Sulfane Sulfur Is a Strong Inducer of the Multiple Antibiotic Resistance Regulator MarR in Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Culture Conditions, and Reagents
2.2. H2Sn Preparation
2.3. Reporter Construction and Test
2.4. Protein Expression and Purification
2.5. Electrophoretic Mobility Shift Assay (EMSA)
2.6. Size Exclusion Chromatography
2.7. LC–MS/MS Analysis
3. Results
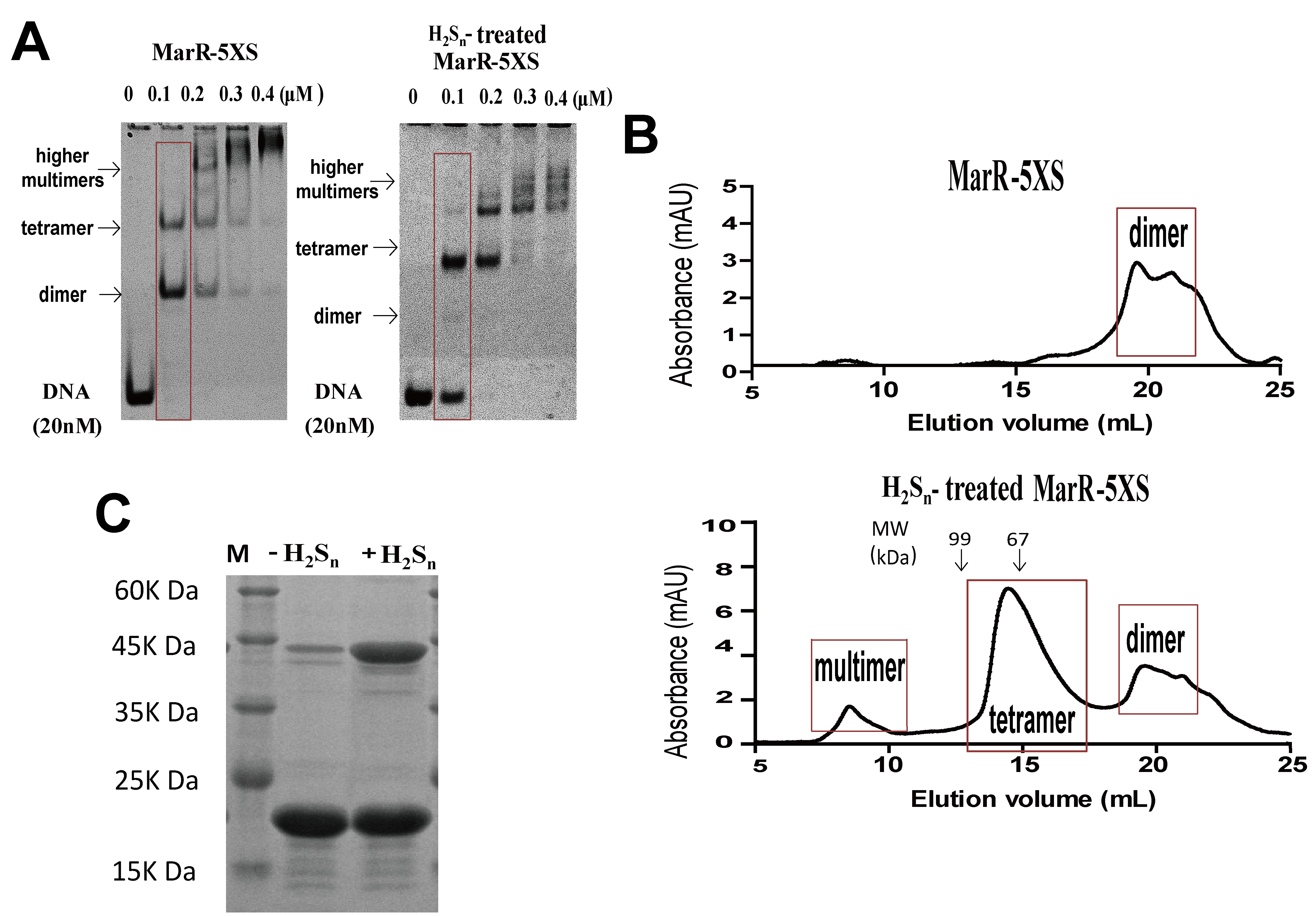
3.1. MarR Sensed Sulfane Sulfur and Decreased Its DNA Binding Affinity
3.2. MarR Responded to H2Sn in An E. Coli Reporter System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, M.; Wang, T.; Shao, M.; Chen, Z.; Liu, H.; Xia, Y.; Xun, L. Sensitive method for reliable quantification of sulfane sulfur in biological samples. Anal. Chem. 2019, 91, 11981–11986. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Liu, H.; Cui, F.; Liu, H.; Xun, L. Recombinant Escherichia coli with sulfide:quinone oxidoreductase and persulfide dioxygenase rapidly oxidises sulfide to sulfite and thiosulfate via a new pathway. Environ. Microbiol. 2016, 18, 5123–5136. [Google Scholar] [CrossRef]
- Lü, C.J.; Xia, Y.Z.; Liu, D.X.; Zhao, R.; Gao, R.; Liu, H.L.; Xun, L.Y. Cupriavidus necator H16 Uses Flavocytochrome c Sulfide Dehydrogenase To Oxidize Self-Produced and Added Sulfide. Appl. Environ. Microbiol. 2017, 83, e01610-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Xin, Y.F.; Xuan, G.H.; Zhao, R.; Liu, H.W.; Xia, Y.Z.; Xun, L.Y. Escherichia coli uses separate enzymes to produce H2S and reactive sulfane sulfur from L-cysteine. Front. Microbiol. 2019, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, N.; Koike, S.; Nirasawa, T.; Kimura, H.; Ogasawara, Y. Alternative pathway of H2S and polysulfides production from sulfurated catalytic-cysteine of reaction intermediates of 3-mercaptopyruvate sulfurtransferase. Biochem. Biophys. Res. Commun. 2018, 496, 648–653. [Google Scholar] [CrossRef]
- Xia, Y.Z.; Lu, C.J.; Hou, N.K.; Xin, Y.F.; Liu, J.H.; Liu, H.L.; Xun, L.Y. Sulfide production and oxidation by heterotrophic bacteria under aerobic conditions. ISME J. 2017, 11, 2754–2766. [Google Scholar] [CrossRef] [Green Version]
- Doka, E.; Pader, I.; Biro, A.; Johansson, K.; Cheng, Q.; Ballago, K.; Prigge, J.R.; Pastor-Flores, D.; Dick, T.P.; Schmidt, E.E.; et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016, 2, e1500968. [Google Scholar] [CrossRef] [Green Version]
- Sato, I.; Shimatani, K.; Fujita, K.; Abe, T.; Shimizu, M.; Fujii, T.; Hoshino, T.; Takaya, N. Glutathione reductase/glutathione is responsible for cytotoxic elemental sulfur tolerance via polysulfide shuttle in fungi. J. Biol. Chem. 2011, 286, 20283–20291. [Google Scholar] [CrossRef] [Green Version]
- Toohey, J.I. Sulfur signaling: Is the agent sulfide or sulfane? Anal. Biochem. 2011, 413, 1–7. [Google Scholar] [CrossRef]
- Xuan, G.; Lu, C.; Xu, H.; Chen, Z.; Li, K.; Liu, H.; Liu, H.; Xia, Y.; Xun, L. Sulfane Sulfur is an intrinsic signal activating MexR-regulated antibiotic resistance in Pseudomonas aeruginosa. Mol. Microbiol. 2020, 114, 1038–1048. [Google Scholar] [CrossRef]
- Lau, N.; Pluth, M.D. Reactive sulfur species (RSS): Persulfides, polysulfides, potential, and problems. Curr. Opin. Chem. Biol. 2019, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Production and physiological effects of hydrogen sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, J.; Lu, C.; Xia, Y.; Xin, Y.; Liu, H.; Xun, L.; Liu, H. FisR activates sigma(54)-dependent transcription of sulfide-oxidizing genes in Cupriavidus pinatubonensis JMP134. Mol. Microbiol. 2017, 105, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Luebke, J.L.; Shen, J.C.; Bruce, K.E.; Kehl-Fie, T.E.; Peng, H.; Skaar, E.P.; Giedroc, D.P. The CsoR-like sulfurtransferase repressor (CstR) is a persulfide sensor in Staphylococcus aureus. Mol. Microbiol. 2014, 94, 1343–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, T.; Shen, J.; Fang, M.; Zhang, Y.; Hori, K.; Trinidad, J.C.; Bauer, C.E.; Giedroc, D.P.; Masuda, S. Sulfide-responsive transcriptional repressor SqrR functions as a master regulator of sulfide-dependent photosynthesis. Proc. Natl. Acad. Sci. USA 2017, 114, 2355–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Cao, Q.; Pang, X.; Xia, Y.; Xun, L.; Liu, H. Sulfane sulfur-activated actinorhodin production and sporulation is maintained by a natural gene circuit in Streptomyces coelicolor. Microb. Biotechnol. 2020, 13, 1917–1932. [Google Scholar] [CrossRef]
- Hou, N.; Yan, Z.; Fan, K.; Li, H.; Zhao, R.; Xia, Y.; Xun, L.; Liu, H. OxyR senses sulfane sulfur and activates the genes for its removal in Escherichia coli. Redox Biol. 2019, 26, 101293. [Google Scholar] [CrossRef] [PubMed]
- Xuan, G.; Lv, C.; Xu, H.; Li, K.; Liu, H.; Xia, Y.; Xun, L. Sulfane Sulfur regulates LasR-mediated quorum sensing and virulence in Pseudomonas Aeruginosa PAO1. Antioxidants 2021, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, Y.; Palmer, L.D.; Kehl-Fie, T.E.; Skaar, E.P.; Trinidad, J.C.; Giedroc, D.P. Hydrogen Sulfide and Reactive Sulfur Species impact Proteome S-Sulfhydration and global virulence regulation in Staphylococcus aureus. ACS Infect. Dis. 2017, 3, 744–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Pande, A.; Sabrin, A.; Thapa, S.S.; Gioe, B.W.; Grove, A. MarR family transcription factors from Burkholderia species: Hidden clues to control of virulence-associated genes. Microbiol. Mol. Biol. Rev. MMBR 2019, 83, e00039-18. [Google Scholar] [CrossRef] [Green Version]
- Deochand, D.K.; Grove, A. MarR family transcription factors: Dynamic variations on a common scaffold. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 595–613. [Google Scholar] [CrossRef]
- Alekshun, M.N.; Levy, S.B. The mar regulon: Multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 1999, 7, 410–413. [Google Scholar] [CrossRef]
- Martin, R.G.; Rosner, J.L. Binding of purified multiple antibiotic-resistance repressor protein (MarR) to mar operator sequences. Proc. Natl. Acad. Sci. USA 1995, 92, 5456–5460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, A.M.; Levy, S.B. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: Involvement of a non-plasmid-determined efflux of tetracycline. J. Bacteriol. 1983, 155, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Ariza, R.R.; Cohen, S.P.; Bachhawat, N.; Levy, S.B.; Demple, B. Repressor mutations in the marRAB operon that activate oxidative stress genes and multiple antibiotic resistance in Escherichia coli. J. Bacteriol. 1994, 176, 143–148. [Google Scholar] [CrossRef] [Green Version]
- White, D.G.; Goldman, J.D.; Demple, B.; Levy, S.B. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J. Bacteriol. 1997, 179, 6122–6126. [Google Scholar] [CrossRef] [Green Version]
- Gambino, L.; Gracheck, S.J.; Miller, P.F. Overexpression of the MarA positive regulator is sufficient to confer multiple antibiotic resistance in Escherichia coli. J. Bacteriol. 1993, 175, 2888–2894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Z.Y.; Lou, H.B.; Zhu, R.F.; Zhu, J.H.; Zhang, D.M.; Zhao, B.X.S.; Zeng, S.Z.; Chen, X.; Chan, J.; He, C.; et al. The multiple antibiotic resistance regulator MarR is a copper sensor in Escherichia coli. Nat. Chem. Biol. 2014, 10, 21–28. [Google Scholar] [CrossRef]
- Kamyshny, A. Improved cyanolysis protocol for detection of zero-valent sulfur in natural aquatic systems. Limnol. Oceanogr. Methods 2009, 7, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Notka, F.; Linde, H.J.; Dankesreiter, A.; Niller, H.H.; Lehn, N. A C-terminal 18 amino acid deletion in MarR in a clinical isolate of Escherichia coli reduces MarR binding properties and increases the MIC of ciprofloxacin. J. Antimicrob. Chemother. 2002, 49, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, K.; Li, J.; Wang, T.; Gu, L.; Xun, L. T5 exonuclease-dependent assembly offers a low-cost method for efficient cloning and site-directed mutagenesis. Nucleic Acids Res. 2019, 47, e15. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.Z.; Chu, W.Q.; Qi, Q.S.; Xun, L.Y. New insights into the QuikChange (TM) process guide the use of Phusion DNA polymerase for site-directed mutagenesis. Nucleic Acids Res. 2015, 43, e12. [Google Scholar] [CrossRef] [Green Version]
- Domain, F.; Levy, S.B. GyrA Interacts with MarR To Reduce Repression of the marRAB Operon in Escherichia coli (Retraction of vol 192, pg 942, 2010). J. Bacteriol. 2011, 193, 2674. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yi, C.Q.; Zhang, J.; Zhang, W.R.; Ge, Z.Y.; Yang, C.G.; He, C.A. Structural insight into the oxidation-sensing mechanism of the antibiotic resistance of regulator MexR. EMBO Rep. 2010, 11, 685–690. [Google Scholar] [CrossRef]
- Chen, H.; Hu, J.; Chen, P.R.; Lan, L.; Li, Z.; Hicks, L.M.; Dinner, A.R.; He, C. The Pseudomonas aeruginosa multidrug efflux regulator MexR uses an oxidation-sensing mechanism. Proc. Natl. Acad. Sci. USA 2008, 105, 13586–13591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosby, H.A.; Schlievert, P.M.; Merriman, J.A.; King, J.M.; Salgado-Pabon, W.; Horswill, A.R. The Staphylococcus aureus global regulator MgrA modulates clumping and virulence by controlling surface protein expression. PloS Pathog. 2016, 12, e1005604. [Google Scholar] [CrossRef]
- Jiang, Q.; Jin, Z.; Sun, B. MgrA negatively regulates biofilm formation and detachment by repressing the expression of psm operons in Staphylococcus aureus. Appl. Environ. Microbiol. 2018, 84, e01008-18. [Google Scholar] [CrossRef] [Green Version]
- Fuangthong, M.; Helmann, J.D. The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative. Proc. Natl. Acad. Sci. USA 2002, 99, 6690–6695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Joachimiak, G.; Bigelow, L.; Babnigg, G.; Joachimiak, A. How Aromatic Compounds block DNA binding of HcaR catabolite regulator. J. Biol. Chem. 2016, 291, 13243–13256. [Google Scholar] [CrossRef] [Green Version]
- Will, W.R.; Brzovic, P.; Le Trong, I.; Stenkamp, R.E.; Lawrenz, M.B.; Karlinsey, J.E.; Navarre, W.W.; Main-Hester, K.; Miller, V.L.; Libby, S.J.; et al. The evolution of SlyA/RovA transcription factors from repressors to Countersilencers in Enterobacteriaceae. mBio 2019, 10, e00009-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.R.; Bae, T.; Williams, W.A.; Duguid, E.M.; Rice, P.A.; Schneewind, O.; He, C. An oxidation-sensing mechanism is used by the global regulator MgrA in Staphylococcus aureus. Nat. Chem. Biol. 2006, 2, 591–595. [Google Scholar] [CrossRef]
- Giedroc, D.P. A new player in bacterial sulfide-inducible transcriptional regulation. Mol. Microbiol. 2017, 105, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, H.J.; Zhang, X.; Chen, Z.G.; Zhao, R.; Hou, N.K.; Liu, J.H.; Xun, L.Y.; Liu, H.W. Developing polysulfide-sensitive GFPs for real-time analysis of polysulfides in live cells and subcellular organelles. Anal. Chem. 2019, 91, 3893–3901. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.M.; Wang, Q.D.; Xia, Y.Z.; Xun, L.Y.; Liu, H.W. A red fluorescent protein-based probe for detection of intracellular reactive sulfane sulfur. Antioxidants 2020, 9, 985. [Google Scholar] [CrossRef]




| Strain/Plasmid | Characteristic | Source |
|---|---|---|
| Escherichia coli strains | ||
| DH5a | Cloning strain | Invitrogen |
| BL21(DE3) | Cloning strain | Invitrogen |
| Plasmids | ||
| pBBR1mcs5 | Gmr, broad host range | [12] |
| pBBR5-MarR-PmarR-mKate | pBBR1mcs5 vector with marR, marR promotor, and mkate genes | This study |
| pET30a | Kmr, expression vector | Invitrogen |
| pET30-MarR | pET30a containing MarR with N terminal his-tag | This study |
| pET30-MarR/5CS | pET30-MarR with Cys47Ser, Cys51Ser, Cys54Ser, Cys108Ser, Cys111Ser | This study |
| Primers a | Sequence (5′-3′) | Usage |
|---|---|---|
| marR-1 | CGACGACGACAAGGCCATGGCTGATGTGAAAAGTACCAGCGATCTG | For pET30-MarR construction |
| marR-2 | CAGTGGTGGTGGTGGTGGTGCTCGATTACGGCAGGACTTTCTTAAG | |
| MarR-5CS-1 | TAATACTCGCCGCGCTGCGGATAGAGCTGAGC | For MarR-5XS mutant construction |
| MarR-5CS-2 | GCTCAGCTCTATCCGCAGCGCGGCGAGTATTA | |
| MarR-5CS-3 | GGCGCGGCAATAAGTGAACAAAGCCAT | |
| MarR-5CS-4 | ATGGCTTTGTTCACTTATTGCCGCGCC | |
| marR-F a | TCTAGAGAAAGAGGAGAAATACTAGGTGAAAAGTACCAGCGATCTGTTCAAT | For pBBR5-MarR-PmarR-mKate construction |
| marR-R | TTGACGGTGGTATTACGGCAGGACTTTCTTAAGCAAATAC | |
| Pmar-F | CCTGCCGTAATACCACCGTCAAAAAAAACGGCGCTTTTTAGCGCCGTTTTTATTTTTCATGAACCGATTTAGCAAAACGTGGC | |
| Pmar-R | CTAGTATTTCTCCTCTTTCTCTAGAATTAGTTGCCCTGGCAAGTAATTAGTT | |
| mKate-F b | TCTAGAGAAAGAGGAGAAATACTAGATGTCAGAATTAATTAAAGAAAATATGCACATG | |
| mKate-R | CTTACAATTTCCATTCGCCATTTCAACGATGTCCTAATTTCGACG | |
| pBBR5-F c | CTAGTATTTCTCCTCTTTCTCTAGACAACATACGAGCCGGAAGCATAAAG | |
| pBBR5-R c | AATGGCGAATGGAAATTGTAAGCG | |
| MarR-emsa-1 | CATCGCATTGAACAAAACTTGAAC | For amplifying the EMSA probe |
| MarR-emsa-1 | GTTGCAGGGGATAATATTGCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Xuan, G.; Liu, H.; Xia, Y.; Xun, L. Sulfane Sulfur Is a Strong Inducer of the Multiple Antibiotic Resistance Regulator MarR in Escherichia coli. Antioxidants 2021, 10, 1778. https://doi.org/10.3390/antiox10111778
Xu H, Xuan G, Liu H, Xia Y, Xun L. Sulfane Sulfur Is a Strong Inducer of the Multiple Antibiotic Resistance Regulator MarR in Escherichia coli. Antioxidants. 2021; 10(11):1778. https://doi.org/10.3390/antiox10111778
Chicago/Turabian StyleXu, Huangwei, Guanhua Xuan, Huaiwei Liu, Yongzhen Xia, and Luying Xun. 2021. "Sulfane Sulfur Is a Strong Inducer of the Multiple Antibiotic Resistance Regulator MarR in Escherichia coli" Antioxidants 10, no. 11: 1778. https://doi.org/10.3390/antiox10111778
APA StyleXu, H., Xuan, G., Liu, H., Xia, Y., & Xun, L. (2021). Sulfane Sulfur Is a Strong Inducer of the Multiple Antibiotic Resistance Regulator MarR in Escherichia coli. Antioxidants, 10(11), 1778. https://doi.org/10.3390/antiox10111778






