, S.; Stoia, M.; Oprean, L. Perspectives on improvement of human health by consumption of food plants rich in antioxidants—Anthocyanins. Acta Med. Transilv. 2011, 2, 59–64. [Google Scholar]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Böhm, H.; Boeing, H.; Hempel, J.; Raab, B.; Kroke, A. Flavonols, Flavone and anthocyanins as natural antioxidants of foods and their possible role in the prevention of chronic disease. Ernahrungswiss 1998, 2, 147–163. [Google Scholar] [CrossRef]
- Sui, X.; Dong, X.; Zhou, W. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef]
- Cisse, M.; Vaillant, F.; Acosta, O.; Mayer, C.D.; Dorner, M. Thermal Degradation Kinetics of Anthocyanins from Blood Orange, Blackberry, and Roselle Using the Arrhenius, Eyring, and Ball Models. J. Agric. Food Chem. 2009, 57, 6285–6291. [Google Scholar] [CrossRef]
- Sadilova, E.; Carle, R.; Stintzing, F.C. Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Mol. Nutr. Food Res. 2007, 51, 1461–1471. [Google Scholar] [CrossRef]
- Nayak, B.; de Berrios, J.; Powers, J.R.; Tang, J. Thermal Degradation of Anthocyanins from Purple Potato (Cv. Purple Majesty) and Impact on Antioxidant Capacity. J. Agric. Food Chem. 2011, 59, 11040–11049. [Google Scholar] [CrossRef]
- Brouillard, R.; Delaporte, B. Chemistry of anthocyanin pigment. 2. Kinetic and thermodynamic study of proton transfer, hydration, and tautomeric reactions of malvidin 3-glucoside. J. Am. Chem. Soc. 1977, 99, 8461–8468. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Piffaut, B.; Kader, F.; Girardin, M.; Metche, M. Comparative degradation pathways of malvidin 3,5-diglucoside after enzymatic and thermal treatments. Food Chem. 1994, 50, 115–120. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Sun, J.; Bai, W.; Zhang, Y.; Liao, X.; Hu, X. Identification of degradation pathways and products of cyanidin-3-sophoroside exposed to pulsed electric field. Food Chem. 2011, 126, 1203–1210. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, H.; Qin, Y.; Mao, Y.; Zhang, B.; Deng, Z. Effects of heat, ultrasound, and microwave processing on the stability and antioxidant activity of delphinidin and petunidin. J. Food Biochem. 2019, e12818. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Fenger, J.-A. The Chemical Reactivity of Anthocyanins and Its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenger, J.-A.; Robbins, R.J.; Collins, T.M.; Dangles, O. The fate of acylated anthocyanins in mildly heated neutral solution. Dyes Pigm. 2020, 178, 108326. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Zhang, H.; Mai, Q.; Zhang, B.; Li, H.; Deng, Z. The degradation rules of anthocyanins from eggplant peel and antioxidant capacity in fortified model food system during the thermal treatments. Food Biosci. 2020, 38, 100701. [Google Scholar] [CrossRef]
- Alasalvar, C.; Shahidi, F. Dried Fruits: Phytochemicals and Health Effects; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 88–89. [Google Scholar]
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, Z.-J.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2016, 214, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Dolan, K.D.; Yang, L.; Trampel, C.P. Nonlinear regression technique to estimate kinetic parameters and confidence intervals in unsteady-state conduction-heated foods. J. Food Eng. 2007, 80, 581–593. [Google Scholar] [CrossRef]
- Labuza, T.P.; Riboh, D. Theory and Application of Arrhenius Kinetics to the Prediction of Nutrient Losses in Foods [Degradation, Keeping, Quality, Temperature, Quality Controls, Analysis, Models]. Food Technol. 1982, 36, 66–74. [Google Scholar]
- Casati, C.B.; Baeza, R.; Sánchez, V. Comparison of the kinetics of monomeric anthocyanins loss and colour changes in thermally treated Blackcurrant, Maqui Berry and Blueberry pulps from Argentina. J. Berry Res. 2017, 7, 85–96. [Google Scholar] [CrossRef]
- Silva, N.L.; Crispim, J.M.S.; Vieira, R.P. Kinetic and thermodynamic analysis of anthocyanin thermal degradation in acerola (Malpighia emarginata d.c.) pulp. J. Food Process. Preserv. 2017, 41, e13053. [Google Scholar] [CrossRef]
- Danişman, E.A.; Toklucu, A.K. Kinetic Analysis of Anthocyanin Degradation and Polymeric Colour Formation in Grape Juice during Heating. Czech J. Food Sci. 2015, 33, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Liu, L.; Pan, S. Thermal Degradation Kinetics of Anthocyanins and Visual Color of Blood Orange Juice. Agric. Sci. China 2011, 10, 1992–1997. [Google Scholar] [CrossRef]
- Kopjar, M.; Piližota, V. Prevention of thermal degradation of anthocyanins in blackberry juice with addition of different sugars. CyTA J. Food 2011, 9, 237–242. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; De Bastiani, G.; Vieira, R.P.; Anjos, C.A.R. Thermal degradation kinetics of total anthocyanins in açaí pulp and transient processing simulations. SN Appl. Sci. 2020, 2, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Kechinski, C.P.; Guimarães, P.V.R.; Noreña, C.P.Z.; Tesaro, I.C.; Marczak, L.D.F. Degradation kinetics of anthocyanin in blueberry juice during thermal treatment. J. Food Sci. 2010, 75, 173–176. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Tao, C.; Liu, M.; Pan, Y.; Lv, Z. Effect of temperature and pH on stability of anthocyanin obtained from blueberry. Food Meas. 2018, 12, 1744–1753. [Google Scholar] [CrossRef]
- Soldatkina, L.M.; Novotna, V.O.; Salamon, I. Degradation kinetics of anthocyanins in acidic aqueous extracts of berries. Odesa Natl. Univ. Herald. Chem. 2017, 22, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Swer, T.L.; Chauhan, K. Stability studies of enzyme aided anthocyanin extracts from Prunus nepalensis L. LWT Food Sci. Technol. 2019, 102, 181–189. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Thermal and pH degradation kinetics of anthocyanins in natural food colorant prepared from black rice bran. J. Food Sci. Technol. 2016, 53, 461–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Song, H.; Zhao, G. Degradation Kinetics of Anthocyanins from Purple Sweet Potato (Ipomoea batatas L.) as Affected by Ascorbic Acid. Food Sci. Biotechnol. 2014, 23, 89–96. [Google Scholar] [CrossRef]
- Park, J.-S.; Bae, J.-O.; Chung, B.-W.; Jung, M.Y.; Choi, D.-S. Degradation Kinetics of Anthocyanin Pigment Solutions from Purple-fleshed Sweet Potato Cultivars. Korean J. Food Nutr. 2011, 24, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Tecucianu, A.C.; Draghici, O.; Oancea, S. An enzyme- enhanced extraction of anthocyanins from red cabbage and their thermal degradation kinetics. Acta Aliment. 2020, 49, 204–213. [Google Scholar] [CrossRef]
- Souza, P.P.; Robazza, W.S.; Galvão, A.C. Thermal degradation of the anthocyanins extracts from jabuticaba peels and red cabbage leaves. Sci. Plena 2020, 16, 1–8. [Google Scholar] [CrossRef]
- Sipahli, S.; Mohanlall, V.; Mellem, J.J. Stability and degradation kinetics of crude anthocyanin extracts from H. sabdariffa. Food Sci. Technol. Camp. 2017, 37, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Guan, Y.; Zhong, Q. Yeast mannoproteins improve thermal stability of anthocyanins at pH 7.0. Food Chem. 2015, 172, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yuan, D.; Wang, Q.; Hu, Z.; Wu, Y.; Cai, J.; Huang, Q.; Li, S.; Liu, G. Maillard-reacted whey protein isolates enhances thermal stability of anthocyanins over a wide pH range. J. Agric. Food Chem. 2018, 66, 9556–9564. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition: Protective effects by intra and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Insfran, D.; Brenes, C.H.; Talcottt, S.T. Phytochemical composition and pigment stability of acai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2004, 52, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Bobbio, F.O.; Bobbio, P.A.; Oliveira, P.A.; Fadelli, S. Stability and stabilization of the anthocyanins from Euterpe oleracea mart. Acta Aliment. 2002, 31, 371–377. [Google Scholar] [CrossRef]
- Oancea, S.; Călin, F. Phenolics content, in vitro antioxidant and anticholinesterase activities of combined berry extracts. Rom. Biotechnol. Lett. 2018, 23, 14025–14034. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Optimization of Extraction of Anthocyanins from Black Currants with Aqueous Ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Ekici, L.; Simsek, Z.; Ozturk, I.; Sagdic, O.; Yetim, H. Effects of Temperature, Time, and pH on the Stability of Anthocyanin Extracts: Prediction of Total Anthocyanin Content Using Nonlinear Models. Food Anal. Methods 2014, 7, 1328–1336. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Angosto, J.M.; Giménez, P.J.; León, G. Thermal Stability of Selected Natural Red Extracts Used as Food Colorants. Plant Foods Hum. Nutr. 2013, 68, 11–17. [Google Scholar] [CrossRef]
- Oancea, S.; Draghici, O. pH and Thermal Stability of Anthocyanin-based Optimised Extracts of Romanian Red Onion Cultivars. Czech J. Food Sci. 2013, 31, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Escher, G.B.; Wen, M.; Zhang, L.; Rosso, N.D.; Granato, D. Phenolic composition by UHPLC-Q-TOF-MS/MS and stability of anthocyanins from Clitoria ternatea L. (butterfly pea) blue petals. Food Chem. 2020, 331, 127341. [Google Scholar] [CrossRef]
- Zozio, S.; Pallet, D.; Dornier, M. Evaluation of anthocyanin stability during storage of a coloured drink made from extracts of the Andean blackberry (Rubus glaucus Benth.), açai (Euterpe oleracea Mart.) and black carrot (Daucus carota L.). Fruits 2011, 66, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Jongen, W. Fruit and Vegetable Processing; CRC Woodhead Publishing Limited Press: Boca Raton, FL, USA, 2002; pp. 188–200. [Google Scholar]
- Gamage, G.C.V.; Lim, Y.Y.; Choo, W.S. Sources and relative stabilities of acylated and nonacylated anthocyanins in beverage systems. J. Food Sci. Technol. 2021, 58, 1–15. [Google Scholar] [CrossRef]
- Yue, X.; Xu, Z. Changes of Anthocyanins, Anthocyanidins, and Antioxidant Activity in Bilberry Extract during Dry Heating. J. Food Sci. 2008, 73. [Google Scholar] [CrossRef]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry products. J. Food Sci. 2008, 73, H72–H79. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Hamouz, K.; Orsak, M.; Pivec, V.; Hejtmankova, K.; Pazderu, K.; Dvorak, P.; Cepl, J. Impact of selected factors—Cultivar, storage, cooking and baking on the content of anthocyanins in coloured-flesh potatoes. Food Chem. 2012, 133, 1107–1116. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Borge, A.; Bengtsson, G.B.; Hansen, M.; Thygese, I.H.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Oancea, S.; Călin, F. Changes in Total Phenolics and Anthocyanins during Blackberry, Raspberry and Cherry Jam Processing and Storage. Rom. Biotechnol. Lett. 2016, 21, 11232–11237. [Google Scholar]
- García-Viguera, C.; Zafrilla, P.; Romero, F.; Abellán, P.; Artés, F.; Tomás-Barberán, F.A. Color Stability of Strawberry Jam as Affected by Cultivar and Storage Temperature. J. Food Sci. 1999, 64, 243–247. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Blackberry Products. J. Agric. Food Chem. 2008, 56, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in Anthocyanins and Polyphenolics During Juice Processing of Highbush Blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–364. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Phenolic Acids, Flavonoids, Vitamin C and Antioxidant Capacity of Strawberry Juices Processed by High-Intensity Pulsed Electric Fields or Heat Treatments. Eur. Food Res. Technol. 2008, 228, 239. [Google Scholar] [CrossRef]
- Hartmann, A.; Patz, C.-D.; Andlauer, W.; Dietrich, H.; Ludwig, M. Influence of Processing on Quality Parameters of Strawberries. J. Agric. Food Chem. 2008, 56, 9484–9489. [Google Scholar] [CrossRef] [PubMed]
- Lohachoompol, V.; Srzednicki, G.; Craske, J. The Change of Total Anthocyanins in Blueberries and Their Antioxidant Effect After Drying and Freezing. J. Biomed. Biotechnol. 2004, 5, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Witrowa-Rajchert, D.; Bawol, A.; Czapski, J.; Kidoń, M. Studies on drying of purple carrot roots. Dry. Technol. 2009, 27, 1325–1331. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, D.; Song, X.; Deng, Y.; Zhao, Y. Degradation kinetics and antioxidant capacity of anthocyanins in air-impingement jet dried purple potato slices. Food Res. Int. 2018, 105, 121–128. [Google Scholar] [CrossRef]
- Morales-Delgado, D.Y.; Téllez-Medina, D.I.; Rivero-Ramírez, N.L.; Arellano-Cárdenas, S.; LópezCortez, S.; Hernández-Sánchez, H.; Gutiérrez-López, G.; Cornejo-Mazón, M. Effect of convective drying on total anthocyanin content, antioxidant activity and cell morphometric parameters of strawberry parenchymal tissue (Fragaria × ananassa Dutch). Rev. Mex. Ing. Química 2014, 13, 179–187. [Google Scholar]
- Ioannou, I.; Hafsa, I.; Hamdi, S.; Charbonnel, C.; Ghoul, M. Review of the effects of food processing and formulation on flavonol and anthocyanin behaviour. J. Food Eng. 2012, 111, 208–217. [Google Scholar] [CrossRef]
- Barti, P.; Albreht, A.; Skrt, M.; Tremlová, B.; Ošťádalová, M.; Šmejkal, K.; Vovk, I.; Ulrih, N.P. Anthocyanins in purple and blue wheat grains and in resulting bread: Quantity, composition, and thermal stability. Int. J. Food Sci. Nutr. 2015, 66, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Yap, P.Y.; Zhou, W. Anthocyanins During Baking: Their Degradation Kinetics and Impacts on Color and Antioxidant Capacity of Bread. Food Bioprocess. Technol. 2015, 8, 983–994. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Perera, C.O.; Waterhouse, G.I.N. Exploring the interactions between blackcurrant polyphenols, pectin and wheat biopolymers in model breads; a FTIR and HPLC investigation. Food Chem. 2012, 131, 802–810. [Google Scholar] [CrossRef]
- Karakaya, S.; Simsek, S.; Eker, A.T.; Pineda-Vadillo, C.; Dupont, D.; Perez, B.; Viadel, B.; Sanz-Buenhombre, M.; Rodriguez, A.G.; Kertész, Z.; et al. Stability and bioaccessibility of anthocyanins in bakery products enriched with anthocyanins. Food Funct. 2016, 7, 3488–3496. [Google Scholar] [CrossRef]
- Sui, X. Impact of Food Procesing on Anthocyanins. Ph.D. Thesis, National University of Singapore, Singapore, January 2017. [Google Scholar]
- Zhang, Y.; Deng, Z.; Li, H.; Zheng, L.; Liu, R.; Zhang, B. Degradation Kinetics of Anthocyanins from Purple Eggplant in a Fortified Food Model System during Microwave and Frying Treatments. J. Agric. Food Chem. 2020, 68, 11817–11828. [Google Scholar] [CrossRef] [PubMed]
- Menchaca-Armenta, M.; Frutos, M.J.; Ramírez-Wong, B.; Quintero-Ramos, A.; Torres-Chávez, P.I.; Valero-Cases, E.; Muelas-Domingo, R.; Ledesma-Osuna, A.I.; Campas-Baypoli, O.N. The Effect of Nixtamalization Extrusion Process and Tortillas Making on the Stability of Anthocyanins from Blue Corn through the Kinetic and Thermodynamic Parameters. Plant Foods Hum. Nutr. 2021, 76. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Guerin-Dubiard, C.; Jardin, J.; Lechevalier, V.; Sanz-Buenhombre, M.; Guadarrama, A.; Tóth, T.; Csavajda, E.; Hingyi, H.; et al. The food matrix affects the anthocyanin profile of fortified egg and dairy matrices during processing and in vitro digestion. Food Chem. 2016, 214, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Andrés, A.I.; Petrón, M.J.; Adámez, J.D.; López, M.; Timón, M. Food byproducts as potential antioxidant and antimicrobial additives in chill stored raw lamb patties. Meat Sci. 2017, 129, 62–70. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domınguez, R.; Barba, F.J.; Putnik, P.; Kovacevic, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Stobnicka, A.; Gniewosz, M. Antimicrobial protection of minced pork meat with the use of Swamp Cranberry (Vaccinium oxycoccos L.) fruit and pomace extracts. J. Food Sci. Technol. 2018, 55, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.D.; Auqui, M.; Mart´, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT Food Sci. Technol. 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.-R.; Xiang, R.; Liu, X.-M.; Zhu, M.-J. The effects of thermal processing and β-cyclodextrin on extractable polyphenols in mulberry juice-enriched dried minced pork slices. LWT Food Sci. Technol. 2019, 116, 108503. [Google Scholar] [CrossRef]
- Parinyapatthanaboot, T.; Pinsirodom, P. Effect of anthocyanins from different plant source on the oxidative stability of vacuum-packed chinese-style sausages during storage. In Proceedings of the 56th International Congress of Meat Science and Technology, Jeju, Korea, 15–20 August 2010; Available online: http://icomst-proceedings.helsinki.fi/papers/2010_04_94.pdf (accessed on 12 August 2021).
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; de Mejia, E.G. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Kopjar, M.; Jakšic´, K.; Piližota, V. Influence of sugars and chlorogenic acid addition on anthocyanin content, antioxidant activity and color of blackberry juice during storage. J. Food Process. Preserv. 2012, 36, 545–552. [Google Scholar] [CrossRef]
- Mollaamin, F.; Monajjemi, M. Thermodynamic and IR Spectral Study of Metal Cations–Anthocyanin Chelation: Mechanism of Formation of Pigments. Russ. J. Phys. Chem. 2020, 94, 1887–1901. [Google Scholar] [CrossRef]
- Tachibana, N.; Kimura, Y.; Ohno, T. Examination of molecular mechanism for the enhanced thermal stability of anthocyanins by metal cations and polysaccharides. Food Chem. 2014, 143, 452–458. [Google Scholar] [CrossRef]
- Charurungsipong, P.; Tangduangdee, C.; Amornraksa, S.; Asavasanti, S.; Lin, J. Improvement of anthocyanin stability in butterfly pea flower extract by co-pigmentation with catechin. In Proceedings of the 2019 Research, Invention, and Innovation Congress, E3S Web of Conferences 2020, Bangkok, Thailand, 11–13 December 2019; Volume 141, p. 03008. [Google Scholar] [CrossRef]
- Cassol, L.; Noreña, C.P.Z. Microencapsulation and accelerated stability testing of bioactive compounds of Hibiscus sabdariffa. Food Meas. 2021, 15, 1599–1610. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A. Micro and Nanoencapsulation of Natural Colors: A Holistic View. Appl. Biochem. Biotechnol. 2021, 193. [Google Scholar] [CrossRef]
- Dos Santos Lima, K.T.; Garcez, J.; dos Santos Alves, M.J.; Monteiro, A.R.; Valencia, G.A. Physicochemical Properties of Modified Starches Obtained by Anti-Solvent Precipitation Containing Anthocyanins from Jambolan (Syzygium cumini) Fruit. Starch Staerke 2020, 73, 2000221. [Google Scholar] [CrossRef]
- Mazuco, R.A.; Cardoso, P.M.M.; Bindaco, É.S. Maltodextrin and Gum Arabic-Based Microencapsulation Methods for Anthocyanin Preservation in Juçara Palm (Euterpe edulis Martius) Fruit Pulp. Plant Foods Hum. Nutr. 2018, 73, 209–215. [Google Scholar] [CrossRef]
- Fernandes, A.; Rocha, M.A.A.; Santos, L.M.N.; Brás, J.; Oliveira, J.; Mateus, N.; de Freitas, V. Blackberry anthocyanins: β-cyclodextrin fortification for thermal and gastrointestinal stabilization. Food Chem. 2017, 245, 426–431. [Google Scholar] [CrossRef]
- Su, K.; Li, Y.; Huang, X.; Zhao, L.; Li, Q.; Li, M.; Li, H.; Zhang, J. Complexion of Kadsura coccinea extract with cyclodextrin: Characterization, thermal stability, antioxidative properties in vitro and the protective effects on kidney damage. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 141–148. [Google Scholar] [CrossRef]
- Chi, J.; Ge, J.; Yue, X.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Preparation of nanoliposomal carriers to improve the stability of anthocyanins. LWT Food Sci. Technol. 2019, 109, 101–107. [Google Scholar] [CrossRef]
- Goncalves, F.; Heyraud, A.; De Pinho, M.N.; Rinaudo, M. Characterization of white wine mannoproteins. J. Agric. Food Chem. 2002, 50, 6097–6101. [Google Scholar] [CrossRef] [PubMed]
- Attaribo, T.; Huang, G.; Xin, X.; Zeng, Q.; Zhang, Y.; Zhang, N.; Tang, L.; Sedjoah, R.-C.A.-A.; Zhang, R.; Lee, K.S.; et al. Effect of the silkworm pupa protein–glucose conjugate on the thermal stability and antioxidant activity of anthocyanins. Food Funct. 2021, 12, 4132–4141. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, P.D.; Mota Ramos, A.; Basílio de Oliveira, E.; Furtado Martins, E.M.; Ribeiro de Barros, F.A.; Ribeiro Vidigal, M.C.T.; de Almeida Costa, N.; Tatagiba da Rocha, C. Increased thermal stability of anthocyanins at pH 4.0 by guar gum in aqueous dispersions and in double emulsions W/O/W. Int. J. Biol. Macromol. 2018, 117, 665–672. [Google Scholar] [CrossRef]
- Li, Y.; Yao, L.; Zhang, L.; Zhang, Y.; Zheng, T.; Liu, L.; Zhang, L. Enhanced physicochemical stabilities of cyanidin-3-O-glucoside via combination with silk fibroin peptide. Food Chem. 2021, 355, 129479. [Google Scholar] [CrossRef]
- Ma, Z.; Jing, P. Stabilization of black rice anthocyanins by self-assembled silk fibroin nanofibrils: Morphology, spectroscopy and thermal protection. Int. J. Biol. Macromol. 2020, 146, 1030–10391. [Google Scholar] [CrossRef]
- Jiang, Q.X.; Ning, K.L.; Yu, D.W.; Xu, Y.-S. Effects of blanching on extraction and stability of anthocyanins from blueberry peel. Food Meas. 2020, 14, 2854–2861. [Google Scholar] [CrossRef]
- Preciado-Iñiga, G.E.; Amador-Espejo, G.G.; Bárcenas, M.E. Blanching and antimicrobial mixture (potassium sorbate–sodium benzoate) impact on the stability of a tamarillo (Cyphomandra betacea) sweet product preserved by hurdle technology. J. Food Sci. Technol. 2018, 55, 740–748. [Google Scholar] [CrossRef]
- Marzuki, S.U.; Pranoto, Y.; Khumsap, T.; Nguyen, L.T. Effect of blanching pretreatment and microwave-vacuum drying on drying kinetics and physicochemical properties of purple fleshed sweet potato. J. Food Sci. Technol. 2021, 58, 2884–2895. [Google Scholar] [CrossRef]
- Merina, M.; Luján, G.; Vejarano, R. Thermal stability of anthocyanins in grape skin extracts from red winemaking residues. In Proceedings of the 6th Brazilian Technology Symposium: Smart Innovation, Systems and Technologies (BTSym 2020), PUC-Campinas, São Paulo, Brazil, 26–28 October 2020; Volume 233, pp. 740–749. [Google Scholar] [CrossRef]
- Li, S.E.; Mu, B.; Wang, X.W.; Kang, Y.R.; Wang, A.Q. A comparative study on color stability of anthocyanin hybrid pigments derived from 1D and 2D clay minerals. Materials 2019, 12, 3287. [Google Scholar] [CrossRef] [Green Version]
- Li, S.E.; Mu, B.; Ding, J.J.; Zhang, H.; Wang, X.W.; Wang, A.Q. Fabrication of anthocyanin/montmorillonite hybrid pigments to enhance their environmental stability and application in allochroic composite films. Clays Clay Miner. 2021, 69, 142–151. [Google Scholar] [CrossRef]
- Ribeiro, H.L.; de Oliveira, A.V.; de Brito, E.S.; Ribeiro, P.R.V.; Souza Filho, M.S.M.; Azeredo, H.M.C. Stabilizing effect of montmorillonite on acerola juice anthocyanins. Food Chem. 2018, 245, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.T.M.; da Silva, K.M.; Silva, C.P.; Rodrigues, A.C.B.; Oake, J.; Gehlen, M.H.; Bohne, C.; Quina, F.H. Highly fluorescent hybrid pigments from anthocyanin- and red wine pyranoanthocyanin-analogs adsorbed on sepiolite clay. Photochem. Photobiol. Sci. 2019, 18, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Chacha, J.S.; Zhang, L.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M.; et al. Revisiting Non-Thermal Food Processing and Preservation Methods—Action Mechanisms, Pros and Cons: A Technological Update (2016–2021). Foods 2021, 10, 1430. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Monteagudo, S.I.; Saldaña, M.D.A. Chemical Reactions in Food Systems at High Hydrostatic Pressure. Food Eng. Rev. 2014, 6, 105–127. [Google Scholar] [CrossRef]

| Source | pH | Heating T (°C) | Monitoring Time | Rate Constant (k) | t½ (h) | Ea (kJ/mol) | Ref. |
|---|---|---|---|---|---|---|---|
| Fruits | |||||||
| Açaí (Euterpe precatoria Mart.) pulp | nr | 60 | 90 min | 0.0005 min−1 | 23 | 42.8 | [32] |
| 70 | 0.0006 min−1 | 19 | |||||
| 80 | 0.0007 min−1 | 16 | |||||
| 90 | 0.002 min−1 | 7 | |||||
| Acerola (Malpighia emarginata D.C.) pulp juice | nr | 60 | 1 h, 30 min | Graphical representation k = f(1/T) | 4 | 68.042 | [28] |
| 70 | 2 | ||||||
| 80 | 1 | ||||||
| 90 | 1/2 | ||||||
| Blackcurrants (Ribes nigrum L.) ethanol extract from thermally treated pulps | 3.07 | 75 | 25 h | 0.0065 h−1 | 21.9 | 94 | [27] |
| 80 | 0.0131 h−1 | 7.2 | |||||
| 90 | 0.0352 h−1 | 1.8 | |||||
| Blueberries (Vaccinium corymbosum L.) ethanol extract from thermally treated pulps | 3.46 | 70 | 25 h | 0.0320 h−1 | 12.7 | 92 | [27] |
| 80 | 0.0536 h−1 | 7.3 | |||||
| 90 | 0.1167 h−1 | 1.4 | |||||
| Blueberries/Rabbiteye (Vaccinium achei) juice | nr | 50 | 25 h | 0.273 × 10−3 min−1 | 42.30 | 80.42 | [33] |
| 60 | 0.457 × 10−3 min−1 | 25.30 | |||||
| 70 | 8 h | 1.350 × 10−3 min−1 | 8.60 | ||||
| 80 | 2.254 × 10−3 min−1 | 5.11 | |||||
| Blueberries (Vaccinium corymbosum L.) methanol extract | 3 | 50 | 10 h | 0.009 h−1 | 74.47 | nd | [34] |
| 60 | 0.026 h−1 | 26.51 | |||||
| 70 | 0.047 h−1 | 14.73 | |||||
| 80 | 0.146 h−1 | 4.73 | |||||
| 6 | 50 | 10 h | 0.044 h−1 | 15.64 | nd | ||
| 60 | 0.111 h−1 | 6.21 | |||||
| 70 | 0.249 h−1 | 2.77 | |||||
| 80 | 0.452 h−1 | 1.53 | |||||
| Blackberries (Rubus spp.) acidified aqueous extracts | 2 | 50 | nr | 1.8 × 10−3 min−1 | 6.4 | 15.0 | [35] |
| 75 | 2.8 × 10−3 min−1 | 4.1 | |||||
| 100 | 3.8 × 10−3 min−1 | 3.0 | |||||
| Chokeberries (Aronia spp.) acidified aqueous extracts | 2 | 50 | nr | 2.8 × 10−3 min−1 | 4.1 | 5.7 | |
| 75 | 3.5 × 10−3 min−1 | 3.3 | |||||
| 100 | 3.7 × 10−3 min−1 | 3.1 | |||||
| Elderberries (Sambucus spp.) acidified aqueous extracts | 2 | 50 | nr | 2.3 × 10−3 min−1 | 5.0 | 10.1 | |
| 75 | 3.3 × 10−3 min−1 | 3.5 | |||||
| 100 | 3.8 × 10−3 min−1 | 3.0 | |||||
| Elderberry (Sambucus nigra L.) pigment isolates from concentrates | 3.5 | 95 | 4 h | nr | 1.96 | nd | [11] |
| Grape (Vitis vinifera cv. of Karasakiz) juice | 3.34 | 70 | 90 min | 1.20 × 10−3 min−1 | 10.03 | 64.89 | [29] |
| 80 | 60 min | 2.40 × 10−3 min−1 | 5.02 | ||||
| 90 | 60 min | 4.20 × 10−3 min−1 | 2.79 | ||||
| Prunus nepalensis L. (Sohiong), freeze dried extracts, in citrate phosphate buffer | 3.5 | 50 | 7 h | 0.018 days−1 (CE) 0.017 days−1 (EAE) | 38.5 (CE) 40.7 (EAE) | nd | [36] |
| 80 | 0.044 days−1 (CE) 0.041 days−1 (EAE) | 15.75 (CE) 16.9 (EAE) | |||||
| Strawberry (Fragaria × ananassa Duch.) pigment isolates from concentrates | 3.5 | 95 | 4 h | nr | 1.95 | nd | [11] |
| Vegetables | |||||||
| Black carrot (Daucus carota L. ssp. sativus var. atrorubens Alef.) pigment isolate from concentrate | 3.5 | 95 | 4 h | nr | 2.81 | nd | [11] |
| Black rice (Oryza sativa L.) bran colorant powder dissolved in acetate buffer | 3 | 60 | 2 h | 0.71 × 10−3 min−1 | 16.3 | 45.05 | [37] |
| 80 | 1.26 × 10−3 min−1 | 9.17 | |||||
| 100 | 4.12 × 10−3 min−1 | 2.8 | |||||
| 4 | 60 | 2.16 × 10−3 min−1 | 5.35 | 21.09 | |||
| 80 | 3.67 × 10−3 min−1 | 3.15 | |||||
| 100 | 4.87 × 10−3 min−1 | 2.37 | |||||
| 5 | 60 | 5.34 × 10−3 min−1 | 2.16 | 17.54 | |||
| 80 | 8.59 × 10−3 min−1 | 1.34 | |||||
| 100 | 12.3 × 10−3 min−1 | 0.94 | |||||
| Purple sweet potato (Ipomoea batatas L.), in citric buffer | 3 | 70 | 6 h | 236 × 10−4 h−1 | 29.4 | 16.46 | [38] |
| 80 | 262 × 10−4 h−1 | 26.5 | |||||
| 90 | 320 × 10−4 h−1 | 21.7 | |||||
| Purple sweet potato (Ipomoea batatas L.) solution 5 cvs.: Mokpo No. 62, Borami, Jami, Sinjami and Ayamurasaki | 3 | 60 | - | 0.04035, 0.03453, 0.03613, 0.03774 and 0.03800 h−1 | 17.2, 20.1, 19.2, 18.4 and 3.2 day | 54.67, 60.93, 71.73, 59.35 and 62.28 | [39] |
| 80 | 0.23364, 0.22338, 0.22222, 0.21935 and 0.21627 h−1 | 3.0, 3.1, 3.1, 3.2 and 3.2 day | |||||
| Red cabbage (Brassica oleracea L. var. capitata f. rubra) ethanol extract | 3.5 | 80 | 7 h | 1.7 × 10−3 min−1 | 6.7 | nd | [40] |
| Red cabbage (Brassica oleracea L. var. capitata f. rubra) aqueous extract | nr | 60 | 30 h | 0.0273 h−1 | 25.3 | nd | [41] |
| 70 | 0.0394 h−1 | 17.6 | |||||
| 80 | 0.0694 h−1 | 10.0 | |||||
| Flowers | |||||||
| Hibiscus calyces (Hibiscus sabdariffa L.) 4 types of ethanol/methanol extracts acidified with HCl, formic acid, citric acid or acetic acid | 3 | 70 | 6 h | 0.0007, 0.0009, 0.0011 and 0.0009 min−1 | 22.0, 26.0, 18.0 and 19.0 | nd | [42] |
| 75 | 0.0005, 0.0006, 0.0011 and 0.0007 min−1 | 18.0, 17.0, 12.0 and 17.0 | |||||
| 80 | 0.0004, 0.0006, 0.0013 and 0.0007 min−1 | 19.0, 17.0, 10.0 and 13.0 | |||||
| 85 | 0.0004, 0.0007, 0.0012 and 0.0005 min−1 | 18.0, 19.0, 16.0 and 16.0 | |||||
| Purified anthocyanins | |||||||
| Colorant—anthocyanins liquid (ColorFruit® Violet 100 WS) | 7 | 80 | 90 min | 0.0114 min−1 (without stabilizer) 0.0027 min−1 (with mannoproteins) | 50.4 min (without stabilizer) 272.4 min (with mannoproteins) | nd | [43] |
| 126 | 0.0271 min−1 (without stabilizer) 0.0051 min−1 (with mannoproteins) | 25.8 min (without stabilizer) 143.4 (with mannoproteins) | |||||
| Cyanidin-3-O-glucoside | 3 | 80 | 2 h | 0.0018 min−1 | 386.3 min | nd | [44] |
| 6 | 80 | 0.0063 min−1 | 109.2 min | ||||
| Source | Type of Extract | T (°C) | Conclusion on Anthocyanin Degradation | Ref. |
|---|---|---|---|---|
| Crude Extracts | ||||
| Black currant (Ribes nigrum cv. Ben Lomond) | Acidified ethanol extract (pH 4.1) | 74 | 16.3% anthocyanin degradation with respect that of extraction conducted at 6 °C | [50] |
| Black grape pomace (Vitis vinifera) | Acidified ethanol extract (pH 3.0) | 70 | 11.82% anthocyanin degradation after 120 min | [51] |
| 80 | 12.36% anthocyanin degradation after 120 min | |||
| 90 | 49.79% anthocyanin degradation after 120 min | |||
| Blueberry (Vaccinium corymbosum L.) | Methanol extract (pH 3.0) | 50 | Good anthocyanin preservation rate: 95% after heating at 50 °C for 10 h | [34] |
| 60 | Anthocyanin preservation rate = 80% after heating at 60 °C for 10 h | |||
| 80 | Low anthocyanin preservation rate: 18.54% after heating at 80 °C for 10 h | |||
| Elderberry (Sambucus Nigra L.) | Aqueous extract (pH 5.5) | 70 | ~80% remaining absorbance at 535 nm after 3 h | [52] |
| 90 | 63.8% remaining absorbance at 535 nm after 6 h | |||
| Black carrot (Daucus carota L.) | Acidified ethanol extract (pH 3.0) | 70 | 9.36% anthocyanin degradation after 120 min | [51] |
| Red cabbage (Brassica oleracea L. var. capitata f. rubra) | Acidified ethanol extract/dried (pH 3.0 and pH 5.0) | 70 | 2.57% anthocyanin degradation after 120 min (pH 3) 6.5% anthocyanin degradation after 120 min (pH 5) | [51] |
| 80 | 5.04% anthocyanin degradation after 120 min (pH 3) 24.64% anthocyanin degradation after 120 min (pH 5) | |||
| 90 | 26.09% anthocyanin degradation after 120 min (pH 3) 33.33% anthocyanin degradation after 120 min (pH 5) | |||
| Aqueous extract (pH 5.5) | 70 | ~70% remaining absorbance at 535 nm after 2 h | [52] | |
| 90 | 46.1% remaining absorbance at 535 nm after 6 h | |||
| Red onion outer skin (waste) (Allium cepa L.) | Ethanol extract; different acidic and alkaline conditions | Differential scanning calorimetry (DSC) method | The onset temperature, Ton, of anthocyanin degradation was 51.74 °C under acidic conditions (pH = 4.5); the Ton was 44.64 °C under alkaline conditions (pH = 9.0) | [53] |
| Clitoria ternatea (butterfly pea) blue petals | Aqueous extract (pH 3.6) | 80 | 90% color retention at 617 nm | [54] |
| 90 | 76.1% color retention at 617 nm | |||
| 100 | 70.2% color retention at 617 nm | |||
| Hibiscus (Hibiscus sabdariffa L.) | Aqueous extract (pH 5.5) | 50 | More than 80% remaining absorbance at 535 nm after 2 h | [52] |
| 70 | Less than 70% remaining absorbance at 535 nm after 2 h | |||
| 90 | 26.7% remaining absorbance at 535 nm after 6 h | |||
| Purified Extracts | ||||
| Black carrot (Daucus carota L.) | Purified anthocyanin powder extract (ColorFruit Carrot 12 WSP) | 50 | Kinetic parameters: k × 10−2 days = 0.92 Ea = 63.2 kJ·mol−1 | [55] |
| Purple potato (Solanum tuberosum cv. Purple Majesty) | Purified anthocyanins from acidified methanolic extract (pH 5.95) | 100 | Kinetic parameters, for 0–60 min: t1/2 = 26.456 min, k (min−1) × 103 = 26.2, Ea = 72.89 kJ·mol−1 | [12] |
| 150 | Kinetic parameters, for 0–60 min: t1/2 = 2.428 min, k (min−1) × 103 = 285.5, Ea = 72.89 kJ°mol−1 | |||
| Starting Raw Material | Final Product | Thermal Processing | Major Conclusions on Anthocyanins | Ref. |
|---|---|---|---|---|
| Blueberries (Vaccinium corymbosum, cv. Bluecrop) | Canned in syrup | Cans were exhausted for 4 min in a steam box at 87.8–93.3 °C; the sealed cans were immersed in boiling water for 15 min | 28% anthocyanin loss | [59] |
| Pigmented potato (Solanum tuberosum L.) (Valfi, Blue Congo, Blaue St. Galler, Violette, Highland Burgundy Red) | Cooked | Cooking of whole tubers: (1) boiled water 15 min, (2) boiled steam 15 min, (3) microwave 9 min. Baking (40 min at 180 °C) | Anthocyanins increased by 4.2–4.5 times when boiled steam and boiled water was involved, and by 3.34 times by baking | [60] |
| Red cabbage (Brassica oleracea L. ssp. capitata f. rubra) | Cooked | Cooking (blanching, boiling, steaming) | 59%, 41% and 29% respectively, loss in the anthocyanin content | [61] |
| Blackberries (Rubus fruticosus L.) | Jam | Boiling for 30 min Composition: 67% fruit, 33% sugar; no addition of pectin and citric acid | 80% total anthocyanins degradation | [62] |
| Red raspberries (Rubus idaeus L.) | Jam | Boiling for 30 min Composition: 67% fruit, 33% sugar; no addition of pectin and citric acid | 66% total anthocyanins degradation | [62] |
| Strawberries (Fragaria x ananassa, cultivars Chandler, Tudla and Oso Grande) | Jam | Thermal treatment for 15 min at 78 °C under vacuum, then heated at 92 °C; addition of pectin and citric acid to fruit composition | 36–43% total anthocyanins degradation | [63] |
| Sweet cherries (Prunus avium L., cultivated and wild) | Jam | Boiling for 30 min Composition: 67% fruit, 33% sugar; no addition of pectin and citric acid | 66% total anthocyanins degradation in cultivated cherries 80% total anthocyanins degradation in wild cherries | [62] |
| Blackberries (Rubus sp., cv. Apache | Juice | Blanching for 3 min at 95 °C, enzymatic treatment, pasteurization at 90 °C | ~67% anthocyanin content decrease | [64] |
| Blueberries (Vaccinium corymbosum L.) | Juice | Thawing, depectinization at 43 °C, pasteurization at 90 °C for 1 min | 32% of anthocyanins recovered in single-strength juice; 53% recovery of chlorogenic acid | [65] |
| Strawberries (Fragaria ananassa Duch, cultivar Camarosa) | Juice | Pasteurization: (a) 30 s at 90 °C (b) 60 s at 90 °C | 4% anthocyanins degradation (30 s at 90 °C) 9% anthocyanins degradation (60 s at 90 °C) | [66] |
| Strawberries (Fragaria ananassa) | Juice | Pasteurization in glass bottles, for 15 min at 85 °C | 21% anthocyanins degradation | [67] |
| Blueberries (Vaccinium corymbosum) | Dried fruits | Drying of whole fruits at 90 °C for 90 min, followed by 70 °C for 120 min, and finally 50 °C for 120 min | 41% total anthocyanins degradation | [68] |
| Purple carrot (Daucus carota L., Deep Purple, Purple Haze) | Dried slices | Drying (1) convective, 70 °C; (2) microwave 40 °C; (3) freeze-drying | 50% total anthocyanins degradation (convective drying) 20% total anthocyanins degradation (microwave/Deep Purple) 30% total anthocyanins degradation loss (freeze-drying/Purple Haze) | [69] |
| Purple potato (Solanum tuberosum) | Dried slices | Air-impingement jet drying of slices at 50, 65 and 80 °C | Kinetic parameters: t1/2 = 103.45 min (drying at 50 °C) t1/2 = 82.52 (drying at 65 °C) t1/2 = 64.78 (drying at 80 °C), | [70] |
| Strawberries (Fragaria x Ananassa, Dutch) | Dried slices | Drying of cut slices at 60, 70, 80 and 90 °C using a hot-air experimental tunnel dryer | Total anthocyanins degradation by: 37.04% (60 °C), 50.17% (70 °C), 52.91% (80 °C) 55.32% (90 °C) | [71] |
| Acerola (Malpighia emarginata D.C.) | Pulp | Industrial pasteurization (at 60, 70, 80 and 90 °C) | 0.805% anthocyanin loss, at 90 °C for low residence time (20 s) | [28] |
| Blueberries (Vaccinium corymbosum, cv. Bluecrop) | Puree | Heating of blended berries at 95 °C, cooling and addition of corn syrup (18° Brix), heating at 92.8 °C, and canning in jars | 43% anthocyanin loss | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oancea, S. A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat. Antioxidants 2021, 10, 1337. https://doi.org/10.3390/antiox10091337
Oancea S. A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat. Antioxidants. 2021; 10(9):1337. https://doi.org/10.3390/antiox10091337
Chicago/Turabian StyleOancea, Simona. 2021. "A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat" Antioxidants 10, no. 9: 1337. https://doi.org/10.3390/antiox10091337
APA StyleOancea, S. (2021). A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat. Antioxidants, 10(9), 1337. https://doi.org/10.3390/antiox10091337