Whey Protein Isolate-Xylose Maillard-Based Conjugates with Tailored Microencapsulation Capacity of Flavonoids from Yellow Onions Skins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ethanolic Ultrasound-Assisted Extraction of Flavonoids from Onion Skins
2.3. Preparation of WPI–X–Flavonoid Conjugates
2.4. Characterization of the Extract and of Microencapsulated Powders
2.5. Browning Index and Grafting Degree Measurement of the Powders
2.6. In Silico Investigations
2.7. Confocal Laser Microscope Spectroscopy
2.8. Formulation of a Value-Added Food Product
2.9. Statistical Analyses
3. Results
3.1. Phytochemical Characterization of the Yellow Onion Skin Extract
3.2. Correlation between Browning Intensity, Grafting Degree and Microencapsulation Efficiency
3.3. Molecular Modeling
3.4. Phytochemical Profile of the Powders
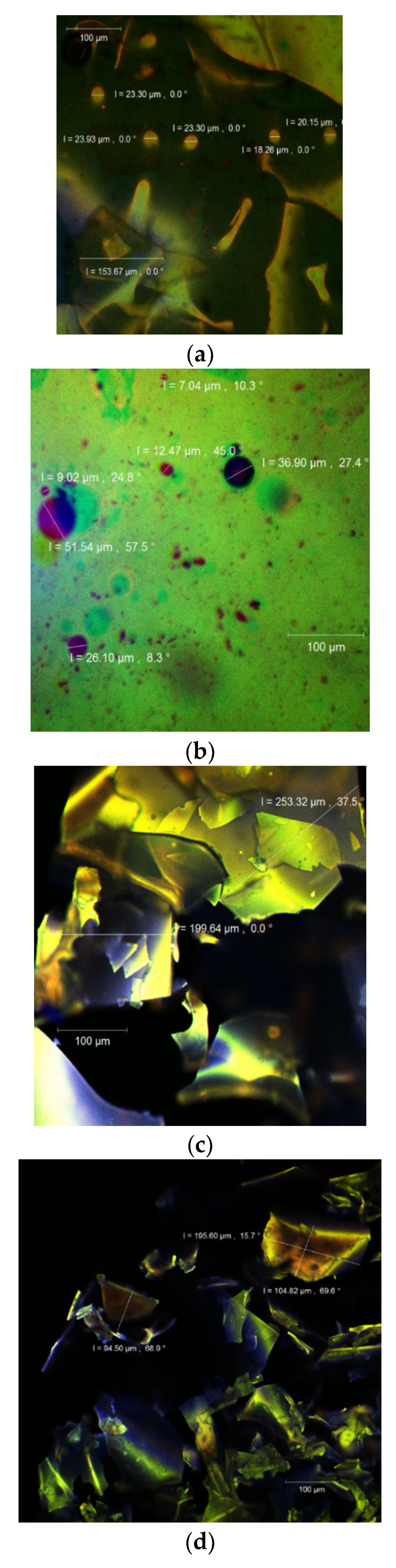
3.5. Structure and Morphology of the Powder
3.6. Characterization of New Formulated Food Product
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mark, R.; Lyu, X.; Lee, J.J.L.; Parra-Saldívar, R.; Chen, W.N. Sustainable production of natural phenolics for functional food applications. J. Funct. Foods 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Conti, V.M.; Guzzetti, L.; Panzeri, D.; De Giuseppe, R.; Coccetti, P.; Labra, M.; Cena, H. Bioactive compounds in legumes: Implications for sustainable nutrition and health in the elderly population. Trends Food Sci. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Choi, I.; Cho, E.; Moon, J.; Bae, H. Onion skin waste as a valorization resource for the by-products quercetin and biosugar. Food Chem. 2015, 188, 537–542. [Google Scholar] [CrossRef]
- Abouzed, T.K.; Contreras, M.D.M.; Sadek, K.M.; Shukry, M.; Abdelhady, D.H.; Gouda, W.M.; Abdo, W.; Nasr, N.E.; Mekky, R.H.; Segura-Carretero, A.; et al. Red onion scales ameliorated streptozotocin-induced diabetes and diabetic nephropathy in Wistar rats in relation to their metabolite fingerprint. Diabetes Res. Clin. Pract. 2018, 140, 253–264. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Nile, S.H.; Lee, Y.R. Economical and environment friendly approaches for usage of onion (Allium cepa L.) wastes. Food Funct. 2016, 7, 3354–3369. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Zhu, D.Y.; Thakur, K.; Wang, C.H.; Wang, H.; Ren, Y.F.; Zhang, J.G.; Wei, Z.J. Antioxidant and antibacterial evaluation of polysaccharides sequentially extracted from onion (Allium cepa L.). Int. J. Biol. Macromol. 2018, 111, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Ko, M.J.; Chung, M.S. Extraction of the flavonol quercetin from onion waste by combined treatment with intense pulsed light and subcritical water extraction. J. Clean. Prod. 2019, 231, 1192–1199. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze-drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef]
- Cam, M.; İçyer, N.C.; Erdoğan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT-Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Milea, A.S.; Aprodu, I.; Vasile, A.M.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Widen the functionality of flavonoids from yellow onion skins through extraction and microencapsulation in whey proteins hydrolysates and different polymers. J. Food Eng. 2019, 251, 29–35. [Google Scholar] [CrossRef]
- Jia, C.; Caoa, D.; Jia, S.; Lina, W.; Zhanga, X.; Muhoza, B. Whey protein isolate conjugated with xylo-oligosaccharides via maillard reaction: Characterization, antioxidant capacity, and application for lycopene microencapsulation. LWT-Food Sci. Technol. 2020, 118, 108837. [Google Scholar] [CrossRef]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-based delivery systems for the nanoencapsulation of food ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef] [Green Version]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Coklar, H.; Akbulut, M. The control of Maillard reaction in white grape molasses by the method of reducing reactant concentration. Food Sci. Technol. 2020, 40, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Chrysina, E.; Brew, K.; Acharya, K. Crystal structures of apo- and holo-bovine α-lactalbumin at 2.2-Å resolution reveal an effect of calcium on inter-lobe interactions. J. Biol. Chem. 2000, 275, 37021–37029. [Google Scholar] [CrossRef] [Green Version]
- Loch, J.I.; Bonarek, P.; Polit, A.; Riès, D.; Dziedzicka-Wasylewska, M.; Lewiński, K. Binding of 18-carbon unsaturated fatty acids to bovine β-lactoglobulin-structural and thermodynamic studies. Int. J. Biol. Macromol. 2013, 57, 226–231. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van Der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Aprodu, I.; Ursache, F.M.; Turturică, M.; Râpeanu, G.; Stănciuc, N. Thermal stability of the complex formed between carotenoids from sea buckthorn (Hippophae rhamnoides L.) and bovine β-lactoglobulin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfson, H.J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33, W363–W367. [Google Scholar] [CrossRef] [Green Version]
- Krissinel, E. Crystal contacts as nature’s docking solutions. J. Comput. Chem. 2010, 31, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Volkamer, A.; Kuhn, D.; Rippmann, F.; Rarey, M. DoGSiteScorer: A web server for automatic binding site prediction, analysis and druggability assessment. Bioinformatics 2012, 28, 2074–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantin, O.E.; Milea, A.Ș.; Bolea, C.A.; Mihalcea, L.; Enachi, E.; Copolovici, D.M.; Copolovici, L.; Munteanu, F.; Bahrim, G.E.; Râpeanu, G. Onion (Allium cepa L.) peel extracts characterization by conventional and modern methods. Int. J. Food Eng. 2021, 17, 485–493. [Google Scholar] [CrossRef]
- Singh, V.; Krishan, P.; Shri, R. Extraction of antioxidant phytoconstituents from onion waste. J. Pharmacog. Phytochem. 2016, 6, 502–505. [Google Scholar]
- Pobłocka-Olech, L.; Głód, D.; Żebrowska, E.M.; Sznitowska, M.; Baranowska, M.K. TLC determination of flavonoids from different cultivars of Allium cepa and Allium ascalonicum. Acta Pharmacol. 2016, 66, 543–554. [Google Scholar] [CrossRef] [Green Version]
- Benito-Román, O.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical Water Extraction of Phenolic Compounds from Onion Skin Wastes (Allium cepa cv. Horcal): Effect of Temperature and Solvent Properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef]
- Galanakis, C.M. Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Creating proteins with novel functionality via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C.; Yang, D.-J.; Jin, S.-J.; Hsu, C.-S.; Chen, S.-L. Kinetics of color development, pH decreasing, and anti-oxidative activity reduction of Maillard reaction in galactose/glycine model systems. Food Chem. 2008, 108, 533–541. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, F.; Ji, W.; Yokoyama, W.; Shoemaker, C.F.; Zhu, S.; Xia, W. Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocoll. 2013, 30, 53–60. [Google Scholar] [CrossRef]
- Jiménez-Castanõ, L.; Villamiel, M.; Lòpez-Fandinõ, R. Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll. 2007, 21, 433–443. [Google Scholar] [CrossRef]
- Suminar, A.T.; Al-Baarri, A.N.; Legowo, A.M. Demonstration of physical phenomena’s and scavenging activity from d-psicose and methionine Maillard reaction products. Potravin. Slovak J. Food Sci. 2017, 11, 417–424. [Google Scholar]
- Liu, L.; Li, X.; Zhu, Y.; Massounga Bora, A.F.; Zhao, Y.; Du, L.; Li, D.; Bi, W. Effect of microencapsulation with Maillard reaction products of whey proteins and isomaltooligosaccharide on the survival of Lactobacillus rhamnosus. LWT 2016, 73, 37–43. [Google Scholar] [CrossRef]
- Xu, Y.; Pitkänen, L.; Maina, N.H.; Coda, R.; Katina, K.; Tenkanen, M. Interactions between fava bean protein and dextrans produced by Leuconostoc pseudomesenteroides DSM 20193 and Weissella cibaria Sj 1b. Carbohydr. Polym. 2018, 190, 315–323. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, H.; Muhoza, B.; Hayat, K.; Hussain, S.; Tahir, M.U.; Zhang, X.; Ho, C.-T. Whey protein isolate-dextran conjugates: Decisive role of glycation time dependent conjugation degree in size control and stability improvement of colloidal nanoparticles. LWT 2021, 148, 111766. [Google Scholar] [CrossRef]
- Shang, J.; Zhong, F.; Zhu, S.; Wang, J.; Huang, D.; Li, Y. Structure and physiochemical characteristics of whey protein isolate conjugated with xylose through Maillard reaction at different degrees. Arab. J. Chem. 2020, 13, 8051–8059. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Cai, J.; Zhang, X.; Su, J.; Li, L. Time effect on coenzyme Q 10 loading and stability of micelles based on glycosylated casein via Maillard reaction. Food Hydrocoll. 2017, 72, 271–280. [Google Scholar] [CrossRef]
- Ghatak, D.; Iyyaswami, R. Selective encapsulation of quercetin from dry onion peel crude extract in reassembled casein particles. Food Bioprod. Process. 2019, 115, 100–109. [Google Scholar] [CrossRef]
- Akdeniz, N.; Sumnu, G.; Sahin, S. Microencapsulation of phenolic compounds extracted from onion (Allium cepa) skin. J. Food Process. Preserv. 2018, 42, e13648. [Google Scholar] [CrossRef]
- Horincar, G.; Aprodu, I.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Interactions of flavonoids from yellow onion skins with whey proteins: Mechanisms of binding and microencapsulation with different combinations of polymers. Spectrochim Acta A Mol. Biomol. Spectrosc. 2019, 215, 158–167. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD-Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Milea, Ș.A.; Vasile, M.A.; Crăciunescu, O.; Prelipcean, A.M.; Bahrim, G.E.; Râpeanu, G.; Oancea, A.; Stănciuc, N. Co-microencapsulation of flavonoids from yellow onion skins and lactic acid bacteria lead to a multifunctional ingredient for foods and pharmaceutics applications. Pharmaceutics 2020, 12, 1053. [Google Scholar] [CrossRef] [PubMed]
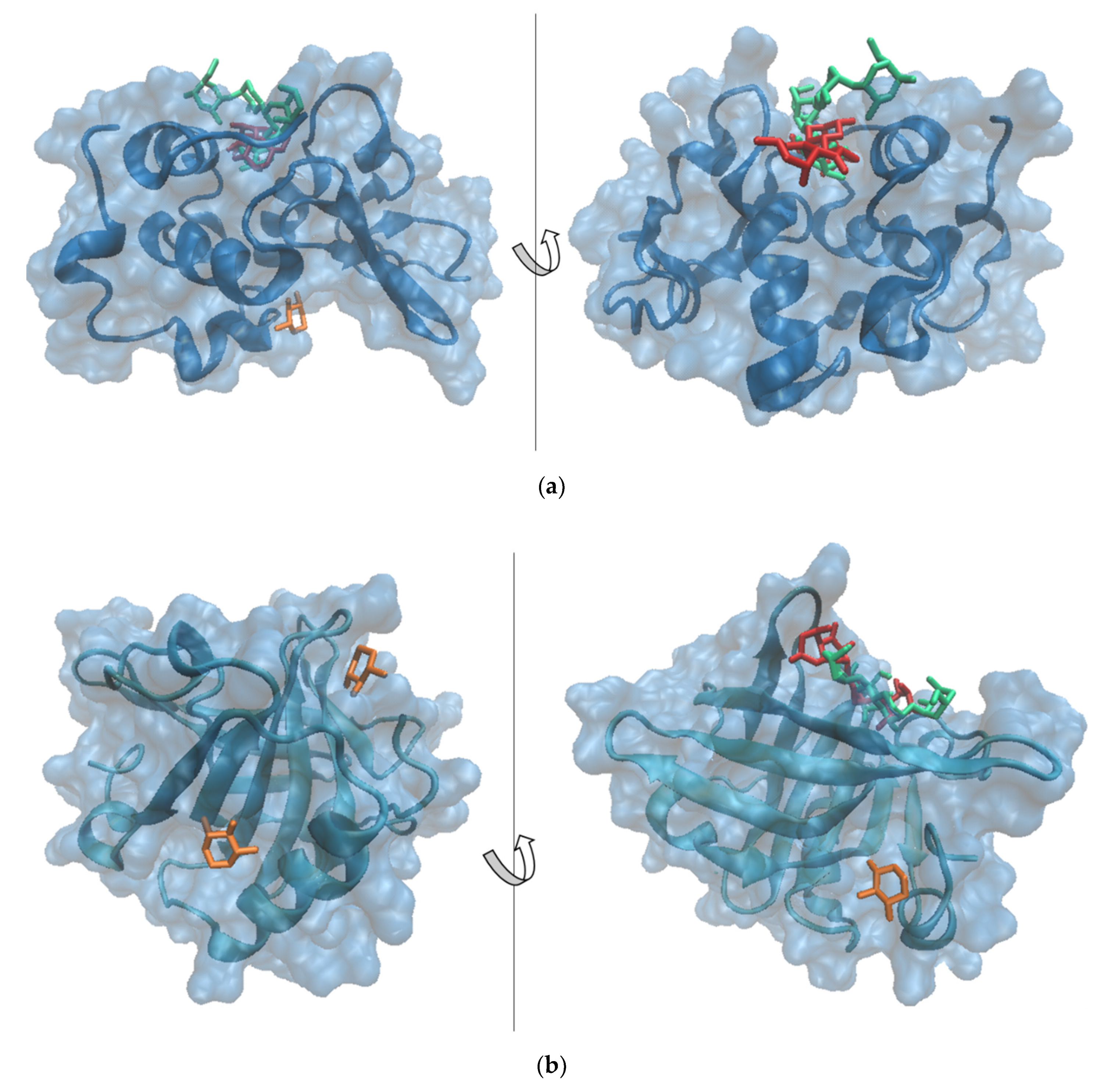
| α-LA—X | α-LA—QMG | α-LA—QDG | |||
|---|---|---|---|---|---|
| Complex 1 | Complex 2 | Complex 3 | Horincar et al. [41] | ||
| Interaction descriptors | |||||
| Amino acids interacting with ligands | Thr33, Glu49, Phe53, Gln54, Tyr103, Trp104, Leu105, Ala106 | Thr33, Val42, Asn44, Glu49, Phe53, Gln54, Tyr103, Trp104, Leu105, Ala106 | Glu11, Leu12, Asp14, Leu15, Thr38, Leu85, Thr86, Ile89, Met90, Lys93 | Leu3, Glu11, Leu12, Lys13, Asp14, Thr38, Leu52, Leu85, Thr86, Asp88, Ile89, Met90, Lys93 | Glu1, Leu3, Arg10, Glu11, Leu12, Lys13, Thr38, Leu52, Asp83, Leu85, Thr86, Asp88, Ile89 |
| Binding energy, kcal/mol | −13.00 | −10.52 | −7.48 | −24.41 | −32.01 |
| Interface area, Å2 | 153.5 | 147.5 | 159.8 | 625.20 | 541.20 |
| Pocket descriptors | |||||
| Volume, Å3 | 339.58 | 339.58 | 381.50 | 435.84 | |
| Depth, Å | 15.72 | 15.72 | 13.18 | 13.94 | |
| Enclosure | 0.16 | 0.16 | 0.20 | 0.30 | |
| β-LG—X | β-LG—QMG | β-LG—QDG | |||
| Complex 1 | Complex 2 | Complex 3 | Horincar et al. [41] | ||
| Interaction descriptors | |||||
| Amino acids interacting with ligands | Tyr20, Tyr42, Val43, Glu44, Gln59, Cys66, Pro126, Leu156, Glu157, Glu158, Gln159, Cys160, His161 | Ala23, Met24, Ala25, Leu133, Phe136, Asp137, Leu140, Arg148, Leu149, Ser150 | Ile2, Leu93, Glu108, Ala111, Gln115, Leu117 | Ala37, Pro38, Leu39, Val41, Leu58, Lys60, Ile71, Ile84, Asp85, Ala86, Leu87, Asn88, Asn90, Met107, Glu108, Asn109 | Pro38, Leu39, Val41, Leu58, Lys60, Glu62, Ala67, Lys69, Ile71, Asn90, Met107, Glu108, Asn109 |
| Binding energy, kcal/mol | −13.37 | −16.41 | −13.00 | −35.05 | −34.37 |
| Interface area, Å2 | 169.1 | 152.8 | 157.6 | 502.10 | 585.90 |
| Pocket descriptors | |||||
| Volume, Å3 | 217.15 | 141.31 | 137.86 | 229.70 | |
| Depth, Å | 9.42 | 7.68 | 10.16 | 13.08 | |
| Enclosure | 0.12 | 0.19 | 0.19 | 0.33 | |
| Selected Phytochemicals | Control | Samples with 3% Addition of Variant 1 | Samples with 3% Addition of Variant 2 | |||
|---|---|---|---|---|---|---|
| Time 0 | After 28 Days | Time 0 | After 28 Days | Time 0 | After 28 Days | |
| Total flavonoids, mg QE/g DW | 0.66 ± 0.02 a | 0.63 ± 0.02 a | 1.04 ± 0.05 a | 0.99 ± 0.01 a | 1.08 ± 0.05 a | 0.98 ± 0.04 b |
| Total polyphenols, mg GAE/g DW | 0.73 ± 0.04 a | 0.75 ± 0.022 | 1.24 ± 0.03 a | 1.22 ± 0.05 a | 1.37 ± 0.19 a | 1.08 ± 0.019 a |
| Antioxidant activity, mMol TE/g DW | 156.07 ± 2.57 b | 197.97 ± 1.74 a | 157.89 ± 1.41 b | 199.49 ± 0.81 a | 158.19 ± 0.48 b | 198.57 ± 0.35 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milea, Ș.A.; Aprodu, I.; Enachi, E.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Whey Protein Isolate-Xylose Maillard-Based Conjugates with Tailored Microencapsulation Capacity of Flavonoids from Yellow Onions Skins. Antioxidants 2021, 10, 1708. https://doi.org/10.3390/antiox10111708
Milea ȘA, Aprodu I, Enachi E, Barbu V, Râpeanu G, Bahrim GE, Stănciuc N. Whey Protein Isolate-Xylose Maillard-Based Conjugates with Tailored Microencapsulation Capacity of Flavonoids from Yellow Onions Skins. Antioxidants. 2021; 10(11):1708. https://doi.org/10.3390/antiox10111708
Chicago/Turabian StyleMilea, Ștefania Adelina, Iuliana Aprodu, Elena Enachi, Vasilica Barbu, Gabriela Râpeanu, Gabriela Elena Bahrim, and Nicoleta Stănciuc. 2021. "Whey Protein Isolate-Xylose Maillard-Based Conjugates with Tailored Microencapsulation Capacity of Flavonoids from Yellow Onions Skins" Antioxidants 10, no. 11: 1708. https://doi.org/10.3390/antiox10111708
APA StyleMilea, Ș. A., Aprodu, I., Enachi, E., Barbu, V., Râpeanu, G., Bahrim, G. E., & Stănciuc, N. (2021). Whey Protein Isolate-Xylose Maillard-Based Conjugates with Tailored Microencapsulation Capacity of Flavonoids from Yellow Onions Skins. Antioxidants, 10(11), 1708. https://doi.org/10.3390/antiox10111708










