Humulus lupulus Cone Extract Efficacy in Alginate-Based Edible Coatings on the Quality and Nutraceutical Traits of Fresh-Cut Kiwifruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
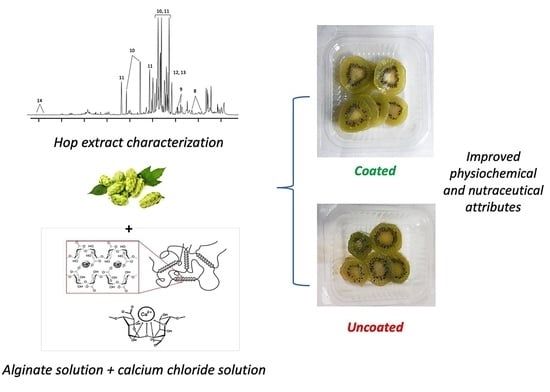
2.3. Preparation and Characterization of Plant Extracts
2.4. Preparation and Application of Functionalized ECs
2.5. Quality Traits of Fresh-Cut Kiwifruit
2.6. Nutraceutical Traits of Fresh-Cut Kiwifruit
2.7. Visual Appearance of Fresh-Cut Kiwifruit
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of Hop Extract
3.2. Influence of Active ECs on the Fresh-Cut Kiwifruit Quality Parameters
3.3. Influence of Active ECs on the Fresh-Cut Kiwifruit Nutraceutical Parameters
3.4. Influence of Active ECs on the Fresh-Cut Kiwifruit Visual Appearance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fruitlogistica. 2020. Available online: https://www.fruitlogistica.com/FRUIT-LOGISTICA/Downloads-Alle-Sprachen/Auf-einen-Blick/European_Statistics_Handbook_2020.pdf (accessed on 12 August 2021).
- Duquesne, B.; Matendo, S.; Lebailly, P. Profiling food consumption: Comparison between USA and EU. In Competitiveness in Agriculture and the Food Industry: US and EU Perspectives; Boll, E., Fanfani, R., Gutierrez, L., Ricci-Maccarini, E., Eds.; Bonomia University Press: Bologna, Italy, 2006; pp. 1–11. [Google Scholar]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Dhumal, C.V.; Sarkar, P. Composite edible films and coatings from food-grade biopolymers. J. Food Sci. Technol. 2018, 55, 4369–4383. [Google Scholar] [CrossRef] [PubMed]
- Sapper, M.; Chiralt, A. Starch-Based Coatings for Preservation of Fruits and Vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Dubey, N.K.; Dubey, R. Edible films and coatings: An update on recent advances. In Biopolymer-Based Formulations; Pal, K., Banerjee, I., Sarkar, P., Kim, D., Deng, W.-P., Dubey, N.K., Majumder, K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 675–695. [Google Scholar]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and Functionalized Films/Coatings—Performances and Perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- Carbone, K.; Macchioni, V.; Petrella, G.; Cicero, D.O. Exploring the potential of microwaves and ultrasounds in the green extraction of bioactive compounds from Humulus lupulus for the food and pharmaceutical industry. Ind. Crop. Prod. 2020, 156, 112888. [Google Scholar] [CrossRef]
- Licciardo, F.; Carbone, K.; Ievoli, C.; Manzo, A.; Tarangioli, S. Outlook Economico-Statistico del Comparto Luppolo. CREA: Rome, Italy, 2021; ISBN 9788833851228. [Google Scholar]
- Bajić, M.; Jalšovec, H.; Travan, A.; Novak, U.; Likozar, B. Chitosan-based films with incorporated supercritical CO2 hop extract: Structural, physicochemical, and antibacterial properties. Carbohydr. Polym. 2019, 219, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, T.; Liu, Y. The physical properties, antioxidant and antimicrobial activity of chitosan–gelatin edible films incorporated with the extract from hop plant. Polym. Bull. 2020, 78, 3607–3624. [Google Scholar] [CrossRef]
- Nieto, C.; Carballo, D.E.; Caro, I.; Quinto, E.J.; Andrés, S.; Mateo, J. Immersing fresh chicken into an aqueous hop (Humulus lupulus) extract to delay spoilage during vacuum refrigerated storage. CyTA J. Food 2020, 18, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2019, 96, 253–267. [Google Scholar] [CrossRef]
- Faostat. Available online: http://faostat3.fao.org/browse/Q/QC/E (accessed on 13 April 2021).
- Ciccoritti, R.; Paliotta, M.; Amoriello, T.; Carbone, K. FT-NIR spectroscopy and multivariate classification strategies for the postharvest quality of green-fleshed kiwifruit varieties. Sci. Hortic. 2019, 257. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Liu, M.; Wang, X.; Zhao, G.; Zong, W. Effect of poly-ε-lysine incorporated into alginate-based edible coatings on microbial and physicochemical properties of fresh-cut kiwifruit. Postharvest Biol. Technol. 2017, 134, 114–121. [Google Scholar] [CrossRef]
- Manzoor, S.; Gull, A.; Wani, S.M.; Ganaie, T.A.; Masoodi, F.A.; Bashir, K.; Malik, A.; Dar, B. Improving the shelf life of fresh cut kiwi using nanoemulsion coatings with antioxidant and antimicrobial agents. Food Biosci. 2021, 41, 101015. [Google Scholar] [CrossRef]
- AOAC, Association of Official Analytical Chemists Official. Methods of Analysis of AOAC International, 17th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Ciccoritti, R.; Paliotta, M.; Centioni, L.; Mencarelli, F.; Carbone, K. The effect of genotype and drying condition on the bioactive compounds of sour cherry pomace. Eur. Food Res. Technol. 2017, 244, 635–645. [Google Scholar] [CrossRef]
- Carbone, K.; Giannini, B.; Picchi, V.; Scalzo, R.L.; Cecchini, F. Phenolic composition and free radical scavenging activity of different apple varieties in relation to the cultivar, tissue type and storage. Food Chem. 2011, 127, 493–500. [Google Scholar] [CrossRef]
- Tinoco, M.B.; Kaulmann, A.; Corte-Real, J.; Rodrigo, D.; Martínez-Navarrete, N.; Bohn, T. Chlorophylls and carotenoids of kiwifruit puree are affected similarly or less by microwave than by conventional heat processing and storage. Food Chem. 2015, 187, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Effects of citrus essential oils incorporated in alginate coating on quality of fresh-cut Jintao kiwifruit. J. Food Nutr. Res. 2019, 58, 177–186. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; Sage Publications, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Macchioni, V.; Carbone, K.; Cataldo, A.; Fraschini, R.; Bellucci, S. Lactic acid-based deep natural eutectic solvents for the extraction of bioactive metabolites of Humulus lupulus L.: Supramolecular organization, phytochemical profiling and biological activity. Sep. Purif. Technol. 2020, 264, 118039. [Google Scholar] [CrossRef]
- Fan, N.; Wang, X.; Sun, J.; Lv, X.; Gu, J.; Zhao, C.; Wang, D. Effects of konjac glucomannan/pomegranate peel extract composite coating on the quality and nutritional properties of fresh-cut kiwifruit and green bell pepper. J. Food Sci. Technol. 2021, 1–11. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M. Relationship between ripe soluble solids concentration (RSSC) and consumer acceptance of high and low acid melting flesh peach and nectarine (Prunus persica (L.) Batsch) cultivars. Postharvest Biol. Technol. 2005, 38, 239–246. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Sepulcre, F.; Pujola, M. Aloe vera based edible coatings improve the quality of minimally processed ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2013, 81, 29–36. [Google Scholar] [CrossRef]
- Diaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate Coatings Preserve Fruit Quality and Bioactive Compounds during Storage of Sweet Cherry Fruit. Food Bioprocess Technol. 2011, 5, 2990–2997. [Google Scholar] [CrossRef]
- Hassani, F.; Garousi, F.; Javanmard, M. Edible coating based on whey protein concentrate-rice bran oil to maintain the physical and chemical properties of the kiwifruit Actinidia deliciosa. Trakia J. Sci. 2012, 10, 26–34. [Google Scholar]
- Baldwin, E.; Nisperos, M.; Chen, X.; Hagenmaier, R. Improving storage life of cut apple and potato with edible coating. Postharvest Biol. Technol. 1996, 9, 151–163. [Google Scholar] [CrossRef]
- Carbone, K.; Amoriello, T.; Iadecola, R. Exploitation of Kiwi Juice Pomace for the Recovery of Natural Antioxidants through Microwave-Assisted Extraction. Agriculture 2020, 10, 435. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef] [Green Version]
- Brosnan, T.; Sun, D.-W. Improving quality inspection of food products by computer vision––A review. J. Food Eng. 2004, 61, 3–16. [Google Scholar] [CrossRef]











| Parameters | Values |
|---|---|
| α acids (%) | 3.3 ± 0.1 |
| β acids (%) | 3.7 ± 0.1 |
| Xanthohumol (mg g−1) | 4.3 ± 0.1 |
| Total phenolic content (mg GAE g−1) | 12.9 ± 0.9 |
| Treatment | Storage Days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | TA 1 | |||||||||
| 0 | 2 | 5 | 7 | 10 | 0 | 2 | 5 | 7 | 10 | |
| CTRL 2 | 3.17 ± 0.01 aA | 3.18 ± 0.04 aB | 3.28 ± 0.01 bC | 3.35 ± 0.01 cC | 3.35 ± 0.03 cC | 1.43 ± 0.05 bA | 1.39 ± 0.02 aA | 1.39 ± 0.04 aA | 1.41 ± 0.05 aB | 1.38 ± 0.03 aA |
| ALG 3 | 3.17 ± 0.01 aA | 3.21 ± 0.01 bC | 3.25 ± 0.01 bB | 3.24 ± 0.01 bB | 3.24 ± 0.01 bB | 1.43 ± 0.07 aA | 1.43 ± 0.05 aB | 1.45 ± 0.06 aB | 1.45 ± 0.02 aA | 1.47 ± 0.05 aB |
| ALG_HE_0.5 4 | 3.17 ± 0.02 aA | 3.15 ± 0.01 aA | 3.16 ± 0.04 aA | 3.18 ± 0.01 aA | 3.17 ± 0.01 aA | 1.43 ± 0.05 aA | 1.41 ± 0.05 aB | 1.41 ± 0.05 aB | 1.45 ± 0.01 aA | 1.43 ± 0.05 aB |
| ALG_HE_1 5 | 3.17 ± 0.01 aA | 3.17 ± 0.01 aA | 3.18 ± 0.02 aA | 3.17 ± 0.01 aA | 3.19 ± 0.02 aA | 1.43 ± 0.04 aA | 1.43 ± 0.02 aB | 1.44 ± 0.02 aB | 1.40 ± 0.04 aA | 1.43 ± 0.05 aB |
| Storage Time (d) | ||
|---|---|---|
| Sample | 0 | 10 |
| CTRL | 7.7 ± 0.3 bA | 3.1 ± 0.1 aA |
| ALG | 8.6 ± 0.1 bB | 6.5 ± 0.1 aB |
| ALG_HE_0.5 | 8.9 ± 0.1 bB | 7.1 ± 0.2 aC |
| ALG_HE_1 | 8.6 ± 0.3 aB | 7.3 ± 0.1 aC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, K.; Macchioni, V.; Petrella, G.; Cicero, D.O.; Micheli, L. Humulus lupulus Cone Extract Efficacy in Alginate-Based Edible Coatings on the Quality and Nutraceutical Traits of Fresh-Cut Kiwifruit. Antioxidants 2021, 10, 1395. https://doi.org/10.3390/antiox10091395
Carbone K, Macchioni V, Petrella G, Cicero DO, Micheli L. Humulus lupulus Cone Extract Efficacy in Alginate-Based Edible Coatings on the Quality and Nutraceutical Traits of Fresh-Cut Kiwifruit. Antioxidants. 2021; 10(9):1395. https://doi.org/10.3390/antiox10091395
Chicago/Turabian StyleCarbone, Katya, Valentina Macchioni, Greta Petrella, Daniel Oscar Cicero, and Laura Micheli. 2021. "Humulus lupulus Cone Extract Efficacy in Alginate-Based Edible Coatings on the Quality and Nutraceutical Traits of Fresh-Cut Kiwifruit" Antioxidants 10, no. 9: 1395. https://doi.org/10.3390/antiox10091395
APA StyleCarbone, K., Macchioni, V., Petrella, G., Cicero, D. O., & Micheli, L. (2021). Humulus lupulus Cone Extract Efficacy in Alginate-Based Edible Coatings on the Quality and Nutraceutical Traits of Fresh-Cut Kiwifruit. Antioxidants, 10(9), 1395. https://doi.org/10.3390/antiox10091395