A Comprehensive Overview of Subacute Combined Degeneration: MRI Diagnostic Challenges and Treatment Pathways
Abstract
1. Introduction
1.1. Etiology
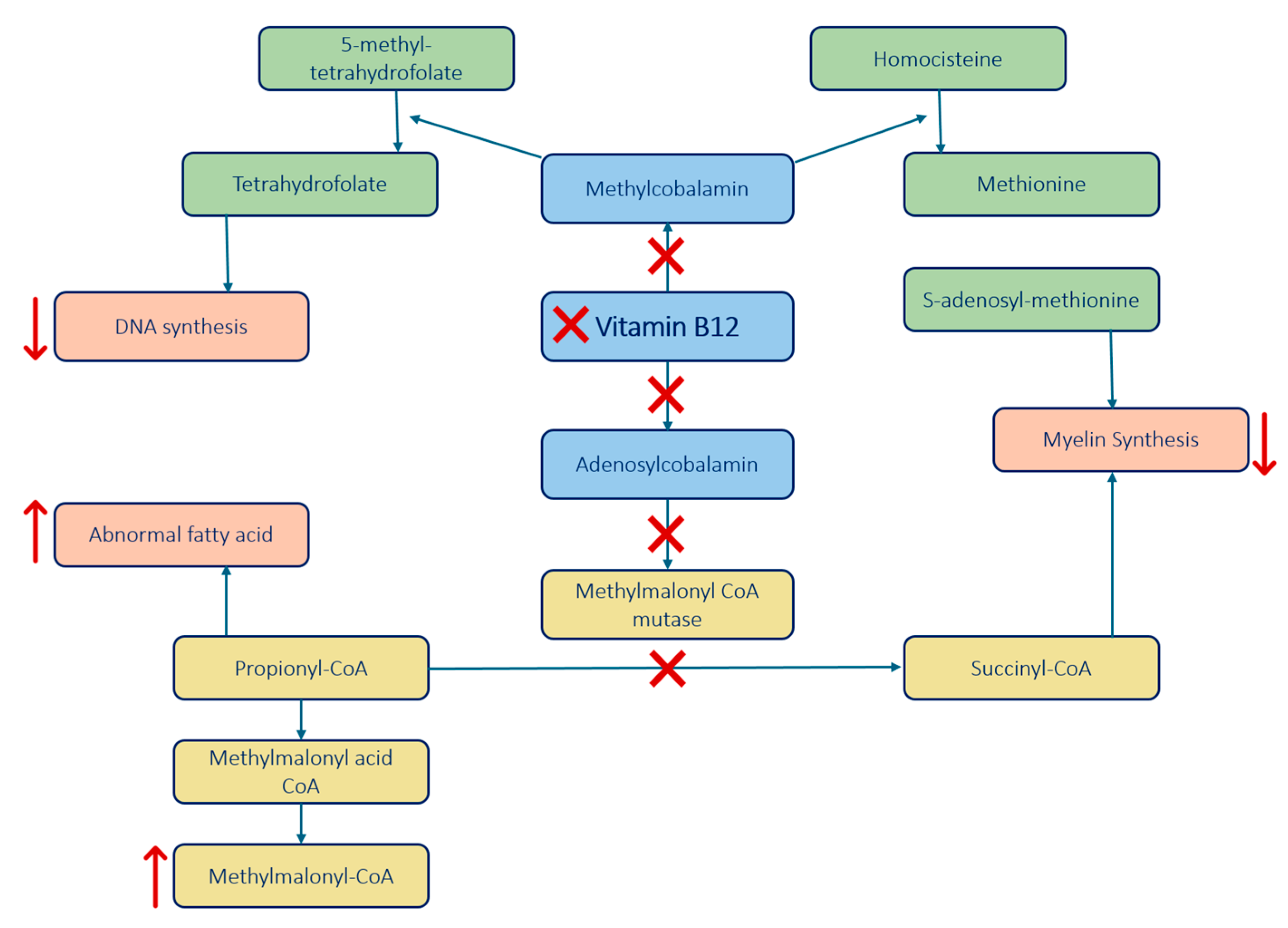
- Function: Converts homocysteine to methionine, which is a precursor to S-adenosyl-methionine (SAM) essential for myelin proteins and lipids methylation.
- Impact of B12 Deficiency: B12 deficiency disrupts the conversion, leading to reduced SAM formation, which impairs the methylation process essential for maintaining the myelin sheath.
- Additionally, this enzyme also helps convert 5-methyl-tetrahydrofolate to tetrahydrofolate, crucial for DNA synthesis. B12 deficiency impairs this demethylation impeding effective DNA synthesis.
- Function: Converts methylmalonyl-CoA to succinyl-CoA, necessary for myelin synthesis.
- Impact of B12 Deficiency: B12 deficiency leads to an accumulation of methylmalonyl-CoA and propionyl-CoA, which disrupts normal myelin synthesis and leads to the build-up of abnormal fatty acids, contributing to demyelination.
- Vitamin B12 is also involved in cytokine imbalance.
- Findings: Elevated levels of tumor necrosis factor-alpha (TNF-α) and reduced levels of epidermal growth factor (EGF) and interleukin-6 (IL-6) could be involved in the process of demyelination.
| Etiology | Description |
|---|---|
| Intake deficiency or increased demand | Cobalamin cannot be synthesized by the human body and must be obtained from diet, mainly animal-derived foods. Strict vegetarians and vegans are at a higher risk, especially during periods of increased demand such as pregnancy or lactation. |
| Gastrointestinal conditions | Stomach: Gastric surgeries (e.g., gastrectomy, bariatric surgery) and conditions such as gastritis or autoimmune gastritis (pernicious anemia), which affect gastric acid and intrinsic factor production, could hamper B12 absorption. Small bowel: Ileal resection, diseases affecting the small intestine (e.g., inflammatory bowel disease—IBD, celiac disease, intestinal motility disorders), bacterial overgrowth, regional enteritis, tropical sprue. These conditions can affect B12 absorption due to reduced absorptive surface or increased bacterial competition. |
| Pancreatic pathologies | Pancreatic insufficiency or chronic pancreatitis disrupts the cleavage of B12 from proteins, impeding its transfer to the intrinsic factor and reducing absorption. |
| Drugs | Certain medications can affect B12 availability or absorption mechanisms through various pathways, including long-term suppression of gastric acid (proton pump inhibitors) or interference with calcium-dependent absorption (metformin). |
| Nitrous oxide (N2O) | Irreversibly oxidizes the cobalt atom in vitamin B12, rendering it inactive. This process inhibits the enzyme methionine synthase, leading to the same metabolic block and neurological consequences as other etiologies. Cessation of N2O use and B12 supplementation are required for neurological recovery. |
| Genetic anomalies | Neonates may inherit conditions (e.g., transcobalamin deficiency), which affect B12 transport and absorption, leading to early-life deficiencies. |
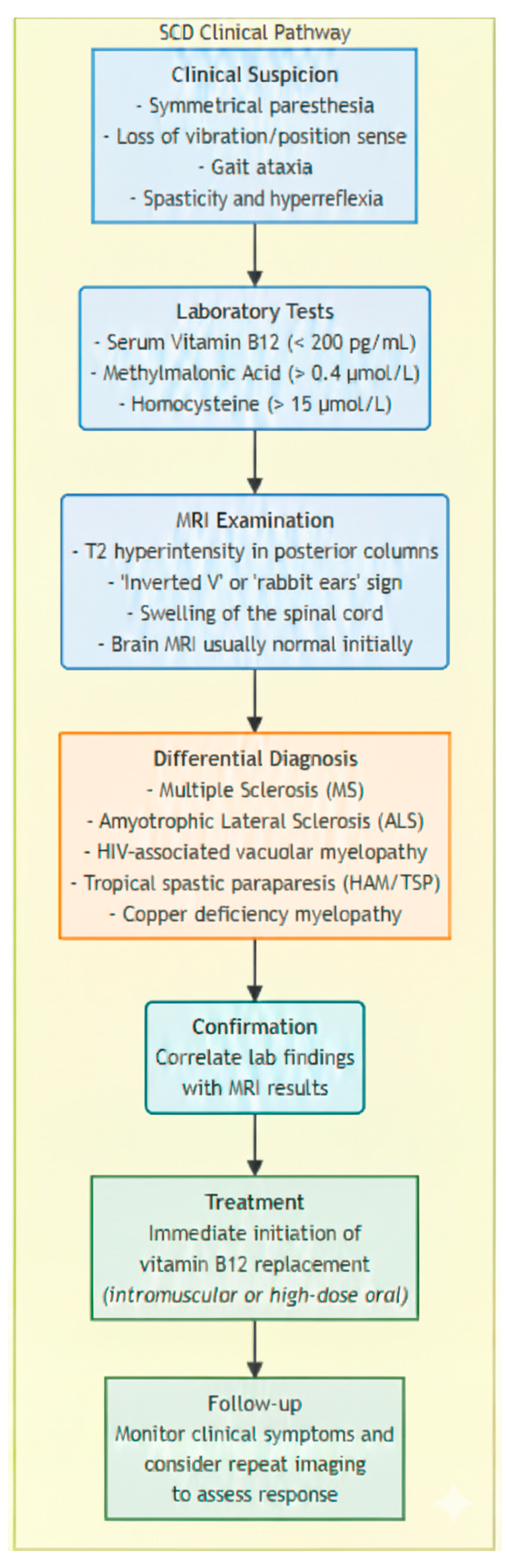
1.2. Clinical and Laboratory Findings
| Affected Area | Symptoms |
|---|---|
| Dorsal Columns | Impairment of proprioception, tactile discrimination, vibration sense; tingling, burning, paresthesia in limbs; difficulty maintaining balance without visual cues. Lhermitte’s sign may be present. |
| Lateral Corticospinal Tracts | Muscle weakness, spasticity, hyperreflexia; initial stiffness, progressing to paraplegia or quadriplegia if untreated; possible sphincter involvement leading to incontinence. |
| Spinocerebellar Tracts | Gait disturbances, sensory ataxia, positive Romberg’s sign. |
| Peripheral Nerves and Others | Peripheral neuropathy, visual deficits, neuropsychiatric issues (depression, dementia). |
- No anomalies.
- Hypersegmented neutrophils, mild leukopenia, or thrombocytopenia.
- Folate Levels: Tested to rule out folate deficiency, which can mimic B12 deficiency signs.
| Evaluation Step | Tools and Tests | Key Indicators and Findings |
|---|---|---|
| Hematological Abnormalities | CBC, Blood Smear |
|
| B12 Deficiency Confirmation | Serum B12 Levels, Metabolite Levels (MMA, Homocysteine) |
|
| Cause of B12 Deficiency | Anti-intrinsic Factor Antibodies, Serum Gastrin Folate |
|
1.3. MRI: Key Diagnostic Challenges and Findings
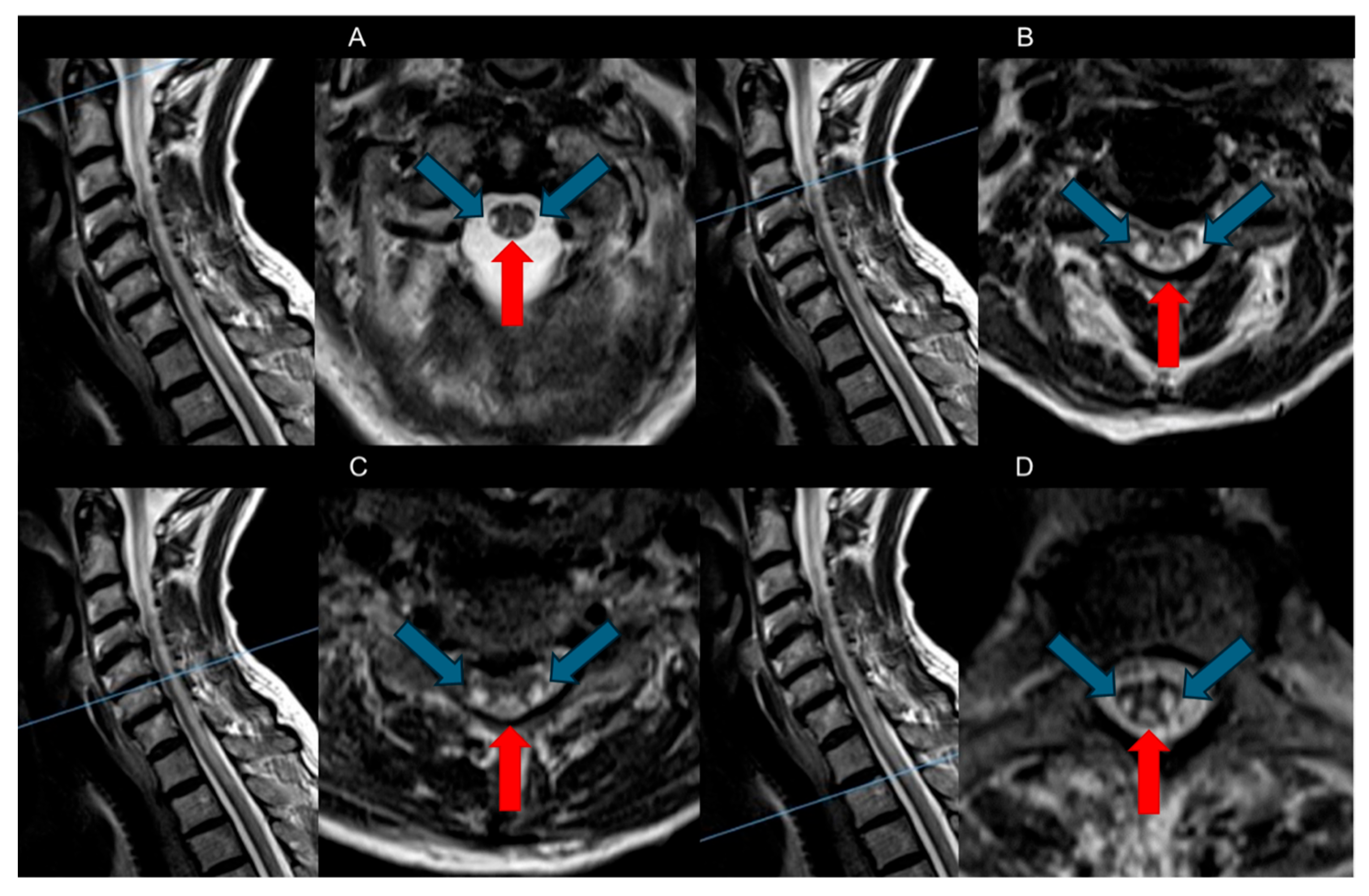
- Dorsal Columns: The most characteristic feature observed in SCD is symmetrical bilateral high T2-weighted signal intensities within the dorsal columns of the spinal cord, often referred to as the “Inverted V” sign or “Inverted rabbit ears” sign. This radiological hallmark typically initiates in the upper thoracic region and may show either ascending or descending progression [8,26].
| Spinal Cord | ||
|---|---|---|
| Dorsal Columns | Bilateral symmetric high signals lesions (inverted “V” sign) in T2-weighted axial sequences |
|
| Lateral Tracts | Involvement possible | |
| Brain | ||
| Cerebral White Matter | Diffuse or patchy T2 hyperintensities | Changes resolve after B12 correction |
Advanced and Quantitative Imaging
1.4. Differential Diagnosis
| Condition | Spinal Involvement | Distinguishing Features |
|---|---|---|
| Nutritional/Metabolic Deficiencies or Toxicity | Dorsal columns, T2 hyperintensity |
|
| Demyelinating Disorders: TM and MS [48] | Variable segments may be asymmetric.
| Younger age, additional CNS symptoms (MS) |
| Infectious Causes (Syphilis) | Posterolateral and dorsal columns |
|
| HIV Vacuolar Myelopathy and HTLV-1-Associated Myelopathy | T2-weighted hyperintensity Dorsal columns of thoracic spinal cord Cerebral involvement: More common |
|
| Friedreich’s Ataxia | Dorsal and spinocerebellar tracts | Autosomal recessive (adolescence), hypertrophic cardiomyopathy |
| LBSL | Entire spinal cord, extends to medulla | Symmetrical involvement, lactate elevation, young age (children) |
| Other Disorders (e.g., Sarcoidosis, Ischemia, Tumors) [31,32] | Variable | Depending on underlying condition |
1.5. Prognosis and Complications
1.6. Treatment and Management
1.7. Monitoring and Response to Therapy
- It is essential to routinely check vitamin B12 levels, particularly in patients with SCD, and continue monitoring until recovery is complete.
- Markers of hemolysis often begin to decrease within a few days, and reticulocyte counts generally increase within the first week. Normalization of hematological parameters—including anemia, neutrophil segmentation, leukopenia, and thrombocytopenia—is typically observed within two to four weeks [55].
- Additionally, it is crucial to monitor for hypokalemia during the initial treatment phases due to the increased uptake of potassium by cells.
- Neurological impairments, including spinal and cerebral alterations, often improve after correcting vitamin B12 deficiency but may take longer (three to twelve months), highlighting the potential for reversing some neurological damage with timely treatment. However, in more severe cases, the neurological deficits may become permanent [21].
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qudsiya, Z.; De Jesus, O. Subacute Combined Degeneration of the Spinal Cord. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Al Ani, A.; Qureshi, P.A.A.A.; Bollineni, V.R. Subacute Combined Degeneration in a Young Female Patient After Sleeve Gastrectomy. Cureus 2023, 15, e50336. [Google Scholar] [CrossRef]
- Barfei, M.; Srinivasan, V.; Venkatramanan, V.; Weston, M.; Dixon, S. The Other Side of Funny Gas (Nitrous Oxide-N2O), a Case Report of Subacute Combined Degeneration of Cord. Clin. Med. 2023, 23, 4–5. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Shi, Y.; Wang, T.; Jin, W. Nitrous Oxide Induced Subacute Combined Degeneration of the Spine Cord: A Case Report. Medicine 2024, 103, e37032. [Google Scholar] [CrossRef] [PubMed]
- Goyne, C.; Kansal, L. Pearls & Oy-Sters: Late-Onset Cobalamin C Deficiency Presenting With Subacute Combined Degeneration. Neurology 2023, 100, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Surtees, R. Biochemical Pathogenesis of Subacute Combined Degeneration of the Spinal Cord and Brain. J. Inherit. Metab. Dis. 1993, 16, 762–770. [Google Scholar] [CrossRef]
- Saji, A.M.; Lui, F.; De Jesus, O. Spinal Cord Subacute Combined Degeneration. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Feng, T.; Wang, B.; Li, F.; Li, F.; Wang, S.; Zhou, R.; Wang, L.; Lin, H.; Ma, Y. High Homocysteine Levels as Potential Indicators of Subacute Combined Degeneration of the Spinal Cord. Am. J. Emerg. Med. 2024, 85, 266.e5–266.e7. [Google Scholar] [CrossRef]
- Sachdev, P.; Parslow, R.; Salonikas, C.; Lux, O.; Wen, W.; Kumar, R.; Naidoo, D.; Christensen, H.; Jorm, A. Homocysteine and the Brain in Midadult Life: Evidence for an Increased Risk of Leukoaraiosis in Men. Arch. Neurol. 2004, 61, 1369–1376. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 Deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Kumar, R.R.; Singh, L.; Thakur, A.; Singh, S.; Kumar, B. Role of Vitamins in Neurodegenerative Diseases: A Review. CNS Neurol. Disord. Drug Targets 2022, 21, 766–773. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, Folate, and the Methionine Remethylation Cycle-Biochemistry, Pathways, and Regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Patel, K.K.; Mejia Munne, J.C.; Gunness, V.R.N.; Hersey, D.; Alshafai, N.; Sciubba, D.; Nasser, R.; Gimbel, D.; Cheng, J.; Nouri, A. Subacute Combined Degeneration of the Spinal Cord Following Nitrous Oxide Anesthesia: A Systematic Review of Cases. Clin. Neurol. Neurosurg. 2018, 173, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.-Y.; Kuo, C.-Y.; Chou, C.-C.; Kong, S.-S.; Hung, P.-C.; Tsai, H.-Y.; Chen, Y.-C.; Lin, J.-J.; Chou, I.-J.; Lin, K.-L.; et al. Recreational Nitrous Oxide Abuse Related Subacute Combined Degeneration of the Spinal Cord in Adolescents—A Case Series and Literature Review. Brain Dev. 2019, 41, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Burlock, B.; Williams, J.P. Recognizing Subacute Combined Degeneration in Patients with Normal Vitamin B12 Levels. Cureus 2021, 13, e15429. [Google Scholar] [CrossRef] [PubMed]
- Holroyd, K.B.; Berkowitz, A.L. Metabolic and Toxic Myelopathies. Continuum 2024, 30, 199–223. [Google Scholar] [CrossRef]
- Bi, Z.; Cao, J.; Shang, K.; Su, Z.; Xu, S.; Liu, C. Correlation between Anemia and Clinical Severity in Subacute Combined Degeneration Patients. J. Clin. Neurosci. 2020, 80, 11–15. [Google Scholar] [CrossRef]
- Lee, E.C.; Lee, D.G. Progressive Lower Extremity Paralysis Caused by Intrathecal MTX-Induced Myelopathy Mimicking Guillain-Barre Syndrome: A Case Report. Diagnostics 2023, 13, 3337. [Google Scholar] [CrossRef]
- Ibraheem, A.; Nashwan, A.J.; Yassin, M.A. Elderly Patient With Hematological and Neurological Manifestations of Undetermined Origin: A Diagnostic Dilemma of Pernicious Anemia. Cureus 2023, 15, e43045. [Google Scholar] [CrossRef]
- Mohammed, H.J.; Hammady, M.M.; Abbas, F.N. A Comparison Between Somatosensory Evoked Potentials and Spine MRI in the Diagnosis of Non-Compressive Myelopathy: Which Is More Accurate? Cureus 2023, 15, e38051. [Google Scholar] [CrossRef]
- Ghazal, F.; Zur, M.; Silver, A. Combined Presentation of Acute Confusion and Severe Pancytopenia in Vitamin B12 Deficiency. Cureus 2023, 15, e40236. [Google Scholar] [CrossRef]
- Zhang, F.; Chang, X.; Liu, T.; Wang, W.; Zhao, X.; Ji, C.; Yang, R.; Guo, H. Prognostic Factors for Long-Term Survival in Patients with Renal-Cell Carcinoma After Radiofrequency Ablation. J. Endourol. 2016, 30, 37–42. [Google Scholar] [CrossRef]
- Wu, L.; Shi, B.; Zhao, M.; Sun, H.; Zhang, F.; Li, J.; Huang, D.; Shi, Z. Rare Anterior Funiculus Lesions in Subacute Combined Degeneration of the Spinal Cord: A Case Report and Literature Review. Int. J. Neurosci. 2020, 130, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Briani, C.; Dalla Torre, C.; Citton, V.; Manara, R.; Pompanin, S.; Binotto, G.; Adami, F. Cobalamin Deficiency: Clinical Picture and Radiological Findings. Nutrients 2013, 5, 4521–4539. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, H.; Xiang, X. Abnormal Brain Changes in a Patient with Vegetarian Diet-induced Subacute Combined Degeneration: A Case Report. Exp. Ther. Med. 2023, 26, 491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, H.; Wang, X.; Qiu, H.; Ren, H.; Zheng, A.; Luo, Q. Psychiatric Symptoms in a Female with Subacute Combined Degeneration of the Spinal Cord (SCD): A Case Report. BMC Psychiatry 2023, 23, 129. [Google Scholar] [CrossRef]
- Cao, J.; Xu, S.; Liu, C. Is Serum Vitamin B12 Decrease a Necessity for the Diagnosis of Subacute Combined Degeneration?: A Meta-Analysis. Medicine 2020, 99, e19700. [Google Scholar] [CrossRef]
- Vitale, V.; Caranci, F.; Pisciotta, C.; Manganelli, F.; Briganti, F.; Santoro, L.; Brunetti, A. Hirayama’s Disease: An Italian Single Center Experience and Review of the Literature. Quant. Imaging Med. Surg. 2016, 6, 364–373. [Google Scholar] [CrossRef]
- Ma, Z.; Ye, Q.; Ma, X.; Chen, C.; Feng, H.-Y.; Zhang, Y.-N. Correlation of Imaging Characteristics of Degenerative Cervical Myelopathy and the Surgical Approach with Improvement for Postoperative Neck Pain and Neural Function: A Retrospective Cohort Study. Quant. Imaging Med. Surg. 2024, 14, 3923–3938. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, T.; Han, D.; Zhou, X.; Wang, Y.; Zhao, F.; Shi, J.; Shi, G. Classification of Cervical Disc Herniation Myelopathy or Radiculopathy: A Magnetic Resonance Imaging-Based Analysis. Quant. Imaging Med. Surg. 2023, 13, 4984–4994. [Google Scholar] [CrossRef]
- Peng, J.-C.; Li, J.; Zhou, M.; Zhu, S.-H.; Li, K.-F. Longitudinally Extensive Myelitis Onset in Subacute Combined Degeneration Combined with a Novel Coronavirus Vaccine Injection. Neurol. India 2023, 71, 1059–1060. [Google Scholar] [CrossRef]
- Douglas, A.G.; Xu, D.J.; Shah, M.P. Approach to Myelopathy and Myelitis. Neurol. Clin. 2022, 40, 133–156. [Google Scholar] [CrossRef]
- Matsushita, T.; Sakamoto, Y.; Tanakami, A.; Shimazu, H.; Kudo, C.; Kida, Y.; Harada, M. A Case of Subacute Combined Spinal Cord Degeneration and Suspected Leukoencephalopathy Associated with Vitamin B12 Deficiency Showing Improved Imaging Findings after Vitamin B12 Administration. J. Med. Investig. 2022, 69, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Jhunjhnuwala, D.; Tanglay, O.; Briggs, N.E.; Yuen, M.T.Y.; Huynh, W. Prognostic Indicators of Subacute Combined Degeneration from B12 Deficiency: A Systematic Review. PM&R 2022, 14, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Kostick, N.; Chen, E.; Eckert, T.; Sirotkin, I.; Baldinger, E.; Frontera, A. Clinical Presentation of Subacute Combined Degeneration in a Patient With Chronic B12 Deficiency. Fed. Pract. 2022, 39, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.G.; Jayakumar, P.N.; Vasudev, M.K.; Taly, A.B.; Chandrashekar, H.S. MRI in Subacute Combined Degeneration of Spinal Cord: A Case Report and Review of Literature. Neurol. India 2002, 50, 310–312. [Google Scholar]
- Cheng, S.-J.; Tsai, P.-H.; Lee, Y.-T.; Li, Y.-T.; Chung, H.-W.; Chen, C.-Y. Diffusion Tensor Imaging of the Spinal Cord. Magn. Reson. Imaging Clin. N. Am. 2021, 29, 195–204. [Google Scholar] [CrossRef]
- Vattipally, V.N.; Jillala, R.R.; Aude, C.A.; Menta, A.K.; Jo, J.; Hughes, L.P.; Khalifeh, J.M.; Azad, T.D. Artificial Intelligence and Machine Learning in the Management of Patients with Degenerative Cervical Myelopathy: A Systematic Review. J. Neurosurg. Sci. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- King, D.; Siau, K.; Senthil, L.; Kane, K.F.; Cooper, S.C. Copper Deficiency Myelopathy After Upper Gastrointestinal Surgery. Nutr. Clin. Pract. 2018, 33, 515–519. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, A.; Pandey, V.P.; Verma, A. Acute Presentation of Subacute Combined Degeneration Mimicking Multiple Sclerosis-Neuromyelitis Optica Spectrum Disorder. J. Assoc. Physicians India 2020, 68, 59. [Google Scholar]
- Maggi, P.; Absinta, M. Emerging MRI Biomarkers for the Diagnosis of Multiple Sclerosis. Mult. Scler. 2024, 30, 1704–1713. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive Impairment in Multiple Sclerosis: Clinical Management, MRI, and Therapeutic Avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Kassubek, J.; Pagani, M. Imaging in Amyotrophic Lateral Sclerosis: MRI and PET. Curr. Opin. Neurol. 2019, 32, 740–746. [Google Scholar] [CrossRef]
- Scalabrino, G.; Veber, D.; Briani, C.; Milani, S.; Terralavoro, A.; Brenna, S.; Valenti, L.; Silani, V.; Morelli, C.; Peracchi, M. Cobalamin as a Regulator of Serum and Cerebrospinal Fluid Levels of Normal Prions. J. Clin. Neurosci. 2013, 20, 134–138. [Google Scholar] [CrossRef]
- Nodera, H.; Izumi, Y.; Kaji, R. Effects of Vitamin B12 in Patients with Amyotrophic Lateral Sclerosis and Peripheral Neuropathy]. Brain Nerve 2015, 67, 1133–1138. [Google Scholar] [CrossRef]
- Rison, R.A.; Beydoun, S.R. Amyotrophic Lateral Sclerosis-Motor Neuron Disease, Monoclonal Gammopathy, Hyperparathyroidism, and B12 Deficiency: Case Report and Review of the Literature. J. Med. Case Rep. 2010, 4, 298. [Google Scholar] [CrossRef] [PubMed]
- Gray, F.; Gherardi, R.; Trotot, P.; Fenelon, G.; Poirier, J. Spinal Cord Lesions in the Acquired Immune Deficiency Syndrome (AIDS). Neurosurg. Rev. 1990, 13, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Barnett, Y.; Garber, J.Y.; Barnett, M.H. MRI Biomarkers of Disease Progression in Multiple Sclerosis: Old Dog, New Tricks? Quant. Imaging Med. Surg. 2020, 10, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Suliman, O.S.M. Sub-Acute Combined Degeneration of the Spinal Cord as First Presentation of Coeliac Disease in a Sudanese Child. Sudan. J. Paediatr. 2023, 23, 98–103. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, X. A Case Report: Subacute Combined Degeneration of the Spinal Cord and Pernicious Anemia Caused by Autoimmune Gastritis. Medicine 2022, 101, e29226. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Lee, J.; An, J.Y. A Case of Gastric Adenocarcinoma With Pernicious Anemia, Polyneuropathy, and Subacute Combined Degeneration Caused by Autoimmune Gastritis. J. Clin. Neurol. 2023, 19, 312–314. [Google Scholar] [CrossRef]
- Taniguchi, M.; Sudo, G.; Sekiguchi, Y.; Nakase, H. Autoimmune Gastritis Concomitant with Gastric Adenoma and Subacute Combined Degeneration of the Spinal Cord. BMJ Case Rep. 2021, 14, e242836. [Google Scholar] [CrossRef]
- Ravina, B.; Loevner, L.A.; Bank, W. MR Findings in Subacute Combined Degeneration of the Spinal Cord: A Case of Reversible Cervical Myelopathy. AJR Am. J. Roentgenol. 2000, 174, 863–865. [Google Scholar] [CrossRef]
- Martinelli, D.; Deodato, F.; Dionisi-Vici, C. Cobalamin C Defect: Natural History, Pathophysiology, and Treatment. J. Inherit. Metab. Dis. 2011, 34, 127–135. [Google Scholar] [CrossRef]
- Hara, D.; Akamatsu, M.; Mizukami, H.; Kato, B.; Suzuki, T.; Oshima, J.; Hasegawa, Y. A Case of Subacute Combined Degeneration of Spinal Cord Diagnosed by Vitamin B12 Administration Lowering Methylmalonic Acid. Case Rep. Neurol. 2020, 12, 27–34. [Google Scholar] [CrossRef]
| Treatment Aspect | Recommendations |
|---|---|
| Initial Treatment Route | Parenteral (intramuscular) preferred for severe cases |
| Oral Dosing | 1000–2000 mcg daily depending on absorption status |
| Parenteral Dosing | 1000 mcg weekly for one month, then monthly |
| Treatment Duration | Lifelong for irreversible causes; until correction for reversible causes |
| Monitoring Frequency | Until recovery |
| Outcome Expectations | Hematological improvement within weeks; neurological over months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernetti, C.; Cea, L.; Buoso, A.; Greco, F.; Rossi, M.; Pilato, F.; Calandrelli, R.; Di Gennaro, G.; Di Lazzaro, V.; Zobel, B.B.; et al. A Comprehensive Overview of Subacute Combined Degeneration: MRI Diagnostic Challenges and Treatment Pathways. Brain Sci. 2025, 15, 972. https://doi.org/10.3390/brainsci15090972
Bernetti C, Cea L, Buoso A, Greco F, Rossi M, Pilato F, Calandrelli R, Di Gennaro G, Di Lazzaro V, Zobel BB, et al. A Comprehensive Overview of Subacute Combined Degeneration: MRI Diagnostic Challenges and Treatment Pathways. Brain Sciences. 2025; 15(9):972. https://doi.org/10.3390/brainsci15090972
Chicago/Turabian StyleBernetti, Caterina, Laura Cea, Andrea Buoso, Federico Greco, Mariagrazia Rossi, Fabio Pilato, Rosalinda Calandrelli, Gianfranco Di Gennaro, Vincenzo Di Lazzaro, Bruno Beomonte Zobel, and et al. 2025. "A Comprehensive Overview of Subacute Combined Degeneration: MRI Diagnostic Challenges and Treatment Pathways" Brain Sciences 15, no. 9: 972. https://doi.org/10.3390/brainsci15090972
APA StyleBernetti, C., Cea, L., Buoso, A., Greco, F., Rossi, M., Pilato, F., Calandrelli, R., Di Gennaro, G., Di Lazzaro, V., Zobel, B. B., & Mallio, C. A. (2025). A Comprehensive Overview of Subacute Combined Degeneration: MRI Diagnostic Challenges and Treatment Pathways. Brain Sciences, 15(9), 972. https://doi.org/10.3390/brainsci15090972









