From Green Technology to Functional Olive Oils: Assessing the Best Combination of Olive Tree-Related Extracts with Complementary Bioactivities
Abstract
1. Introduction
2. Materials and Methods
2.1. Extracts of Bioactive Components of the Olive Tree
2.1.1. Phenolic Compound Extracts
2.1.2. Triterpenic Acid Solutions
2.1.3. Tested Concentrations
2.2. In Vitro Bioactivities
2.2.1. Antioxidant Properties
2.2.2. Vasoactive Properties
2.2.3. Anti-Inflammatory and Antithrombotic Properties
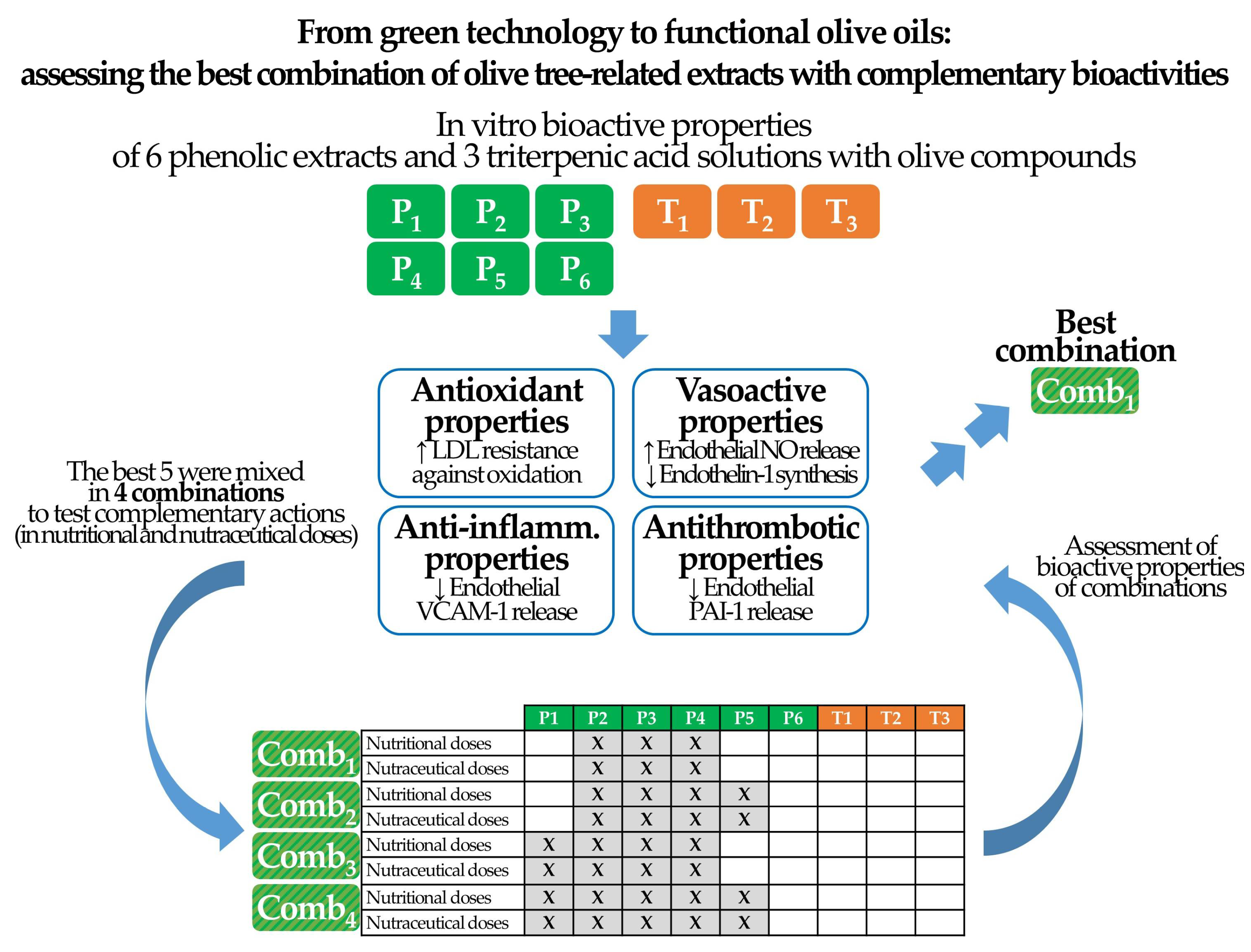
2.3. Evidence-Based Combination of Extracts
3. Results
3.1. Dose-Dependent Bioactivities of Individual Extracts
3.2. Combinations of Extracts
3.3. Effects of Extract Combinations
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Bouknana, D.; Jodeh, S.; Sbaa, M.; Hammouti, B.; Arabi, M.; Darmous, A.; Slamini, M.; Haboubi, K. A phytotoxic impact of phenolic compounds in olive oil mill wastewater on fenugreek “Trigonella foenum-graecum”. Environ. Monit. Assess. 2019, 191, 405. [Google Scholar] [CrossRef] [PubMed]
- Casazza, A.A.; Aliakbarian, B.; Perego, P. Recovery of phenolic compounds from grape seeds: Effect of extraction time and solid-liquid ratio. Nat. Prod. Res. 2011, 25, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Caboni, M.F.; Gianotti, A.; Bordoni, A.; Capozzi, F. Olive oil industry by-products. Effects of a polyphenol-rich extract on the metabolome and response to inflammation in cultured intestinal cell. Food Res. Int. 2018, 113, 392–400. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Gaforio, J.J.; Visioli, F.; Alarcón-De-la-lastra, C.; Castañer, O.; Delgado-Rodríguez, M.; Fitó, M.; Hernández, A.F.; Huertas, J.R.; Martínez-González, M.A.; Menendez, J.A.; et al. Virgin olive oil and health: Summary of the iii international conference on virgin olive oil and health consensus report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039. [Google Scholar] [CrossRef]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Boronat, A.; Mateus, J.; Soldevila-Domenech, N.; Guerra, M.; Rodríguez-Morató, J.; Varon, C.; Muñoz, D.; Barbosa, F.; Morales, J.C.; Gaedigk, A.; et al. Cardiovascular benefits of tyrosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free Radic. Biol. Med. 2019, 143, 471–481. [Google Scholar] [CrossRef]
- Moreno-Luna, R.; Muñoz-Hernandez, R.; Miranda, M.L.; Costa, A.F.; Jimenez-Jimenez, L.; Vallejo-Vaz, A.J.; Muriana, F.J.G.G.G.; Villar, J.; Stiefel, P. Olive Oil Polyphenols Decrease Blood Pressure and Improve Endothelial Function in Young Women with Mild Hypertension. Am. J. Hypertens. 2012, 25, 1299–1304. [Google Scholar] [CrossRef]
- Pedret, A.; Fernández-Castillejo, S.; Valls, R.-M.; Catalán, Ú.; Rubió, L.; Romeu, M.; Macià, A.; López de las Hazas, M.C.; Farràs, M.; Giralt, M.; et al. Cardiovascular Benefits of Phenol-Enriched Virgin Olive Oils: New Insights from the Virgin Olive Oil and HDL Functionality (VOHF) Study. Mol. Nutr. Food Res. 2018, 62, 1800456. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Osada, J. Could squalene be an added value to use olive by-products? J. Sci. Food Agric. 2020, 100, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the Prevention of the Metabolic Syndrome and Related Disorders. Nutrients 2017, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Virruso, C.; Accardi, G.; Colonna-Romano, G.; Candore, G.; Vasto, S.; Caruso, C. Nutraceutical properties of extra-virgin olive oil: A natural remedy for age-related disease? Rejuvenation Res. 2014, 17, 217–220. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Fernández-Bolaños Guzmán, J.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Senent-Rubio, F.; Fernández-Bolaños Guzmán, J.M.; Maya-Castilla, I.; López-López, Ó.; Marset-Castro, A. Patent PCT/ES2012/070491. Method for Obtaining Hydroxytyrosol Extract, Mixture of Hydroxytyrosol and 3,4-dihydroxyphenylglycol Extract, and Hydroxytyrosyl Acetate Extract, from by-Products of the Olive Tree, and the Purification Thereof. 2013. Available online: https://patentscope.wipo.int/search/es/detail.jsf;jsessionid=A98E18EC359BACA7E49A8EFD65AC4A03.wapp2nA?docId=WO2013007850&recNum=105&office=&queryString=%28ANA%3AES%29&prevFilter=&sortOption=PubDateDesc&maxRec=32493 (accessed on 21 December 2020).
- Fernandez-Bolaños Guzman, J.; Heredia Moreno, A.; Rodriguez Gutierrez, G.; Rodriguez Arcos, R.; Jimenez Araujo, A.; Guillen Bejarano, R. Patent PCT/ES2002/000058. Method for Obtaining Purified Hydroxytyrosol from Products and By-Products Derived from the Olive Tree. 2002. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2002064537 (accessed on 21 December 2020).
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Heredia, A.; Guillén, R.; Jimínez, A. Production in large quantities of highly purified hydroxytyrosol from liquid-solid waste of two-phase olive oil processing or “alperujo”. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and Hydrolyzable Phenolic Compounds in Virgin Olive Oil. 1. Their Extraction, Separation, and Quantitative and Semiquantitative Evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Vulcano, I.; Halabalaki, M.; Skaltsounis, L.; Ganzera, M. Quantitative analysis of pungent and anti-inflammatory phenolic compounds in olive oil by capillary electrophoresis. Food Chem. 2015, 169, 381–386. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Miro-Casas, E.; Covas, M.-I.I.; Farre, M.; Fito, M.; Ortuño, J.; Weinbrenner, T.; Roset, P.; De La Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef]
- Catalán, Ú.; López de las Hazas, M.C.; Rubió, L.; Fernández-Castillejo, S.; Pedret, A.; de la Torre, R.; Motilva, M.J.; Solà, R. Protective effect of hydroxytyrosol and its predominant plasmatic human metabolites against endothelial dysfunction in human aortic endothelial cells. Mol. Nutr. Food Res. 2015, 59, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- Pozo, O.J.; Pujadas, M.; Gleeson, S.B.; Mesa-García, M.D.; Pastor, A.; Kotronoulas, A.; Fitó, M.; Covas, M.I.; Navarro, J.R.F.; Espejo, J.A.; et al. Liquid chromatography tandem mass spectrometric determination of triterpenes in human fluids: Evaluation of markers of dietary intake of olive oil and metabolic disposition of oleanolic acid and maslinic acid in humans. Anal. Chim. Acta 2017, 990, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, A.; Remaley, A.T.A.T.; Farràs, M.; Fernández-Castillejo, S.; Subirana, I.; Schröder, H.; Fernández-Mampel, M.; Muñoz-Aguayo, D.; Sampson, M.; Solà, R.; et al. Olive Oil Polyphenols Decrease LDL Concentrations and LDL Atherogenicity in Men in a Randomized Controlled Trial. J. Nutr. 2015, 145, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, Á.; Castañer, O.; Goday, A.; Ros, E.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; et al. The Mediterranean Diet decreases LDL atherogenicity in high cardiovascular risk individuals: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1601015. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, A.; Fernández-Castillejo, S.; Farràs, M.; Catalán, U.; Subirana, I.; Montes, R.; Solà, R.; Muñoz-Aguayo, D.; Gelabert-Gorgues, A.; Díaz-Gil, O.; et al. Olive oil polyphenols enhance high-density lipoprotein function in humans: A randomized controlled trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, A.; Castañer, O.; Elosua, R.; Pinto, X.; Estruch, R.R.; Salas-Salvado, J.; Corella, D.; Aros, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals: A Randomized Controlled Trial. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Barnett, J.; Fong, L.G. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim. Biophys. Acta 1990, 1044, 275–283. [Google Scholar] [CrossRef]
- Räthel, T.R.; Leikert, J.F.; Vollmar, A.M.; Dirsch, V.M. Application of 4,5-diaminofluorescein to reliably measure nitric oxide released from endothelial cells in vitro. Biol. Proced. Online 2003, 5, 136–142. [Google Scholar] [CrossRef]
- Rapoport, R.M. Nitric oxide inhibition of endothelin-1 release in the vasculature: In vivo relevance of in vitro findings. Hypertension 2014, 64, 908–914. [Google Scholar] [CrossRef]
- Carlos, T.M.; Schwartz, B.R.; Kovach, N.L.; Yee, E.; Rosa, M.; Osborn, L.; Chi-Rosso, G.; Newman, B.; Lobb, R.; Rosso, M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood 1990, 76, 965–970. [Google Scholar] [CrossRef]
- van Hinsbergh, V.W.; Kooistra, T.; van den Berg, E.A.; Princen, H.M.; Fiers, W.; Emeis, J.J. Tumor necrosis factor increases the production of plasminogen activator inhibitor in human endothelial cells in vitro and in rats in vivo. Blood 1988, 72, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Castañer, O.; Martín-Peláez, S.; Hernáez, Á.; Schröder, H.; Subirana, I.; Muñoz-Aguayo, D.; Gaixas, S.; de la Torre, R.; Farré, M.; et al. Complementary phenol-enriched olive oil improves HDL characteristics in hypercholesterolemic subjects. A randomized, double-blind, crossover, controlled trial. The VOHF study. Mol. Nutr. Food Res. 2015, 59, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Pedret, A.; Catalán, Ú.; Fernández-Castillejo, S.; Farràs, M.; Valls, R.-M.; Rubió, L.; Canela, N.; Aragonés, G.; Romeu, M.; Castañer, O.; et al. Impact of Virgin Olive Oil and Phenol-Enriched Virgin Olive Oils on the HDL Proteome in Hypercholesterolemic Subjects: A Double Blind, Randomized, Controlled, Cross-Over Clinical Trial (VOHF Study). PLoS ONE 2015, 10, e0129160. [Google Scholar] [CrossRef]
- Fernández-Castillejo, S.; Rubió, L.; Hernáez, A.; Catalán, U.; Pedret, A.; Valls, R.-M.; Mosele, J.I.; Covas, M.-I.; Remaley, A.T.; Castañer, O.; et al. Determinants of HDL Cholesterol Efflux Capacity after Virgin Olive Oil Ingestion: Interrelationships with Fluidity of HDL Monolayer. Mol. Nutr. Food Res. 2017, 61, 1700445. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Fernández-Castillejo, S.; Rubió, L.; Arranz, S.; Catalán, Ú.; Subirana, I.; Romero, M.-P.; Castañer, O.; Pedret, A.; Blanchart, G.; et al. Phenol-enriched olive oils improve HDL antioxidant content in hypercholesterolemic subjects. A randomized, double-blind, cross-over, controlled trial. J. Nutr. Biochem. 2018, 51, 99–104. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Duthie, G.G.; Wood, S.; Morrice, P.; Nicol, F.; Reid, M.; Cantlay, L.L.; Kelder, T.; Horgan, G.W.; Fernández-Bolaños Guzmán, J.; et al. Alperujo extract, hydroxytyrosol, and 3,4-dihydroxyphenylglycol are bioavailable and have antioxidant properties in vitamin E-deficient rats-a proteomics and network analysis approach. Mol. Nutr. Food Res. 2012, 56, 1131–1147. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sánchez-Fidalgo, S.; González-Benjumea, A.; Maya, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Naturally occurring hydroxytyrosol derivatives: Hydroxytyrosyl acetate and 3,4-dihydroxyphenylglycol modulate inflammatory response in murine peritoneal macrophages. potential utility as new dietary supplements. J. Agric. Food Chem. 2015, 63, 836–846. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Resende, D.; Monteiro, M.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Application of hydroxytyrosol in the functional foods field: From ingredient to dietary supplements. Antioxidants 2020, 9, 1246. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Young, M.; Tsiani, E. Antidiabetic effects of hydroxytyrosol: In vitro and in vivo evidence. Antioxidants 2019, 8, 188. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a bioactive compound from olea europaea l., as a potential preventive and therapeutic agent in non-communicable diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- De Roos, B.; Zhang, X.; Rodriguez Gutierrez, G.; Wood, S.; Rucklidge, G.J.; Reid, M.D.; Duncan, G.J.; Cantlay, L.L.; Duthie, G.G.; O’Kennedy, N. Anti-platelet effects of olive oil extract: In vitro functional and proteomic studies. Eur. J. Nutr. 2011, 50, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castellón, J.; López-Yerena, A.; Rinaldi de Alvarenga, J.F.; Romero del Castillo-Alba, J.; Vallverdú-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Kicel, A.; Owczarek, A.; Kapusta, P.; Kolodziejczyk-Czepas, J.; Olszewska, M.A. Contribution of individual polyphenols to antioxidant activity of cotoneaster bullatus and Cotoneaster zabelii leaves—Structural relationships, synergy effects and application for quality control. Antioxidants 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.; Beloborodov, V.; Antonov, D.; Dubrovskaya, A.; Terekhov, R.; Zhevlakova, A.; Saydasheva, A.; Evteev, V.; Selivanova, I. Flavonoids with glutathione antioxidant synergy: Influence of free radicals inflow. Antioxidants 2020, 9, 695. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, R.; Carbó, M.; Pujadas, M.; Biel, S.; Mesa, M.D.; Covas, M.I.; Expósito, M.; Espejo, J.A.; Sanchez-Rodriguez, E.; Díaz-Pellicer, P.; et al. Pharmacokinetics of maslinic and oleanolic acids from olive oil—Effects on endothelial function in healthy adults. A randomized, controlled, dose–response study. Food Chem. 2020, 322, 126676. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.-J.; Macià, A.; Ramo, T.; Romero, M.-P. Development of a phenol-enriched olive oil with both its own phenolic compounds and complementary phenols from thyme. J. Agric. Food Chem. 2012, 60, 3105–3112. [Google Scholar] [CrossRef]
- Vazquez, A.; Sanchez-Rodriguez, E.; Vargas, F.; Montoro-Molina, S.; Romero, M.; Espejo-Calvo, J.A.; Vilchez, P.; Jaramillo, S.; Olmo-García, L.; Carrasco-Pancorbo, A.; et al. Cardioprotective effect of a virgin olive oil enriched with bioactive compounds in spontaneously hypertensive rats. Nutrients 2019, 11, 1728. [Google Scholar] [CrossRef]
| Antioxidant Properties | Vasoactive Properties | Anti-Inflammatory Properties | Anticoagulant Properties | Score Sum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Increase in LDL Lag Time | Decrease in LDL Oxidation Rate | Weighted Score | Increase in Nitric Oxide Release | Decrease in Endothelin-1 Release | Weighted Score | Decrease in VCAM-1 Release | Decrease in PAI-1 Release | ||||||||
| % [95% CI] | Score | % [95% CI] | Score | % [95% CI] | Score | % [95% CI] | Score | % [95% CI] | Score | % [95% CI] | Score | ||||
| P1 | 168 | 7 | −67.4 | 8 | 7.33 | 10.3 | 9 | −0.33 | 0 | 6 | −0.97 | 4 | −1.05 | 0 | 17.33 |
| [133; 203] | [−79.1; −55.7] | [8.79; 11.8] | [−2.63; 1.97] | [−1.15; −0.78] | [−2.94; 0.85] | (4th) | |||||||||
| P2 | 171 | 8 | −59.7 | 7 | 7.67 | 9.77 | 8 | −0.94 | 8 | 8 | −1.62 | 5 | −0.47 | 0 | 20.67 |
| [145; 197] | [−66.5; −52.9] | [8.71; 10.8] | [−1.27; −0.62] | [−2.19; −1.05] | [−1.37; 0.43] | (3rd) | |||||||||
| P3 | 70.8 | 6 | −26.9 | 6 | 6 | −0.2 | 0 | −0.39 | 7 | 2.33 | −1.93 | 6 | 0.41 | 0 | 14.33 |
| [34.8; 107] | [−43.2; −10.6] | [−0.74; 0.33] | [−0.65; −0.12] | [−3.27; −0.60] | [−0.43; 1.25] | (5th) | |||||||||
| P4 | 210 | 9 | −71.1 | 9 | 9 | 4.75 | 7 | 0.33 | 0 | 4.67 | −2.05 | 7 | −0.51 | 8 | 28.67 |
| [126; 294] | [−92.7; −49.5] | [4.11; 5.40] | [−0.49; 1.16] | [−2.45; −1.66] | [−0.90; −0.11] | (2nd) | |||||||||
| P5 | 21.9 | 4 | −12.2 | 5 | 4.33 | 0.86 | 6 | −4.8 | 9 | 7 | −3.74 | 9 | −5.78 | 9 | 29.33 |
| [19.7; 24.1] | [−17.4; −6.91] | [0.51; 1.21] | [−6.17; −3.43] | [−7.22; −0.26] | [−6.29; −5.27] | (1st) | |||||||||
| P6 | 67.3 | 5 | −11.1 | 4 | 4.67 | −0.39 | 0 | 0.024 | 0 | 0 | −2.49 | 8 | 1.51 | 0 | 12.67 |
| [35.5; 99.0] | [−12.6; −9.61] | [−0.88; 0.087] | [−0.32; 0.37] | [−4.71; −0.28] | [0.52; 2.50] | (6th) | |||||||||
| T1 | −0.62 | 0 | 1.17 | 0 | 0 | 0.24 | 0 | 0.77 | 0 | 0 | 0.96 | 0 | −0.76 | 0 | 0 |
| [−1.07; −0.17] | [0.40; 1.94] | [−0.36; 0.84] | [0.40; 1.14] | [−2.38; 4.30] | [−4.94; 3.42] | (7th) | |||||||||
| T2 | −0.042 | 0 | 0.82 | 0 | 0 | −0.22 | 0 | −0.96 | 0 | 0 | −1.51 | 0 | −0.66 | 0 | 0 |
| [−0.33; 0.25] | [0.31; 1.32] | [−0.52; 0.070] | [−3.12; 1.20] | [−4.62; 1.60] | [−1.47; 0.15] | (7th) | |||||||||
| T3 | −0.042 | 0 | 1.13 | 0 | 0 | −0.13 | 0 | 0.79 | 0 | 0 | −2.27 | 0 | −2.59 | 0 | 0 |
| [−0.33; 0.25] | [−0.030; 2.30] | [−0.97; 0.71] | [0.59; 0.99] | [−8.95; 4.40] | [−7.22; 2.04] | (7th) | |||||||||
| P1 (μmol/L) | P2 (μmol/L) | P3 (μmol/L) | P4 (μmol/L) | P5 (μmol/L) | P6 (μmol/L) | T1 (μmol/L) | T2 (μmol/L) | T3 (μmol/L) | Sum | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | Nutritional doses | 0.05 | 0.05 | 0.05 | 0.15 μmol/L | ||||||
| Nutraceutical doses | 1 | 1 | 1 | 3 μmol/L | |||||||
| C2 | Nutritional doses | 0.05 | 0.05 | 0.05 | 0.05 | 0.2 μmol/L | |||||
| Nutraceutical doses | 1 | 1 | 1 | 1 | 4 μmol/l | ||||||
| C3 | Nutritional doses | 0.05 | 0.05 | 0.05 | 0.05 | 0.2 μmol/L | |||||
| Nutraceutical doses | 1 | 1 | 1 | 1 | 4 μmol/l | ||||||
| C4 | Nutritional doses | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.25 μmol/L | ||||
| Nutraceutical doses | 1 | 1 | 1 | 1 | 1 | 5 μmol/L |
| Antioxidant properties | Vasoactive properties | Anti-Inflammatory Properties | Anticoagulant Properties | Score Sum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Increase in LDL Lag Time | Decrease in LDL Oxidation Rate | Weighted Score | Increase in Nitric Oxide Release | Decrease in endothelin-1 Release | Weighted Score | Decrease in VCAM-1 Release | Decrease in PAI-1 Release | ||||||||
| % | Score | % | Score | % | Score | % | Score | % | Score | % | Score | ||||
| Nutritional doses | |||||||||||||||
| C1 | 606.1 | 4 | −14.3 | 4 | 4 | 3.7 | 4 | −6.0 | 2 | 3.33 | 2.5 | 0 | −5.3 | 4 | 11.33 (1st) |
| C2 | 287.6 | 2 | −12.4 | 2 | 2 | 1.8 | 1 | −8.9 | 4 | 2.00 | −14.4 | 4 | −5.0 | 3 | 11.00 (2nd) |
| C3 | 390.4 | 3 | −12.5 | 3 | 3 | 2.2 | 3 | −2.7 | 1 | 2.33 | 0.9 | 0 | 5.8 | 0 | 5.33 (4th) |
| C4 | 158.6 | 1 | −12.0 | 1 | 1 | 2.1 | 2 | −8.4 | 3 | 2.33 | −8.9 | 3 | −3.6 | 2 | 8.33 (3rd) |
| Nutraceutical doses | |||||||||||||||
| C1 | 180.6 | 1 | −48.2 | 4 | 2.00 | 40.3 | 4 | −35 | 3 | 3.67 | −53.8 | 4 | −77.3 | 4 | 13.67 (1st) |
| C2 | 181.9 | 2 | −38.7 | 2.5 | 2.17 | 24.4 | 3 | −7.6 | 2 | 2.67 | −32.9 | 3 | −24.8 | 3 | 10.83 (2nd) |
| C3 | 237.8 | 4 | 8.7 | 0 | 2.67 | 15.6 | 2 | 18.9 | 0 | 1.33 | 155.9 | 0 | 728.8 | 0 | 4.00 (4th) |
| C4 | 193.8 | 3 | −38.7 | 2.5 | 2.83 | 7.2 | 1 | −53 | 4 | 2.00 | 37.8 | 0 | −6.6 | 2 | 6.83 (3rd) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernáez, Á.; Jaramillo, S.; García-Borrego, A.; Espejo-Calvo, J.A.; Covas, M.-I.; Blanchart, G.; de la Torre, R.; Carrasco-Pancorbo, A.; Mesa, M.D.; Fernández-Prior, M.Á.; et al. From Green Technology to Functional Olive Oils: Assessing the Best Combination of Olive Tree-Related Extracts with Complementary Bioactivities. Antioxidants 2021, 10, 202. https://doi.org/10.3390/antiox10020202
Hernáez Á, Jaramillo S, García-Borrego A, Espejo-Calvo JA, Covas M-I, Blanchart G, de la Torre R, Carrasco-Pancorbo A, Mesa MD, Fernández-Prior MÁ, et al. From Green Technology to Functional Olive Oils: Assessing the Best Combination of Olive Tree-Related Extracts with Complementary Bioactivities. Antioxidants. 2021; 10(2):202. https://doi.org/10.3390/antiox10020202
Chicago/Turabian StyleHernáez, Álvaro, Sara Jaramillo, Aránzazu García-Borrego, Juan Antonio Espejo-Calvo, María-Isabel Covas, Gemma Blanchart, Rafael de la Torre, Alegría Carrasco-Pancorbo, María Dolores Mesa, Maria África Fernández-Prior, and et al. 2021. "From Green Technology to Functional Olive Oils: Assessing the Best Combination of Olive Tree-Related Extracts with Complementary Bioactivities" Antioxidants 10, no. 2: 202. https://doi.org/10.3390/antiox10020202
APA StyleHernáez, Á., Jaramillo, S., García-Borrego, A., Espejo-Calvo, J. A., Covas, M.-I., Blanchart, G., de la Torre, R., Carrasco-Pancorbo, A., Mesa, M. D., Fernández-Prior, M. Á., Castañer, O., & Fitó, M. (2021). From Green Technology to Functional Olive Oils: Assessing the Best Combination of Olive Tree-Related Extracts with Complementary Bioactivities. Antioxidants, 10(2), 202. https://doi.org/10.3390/antiox10020202








