Neural Oscillation Associated with Contagious Itch in Patients with Atopic Dermatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
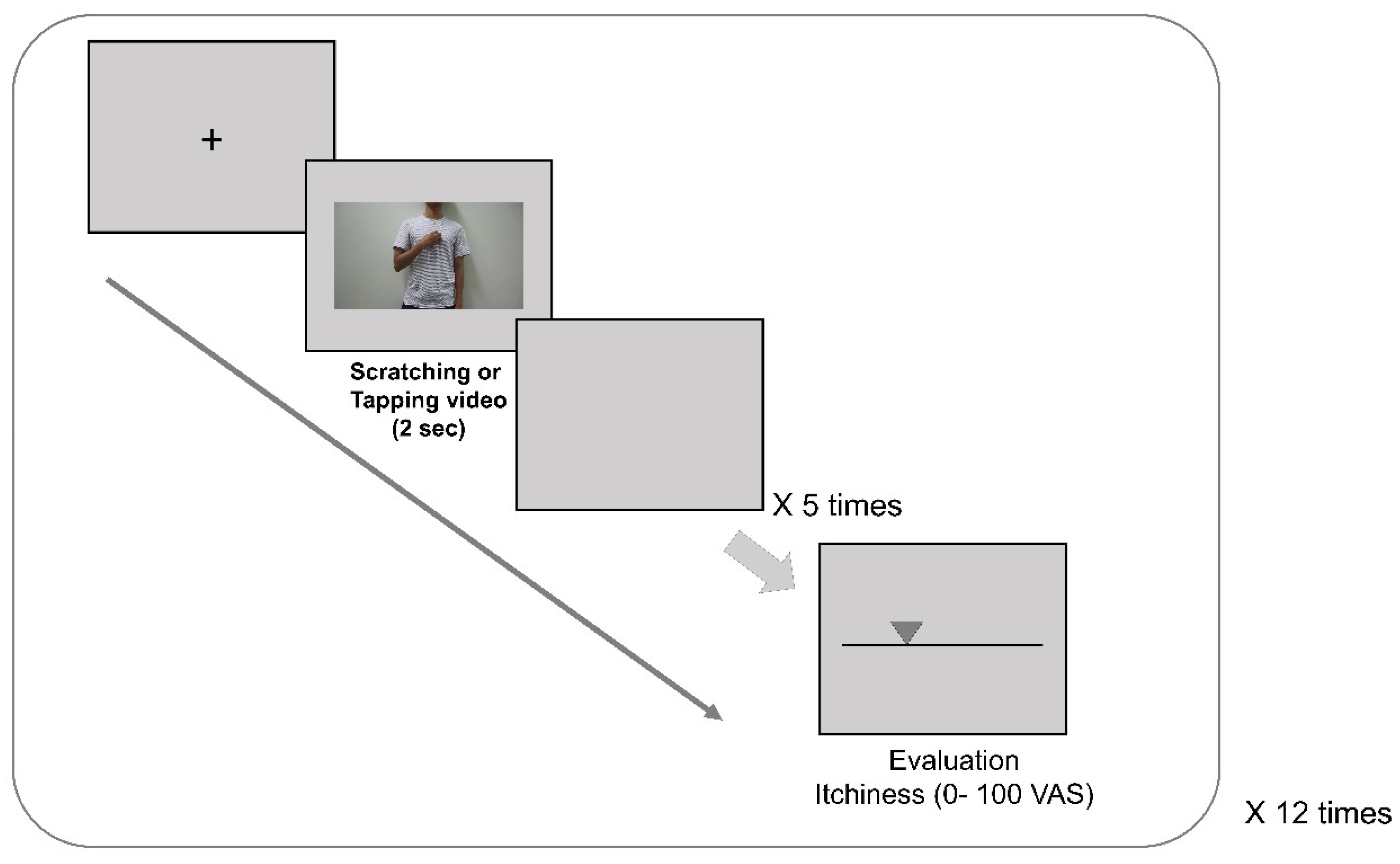
2.2. Experimental Design and Procedures
2.3. EEG Recording and Analysis
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Contagious Itch Ratings in Response to Visual Stimuli
3.3. Mu Suppression during Contagious Itch
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savin, J.A. How should we define itching? J. Am. Acad. Dermatol. 1998, 39, 268–269. [Google Scholar] [CrossRef]
- Wahlgren, C.F. Itch and atopic dermatitis: An overview. J. Dermatol. 1999, 26, 770–779. [Google Scholar] [CrossRef]
- Leknes, S.G.; Bantick, S.; Willis, C.M.; Wilkinson, J.D.; Wise, R.G.; Tracey, I. Itch and motivation to scratch: An investigation of the central and peripheral correlates of allergen- and histamine-induced itch in humans. J. Neurophysiol. 2007, 97, 415–422. [Google Scholar] [CrossRef]
- Mochizuki, H.; Tashiro, M.; Kano, M.; Sakurada, Y.; Itoh, M.; Yanai, K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain 2003, 105, 339–346. [Google Scholar] [CrossRef]
- Napadow, V.; Li, A.; Loggia, M.L.; Kim, J.; Schalock, P.C.; Lerner, E.; Tran, T.N.; Ring, J.; Rosen, B.R.; Kaptchuk, T.J.; et al. The brain circuitry mediating antipruritic effects of acupuncture. Cereb. Cortex 2014, 24, 873–882. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.D.; Zubieta, J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463–484. [Google Scholar] [CrossRef]
- Mochizuki, H.; Sadato, N.; Saito, D.N.; Toyoda, H.; Tashiro, M.; Okamura, N.; Yanai, K. Neural correlates of perceptual difference between itching and pain: A human fMRI study. Neuroimage 2007, 36, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Najafi, P.; Carre, J.L.; Ben Salem, D.; Brenaut, E.; Misery, L.; Dufor, O. Central mechanisms of itch: A systematic literature review and meta-analysis. J. Neuroradiol. 2019, 47, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.A.; Giesbrecht, T.; Stancak, A.; Fallon, N.; Thomas, A.; Kirkham, T.C. Where is itch represented in the brain, and how does it differ from pain? An activation likelihood estimation meta-analysis of experimentally-induced itch. J. Investig. Dermatol. 2019, 139, 2245–2248.e3. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Kakigi, R. Central mechanisms of itch. Clin. Neurophysiol. 2015, 126, 1650–1660. [Google Scholar] [CrossRef]
- Papoiu, A.D.; Wang, H.; Coghill, R.C.; Chan, Y.H.; Yosipovitch, G. Contagious itch in humans: A study of visual ’transmission’ of itch in atopic dermatitis and healthy subjects. Br. J. Dermatol. 2011, 164, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Schut, C.; Grossman, S.; Gieler, U.; Kupfer, J.; Yosipovitch, G. Contagious itch: What we know and what we would like to know. Front. Hum. Neurosci. 2015, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Ishiuji, Y.; Coghill, R.C.; Patel, T.S.; Oshiro, Y.; Kraft, R.A.; Yosipovitch, G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br. J. Dermatol. 2009, 161, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Valet, M.; Pfab, F.; Sprenger, T.; Woller, A.; Zimmer, C.; Behrendt, H.; Ring, J.; Darsow, U.; Tolle, T.R. Cerebral processing of histamine-induced itch using short-term alternating temperature modulation--an FMRI study. J. Investig. Dermatol. 2008, 128, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Holle, H.; Warne, K.; Seth, A.K.; Critchley, H.D.; Ward, J. Neural basis of contagious itch and why some people are more prone to it. Proc. Natl. Acad. Sci. USA 2012, 109, 19816–19821. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Baumgartner, U.; Kamping, S.; Ruttorf, M.; Schad, L.R.; Flor, H.; Kakigi, R.; Treede, R.D. Cortico-subcortical activation patterns for itch and pain imagery. Pain 2013, 154, 1989–1998. [Google Scholar] [CrossRef]
- Schut, C.; Bosbach, S.; Gieler, U.; Kupfer, J. Personality traits, depression and itch in patients with atopic dermatitis in an experimental setting: A regression analysis. Acta Derm. Venereol. 2014, 94, 20–25. [Google Scholar] [CrossRef]
- Schut, C.; Mochizuki, H.; Grossman, S.K.; Lin, A.C.; Conklin, C.J.; Mohamed, F.B.; Gieler, U.; Kupfer, J.; Yosipovitch, G. Brain Processing of contagious itch in patients with atopic dermatitis. Front. Psychol. 2017, 8, 1267. [Google Scholar] [CrossRef]
- Hari, R.; Salmelin, R. Human cortical oscillations: A neuromagnetic view through the skull. Trends Neurosci. 1997, 20, 44–49. [Google Scholar] [CrossRef]
- Pineda, J.A. The functional significance of mu rhythms: Translating seeing and hearing into doing. Brain Res. Rev. 2005, 50, 57–68. [Google Scholar] [CrossRef]
- Ohara, S.; Crone, N.E.; Weiss, N.; Lenz, F.A. Attention to a painful cutaneous laser stimulus modulates electrocorticographic event-related desynchronization in humans. Clin. Neurophysiol. 2004, 115, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Ploner, M.; Gross, J.; Timmermann, L.; Pollok, B.; Schnitzler, A. Pain suppresses spontaneous brain rhythms. Cereb. Cortex 2006, 16, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yang, C.Y.; Lin, C.P.; Lee, P.L.; Decety, J. The perception of pain in others suppresses somatosensory oscillations: A magnetoencephalography study. Neuroimage 2008, 40, 1833–1840. [Google Scholar] [CrossRef]
- Hari, R.; Forss, N.; Avikainen, S.; Kirveskari, E.; Salenius, S.; Rizzolatti, G. Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc. Natl. Acad. Sci. USA 1998, 95, 15061–15065. [Google Scholar] [CrossRef]
- Cho, H.; Ahn, M.; Ahn, S.; Kwon, M.; Jun, S.C. EEG datasets for motor imagery brain-computer interface. Gigascience 2017, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.M.; Hubbard, E.M.; McCleery, J.P.; Altschuler, E.L.; Ramachandran, V.S.; Pineda, J.A. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn. Brain Res. 2005, 24, 190–198. [Google Scholar] [CrossRef]
- Oberman, L.M.; Pineda, J.A.; Ramachandran, V.S. The human mirror neuron system: A link between action observation and social skills. Soc. Cogn. Affect. Neurosci. 2007, 2, 62–66. [Google Scholar] [CrossRef]
- Jordan, J.M.; Whitlock, F.A. Emotions and the skin: The conditioning of scratch responses in cases of atopic dermatitis. Br. J. Dermatol. 1972, 86, 574–585. [Google Scholar] [CrossRef]
- Jordan, J.M.; Whitlock, F.A. Atopic dermatitis anxiety and conditioned scratch responses. J. Psychosom. Res. 1974, 18, 297–299. [Google Scholar] [CrossRef]
- Ikoma, A.; Fartasch, M.; Heyer, G.; Miyachi, Y.; Handwerker, H.; Schmelz, M. Painful stimuli evoke itch in patients with chronic pruritus: Central sensitization for itch. Neurology 2004, 62, 212–217. [Google Scholar] [CrossRef]
- Schneider, G.; Stander, S.; Burgmer, M.; Driesch, G.; Heuft, G.; Weckesser, M. Significant differences in central imaging of histamine-induced itch between atopic dermatitis and healthy subjects. Eur. J. Pain 2008, 12, 834–841. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Li, H.; Yang, J.; Yuan, J. The impact of mood on empathy for pain: Evidence from an EEG study. Psychophysiology 2017, 54, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Gallese, V.; Goldman, A. Mirror neurons and the simulation theory of mind-reading. Trends Cogn. Sci. 1998, 2, 493–501. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.-S.; Kim, K.; Park, H.-J.; Lee, H.; Jung, W.-M.; Kim, D.-W.; Chae, Y. Neural Oscillation Associated with Contagious Itch in Patients with Atopic Dermatitis. Brain Sci. 2021, 11, 438. https://doi.org/10.3390/brainsci11040438
Lee I-S, Kim K, Park H-J, Lee H, Jung W-M, Kim D-W, Chae Y. Neural Oscillation Associated with Contagious Itch in Patients with Atopic Dermatitis. Brain Sciences. 2021; 11(4):438. https://doi.org/10.3390/brainsci11040438
Chicago/Turabian StyleLee, In-Seon, Kyuseok Kim, Hi-Joon Park, Hyangsook Lee, Won-Mo Jung, Do-Won Kim, and Younbyoung Chae. 2021. "Neural Oscillation Associated with Contagious Itch in Patients with Atopic Dermatitis" Brain Sciences 11, no. 4: 438. https://doi.org/10.3390/brainsci11040438
APA StyleLee, I.-S., Kim, K., Park, H.-J., Lee, H., Jung, W.-M., Kim, D.-W., & Chae, Y. (2021). Neural Oscillation Associated with Contagious Itch in Patients with Atopic Dermatitis. Brain Sciences, 11(4), 438. https://doi.org/10.3390/brainsci11040438





