Application of Cornelian Cherry (Cornus mas L.) Peel in Probiotic Ice Cream: Functionality and Viability during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ice Creams
2.3. Analysis of Proximate Composition and Mineral Content
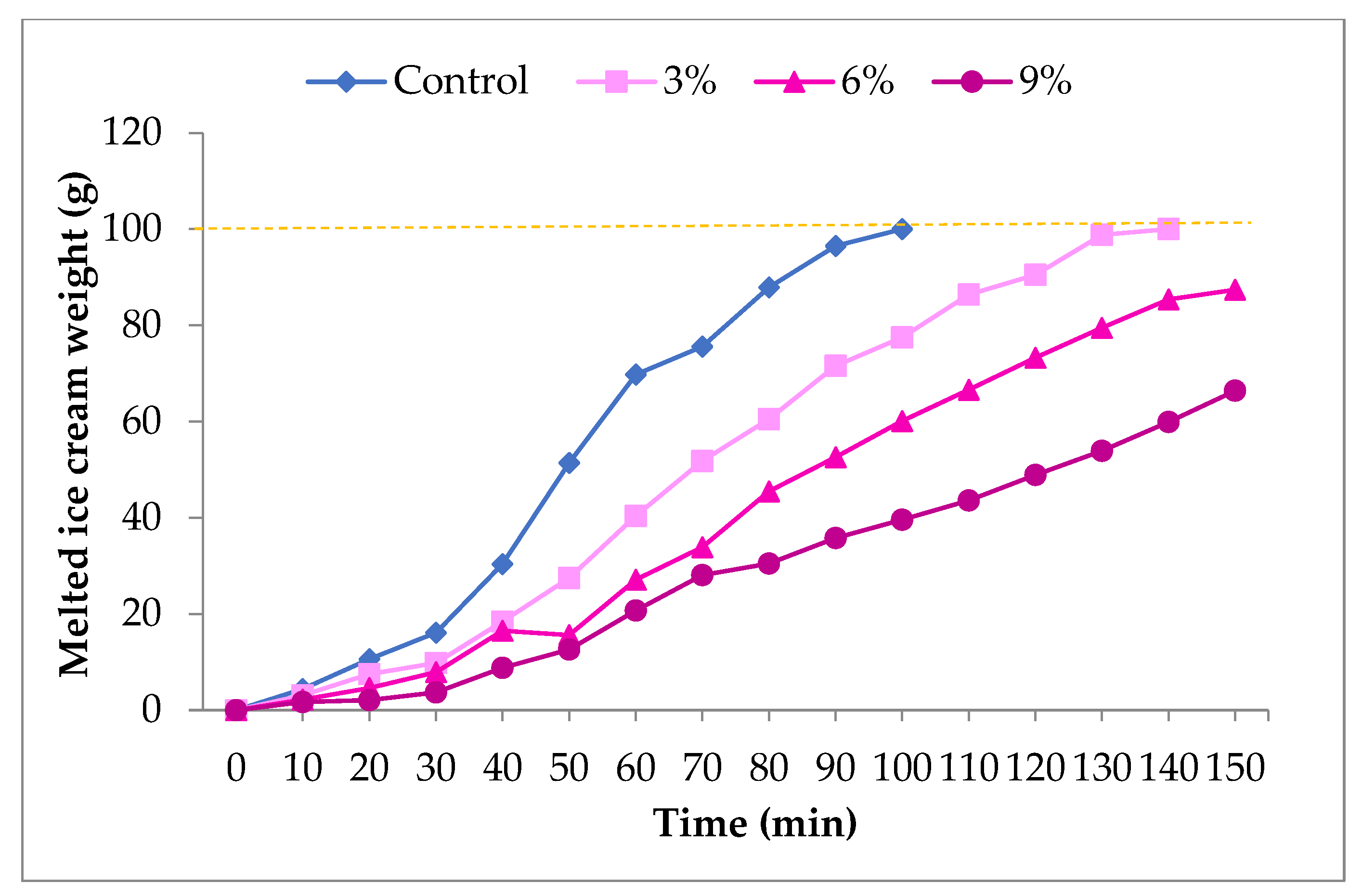
2.4. Physicochemical Properties of Ice Cream
2.5. Determination of Bioactive Compounds
2.6. Determination of Antioxidant Activity
2.7. Gastrointestinal Resistance of Probiotic Ice Cream
2.8. Viability of B. lactis
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition and Mineral Content of Ice Cream
3.2. Physicochemical Properties of Ice Creams
3.3. Bioactive and Antioxidant Activity of Ice Creams during Storage
3.4. Viability of B. lactis after Simulated Gastrointestinal Stress and during Storage
3.5. Sensory Analysis of Ice Cream
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Öztürk, H.İ.; Demirci, T.; Akın, N. Production of functional probiotic ice creams with white and dark blue fruits of Myrtus communis: The comparison of the prebiotic potentials on Lactobacillus casei 431 and functional characteristics. LWT-Food Sci. Technol. 2018, 90, 339–345. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Shyu, Y.S.; Hsu, C.K. Grape wine lees improves the rheological and adds antioxidant properties to ice cream. LWT-Food Sci. Technol. 2009, 42, 312–318. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Hsu, C.L.; Fang, S.C.; Liu, C.W.; Chen, Y.F. Inhibitory effects of new varieties of bitter melon on lipopolysaccharide-stimulated inflammatory response in RAW 264.7 cells. J. Funct. Foods 2013, 5, 1829–1837. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Albuquerque, T.M.R.; dos Santos, A.S.; Massa, N.M.L.; de Brito Alves, J.L. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities–A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef]
- Hadidi, M.; Majidiyan, N.; Jelyani, A.Z.; Moreno, A.; Hadian, Z.; Khanegah, A.M. Alginate/fish gelatin-encapsulated Lactobacillus acidophilus: A study on viability and technological quality of bread during baking and storage. Foods 2021, 10, 2215. [Google Scholar] [CrossRef] [PubMed]
- Parussolo, G.; Busatto, R.T.; Schmitt, J.; Pauletto, R.; Schons, P.F.; Ries, E.F. Synbiotic ice cream containing yacon flour and Lactobacillus acidophylus NCFM. LWT-Food Sci. Technol. 2017, 82, 192–198. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Hadidi, M.; Amoli, P.I.; Jelyani, A.Z.; Hasiri, Z.; Rouhafza, A.; Ibarz, A.; Khaksar, F.B.; Tabrizi, S.T. Polysaccharides from pineapple core as a canning by-product: Extraction optimization, chemical structure, antioxidant and functional properties. Int. J. Biol. Macromol. 2020, 163, 2357–2364. [Google Scholar] [CrossRef]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B. Extraction, characterization and potential health benefits of bioactive compounds from selected cornus fruits. In Fruit and Pomace Extracts: Biological Activity, Potential Applications and Beneficial Health Effects; Owen, J.P., Ed.; Nova Science Publishers: New York, NY, USA, 2015; pp. 157–188. [Google Scholar]
- AOAC Official Methods of Analysis of the Association of the Official Analytical Chemists. Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ (accessed on 5 November 2019).
- Erkaya, T.; Daǧdemir, E.; Sengül, M. Influence of Cape gooseberry (Physalis peruviana L.) addition on the chemical and sensory characteristics and mineral concentrations of ice cream. Food Res. Int. 2012, 45, 331–335. [Google Scholar] [CrossRef]
- Martinou-Voulasiki, I.S.; Zerfiridis, G.K. Effect of some stabilizers on textural and sensory characteristics of yogurt ice cream from sheep’s milk. J. Food Sci. 1990, 55, 703–707. [Google Scholar] [CrossRef]
- Çakmakçi, S.; Topdaş, E.F.; Kalin, P.; Han, H.; Şekerci, P.; Köse, L.; Gülçin, I. Antioxidant capacity and functionality of oleaster (Elaeagnus angustifolia L.) flour and crust in a new kind of fruity ice cream. Int. J. Food Sci. Technol. 2015, 50, 472–481. [Google Scholar] [CrossRef]
- Felicio, T.L.; Esmerino, E.A.; Vidal, V.A.S.; Cappato, L.P.; Garcia, R.K.A.; Cavalcanti, R.N.; Freitas, M.Q.; Conte, C.A., Jr.; Padilha, M.C.; Silva, M.C.; et al. Physico-chemical changes during storage and sensory acceptance of low sodium probiotic Minas cheese added with arginine. Food Chem. 2016, 196, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Krośniak, M.; Ga̧sto, M.; Szałkowski, M.; Zagrodzki, P.; Derwisz, M. Cornelian cherry (Cornus mas L.) juices as a source of minerals in human diet. J. Toxicol. Environ. Health-Part A Curr. Issues 2010, 73, 1155–1158. [Google Scholar] [CrossRef]
- Szefer, P.; Nriagu, J.O. Mineral Components in Foods; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2006; ISBN 9780849322341. [Google Scholar]
- Sun-Waterhouse, D.; Edmonds, L.; Wadhwa, S.S.; Wibisono, R. Producing ice cream using a substantial amount of juice from kiwifruit with green, gold or red flesh. Food Res. Int. 2013, 50, 647–656. [Google Scholar] [CrossRef]
- Wang, C.; He, X.W.; Huang, Q.; Fu, X.; Liu, S. Physicochemical properties and application of micronized cornstarch in low fat cream. J. Food Eng. 2013, 116, 881–888. [Google Scholar] [CrossRef]
- Yilmaz, K.U.; Ercisli, S.; Zengin, Y.; Sengul, M.; Kafkas, E.Y. Preliminary characterisation of cornelian cherry (Cornus mas L.) genotypes for their physico-chemical properties. Food Chem. 2009, 114, 408–412. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar, R.; Azad, Z.R.A.A.; Adsule, P.G. Use of fine wine lees for value addition in ice cream. J. Food Sci. Technol. 2015, 52, 592–596. [Google Scholar] [CrossRef]
- Akalin, A.S.; Erişir, D. Effects of inulin and oligofructose on the rheological characteristics and probiotic culture survival in low-fat probiotic ice cream. J. Food Sci. 2008, 73, M184–M188. [Google Scholar] [CrossRef]
- Jaćimović, V.; Božović, D.; Ercisli, S.; Ognjanov, V.; Bosančić, B. Some Fruit Characteristics of Selected Cornelian Cherries (Cornus mas L.) from Montenegro. Erwerbs-Obstbau 2015, 57, 119–124. [Google Scholar] [CrossRef]
- Kunyanga, C.N.; Imungi, J.K.; Okoth, M.W.; Biesalski, H.K.; Vadivel, V. Total phenolic content, antioxidant and antidiabetic properties of methanolic extract of raw and traditionally processed Kenyan indigenous food ingredients. LWT-Food Sci. Technol. 2012, 45, 269–276. [Google Scholar] [CrossRef]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Kavaz Yuksel, A. The Effects of Blackthorn (Prunus spinosa L.) addition on certain quality characteristics of ice cream. J. Food Qual. 2015, 38, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Vital, A.C.P.; Santos, N.W.; Matumoto-Pintro, P.T.; da Silva Scapim, M.R.; Madrona, G.S. Ice cream supplemented with grape juice residue as a source of antioxidants. Int. J. Dairy Technol. 2018, 71, 183–189. [Google Scholar] [CrossRef]
- Popović, B.M.; Štajner, D.; Slavko, K.; Sandra, B. Antioxidant capacity of cornelian cherry (Cornus mas L.)-Comparison between permanganate reducing antioxidant capacity and other antioxidant methods. Food Chem. 2012, 134, 734–741. [Google Scholar] [CrossRef]
- Skrede, G. Freezing Effects on Food Quality. In Freezing Effects on Food Quality; Jeremiah, L.E., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 183–245. ISBN 9780367401405. [Google Scholar]
- Sagdic, O.; Ozturk, I.; Cankurt, H.; Tornuk, F. Interaction between some phenolic compounds and probiotic bacterium in functional ice cream production. Food Bioprocess Technol. 2012, 5, 2964–2971. [Google Scholar] [CrossRef]
- dos Santos Cruxen, C.E.; Hoffmann, J.F.; Zandoná, G.P.; Fiorentini, Â.M.; Rombaldi, C.V.; Chaves, F.C. Probiotic butiá (Butia odorata) ice cream: Development, characterization, stability of bioactive compounds, and viability of Bifidobacterium lactis during storage. LWT-Food Sci. Technol. 2017, 75, 379–385. [Google Scholar] [CrossRef]
- Akca, S.; Akpinar, A. The Effects of grape, pomegranate, sesame seed powder and their oils on probiotic ice cream: Total phenolic contents, antioxidant activity and probiotic viability. Food Biosci. 2021, 42, 101203. [Google Scholar] [CrossRef]
- Costa, M.G.M.; Ooki, G.N.; Vieira, A.D.S.; Bedani, R.; Saad, S.M.I. Synbiotic amazonian palm berry (açai, Euterpe oleracea Mart.) ice cream improved Lactobacillus rhamnosus GG survival to simulated gastrointestinal stress. Food Funct. 2017, 8, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, C.F.; Silva, H.L.A.; Esmerino, E.A.; Rocha, R.S.; Moraes, J.; Carmo, M.A.V.; Azevedo, L.; Camps, I.; K.D Abud, Y.; Sant’Anna, C.; et al. The addition of inulin and Lactobacillus casei 01 in sheep milk ice cream. Food Chem. 2018, 246, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S.; Toker, Ö.S.; Yüksel, F.; Çam, M.; Kayacier, A.; Dogan, M. Physicochemical, bioactive, and sensory properties of persimmon-based ice cream: Technique for order preference by similarity to ideal solution to determine optimum concentration. J. Dairy Sci. 2014, 97, 97–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Compounds | Level (Unit) |
|---|---|
| Total soluble solids | 14.8 ± 0.3% |
| Fat | 0.28 ± 0.01% |
| Protein | 2.46 ± 0.08% |
| Ash | 1.08 ± 0.02% |
| Vitamin C | 97.43 ± 4.19 mg/100 g |
| Total phenolic content | 638.2 ± 17.5 mg gallic acid/100 g |
| Component | Treatments | |||
|---|---|---|---|---|
| Control | 3% | 6% | 9% | |
| Total solid (%) | 33.4 ± 0.6 c | 34.6 ± 0.6 b | 35.7 ± 0.5 b | 36.5 ± 0.7 a |
| Fat (%) | 5.95 ± 0.1 a | 5.55 ± 0.2 b | 4.96 ± 0.7 c | 4.49 ± 0.1 d |
| Protein (%) | 4.93 ± 0.5 a | 4.64 ± 0.1 a | 4.15 ±0.2 b | 3.96 ± 0.3 b |
| Ash (%) | 0.96 ± 0.1 b | 1.12 ± 0.1 b | 1.34 ± 0.1 a | 1.42 ± 0.2 a |
| Ca (mg/kg) | 2481.2 ± 21 a | 2352.7 ± 18 b | 2211.5 ± 25 c | 2019 ± 14 d |
| Mg (mg/kg) | 315.8± 11 c | 398.8 ± 14 b | 437.4 ± 12 ab | 478.2 ± 9 a |
| P (mg/kg) | 1018.8 ± 25 a | 901.3 ± 18 ab | 779.3 ± 38 b | 624.2 ± 18 c |
| K (mg/kg) | 2321.4± 37 d | 2547.2 ± 28 c | 2787 ± 20 b | 3013.1 ± 33 a |
| Na (mg/kg) | 670.3 ± 35 a | 654.1 ± 18 a | 649.3 ± 33 a | 630 ± 24 a |
| Component | Treatments | |||
|---|---|---|---|---|
| Control | 3% | 6% | 9% | |
| Overrun (%) | 56.56 ± 2.3 a | 53.53 ± 1.7 b | 49.13 ± 0.4 c | 47.46 ± 0.2 c |
| Viscosity (cP) | 414.1 ± 26.1 d | 567.3 ± 35.2 c | 656.3 ± 13.3 b | 771.5 ± 32.4 a |
| Firmness (g) | 322.6 ± 45.5 a | 297.8 ± 36.2 a | 235.3 ± 25.8 b | 182.5 ± 21.7 c |
| Cohesiveness | 0.28 ± 0.03 a | 0.26 ± 0.04 ab | 0.24 ± 0.02 b | 0.20 ± 0.01 c |
| pH | 6.8 ± 0.4 a | 6.6 ± 0.1 b | 6.2 ± 0.2 c | 5.7 ± 0.1 d |
| L* | 97.20 ± 1.7 a | 88.43 ± 2.4 b | 82.90 ± 3.1 c | 80.90 ± 2.6 c |
| a* | 0.36 ± 0.1 d | 18.73 ± 0.2 c | 23.40 ± 0.9 b | 27.6 ± 1.2 a |
| b* | 1.43 ± 0.4 d | −2.46 ± 0.1 c | −3.66 ± 0.1 b | −4.20 ± 0.3 a |
| Samples | Days | Ascorbic Acid | Total Polyphenol | Total Anthocyanins | DPPH |
|---|---|---|---|---|---|
| (mg/kg DM) | (mg/mL) | (mg/L) | (%) | ||
| Control | 0 | n.d | 1.120 ± 0.047 e | n.d | 8.1 ± 0.5 i |
| 1 | n.d | 1.023 ± 0.028 e | n.d | 8.3 ± 2.4 i | |
| 60 | n.d | 0.975 ±0.050 e | n.d | 8.1 ± 0.9 i | |
| 120 | n.d | 1.071 ± 0.079 e | n.d | 7.9 ± 1.3 i | |
| 3% | 0 | 7.93 ± 0.57 g | 3.349 ± 0.325 d | 0.097 ± 0.004 f | 24.8 ± 0.7 g |
| 1 | 6.83 ± 0.81 h | 3.407 ± 0.101 d | 0.135 ± 0.016 e | 25.5 ± 1.2 g | |
| 60 | 6.26 ± 0.24 hi | 3.257 ± 0.264 d | 0.142 ± 0.011 e | 22.1 ± 0.8 h | |
| 120 | 5.79 ± 0.38 i | 3.224 ± 0.097 d | 0.140 ± 0.007 e | 21.9 ± 2.7 h | |
| 6% | 0 | 14.18 ± 0.85 d | 4.596 ± 0.091 c | 0.191 ± 0.025 d | 45.3 ± 1.5 d |
| 1 | 13.07 ± 1.32 e | 4.686 ± 0.166 c | 0.216 ± 0.008 c | 45.7 ± 3.1 d | |
| 60 | 11.02 ± 1.87 f | 4.445 ± 0.207 c | 0.210 ± 0.013 c | 41.7 ± 1.2 e | |
| 120 | 10.85 ± 0.96 f | 4.298 ± 0.101 c | 0.203 ± 0.019 c | 37.7 ± 2.9 f | |
| 9% | 0 | 20.11 ± 1.76 a | 6.057 ± 0.220 a | 0.332 ± 0.019 b | 59.7 ± 2.2 a |
| 1 | 17.74 ± 1.38 b | 6.139 ± 0.112 a | 0.405 ± 0.016 a | 60.5 ± 3.7 a | |
| 60 | 16.14 ± 0.84 c | 5.762 ± 0.319 ab | 0.427 ± 0.005 a | 54.5 ± 1.5 b | |
| 120 | 13.59 ± 2.09 de | 5.347 ± 0.095 b | 0.398 ± 0.021 a | 52.5 ± 0.7 c |
| Samples | Days | Viability (Log CFU/g) | |
|---|---|---|---|
| In Ice Cream | After Simulated Gastrointestinal Digestion | ||
| Control | 0 | 8.86 ± 0.16 a | - |
| 1 | 7.62 ± 0.12 c | 6.10 ± 0.12 b | |
| 60 | 7.29 ± 0.10 de | 5.24 ± 0.12 d | |
| 120 | 7.16 ± 0.10 e | 5.32 ± 0.12 cd | |
| 3% | 0 | 8.81 ± 0.08 a | - |
| 1 | 7.55 ± 0.11 c | 5.97 ± 0.11 b | |
| 60 | 7.42 ± 0.19 cd | 5.35 ± 0.11 cd | |
| 120 | 7.32 ± 0.11 de | 5.49 ± 0.11 c | |
| 6% | 0 | 8.95 ± 0.24 a | - |
| 1 | 8.01 ± 0.22 b | 6.43 ± 0.22 a | |
| 60 | 7.55 ± 0.08 c | 6.04 ± 0.22 b | |
| 120 | 7.41 ± 0.05 cd | 6.13 ± 0.22 b | |
| 9% | 0 | 8.81 ± 0.19 a | - |
| 1 | 7.89 ± 0.11 b | 6.31 ± 0.11 a | |
| 60 | 7.60 ± 0.27 c | 6.35 ± 0.11 a | |
| 120 | 7.45 ± 0.07 cd | 6.05 ± 0.11 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghani, S.; Hadidi, M.; Pouramin, S.; Adinepour, F.; Hasiri, Z.; Moreno, A.; Munekata, P.E.S.; Lorenzo, J.M. Application of Cornelian Cherry (Cornus mas L.) Peel in Probiotic Ice Cream: Functionality and Viability during Storage. Antioxidants 2021, 10, 1777. https://doi.org/10.3390/antiox10111777
Haghani S, Hadidi M, Pouramin S, Adinepour F, Hasiri Z, Moreno A, Munekata PES, Lorenzo JM. Application of Cornelian Cherry (Cornus mas L.) Peel in Probiotic Ice Cream: Functionality and Viability during Storage. Antioxidants. 2021; 10(11):1777. https://doi.org/10.3390/antiox10111777
Chicago/Turabian StyleHaghani, Shaghayegh, Milad Hadidi, Shiva Pouramin, Fateme Adinepour, Zahra Hasiri, Andrés Moreno, Paulo E. S. Munekata, and José M. Lorenzo. 2021. "Application of Cornelian Cherry (Cornus mas L.) Peel in Probiotic Ice Cream: Functionality and Viability during Storage" Antioxidants 10, no. 11: 1777. https://doi.org/10.3390/antiox10111777
APA StyleHaghani, S., Hadidi, M., Pouramin, S., Adinepour, F., Hasiri, Z., Moreno, A., Munekata, P. E. S., & Lorenzo, J. M. (2021). Application of Cornelian Cherry (Cornus mas L.) Peel in Probiotic Ice Cream: Functionality and Viability during Storage. Antioxidants, 10(11), 1777. https://doi.org/10.3390/antiox10111777










