Coffee Infusions: Can They Be a Source of Microelements with Antioxidant Properties?
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
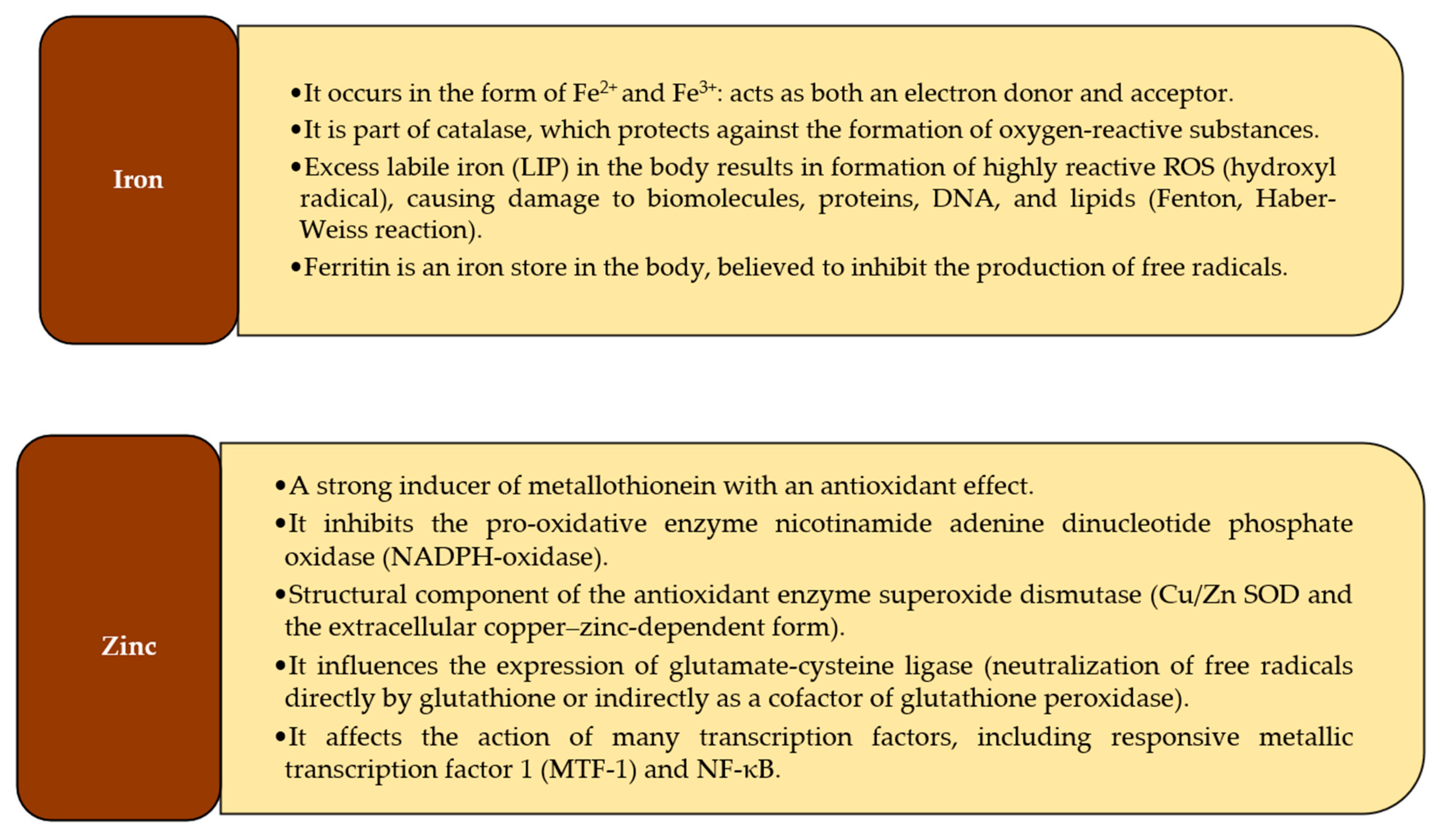
3.1. Zinc
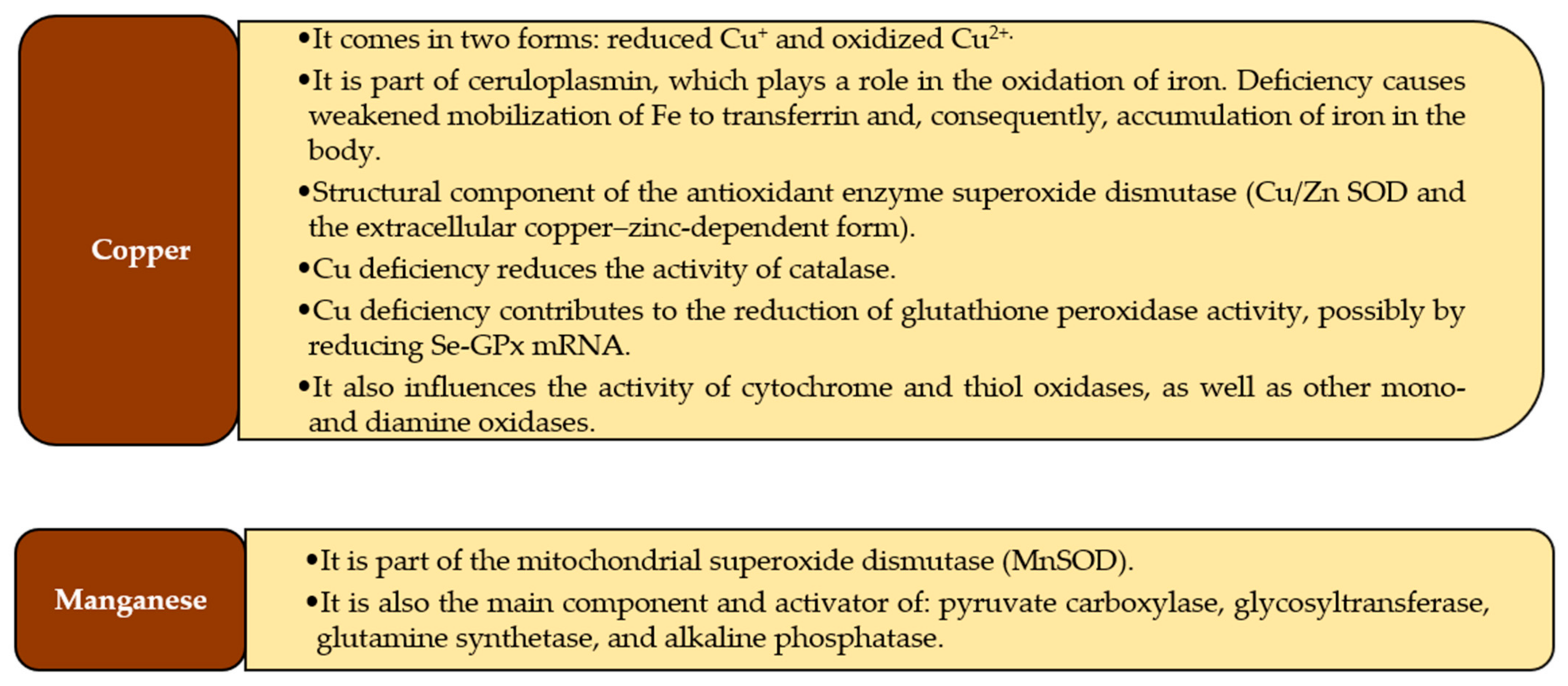
3.2. Copper
3.3. Manganese
3.4. Iron
4. Other Microelements in Coffee Brews
4.1. Cobalt
4.2. Chromium
4.3. Fluoride
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, F.; Tanokura, M. Organic Compounds in Green Coffee Beans. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: New York, NY, USA, 2015; pp. 149–162. [Google Scholar]
- International Coffee Organization. World Coffee Consumption. Available online: https://www.ico.org/prices/new-consumption-table.pdf (accessed on 26 June 2021).
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef]
- Gökcen, B.B.; Şanlier, N. Coffee consumption and disease correlations. Crit. Rev. Food Sci. Nutr. 2019, 59, 336–348. [Google Scholar] [CrossRef]
- Shang, F.; Li, X.; Jiang, X. Coffee consumption and risk of the metabolic syndrome: A meta-analysis. Diabetes Metab. 2016, 42, 80–87. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y.; Giovannucci, E. Coffee consumption and all-cause and cause-specific mortality: A meta-analysis by potential modifiers. Eur. J. Epidemiol. 2019, 34, 731–752. [Google Scholar] [CrossRef] [PubMed]
- Je, Y.; Giovannucci, E. Coffee consumption and total mortality: A meta-analysis of twenty prospective cohort studies. Br. J. Nutr. 2014, 111, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M.; Larsson, S.C. Coffee consumption and reduced risk of developing type 2 diabetes: A systematic review with meta-analysis. Nutr. Rev. 2018, 76, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Park, J.H.; Im, S.S.; Song, D.K. Coffee and health. Integr. Med. Res. 2014, 3, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Braffett, B.H.; Simmens, S.J.; Young, H.A.; Ogden, C.L. Dietary Polyphenol Intake in US Adults and 10-Year Trends: 2007–2016. J. Acad. Nutr. Diet. 2020, 120, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Carnauba, R.A.; Hassimotto, N.M.A.; Lajolo, F.M. Estimated dietary polyphenol intake and major food sources of the Brazilian population. Br. J. Nutr. 2021, 126, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, C.; Fukushima, Y.; Kishimoto, Y.; Suzuki-Sugihara, N.; Saita, E.; Takahashi, Y.; Kondo, K. Estimated Dietary Polyphenol Intake and Major Food and Beverage Sources among Elderly Japanese. Nutrients 2015, 7, 10269–10281. [Google Scholar] [CrossRef] [PubMed]
- De Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical composition and health properties of coffee and coffee by-products. Adv. Food Nutr. Res. 2020, 91, 65–96. [Google Scholar]
- Janda, K.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Kapczuk, P.; Kochman, J.; Rębacz-Maron, E.; Gutowska, I. Mineral Composition and Antioxidant Potential of Coffee Beverages Depending on the Brewing Method. Foods 2020, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ramos, S.; Delerue-Matos, C.; Morais, S. Espresso beverages of pure origin coffee: Mineral characterization, contribution for mineral intake and geographical discrimination. Food Chem. 2015, 177, 330–338. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Socha, K.; Zujko, M.E. Coffee Brews: Are They a Source of Macroelements in Human Nutrition? Foods 2021, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Donangelo, C.M. Chapter 21—Minerals. In Coffee: Production, Quality and Chemistry; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 505–516. [Google Scholar]
- Gropper, S.S.; Smith, J.L.; Carr, T.P. Essential Trace and Ultratrace Minerals. In Advanced Nutrition and Human Metabolism, 7th ed.; Gropper, S.S., Smith, J.L., Eds.; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Shenkin, A. The key role of micronutrients. Clin. Nutr. 2006, 25, 1–13. [Google Scholar] [CrossRef]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R. Chapter 1: The Role of Antioxidants in Human Health. In Oxidative Stress: Diagnostics, Prevention and Therapy; Andreescu, S., Hepel, M., Eds.; American Chemical Society: Washington, DC, USA, 2011; pp. 1–37. [Google Scholar]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of selected food. Int. J. Food Prop. 2011, 14, 300–308. [Google Scholar] [CrossRef]
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of beverages, chocolates, nuts, and seeds. Int. J. Food Prop. 2014, 17, 86–92. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Santato, A.; Bertoldi, D.; Perini, M.; Camin, F.; Larcher, R. Using elemental profiles and stable isotopes to trace the origin of green coffee beans on the global market. J. Mass Spectrom. 2012, 47, 1132–1140. [Google Scholar] [CrossRef]
- Rodrigues, C.; Brunner, M.; Steiman, S.; Bowen, G.J.; Nogueira, J.M.; Gautz, L.; Prohaska, T.; Máguas, C. Isotopes as tracers of the Hawaiian coffee-producing regions. J. Agric. Food Chem. 2011, 59, 10239–10246. [Google Scholar] [CrossRef]
- Juniora, J.B.D.S.E.; da Silvaa, G.B.M.D.; Bastos, R.; Furlong, E.; Carapelli, R. Evaluation of the influence of cultivation on the total magnesium concentration and infusion extractability in commercial Arabica coffee. Food Chem. 2020, 327, 127012. [Google Scholar] [CrossRef]
- Cruz, R.; Morais, S.; Casal, S. Chapter 66—Mineral Composition Variability of Coffees: A Result of Processing and Production. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: New York, NY, USA, 2015. [Google Scholar]
- Mehari, B.; Redi-Abshiro, M.; Chandravanshi, B.S.; Combrinck, S.; McCrindle, R. Characterization of the cultivation region of Ethiopian coffee by elemental analysis. Anal. Lett. 2016, 49, 2474–2489. [Google Scholar] [CrossRef]
- Endaye, M.; Atlabachew, M.; Mehari, B.; Alemayehu, M.; Mengistu, D.A.; Kerisew, B. Combining Multi-Element Analysis with Statistical Modeling for Tracing the Origin of Green Coffee Beans from Amhara Region, Ethiopia. Biol. Trace Elem Res. 2020, 195, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Feleke, H.M.; Srinivasulu, A.; Surendra, K.; Aruna, B.; Biswas, J.; Sudershan, M.; Rao, A.D.P.; Narayana, P.V.L. Estimation of elemental concentrations of Ethiopia Coffee Arabica on different coffee bean varieties (subspecies) using Energy Dispersive X-ray Florescence. Int. J. Eng. Res. 2018, 9, 148–165. [Google Scholar]
- Pereira, P.V.; da Silveira, D.L.; Schwan, R.F.; Assis, D.; Silva, S.; Coelho, J.M.; Bernardes, P.C. Effect of altitude and terrain aspect on the chemical composition of Coffea canephora cherries and sensory characteristics of the beverage. J. Sci. Food Agric. 2021, 101, 2570–2575. [Google Scholar] [CrossRef] [PubMed]
- Severini, C.; Degrossi, A.; Ricci, I.; Fiore, A.G.; Caporizzi, R. How Much Caffeine in Coffee Cup? Effects of Processing Operations, Extraction Methods and Variables. In The Question of Caffeine; Latosinska, J.N., Latosinska, M., Eds.; Intechopen: London, UK, 2017; pp. 45–85. [Google Scholar]
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.G.; Grauwet, T. Barriers impairing mineral bioaccessibility and bioavailability in plant-based foods and the perspectives for food processing. Crit. Rev. Food Sci. Nutr. 2020, 60, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, E.; Pohl, P.; Szymczycha-Madeja, A. The suitability of the simplified method of the analysis of coffee infusions on the content of Ca, Cu, Fe, Mg, Mn and Zn and the study of the effect of preparation conditions on the leachability of elements into the coffee brew. Food Chem. 2013, 141, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Puntarulo, S. Iron, oxidative stress and human health. Mol. Asp. Med. 2005, 26, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Moreira, P.I.; Perry, G.; Zhu, X. The role of iron as a mediator of oxidative stress in Alzheimer disease. Biofactors 2012, 38, 133–138. [Google Scholar] [CrossRef]
- Bresgen, N.; Eckl, P.M. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 2015, 5, 808–847. [Google Scholar] [CrossRef]
- Cherayil, B.J. Iron and immunity: Immunological consequences of iron deficiency and overload. Arch. Immunol. Ther. Exp. 2010, 58, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cel death. Nat. Chem. Biol. 2013, 10, 9–17. [Google Scholar] [CrossRef]
- Jomovaa, K.; Valkoa, M. Importance of Iron Chelation in Free Radical-Induced Oxidative Stress and Human Disease. Curr. Pharm. Des. 2011, 17, 3460–3473. [Google Scholar] [CrossRef] [PubMed]
- Gudjoncik, A.; Guenancia, C.; Zeller, M.; Cottin, Y.; Vergely, C.; Rochette, L. Iron, oxidative stress, and redox signaling in the cardiovascular system. Mol. Nutr. Food Res. 2014, 58, 1721–1738. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef]
- Lee, S.R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.D.; Cruz, K.J.; Morais, J.B.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Qin, P.; Liu, R.; Li, J.; Zhang, F. Molecular Mechanism on Two Fluoroquinolones-induced Oxidative Stress: Evidences from Copper/zinc Superoxide Dismutase. RSC Adv. 2016, 6, 91141–91149. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Eskici, G.; Axelsen, P.H. Copper and oxidative stress in the pathogenesis of Alzheimer’s disease. Biochemistry 2012, 51, 6289–6311. [Google Scholar] [CrossRef] [PubMed]
- Latorre, M.; Troncoso, R.; Uauy, R. Chapter 4—Biological Aspects of Copper. In In Clinical and Translational Perspectives on Wilson Disease; Kerkar, N., Roberts, E.A., Eds.; Academic Press: London, UK, 2019; pp. 25–31. [Google Scholar]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Gaware, V.; Kotade, K.; Dhamak, K.; Somawanshi, S. Ceruloplasmin its role and significance: A review. Int. J. Biomed. Res. 2011, 1, 153–162. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Bresciani, G.; da Cruz, I.B.; González-Gallego, J. Manganese superoxide dismutase and oxidative stress modulation. Adv. Clin. Chem. 2015, 68, 87–130. [Google Scholar] [CrossRef]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St Clair, D.K. Manganese superoxide dismutase: Guardian of the powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, J. Copper and zinc, biological role and significance of copper/zinc imbalance. Clin. Toxicol. 2011, 3, 0495. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Yuce, K.; Mogulkoc, R. Zinc Metabolism and Metallothioneins. Biol. Trace Elem. Res. 2018, 183, 22–31. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef]
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, M.; Malinowska, E.; Szefer, P. Differentiation of market coffee and its infusions in view of their mineral composition. Sci. Total Environ. 2007, 383, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ashu, R.; Chandravanshi, B.S. Concentration levels of metals in commercially available Ethiopian roasted coffee powders and their infusions. Bull. Chem. Soc. Ethiop. 2011, 25, 11–24. [Google Scholar] [CrossRef]
- Adler, G.; Nędzarek, A.; Tórz, A. Concentrations of Selected Metals (NA, K, CA, MG, FE, CU, ZN, AL, NI, PB, CD) in Coffee. Zdr. Varst. 2019, 58, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Jaganyi, D.; Madlala, S.P. Kinetics of coffee infusion: A comparative study on the extraction kinetics of mineral ions and caffeine from several types of medium roasted coffees. J. Sci. Food. Agric. 2000, 80, 85–90. [Google Scholar] [CrossRef]
- Özdestan, Ö. Evaluation of bioactive amine and mineral levels in Turkish coffee. Food Res. Int. 2014, 61, 167–175. [Google Scholar] [CrossRef]
- Da Silva, S.A.; Mendes, F.Q.; Reis, M.R.; Passos, F.R.; de Carvalho, A.M.X.; de Oliveira Rocha, K.R.; Pinto, F.G. Determination of heavy metals in the roasted and ground coffee beans and brew. Afr. J. Agric. Res. 2017, 12, 221–228. [Google Scholar] [CrossRef]
- Gogoaşă, I.; Sipos, L.; Negrea, A.; Alda, L.M.; Costescu, C.; Rada, M.; Velimirovici, D.; Draghici, G.A.; Ostan, M.; Bordean, D.M.; et al. Study regarding coffee brew metal content. In Proceedings of the 22nd International Symposium on Analytical and Environmental Problems, Szeged, Hungary, 10 October 2016; pp. 164–167. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum. Available online: https://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/water-safety-and-quality/drinking-water-quality-guidelines (accessed on 3 July 2021).
- Świetlik, R.; Trojanowska, M. Specjacja fizyczna metali ciężkich w naparach kawy [Physical speciation of heavy metals in coffee infusions]. Bromat. Chem. Toksykol. [Bromat. Toxicol. Chem.] 2014, 47, 82–88. [Google Scholar]
- Gebretsadik, A.T.; Berhanu, T.; Kefarge, B. Levels of selected ssential and nonessential metals in roasted coffee beans of Yirgacheffe and Sidama, Ethiopia. Am. J. Environ. Prot. 2015, 4, 188–192. [Google Scholar] [CrossRef][Green Version]
- Habte, G.; Hwang, I.M.; Kim, J.S.; Hong, J.H.; Hong, Y.S.; Choi, J.Y.; Nho, E.Y.; Jamila, N.; Khan, N.; Kim, K.S. Elemental profiling and geographical differentiation of Ethiopian coffee samples through inductively coupled plasma-optical emission spectroscopy (ICP-OES), ICP-mass spectrometry (ICP-MS) and direct mercury analyzer (DMA). Food Chem. 2016, 212, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Gure, A.; Chandravanshi, B.S.; Godeto, T.W. Assessment of metals in roasted indigenous coffee varieties of Ethiopia. Bull. Chem. Soc. Ethiop. 2018, 32, 27–38. [Google Scholar] [CrossRef]
- European Food Safety Authority. Dietary Reference Values for Nutrients Summary Report. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2017.e15121 (accessed on 31 March 2021).
- Brnić, M.; Wegmüller, R.; Zeder, C.; Senti, G.; Hurrell, R.F. Influence of phytase, EDTA, and polyphenols on zinc absorption in adults from porridges fortified with zinc sulfate or zinc oxide. J. Nutr. 2014, 144, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Pai, T.K.; Han, O. Effect of bioactive dietary polyphenols on zinc transport across the intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2011, 59, 3606–3612. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, K.; Tas, S.; Robberecht, H.; Deelstra, H. The influence of different food components on the in vitro availability of iron, zinc and calcium from a composed meal. Int. J. Food Sci. Nutr. 1996, 47, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Debastiani, R.; Iochims Dos Santos, C.E.; Maciel Ramos, M.; Sobrosa Souza, V.; Amaral, L.; Yoneama, M.L.; Ferraz Dias, J. Elemental analysis of Brazilian coffee with ion beam techniques: From ground coffee to the final beverage. Food Res. Int. 2019, 119, 297–304. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Stanisz, E.; De Peña, M.P. Relationship between antioxidant capacity, chlorogenic acids and elemental composition of green coffee. LWT 2016, 73, 243–250. [Google Scholar] [CrossRef]
- Martín, M.J.; Pablos, F.; González, A.G. Characterization of green coffee varieties according to their metal content. Anal. Chim. Acta 1998, 358, 177–183. [Google Scholar] [CrossRef]
- Martín, M.J.; Pablos, F.; González, A.G. Characterization of arabica and robusta roasted coffee varieties and mixture resolution according to their metal content. Food Chem. 1999, 66, 365–370. [Google Scholar] [CrossRef]
- Pietsch, A. Chapter 10: Decaffeination—Process and Quality. In The Craft and Science of Coffee; Folmer, B., Ed.; Academic Press: New York, NY, USA, 2017; pp. 225–243. [Google Scholar]
- Van Cuong, T.; Ling, L.H.; Quan, G.K.; Jin, S.; Jie, S.S.; Linh, T.L.; Tiep, T.D. Effect of roasting conditions on concentration in elements of Vietnam Robusta coffee. Acta Univ. Cibiniensis Ser. E Food Technol. 2014, 18, 19–34. [Google Scholar] [CrossRef]
- Rodrigues, C.I.; Maia, R.; Miranda, M.; Ribeirinho, M.; Nogueira, J.M.F.; Maguas, C. Stable isotope analysis for green coffee bean: A possible method for geographic origin discrimination. J. Food Compos. Anal. 2009, 22, 463–471. [Google Scholar] [CrossRef]
- Bertrand, B.; Villarreal, D.; Laffargue, A.; Posada, H.; Lashermes, P.; Dussert, S. Comparison of the effectiveness of fatty acids, chlorogenic acids, and elements for the chemometric discrimination of coffee (Coffea arabica L.) varieties and growing origins. J. Agric. Food Chem. 2008, 56, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.S.; dos Santos, M.L.P.; Conti, M.M. Comparative study of metal contents in Brazilian coffees cultivated by conventional and organic agriculture applying principal component analysis. J. Braz. Chem. Soc. 2010, 21, 1468–1476. [Google Scholar] [CrossRef]
- Şemen, S.; Mercan, S.; Yayla, M.; Açıkkol, M. Elemental composition of green coffee and its contribution to dietary intake. Food Chem. 2017, 215, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Finley, E.J.; Gavin, C.E.; Aschner, M.; Gunter, T.E. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic. Biol. Med. 2013, 62, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Nędzarek, A.; Tórz, A.; Karakiewicz, B.; Clark, J.S.; Laszczyńska, M.; Kaleta, A.; Adler, G. Concentrations of heavy metals (Mn, Co, Ni, Cr, Ag, Pb) in coffe. Acta Biochim. Pol. 2013, 60, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Tidemann-Andersen, I.; Acham, H.; Maage, A.; Malde, M.K. Iron and zinc content of selected foods in the diet of schoolchildren in Kumi district, east of Uganda: A cross-sectional study. Nutr. J. 2011, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Skolmowska, D.; Głąbska, D. Analysis of Heme and Non-Heme Iron Intake and Iron Dietary Sources in Adolescent Menstruating Females in a National Polish Sample. Nutrients 2019, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferro, F.S.; De Nadai Fernandes, E.A.; Bacchi, M.A.; Bode, P.; Joacir De França, E. Can impurities from soil-contaminated coffees reach the cup? J. Radioanal. Nucl. Chem. 2007, 271, 371–375. [Google Scholar] [CrossRef]
- Gizińska, M.; Pytka, A.; Skwarzyńska, A.; Micek, A.; Jóźwiakowski, K.; Marzec, M.; Sosnowska, B. Porównanie skuteczności działania i żywotności filtrów dzbankowych do wody [in Polish, Comparison of the effectiveness and service life of water jug filters]. Technol. Wody 2014, 2, 25–29. [Google Scholar]
- Anderson, K.A.; Smith, B.W. Chemical profiling to differentiate geographic growing origins of coffee. J. Agric. Food Chem. 2002, 50, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Fercan, M.M.; Kipcak, A.S.; Ozdemir, O.D.; Piskin, M.B.; Derun, E.M. Determination of the element contents in Turkish coffee and effect of sugar addition. Eng. Technol. Int. J. Chem. Mol. Eng. 2016, 10, 112–115. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef]
- Dasa, F.; Abera, T. Factors affecting iron absorption and mitigation mechanisms: A review. Int. J. Agric. Sci. Food Technol. 2018, 4, 024–030. [Google Scholar] [CrossRef]
- Morck, T.A.; Lynch, S.R.; Cook, J.D. Inhibition of food iron absorption by coffee. Am. J. Clin. Nutr. 1983, 37, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Brune, M.; Rossander, L.; Hallberg, L. Iron absorption and phenolic compounds: Importance of different phenolic structures. Eur. J. Clin. Nutr. 1989, 43, 547–557. [Google Scholar] [PubMed]
- Layrisse, M.; García-Casal, M.N.; Solano, L.; Barón, M.A.; Arguello, F.; Llovera, D.; Ramírez, J.; Leets, I.; Tropper, E. Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J. Nutr. 2000, 130, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A.K. Selected aspects of the action of cobalt ions in the human body. Cent. Eur. J. Immunol. 2015, 40, 236–242. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology—A brief update. Sci. Total Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.; van der Ent, A.; Baker, A.J.; Echevarria, G.; Mahy, G.; Malaisse, F.; Meerts, P.; Pourret, O.; Verbruggen, N.; Faucon, M.P. Copper and cobalt accumulation in plants: A critical assessment of the current state of knowledge. New Phytol. 2017, 213, 537–551. [Google Scholar] [CrossRef] [PubMed]
- National Minerals Information Center. Cobalt Statistics and Information. Available online: https://www.usgs.gov/centers/nmic/cobalt-statistics-and-information (accessed on 26 June 2021).
- Donaldson, J.D.; Beyersmann, D. Cobalt and Cobalt Compounds; Ley, C., Ed.; Wiley-VCH: Weinheim, Germany, 2005; pp. 429–466. [Google Scholar]
- Martin, M.; Lenglet, S.; Gilardi, F.; Thomas, A.; Augsburger, M.; Alvarez, J.C. Determination of lethal dose (LD50) of chromium (Cr), cobalt (Co) and nickel (Ni) in HepaRG cell. Comparison to concentrations found in liver from autopsied prosthesis-bearing patients. Toxicol. Anal. Clin. 2019, 31, S56. [Google Scholar] [CrossRef]
- Finley, B.L.; Monnot, A.D.; Paustenbach, D.J.; Gaffney, S.H. Derivation of a chronic oral reference dose for cobalt. Regul. Toxicol. Pharmacol. 2012, 64, 491–503. [Google Scholar] [CrossRef]
- Lison, D. Chapter 34—Cobalt. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F.B., Nordberg, M., Eds.; Academic Press: London, UK, 2014; Volume 2, pp. 743–763. [Google Scholar]
- Filippini, T.; Tancredi, S.; Malagoli, C.; Malavolti, M.; Bargellini, A.; Vescovi, L.; Nicolini, F.; Vinceti, M. Dietary estimated intake of trace elements: Risk assessment in an Italian population. Expo. Health 2020, 12, 641–655. [Google Scholar] [CrossRef]
- Swaroop, A.; Bagchi, M.; Preuss, H.G.; Zafra-Stone, S.; Ahmad, T.; Bagchi, D. Benefits of Chromium (III) Complexes in Animal and Human Health. In The Nutritional Biochemistry of Chromium (III), 2nd ed.; Vincent, J.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 251–278. [Google Scholar]
- Caporaso, N.; Genovese, A.; Canela, M.D.; Civitella, A.; Sacchi, R. Neapolitan coffee brew chemical analysis in comparison to espresso, moka and American brews. Food Res. Int. 2014, 61, 152–160. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.; Crozier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Wolska, J.; Janda, K.; Jakubczyk, K.; Szymkowiak, M.; Chlubek, D.; Gutowska, I. Levels of Antioxidant Activity and Fluoride Content in Coffee Infusions of Arabica, Robusta and Green Coffee Beans in According to their Brewing Methods. Biol. Trace Elem. Res. 2017, 179, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Abulmohammadi, P.; Teymouri, P.; Zandi, S.; Daraei, H.; Mahvi, A.H.; Shahsawari, S. Effect of brewing time and water hardness on fluoride release from different Iranian teas. Fluoride 2016, 49, 263–273. [Google Scholar]
- Zhu, J.J.; Tang, A.T.H.; Matinlinna, J.P.; Tsoi, J.K.H.; Hägg, U. Potentiometric determination of fluoride release from three types of tea leaves. Int. J. Electrochem. Sci. 2013, 8, 11142–11150. [Google Scholar]
- Zerabruk, S.; Chandravanshi, B.S.; Zewge, F. Fluoride in black and green tea (Camellia sinensis) infusions in Ethiopia: Measurement and safety evaluation. Bull. Chem. Soc. Ethiop. 2010, 24, 327–338. [Google Scholar] [CrossRef]
- Chan, J.T.; Qui, C.C.; Whitford, G.M.; Weatherred, J.G. Influence of coffee on fluoride metabolism in rats. Proc. Soc. Exp. Biol. Med. 1990, 194, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Whitford, G.M. Lack of significant effect of coffee and caffeine on fluoride metabolism in rats. J. Dent. Res. 1994, 73, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
| Content Av. ± SD (Min–Max) (µg/100 mL or 100 g) | Method of Brewing | Time (min) | Coffee (g) | Water (mL) | Cup Volume (mL) | Type of Water | Pressure (Ba) | Temperature of Water (°C) | Species | Degree of Roasting | Type of Coffee | Origin | Method of Analysis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 292 ± 80 | Turkish coffee | nd | 5 | 65 | nd | ultrapure distilled water | nd | nd | Arabica | roasted | fine ground | nd | HR-CS-FAAS | [67] |
| 35 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 29 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 26.2 ± 11.8 | pour-over | nd | 12 | 100 | nd | nd | nd | 95–100 | Arabica | medium roasted | fresh ground | Brazil (Cerrado Mineiro) | FS-FAAS | [68] |
| 26 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 25 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Sumatra | GF-AAS | [71] |
| 23.5 | coffee machine | nd | 17 | nd | 250 | filtered | 9 | 92 | Arabica | roasted | fine ground | nd | ICP-OES | [15] |
| 23 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 22 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 21.0 ± 0.9–30.0 ± 1.2 | pour-over | 5 | 6 | 200 | nd | nd | nd | 100 | nd | nd | ground | Ethiopia | FAAS | [64] |
| 17 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 17 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Brazil | GF-AAS | [71] |
| 15 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Ethiopia | GF-AAS | [71] |
| 14.6 (12.4–16.3) | pour-over | 10 | 6 | 150 | nd | distilled water | nd | 100 | nd | nd | powder coffee | nd | FAAS | [69] |
| 13.5 | pour-over | 5 | 17 | 250 | nd | filtered | nd | 92 | Arabica | roasted | very fine ground | nd | ICP-OES | [15] |
| 13 | drip | 2,5 | 18 | 300 | nd | filtered | nd | 92 | Arabica | roasted | medium coarse ground | nd | ICP-OES | [15] |
| 12.5 | French press | 5 | 17 | 300 | nd | filtered | 1–2 | 92 | Arabica | roasted | medium ground | nd | ICP-OES | [15] |
| 12.3 | Aeropress | 2 | 18 | nd | 250 | filtered | 2–4 | 93 | Arabica | roasted | coarse ground | nd | ICP-OES | [15] |
| 8 ± 2.9 (5.53–13.17) | Turkish coffee | 5 | 10 | 200 | nd | nd | nd | 100 | nd | nd | fresh ground | nd | FAAS | [65] |
| 66 * (52–76) | pour-over | 10 | 6 | 200 | nd | re-distilled water | nd | 100 | Arabica & Robusta mix | roasted | ground | nd | FAAS | [38] |
| 6 * (11–156) | pour-over | 10 | 6 | 200 | nd | re-distilled water | nd | 100 | Arabica | roasted | ground | nd | FAAS | [38] |
| Content Av. ± SD (Min–Max) (µg/100 mL or 100 g) | Method of Brewing | Time (min) | Coffee (g) | Water (mL) | Cup Volume (mL) | Type of Water | Pressure (Ba) | Temperature of Water (°C) | Species | Degree of Roasting | Type of Coffee | Origin | Method of Analysis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 23 (11.5–34.4) | pour-over | 15 | 0.5 | 20 | nd | double distilled water | nd | 95 | Robusta | green | fresh ground | Vietnam, India Cherry Laos FAQ Indonesia Uganda SC12 Uganda Bugishu | ET AAS | [80] |
| 18.1 | pour-over | 15 | 0.5 | 20 | nd | double distilled water | nd | 95 | Robusta (decaffeinated coffee) | green | fresh ground | Vietnam | ET AAS | [80] |
| 14.6–20.5 | pour-over | 15 | 0.5 | 20 | nd | double distilled water | nd | 95 | Arabica | green | fresh ground | Brazil TG Rwanda Ordinary China Laos Guatemala Peru HB | ET AAS | [80] |
| 9.68 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 8.85 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 8.5 | coffee machine | nd | 17 | nd | 250 | filtered water | 9 | 92 | Arabica | roasted | fine ground | nd | ICP-OES | [15] |
| ~8 | French press | 5 | 17 | 300 | nd | filtered water | 1–2 | 92 | Arabica | roasted | medium ground | nd | ICP-OES | [15] |
| 6.53 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Sumatra | GF-AAS | [71] |
| 5.63 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Ethiopia | GF-AAS | [71] |
| 5.08 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Brazil | GF-AAS | [71] |
| 3.04 ± 1.95 (1.20–6.86) | Turkish coffee | 5 | 10 | 200 | nd | nd | nd | 100 | nd | nd | fresh ground | nd | FAAS | [65] |
| 2.7 (2.3–3.2) | pour-over | 10 | 6 | 150 | nd | distilled water | nd | 100 | nd | nd | powder coffee | nd | FAAS | [69] |
| 2.53 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 2.36 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 2.1 ± 0.1–4.2 ± 0.6 | pour-over | 5 | 6 | 200 | nd | nd | nd | 100 | nd | nd | ground | Ethiopia | FAAS | [64] |
| ~2 | pour-over | 5 | 17 | 250 | nd | filtered water | nd | 92 | Arabica | roasted | very fine ground | nd | ICP-OES | [15] |
| 1.93 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 1.62 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 0.4 | pour-over | nd | 12 | 100 | nd | nd | nd | 95–100 | Arabica | medium roasted | fresh ground | Brazil (Cerrado Mineiro) | FS-FAAS | [68] |
| below the detection limit | Aeropress | 2 | 18 | nd | 250 | filtered water | 2–4 | 93 | Arabica | roasted | coarse ground | nd | ICP-OES | [15] |
| below the detection limit | drip | 2,5 | 18 | 300 | nd | filtered water | nd | 92 | Arabica | roasted | medium coarse ground | nd | ICP-OES | [15] |
| 135 * (112–165) | pour-over | 10 | 6 | 200 | nd | re-distilled water | nd | 100 | Arabica & Robusta mix | roasted | ground | nd | FAAS | [38] |
| <30–51 * | pour-over | 10 | 6 | 200 | nd | re-distilled water | nd | 100 | Arabica | roasted | ground | nd | FAAS | [38] |
| Content Av. ± SD (Min–Max) (µg/100 mL or 100 g) | Method of Brewing | Time (min) | Coffee (g) | Water (mL) | Cup Volume (mL) | Type of Water | Pressure (Ba) | Temperature of Water (°C) | Species | Degree of Roasting | Type of Coffee | Origin | Method of Analysis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 273.6 ± 71.1 (144.7 ± 3–414.6 ± 19) | Turkish coffee | nd | 5 | 65 | nd | ultrapure distilled water | nd | nd | Arabica | roasted | fine ground | nd | HR-CS-FAAS | [67] |
| ~65 | pour-over | 5 | 17 | 250 | nd | filtered water | nd | 92 | Arabica | roasted | very fine ground | nd | ICP-OES | [15] |
| ~64 | Aeropress | 2 | 18 | nd | 250 | filtered water | 2–4 | 93 | Arabica | roasted | coarse ground | nd | ICP-OES | [15] |
| ~60 | drip | 2.5 | 18 | 300 | nd | filtered water | nd | 92 | Arabica | roasted | medium coarse ground | nd | ICP-OES | [15] |
| ~50 | coffee machine | nd | 17 | nd | 250 | filtered water | 9 | 92 | Arabica | roasted | very fine ground | nd | ICP-OES | [15] |
| 45.8 | pour-over | nd | 12 | 100 | nd | nd | nd | 95–100 | Arabica | medium roasted | fresh ground | Brazil (Cerrado Mineiro) | FS-FAAS | [68] |
| 44.3 | French press | 5 | 17 | 300 | nd | filtered water | 1–2 | 92 | Arabica | roasted | medium ground | nd | ICP-OES | [15] |
| 32.6 (18–75) | pour-over | 15 | 0.5 | 20 | nd | double distilled water | nd | 95 | Arabica | green | fresh ground | Brazil TG, Rwanda Ordinary, China, Laos, Guatemala Peru HB | ET AAS | [80] |
| 28 ± 2 (18.9 ± 1.2–27.9 ± 1.5) | pour-over | 5 | 6 | 200 | nd | nd | nd | 100 | nd | nd | ground | Ethiopia | FAAS | [64] |
| 20.6 (17.5–25.7) | pour-over | 10 | 6 | 150 | nd | distilled water | nd | 100 | nd | nd | powder coffee | nd | FAAS | [69] |
| 17.6 | pour-over | 15 | 0.5 | 20 | nd | double distilled water | nd | 95 | Robusta (decaffeinated coffee) | green | fresh ground | Vietnam | ET AAS | [80] |
| 15 (5.2–25.4) | pour-over | 15 | 0.5 | 20 | nd | double distilled water | nd | 95 | Robusta | green | fresh ground | Vietnam, India Cherry Laos FAQ Indonesia Uganda SC12 Uganda Bugishu | ET AAS | [80] |
| 1210 * (949–1460) | pour-over | 10 | 6 | 200 | nd | re-distilled water | nd | 100 | Arabica | roasted | ground | nd | FAAS | [38] |
| 913 * (578–1330) | pour-over | 10 | 6 | 200 | nd | re-distilled water | nd | 100 | Arabica & Robusta mix | roasted | ground | nd | FAAS | [38] |
| 628 ± 101 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| 619 ± 2 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| 502 ± 52 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| 497 ± 10 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| Content Av. ± SD (Min–Max) (µg/100 mL or 100 g) | Method of Brewing | Time (min) | Coffee (g) | Water (mL) | Cup Volume (mL) | Type of Water | Pressure (Ba) | Temperature of Water (°C) | Species | Degree of Roasting | Type of Coffee | Origin | Method of Analysis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ~44 | pour-over | 5 | 17 | 250 | nd | filtered | nd | 92 | Arabica | roasted | very fine ground | nd | ICP-OES | [15] |
| 43.9 | drip | 2.5 | 18 | 300 | nd | filtered | nd | 92 | Arabica | roasted | medium coarse ground | nd | ICP-OES | [15] |
| ~43 | coffee machine | nd | 17 | nd | 250 | filtered | 9 | 92 | Arabica | roasted | very fine ground | nd | ICP-OES | [15] |
| ~42.5 | Aeropress | 2 | 18 | - | 250 | filtered | 2–4 | 93 | Arabica | roasted | coarse ground | nd | ICP-OES | [15] |
| 34.6 | French press | 5 | 17 | 300 | nd | filtered | 1–2 | 92 | Arabica | roasted | medium ground | nd | ICP-OES | [15] |
| 33.9 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 28.7 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 28.5 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 26.2 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 23.8 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 22.9 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Ethiopia | GF-AAS (3100 Perkin Elmer) | [71] |
| 19.4 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Brazil | GF-AAS | [71] |
| 18.7 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 18.7 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Sumatra | GF-AAS | [71] |
| 18.3 (13.8 ± 1.2–21.0 ± 1.8) | pour-over | 5 | 6 | 200 | nd | nd | nd | 100 | nd | nd | ground | Ethiopia | FAAS | [64] |
| 15.8 (9.1–18.4) | pour-over | 10 | 6 | 150 | nd | distilled water | nd | 100 | nd | nd | powder coffee | nd | FAAS | [69] |
| 393 * (322–430) | pour-over | 10 | 6 | 200 | nd | re-distilled water | nd | 100 | Arabica & Robusta mix | roasted | ground | nd | FAAS | [38] |
| 227 * (113–324) | pour-over | 10 | 6 | 200 | nd | re-distilled water | nd | 100 | Arabica | roasted | ground | nd | FAAS | [38] |
| 15.33 ± 533 * (8.93–24.50) | Turkish coffee | 5 | 10 ± 0.1 | 200 | nd | nd | nd | 100 | nd | nd | fresh ground | nd | FAAS | [65] |
| 0.8 * | Turkish coffee | nd | 2 | 100 | nd | distilled water | nd | nd | Arabica | roasted | nd | nd | ICP-OES | [96] |
| Content Av. ± SD (µg/100 mL or 100 g) | Method of Brewing | Time (min) | Coffee (g) | Water (mL) | Cup Volume (mL) | Type of Water | Pressure (Ba) | Temperature of Water (°C) | Species | Degree of Roasting | Type of Coffee | Origin | Method of Analysis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.5 ± 0.1–2.4 ± 0.1 | pour-over | 5 | 6 | 200 | nd | nd | nd | 100 | nd | nd | ground | Ethiopia | FAAS | [64] |
| 1.2 | pour-over | 5 | 17 | 250 | nd | filtered water | nd | 92 | Arabica | roasted | very fine ground | nd | ICP-OES | [15] |
| 1.12 | drip | 2.5 | 18 | 300 | nd | filtered water | nd | 92 | Arabica | roasted | medium coarse ground | nd | ICP-OES | [15] |
| 0.9 | Aeropress | 2 | 18 | nd | 250 | filtered water | 2–4 | 93 | Arabica | roasted | coarse ground | nd | ICP-OES | [15] |
| 0.68 | French press | 5 | 17 | 300 | nd | filtered water | 1–2 | 92 | Arabica | roasted | medium ground | nd | ICP-OES | [15] |
| 0.6 | coffee machine | nd | 17 | nd | 250 | filtered water | 9 | 92 | Arabica | roasted | very fine ground | nd | ICP-OES | [15] |
| 7 ± 2 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| 6.6 ± 0.7 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| 6 ± 2 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| 6 ± 2 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| Content Av. ± SD (µg/100 mL or 100 g) | Method of Brewing | Time (min) | Coffee (g) | Water (mL) | Cup Volume (mL) | Type of Water | Pressure (Ba) | Temperature of Water (°C) | Species | Degree of Roasting | Type of Coffee | Origin | Method of Analysis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.7 | Aeropress | 2 | 18 | nd | 250 | filtered | 2–4 | 93 | Arabica | roasted | coarse ground | nd | ICP-OES | [15] |
| ~3.4 | pour-over | 5 | 17 | 250 | nd | filtered | nd | 92 | Arabica | roasted | very fine ground | nd | ICP-OES | [15] |
| ~3.3 | drip | 2.5 | 18 | 300 | nd | filtered | nd | 92 | Arabica | roasted | medium coarse ground | nd | ICP-OES | [15] |
| ~3.2 | French press | 5 | 17 | 300 | nd | filtered | 1–2 | 92 | Arabica | roasted | medium ground | nd | ICP-OES | [15] |
| ~2.7 | coffee machine | nd | 17 | nd | 250 | filtered | 9 | 92 | Arabica | roasted | very fine ground | nd | ICP-OES | [15] |
| 2.2 | pour-over | nd | 12 | 100 | nd | nd | nd | 95–100 | Arabica | medium roasted | fresh ground | Brazil (Cerrado Mineiro) | FS-FAAS | [68] |
| 0.401 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Ethiopia | GF-AAS | [71] |
| 0.362 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 0.291 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 0.260 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 0.228 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Sumatra | GF-AAS | [71] |
| 0.220 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 0.211 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 0.183 | filter coffee machine | nd | 9 | 75 | nd | nd | nd | nd | Arabica | roasted | fresh ground | Brazil | GF-AAS | [71] |
| 0.170 | coffee machine | nd | 9 | 75 | nd | nd | nd | nd | nd | roasted | ground | nd | GF-AAS | [71] |
| 6 ± 4 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| 5 ± 2 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| 4 ± 2 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| 3.5 ± 0.1 * | pour-over | nd | 1 | nd | 27 | nd | nd | nd | nd | roasted | ground | nd | ICP-MS | [90] |
| Content Av. ± SD (µg/100 mL or 100 g) | Method of Brewing | Time (min) | Coffee (g) | Water (mL) | Cup Volume (mL) | Type of Water | Pressure (Ba) | Temperature of Water (°C) | Species | Degree of Roasting | Type of Coffee | Origin | Method of Analysis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ~50 | Turkish coffee | 5 | 1.5 | 150 | nd | nd | nd | 100 | nd | green | fresh ground | Guatemala (Antigua region) | ISE | [115] |
| ~14 | coffee percolator | 5 | 1.5 | 150 | nd | nd | nd | 100 | nd | green | fresh ground | Guatemala (Antigua region) | ISE | [115] |
| ~8 | pour-over | 5 | 1.5 | 150 | nd | nd | nd | 100 | nd | green | fresh ground | Guatemala (Antigua region) | ISE | [115] |
| ~7.5 | filter coffee machine | 5 | 1.5 | 150 | nd | nd | nd | 100 | Arabica | roasted | fresh ground | Guatemala (Antigua region) | ISE | [115] |
| ~7 | filter coffee machine | 5 | 1.5 | 150 | nd | nd | nd | 100 | nd | green | fresh ground | Guatemala (Antigua region) | ISE | [115] |
| ~4.7 | French press | 5 | 1.5 | 150 | nd | nd | nd | 100 | nd | green | fresh ground | Guatemala (Antigua region) | ISE | [115] |
| ~4 | pour-over | 5 | 1.5 | 150 | nd | nd | nd | 100 | Robusta | roasted | fresh ground | India | ISE | [115] |
| ~3.7 | coffee percolator | 5 | 1.5 | 150 | nd | nd | nd | 100 | Robusta | roasted | fresh ground | India | ISE | [115] |
| ~3 | pour-over | 5 | 1.5 | 150 | nd | nd | nd | 100 | Arabica | roasted | fresh ground | Guatemala (Antigua region) | ISE | [115] |
| ~2.2 | Turkish coffee | 5 | 1.5 | 150 | nd | nd | nd | 100 | Arabica | roasted | fresh ground | Guatemala (Antigua region) | ISE | [115] |
| ~2 | French press | 5 | 1.5 | 150 | nd | nd | nd | 100 | Arabica | roasted | fresh ground | Guatemala (Antigua region) | ISE | [115] |
| ~2 | coffee percolator | 5 | 1.5 | 150 | nd | nd | nd | 100 | Arabica | roasted | fresh ground | Guatemala (Antigua region) | ISE | [115] |
| ~2 | filter coffee machine | 5 | 1.5 | 150 | nd | nd | nd | 100 | Robusta | roasted | fresh ground | India | ISE | [115] |
| ~1.8 | French press | 5 | 1.5 | 150 | nd | nd | nd | 100 | Robusta | roasted | fresh ground | India | ISE | [115] |
| ~1 | Turkish coffee | 5 | 1.5 | 150 | nd | nd | nd | 100 | Robusta | roasted | fresh ground | India | ISE | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olechno, E.; Puścion-Jakubik, A.; Socha, K.; Zujko, M.E. Coffee Infusions: Can They Be a Source of Microelements with Antioxidant Properties? Antioxidants 2021, 10, 1709. https://doi.org/10.3390/antiox10111709
Olechno E, Puścion-Jakubik A, Socha K, Zujko ME. Coffee Infusions: Can They Be a Source of Microelements with Antioxidant Properties? Antioxidants. 2021; 10(11):1709. https://doi.org/10.3390/antiox10111709
Chicago/Turabian StyleOlechno, Ewa, Anna Puścion-Jakubik, Katarzyna Socha, and Małgorzata Elżbieta Zujko. 2021. "Coffee Infusions: Can They Be a Source of Microelements with Antioxidant Properties?" Antioxidants 10, no. 11: 1709. https://doi.org/10.3390/antiox10111709
APA StyleOlechno, E., Puścion-Jakubik, A., Socha, K., & Zujko, M. E. (2021). Coffee Infusions: Can They Be a Source of Microelements with Antioxidant Properties? Antioxidants, 10(11), 1709. https://doi.org/10.3390/antiox10111709









