Quantitative Analysis of Intracranial Atherosclerosis and Its Correlation with Ischemic Cerebrovascular Disease and Prognosis
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Baseline Assessment
2.3. Follow-Up Assessment
2.4. Statistical Analysis
3. Results
3.1. Comparison of the Features of Culprit Plaque and Non-Culprit Plaque
3.2. Univariate Analysis of Favorable Prognosis and Poor Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhao, X.; Liu, L.; Soo, Y.O.; Pu, Y.; Pan, Y.; Wang, Y.; Zou, X.; Leung, T.W.; Cai, Y.; et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: The Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014, 45, 663–669. [Google Scholar] [CrossRef]
- Holmstedt, C.A.; Turan, T.N.; Chimowitz, M.I. Atherosclerotic intracranial arterial stenosis: Risk factors, diagnosis, and treatment. Lancet Neurol. 2013, 12, 1106–1114. [Google Scholar] [CrossRef]
- Mazighi, M.; Tanasescu, R.; Ducrocq, X.; Vicaut, E.; Bracard, S.; Houdart, E.; Woimant, F. Prospective study of symptomatic atherothrombotic intracranial stenoses: The GESICA study. Neurology 2006, 66, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, C.; Kernan, W.N.; Sharrief, A.Z.; Chaturvedi, S.; Cole, J.W.; Cornwell, W.K., 3rd; Cosby-Gaither, C.; Doyle, S.; Goldstein, L.B.; Lennon, O.; et al. 2024 Guideline for the Primary Prevention of Stroke: A Guideline From the American Heart Association/American Stroke Association. Stroke 2024, 55, e344–e424. [Google Scholar] [CrossRef]
- Tjoumakaris, S.I.; Roy, J.M.; Amin-Hanjani, S.; Charbel, F.T.; Dabus, G.; Fisher, M.; Gounis, M.; Hoh, B.L.; Liebeskind, D.S.; Linfante, I.; et al. ARISE II Consensus on the Management of Intracranial Atherosclerotic Disease. Stroke 2025, 56, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Spagnolo-Allende, A.; Yang, D.; Qiao, Y.; Gutierrez, J. Epidemiology, Pathophysiology, and Imaging of Atherosclerotic Intracranial Disease. Stroke 2024, 55, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Savastano, L.; Brinjikji, W.; Lutsep, H.; Chen, H.; Chaturvedi, S. Symptomatic Nonstenotic Carotids: A Topical Review. Stroke 2024, 55, 2921–2931. [Google Scholar] [CrossRef]
- Hoh, B.L.; Chimowitz, M.I. Focused Update on Intracranial Atherosclerosis: Introduction, Highlights, and Knowledge Gaps. Stroke 2024, 55, 305–310. [Google Scholar] [CrossRef]
- Tritanon, O.; Mataeng, S.; Apirakkan, M.; Panyaping, T. Utility of high-resolution magnetic resonance vessel wall imaging in differentiating between atherosclerotic plaques, vasculitis, and arterial dissection. Neuroradiology 2023, 65, 441–451. [Google Scholar] [CrossRef]
- Huang, J.; Liu, C.; Jiao, S.; Chen, Y.; Xu, L.; Gong, T.; Zhu, C.; Song, Y. Application of high-resolution MRI in evaluating statin efficacy on symptomatic intracranial atherosclerosis. Eur. Radiol. 2025, 35, 441–452. [Google Scholar] [CrossRef]
- Khenkina, N.; Aimo, A.; Fabiani, I.; Masci, P.G.; Sagris, D.; Williams, S.E.; Mavraganis, G.; Chen, H.S.; Wintermark, M.; Michel, P.; et al. Magnetic resonance imaging for diagnostic workup of embolic stroke of undetermined source: A systematic review. Int. J. Stroke Off. J. Int. Stroke Soc. 2024, 19, 293–304. [Google Scholar] [CrossRef]
- Mazzacane, F.; Mazzoleni, V.; Scola, E.; Mancini, S.; Lombardo, I.; Busto, G.; Rognone, E.; Pichiecchio, A.; Padovani, A.; Morotti, A.; et al. Vessel Wall Magnetic Resonance Imaging in Cerebrovascular Diseases. Diagnostics 2022, 12, 258. [Google Scholar] [CrossRef]
- Vranic, J.E.; Hartman, J.B.; Mossa-Basha, M. High-Resolution Magnetic Resonance Vessel Wall Imaging for the Evaluation of Intracranial Vascular Pathology. Neuroimaging Clin. N. Am. 2021, 31, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Turan, T.N.; Rumboldt, Z.; Granholm, A.C.; Columbo, L.; Welsh, C.T.; Lopes-Virella, M.F.; Spampinato, M.V.; Brown, T.R. Intracranial atherosclerosis: Correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis 2014, 237, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Seyedsaadat, S.M.; Rizvi, A.; Alzuabi, M.; Dugani, S.B.; Murad, M.H.; Huston, J., 3rd; Saba, L.; Brinjikji, W. Correlation of MRI-detected vulnerable carotid plaques with clinical presentation: A systematic review and meta-analysis. J. Neurosurg. Sci. 2020, 64, 263–271. [Google Scholar] [CrossRef]
- Gutierrez, J.; Turan, T.N.; Hoh, B.L.; Chimowitz, M.I. Intracranial atherosclerotic stenosis: Risk factors, diagnosis, and treatment. Lancet Neurol. 2022, 21, 355–368. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators; Barnett, H.J.M.; Taylor, D.W.; Haynes, R.B.; Sackett, D.L.; Peerless, S.J.; Ferguson, G.G.; Fox, A.J.; Rankin, R.N.; Hachinski, V.C.; et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar] [CrossRef]
- Hartman, J.B.; Watase, H.; Sun, J.; Hippe, D.S.; Kim, L.; Levitt, M.; Sekhar, L.; Balu, N.; Hatsukami, T.; Yuan, C.; et al. Intracranial aneurysms at higher clinical risk for rupture demonstrate increased wall enhancement and thinning on multicontrast 3D vessel wall MRI. Br. J. Radiol. 2019, 92, 20180950. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.M.; Hatsukami, T.S.; Ferguson, M.S.; Small, R.; Polissar, N.L.; Yuan, C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002, 106, 1368–1373. [Google Scholar] [CrossRef]
- Olthuis, S.G.H.; Pirson, F.A.V.; Pinckaers, F.M.E.; Hinsenveld, W.H.; Nieboer, D.; Ceulemans, A.; Knapen, R.; Robbe, M.M.Q.; Berkhemer, O.A.; van Walderveen, M.A.A.; et al. Endovascular treatment versus no endovascular treatment after 6-24 h in patients with ischaemic stroke and collateral flow on CT angiography (MR CLEAN-LATE) in the Netherlands: A multicentre, open-label, blinded-endpoint, randomised, controlled, phase 3 trial. Lancet 2023, 401, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Schneider, D.J.; Feldmann, E.; Liebeskind, D.S. Intracranial atherosclerosis: Review of imaging features and advances in diagnostics. Int. J. Stroke Off. J. Int. Stroke Soc. 2022, 17, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D.; Hackam, D.G. Treating arteries instead of risk factors: A paradigm change in management of atherosclerosis. Stroke 2010, 41, 1193–1199. [Google Scholar] [CrossRef]
- Amarenco, P.; Kim, J.S.; Labreuche, J.; Charles, H.; Abtan, J.; Bejot, Y.; Cabrejo, L.; Cha, J.K.; Ducrocq, G.; Giroud, M.; et al. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N. Engl. J. Med. 2020, 382, 9. [Google Scholar] [CrossRef]
- Pirillo, A.; Tokgozoglu, L.; Catapano, A.L. European Lipid Guidelines and Cardiovascular Risk Estimation: Current Status and Future Challenges. Curr. Atheroscler. Rep. 2024, 26, 133–137. [Google Scholar] [CrossRef]
- Kang, C.K.; Park, C.W.; Han, J.Y.; Kim, S.H.; Park, C.A.; Kim, K.N.; Hong, S.M.; Kim, Y.B.; Lee, K.H.; Cho, Z.H. Imaging and analysis of lenticulostriate arteries using 7.0-Tesla magnetic resonance angiography. Magn. Reason. Med. 2009, 61, 136–144. [Google Scholar] [CrossRef]
- Marinkovic, S.; Gibo, H.; Milisavljevic, M.; Cetkovic, M. Anatomic and clinical correlations of the lenticulostriate arteries. Clin. Anat. 2001, 14, 190–195. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, K.M.; Kim, H.Y.; Kim, Y.S.; Koh, S.H.; Heo, S.H.; Chang, D.I. Basilar Artery Plaque and Pontine Infarction Location and Vascular Geometry. J. Stroke 2018, 20, 92–98. [Google Scholar] [CrossRef]
- Yao, W.; Chen, H.; Huang, K.; Peng, W.; Zhang, X.; Yang, D.; Teng, Z.; Shen, J.; Yang, J.; Cheng, X.; et al. Atherosclerotic plaque evolution predicts cerebral ischemic events in patients with intracranial atherosclerosis: A multicentre longitudinal study using high-resolution MRI. Eur. Radiol. 2025, 35, 3238–3248. [Google Scholar] [CrossRef]
- Teng, Z.; He, J.; Degnan, A.J.; Chen, S.; Sadat, U.; Bahaei, N.S.; Rudd, J.H.; Gillard, J.H. Critical mechanical conditions around neovessels in carotid atherosclerotic plaque may promote intraplaque hemorrhage. Atherosclerosis 2012, 223, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Millon, A.; Boussel, L.; Brevet, M.; Mathevet, J.L.; Canet-Soulas, E.; Mory, C.; Scoazec, J.Y.; Douek, P. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke 2012, 43, 3023–3028. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, X.; Yan, Z.; Wu, B.; Lu, B.; Chen, B.; Tian, D.; Du, A.; Li, L.; Liu, C.; et al. Association between high-resolution magnetic resonance vessel wall imaging characteristics and recurrent stroke in patients with intracranial atherosclerotic steno-occlusive disease: A prospective multicenter study. Int. J. Stroke Off. J. Int. Stroke Soc. 2024, 19, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Geng, D.; Zhao, W.; Li, S.; Du, X.; Zhang, S.; Wang, H. Differences in intracranial atherosclerosis plaque between posterior circulation and anterior circulation on high-resolution magnetic resonance imaging: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2024, 33, 107616. [Google Scholar] [CrossRef]
- Qiao, Y.; Anwar, Z.; Intrapiromkul, J.; Liu, L.; Zeiler, S.R.; Leigh, R.; Zhang, Y.; Guallar, E.; Wasserman, B.A. Patterns and Implications of Intracranial Arterial Remodeling in Stroke Patients. Stroke 2016, 47, 434–440. [Google Scholar] [CrossRef]
- Yu, M.; Yang, D.; Zhang, R.; Jiang, Y.; Qiao, H.; Zhao, X.; Liu, G.; Wang, Y. Carotid atherosclerotic plaque predicts progression of intracranial artery atherosclerosis: A MR imaging-based community cohort study. Eur. J. Radiol. 2024, 172, 111300. [Google Scholar] [CrossRef]
- Rondina, J.; Nachev, P. Artificial intelligence and stroke imaging. Curr. Opin. Neurol. 2025, 38, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Shurrab, S.; Guerra-Manzanares, A.; Magid, A.; Piechowski-Jozwiak, B.; Atashzar, S.F.; Shamout, F.E. Multimodal Machine Learning for Stroke Prognosis and Diagnosis: A Systematic Review. IEEE J. Biomed. Health Inform. 2024, 28, 6958–6973. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Yoon, J.G.; Park, H.; Kim, Y.D.; Nam, H.S.; Heo, J.H. Machine Learning-Based Model for Prediction of Outcomes in Acute Stroke. Stroke 2019, 50, 1263–1265. [Google Scholar] [CrossRef]
- Al-Janabi, O.M.; El Refaei, A.; Elgazzar, T.; Mahmood, Y.M.; Bakir, D.; Gajjar, A.; Alateya, A.; Jha, S.K.; Ghozy, S.; Kallmes, D.F.; et al. Current Stroke Solutions Using Artificial Intelligence: A Review of the Literature. Brain Sci. 2024, 14, 1182. [Google Scholar] [CrossRef]
- Romoli, M.; Caliandro, P. Artificial intelligence, machine learning, and reproducibility in stroke research. Eur. Stroke J. 2024, 9, 518–520. [Google Scholar] [CrossRef]


| Characteristics | Mean ± SD/% | Total | χ2/T | p | |
|---|---|---|---|---|---|
| Symptomatic | Asymptomatic | ||||
| Male | 101 (78.29) | 26 (61.90) | 127 (74.27) | 4.454 | 0.035 |
| Age | 56.99 ± 12.78 | 58.52 ± 10.18 | −0.793 | 0.430 | |
| Smoking | 58 (44.96) | 10 (23.81) | 68 (39.77) | 5.918 | 0.015 |
| Family history | 14 (10.85) | 1 (2.56) | 15 (8.93) | 2.53 | 0.112 |
| Hypertension | 90 (69.77) | 20 (47.62) | 110 (64.33) | 6.773 | 0.009 |
| Diabetes mellitus | 43 (33.33) | 4 (9.52) | 47 (27.49) | 9.012 | 0.003 |
| Hyperlipemia | 95 (73.64) | 28 (66.67) | 123 (71.93) | 0.764 | 0.382 |
| Carotid atherosclerosis | 101 (78.91) | 30 (71.43) | 131 (77.06) | 1.000 | 0.317 |
| MCA Location | |||||
| Superior wall | 38 (32.76) | 14 (37.84) | 52 (33.99) | ||
| Inferior wall | 22 (18.97) | 16 (43.24) | 38 (24.84) | 5.103 | 0.151 * |
| Ventral wall | 20 (17.24) | 5 (13.51) | 25 (16.34) | ||
| Dorsal wall | 4 (3.45) | 0 (0.00) | 4 (2.61) | ||
| BA Location | |||||
| Ventral wall | 9 (7.76) | 1 (2.70) | 10 (6.54) | ||
| Dorsal wall | 7 (6.03) | 0 (0.00) | 7 (4.58) | ||
| Right lateral wall | 5 (4.31) | 0 (0.00) | 5 (3.27) | 1.436 | 1.00 * |
| Left lateral wall | 11 (9.48) | 1 (2.70) | 12 (7.84) | ||
| Enhancement | |||||
| Grade 1 | 6 (4.65) | 6 (14.29) | 12 (7.02) | ||
| Grade 2 | 62 (48.06) | 18 (42.86) | 80 (46.78) | 4.509 | 0.105 |
| Grade 3 | 61 (47.29) | 18 (42.86) | 79 (46.20) | ||
| IPH | 23 (17.80) | 2 (4.8%) | 25 (14.60) | 5.261 | 0.022 |
| Vulnerable | 126 (97.7) | 27 (64.3) | 153 (89.5) | 37.503 | <0.001 |
| Thickness | 0.22 ± 0.11 | 0.20 ± 0.14 | 1.304 | 0.194 | |
| Length | 0.96 ± 2.92 | 0.73 ± 1.24 | 0.475 | 0.635 | |
| Stenosis | 59.31 ± 24.30 | 47.75 ± 24.36 | 2.675 | 0.008 | |
| Characteristics | Group | Total | χ2 | p | |
|---|---|---|---|---|---|
| Non-Culprit | Culprit | ||||
| MCA Location | 15.561 | 0.001 * | |||
| Superior wall | 9 (16.36) | 39 (34.51) | 48 (28.57) | ||
| Inferior wall | 21 (38.18) | 21 (18.58) | 42 (25.00) | ||
| Ventral wall | 7 (12.73) | 19 (16.81) | 26 (15.48) | ||
| Dorsal wall | 7 (12.73) | 3 (2.65) | 10 (5.95) | ||
| BA location | 34.138 | <0.001 * | |||
| Ventral wall | 1 (1.82) | 9 (7.96) | 10 (5.95) | ||
| Dorsal wall | 4 (7.27) | 7 (6.19) | 11 (6.55) | ||
| Right lateral wall | 2 (3.64) | 5 (4.42) | 7 (4.17) | ||
| Left lateral wall | 4 (7.27) | 10 (8.85) | 14 (8.33) | ||
| Enhancement | 23.077 | <0.001 | |||
| 1 | 18 (26.87) | 6 (4.80) | 24 (12.50) | ||
| 2 | 33 (49.25) | 60 (48.00) | 93 (48.44) | ||
| 3 | 16 (23.88) | 59 (47.20) | 75 (39.06) | ||
| Modified AHA type | 62.038 | <0.001 | |||
| IV | 33 (49.25) | 89 (71.20) | 122 (63.54) | ||
| V | 0 (0.00) | 1 (0.80) | 1 (0.52) | ||
| VI | 3 (4.48) | 32 (25.60) | 35 (18.23) | ||
| VII | 6 (8.96) | 1 (0.80) | 7 (3.65) | ||
| VIII | 25 (37.31) | 2 (1.60) | 27 (14.06) | ||
| IPH | 4 (5.97) | 22 (17.60) | 26 (13.54) | 5.039 | 0.025 |
| Vulnerable | 36 (53.73) | 122 (97.60) | 158 (82.29) | 57.605 | <0.001 |
| Thickness | 0.17 ± 0.09 | 0.26 ± 0.38 | −1.926 | 0.056 | |
| Length | 0.42 ± 0.25 | 0.95 ± 2.98 | −1.459 | 0.146 | |
| Stenosis | 40.04 ± 18.89 | 58.28 ± 24.44 | −5.739 | <0.001 | |
| Characteristics | Group (%/x ± SD) | Total | χ2/T | p | |
|---|---|---|---|---|---|
| Favorable | Poor | ||||
| Male | 82 (76.64) | 19 (86.36) | 101 (78.29) | 1.016 | 0.313 |
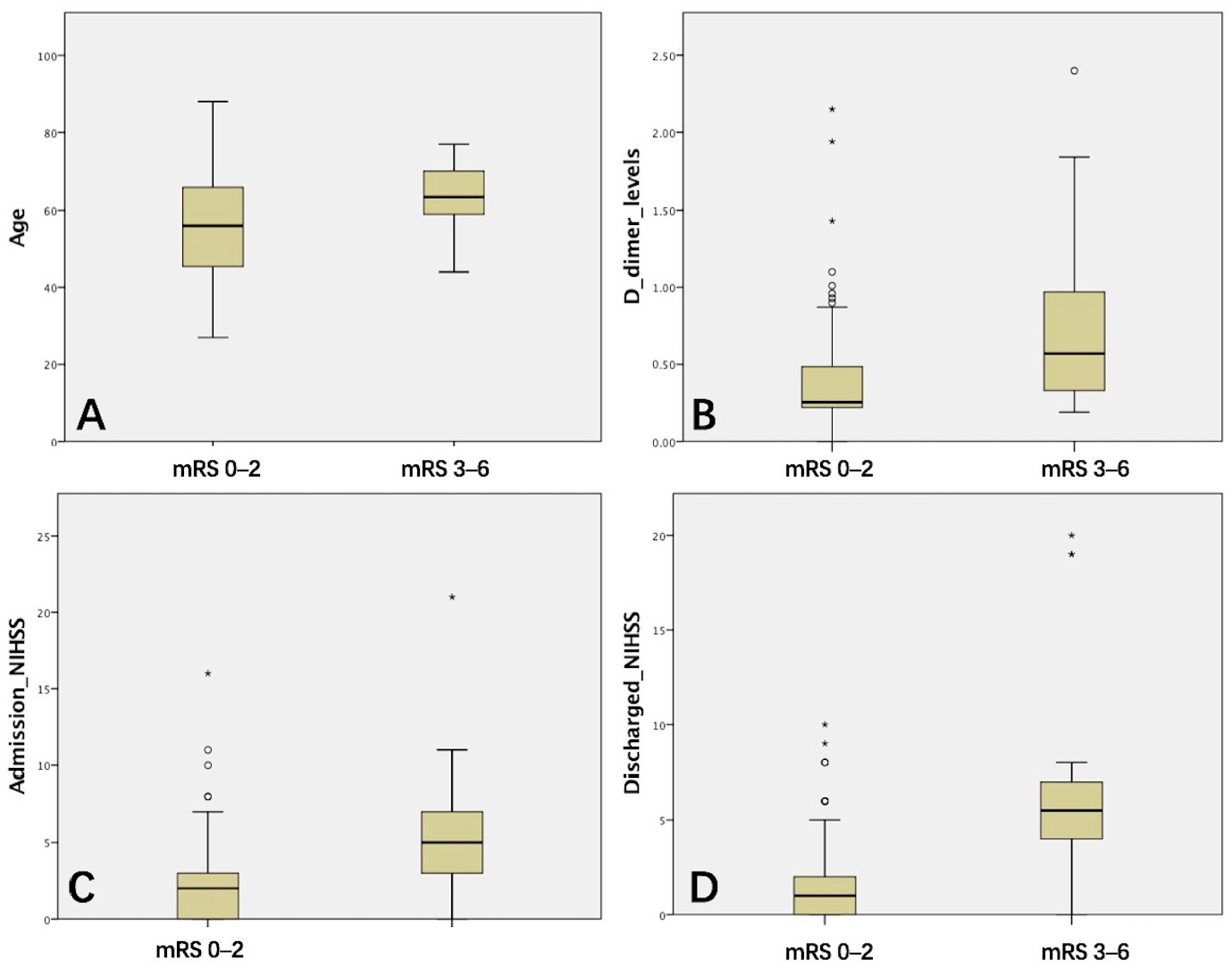
| Age | 55.63 ± 13.15 | 63.64 ± 8.19 | −3.709 | 0.001 ** | |
| Smoking | 51 (47.66) | 7 (31.82) | 58 (44.96) | 1.851 | 0.174 |
| Family history | 14 (13.08) | 0 (0.00) | 14 (10.85) | 3.229 | 0.072 |
| Hypertension | 73 (68.22) | 17 (77.27) | 90 (69.77) | 0.708 | 0.400 |
| Diabetes mellitus | 33 (30.84) | 10 (45.45) | 43 (33.33) | 1.754 | 0.185 |
| Hyperlipemia | 80 (74.77) | 15 (68.18) | 95 (73.64) | 0.408 | 0.523 |
| Carotid atherosclerosis | 80 (75.47) | 21 (95.45) | 101 (78.91) | 4.371 | 0.037 * |
| MCA Location | |||||
| Superior wall | 31 (31.96) | 7 (36.84) | 38 (32.76) | 1.159 | 0.789 |
| Inferior wall | 20 (20.62) | 2 (10.53) | 22 (18.97) | ||
| Ventral wall | 17 (17.53) | 3 (15.79) | 20 (17.24) | ||
| Dorsal wall | 4 (4.12) | 0 (0.00) | 4 (3.45) | ||
| VA Location | 89.086 | <0.001 ** | |||
| Ventral wall | 6 (6.19) | 3 (15.79) | 9 (7.76) | ||
| Dorsal wall | 5 (5.15) | 2 (10.53) | 7 (6.03) | ||
| Right lateral wall | 4 (4.12) | 1 (5.26) | 5 (4.31) | ||
| Left lateral wall | 10 (10.31) | 1 (5.26) | 11 (9.48) | ||
| Enhancement | |||||
| Grade 1 | 6 (5.61) | 0 (0.00) | 6 (4.65) | 1.484 | 0.476 |
| Grade 2 | 50 (46.73) | 12 (54.55) | 62 (48.06) | ||
| Grade 3 | 51 (47.66) | 10 (45.45) | 61 (47.29) | ||
| IPH | 15 (14.29) | 7 (31.82) | 22 (17.32) | 3.904 | 0.048 * |
| Vulnerable | 102 (96.23) | 22 (100.00) | 124 (96.88) | 0.857 | 0.355 |
| Thickness | 0.26 ± 0.41 | 0.24 ± 0.13 | 0.232 | 0.817 | |
| Length | 0.92 ± 3.22 | 1.14 ± 1.22 | −0.316 | 0.753 | |
| Stenosis | 58.44 ± 24.64 | 63.82 ± 23.73 | −0.937 | 0.351 | |
| Triglyceride | 1.70 ± 0.92 | 1.60 ± 0.70 | 0.449 | 0.654 | |
| Total cholesterol | 4.22 ± 1.17 | 4.54 ± 1.03 | −1.101 | 0.273 | |
| HDL | 1.08 ± 0.32 | 1.06 ± 0.22 | 0.278 | 0.781 | |
| LDL | 2.64 ± 1.03 | 2.93 ± 0.94 | −1.115 | 0.267 | |
| HCY | 11.85 ± 5.16 | 12.02 ± 4.35 | −0.128 | 0.898 | |
| Renal insufficiency | 7 (6.5) | 1 (4.5) | 0.195 | ||
| Leukocyte Count | 7.27 ± 2.41 | 8.04 ± 1.29 | −1.424 | 0.157 | |
| CRP | 3.13 ± 4.19 | 6.34 ± 7.69 | −1.563 | 0.138 | |
| Platelet Count | 240.03 ± 61.61 | 247.10 ± 65.46 | −0.475 | 0.636 | |
| INR | 0.92 ± 0.07 | 0.94 ± 0.08 | −0.907 | 0.366 | |
| Plasma fibrinogen levels | 3.41 ± 2.41 | 3.51 ± 0.89 | −0.183 | 0.855 | |
| D-dimer levels | 0.40 ± 0.36 | 0.77 ± 0.60 | −2.489 | 0.022 * | |
| Admission NIHSS | 2.24 ± 2.75 | 5.90 ± 4.84 | −3.356 | 0.003 ** | |
| Discharged NIHSS | 1.75 ± 2.19 | 6.81 ± 5.68 | −4.013 | 0.001 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Chen, S.; Hu, S.; Ren, L.; Xu, G. Quantitative Analysis of Intracranial Atherosclerosis and Its Correlation with Ischemic Cerebrovascular Disease and Prognosis. Brain Sci. 2025, 15, 1009. https://doi.org/10.3390/brainsci15091009
Cai J, Chen S, Hu S, Ren L, Xu G. Quantitative Analysis of Intracranial Atherosclerosis and Its Correlation with Ischemic Cerebrovascular Disease and Prognosis. Brain Sciences. 2025; 15(9):1009. https://doi.org/10.3390/brainsci15091009
Chicago/Turabian StyleCai, Jingjing, Sizhan Chen, Shiyu Hu, Lijie Ren, and Gelin Xu. 2025. "Quantitative Analysis of Intracranial Atherosclerosis and Its Correlation with Ischemic Cerebrovascular Disease and Prognosis" Brain Sciences 15, no. 9: 1009. https://doi.org/10.3390/brainsci15091009
APA StyleCai, J., Chen, S., Hu, S., Ren, L., & Xu, G. (2025). Quantitative Analysis of Intracranial Atherosclerosis and Its Correlation with Ischemic Cerebrovascular Disease and Prognosis. Brain Sciences, 15(9), 1009. https://doi.org/10.3390/brainsci15091009








