A New Palmitoylethanolamide Form Combined with Antioxidant Molecules to Improve Its Effectivess on Neuronal Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Agents Preparation
2.2. Astrocytes
2.3. Intestinal Barrier
2.4. Cell Viability Test
2.5. ROS Production
2.6. Measurement of NO Production
2.7. MAPK Activity Assay
2.8. p53 Assay Kit
2.9. PPARα Assay Kit
2.10. CNR1 ELISA Test
2.11. CNR2 ELISA Test
2.12. NFKB Assay
2.13. Western Blot
2.14. Statistical Analysis
3. Results
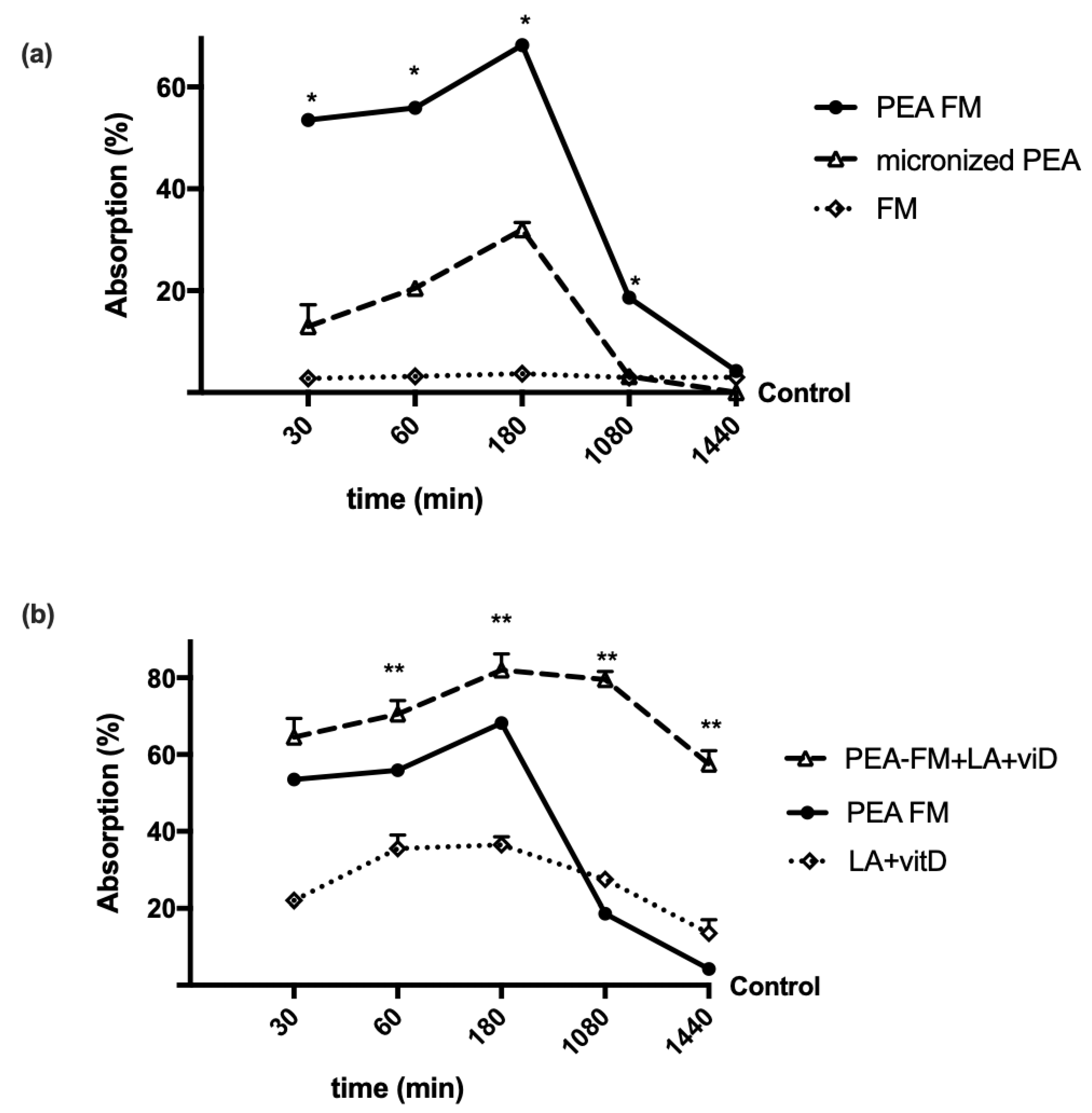
3.1. Time-Dependent Permeability on Caco-2 Cells Treated with PEA Alone and Combined
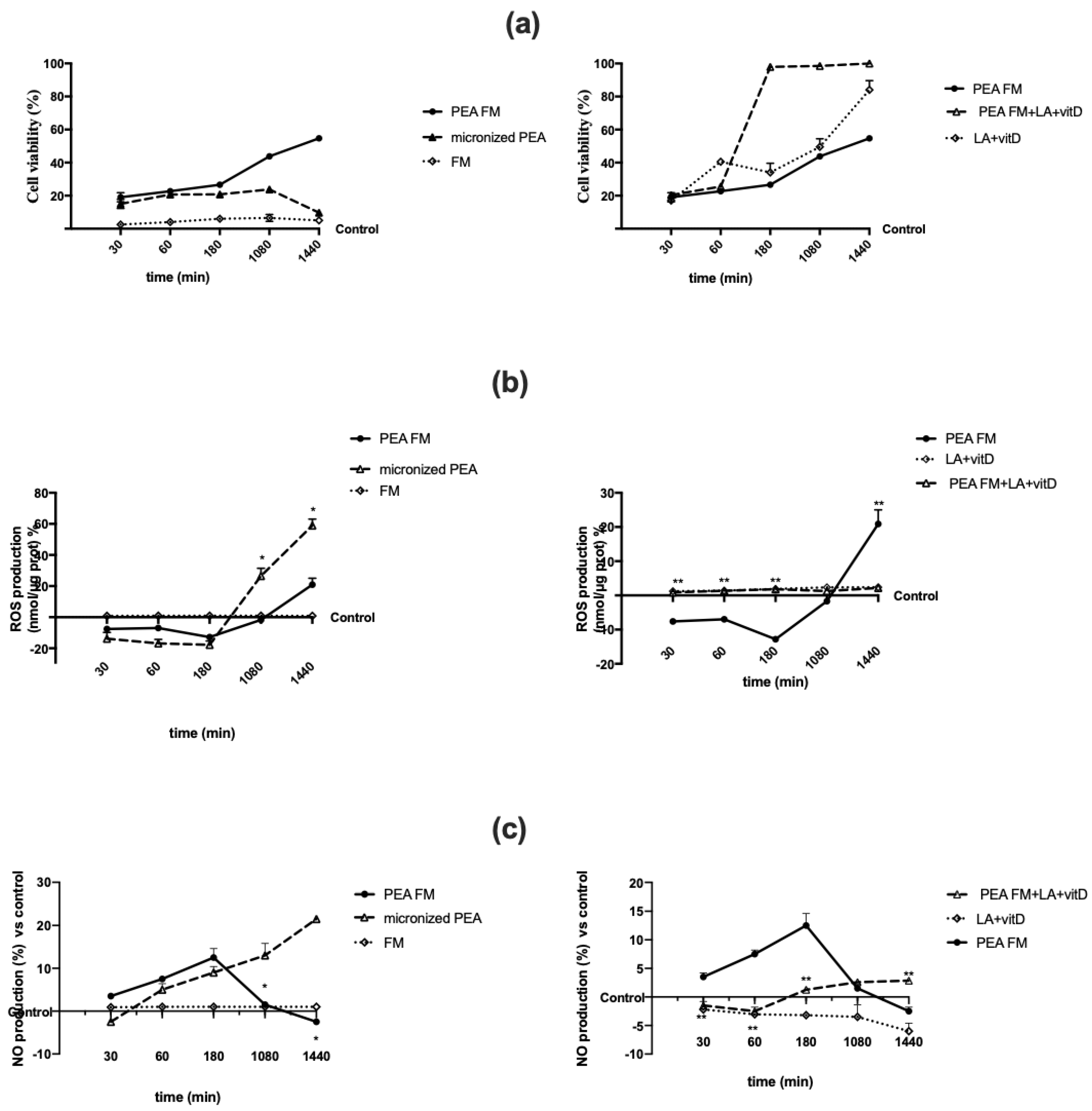
3.2. Direct Effect of Different PEA Forms on Astrocytes during Time
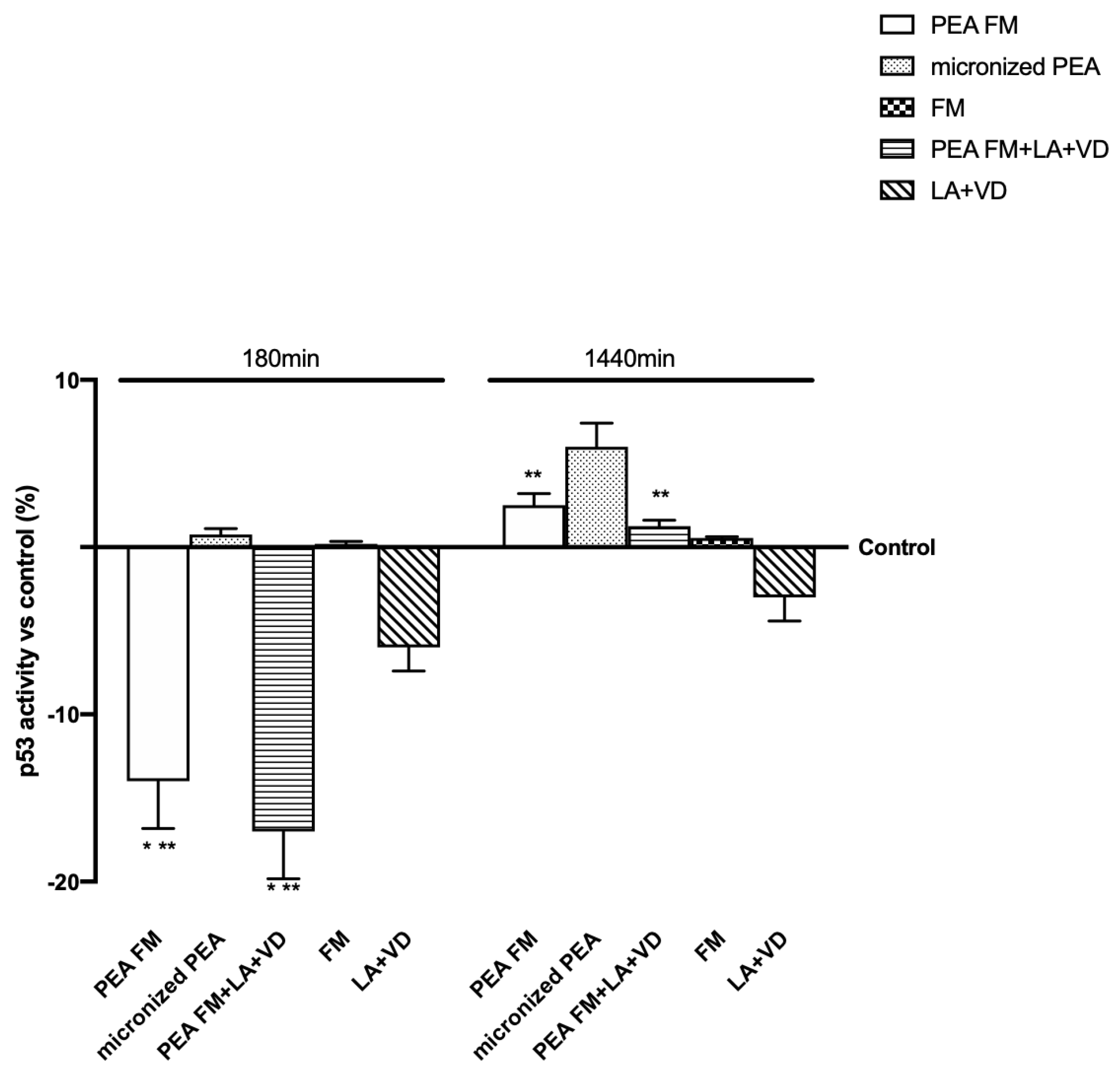
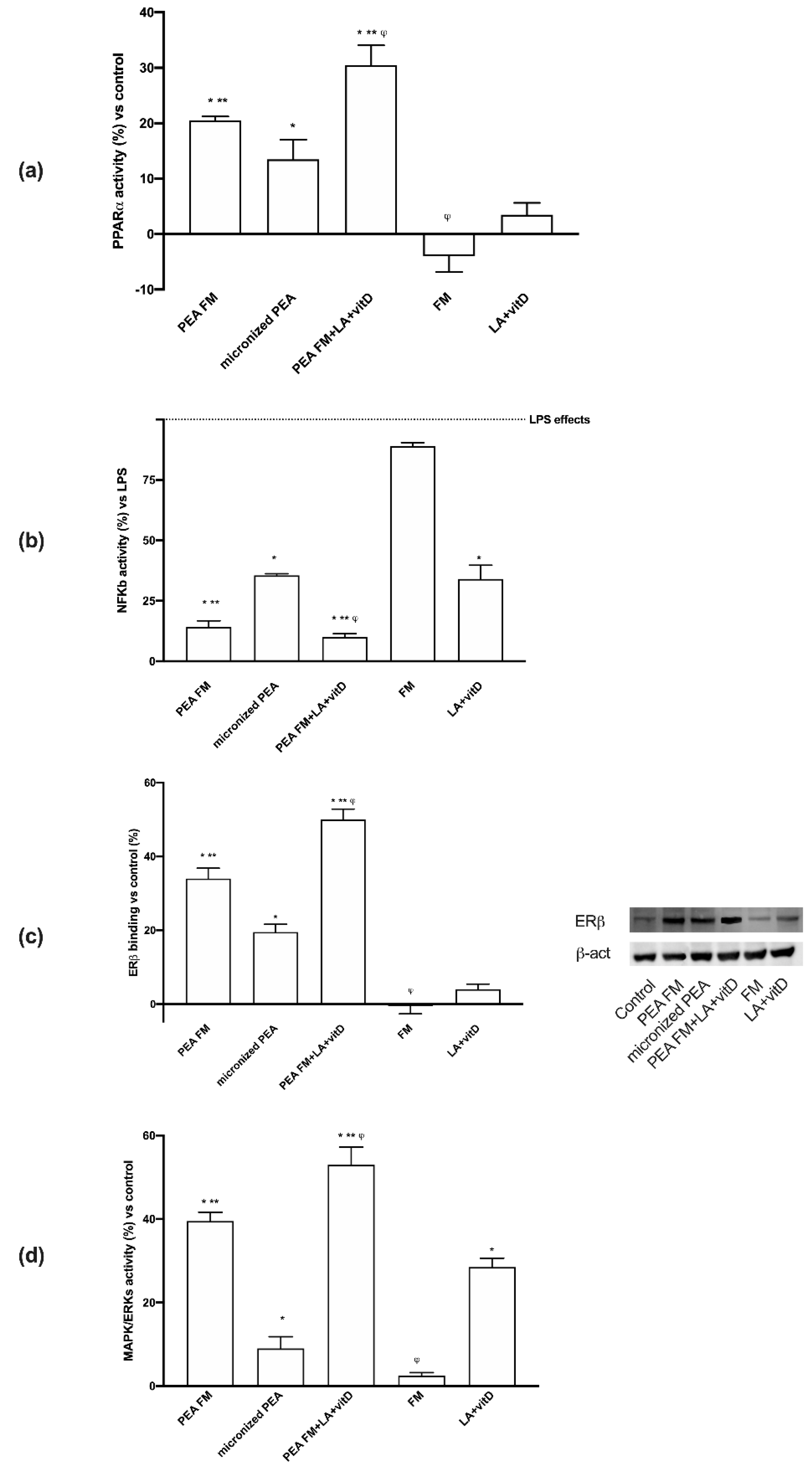
3.3. Molecular Mechanism Activated by Different PEA Forms on Astrocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Gabrielsson, L.; Mattsson, S.; Fowler, C.J. Palmitoylethanolamide for the treatment of pain: Pharmacokinetics, safety and efficacy. Br. J. Clin. Pharmacol. 2016, 82, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Boccella, S.; Cristiano, C.; Romano, R.; Iannotta, M.; Belardo, C.; Farina, A.; Guida, F.; Piscitelli, F.; Palazzo, E.; Mazzitelli, M.; et al. Ultra-micronized palmitoylethanolamide rescues the cognitive decline-associated loss of neural plasticity in the neuropathic mouse entorhinal cortex-dentate gyrus pathway. Neurobiol. Dis. 2019, 121, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Luongo, L.; Marmo, F.; Romano, R.; Iannotta, M.; Napolitano, F.; Belardo, C.; Marabese, I.; D’Aniello, A.; De Gregorio, D.; et al. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol. Brain. 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Boccella, S.; Iannotta, M.; De Gregorio, D.; Giordano, C.; Belardo, C.; Romano, R.; Palazzo, E.; Scafuro, M.A.; Serra, N.; et al. Palmitoylethanolamide Reduces Neuropsychiatric Behaviors by Restoring Cortical Electrophysiological Activity in a Mouse Model of Mild Traumatic Brain Injury. Front. Pharmacol. 2017, 8, 95. [Google Scholar] [CrossRef]
- Al-Ghoul, W.M.; Li Volsi, G.; Weinberg, R.J.; Rustioni, A. Glutamate immunocytochemistry in the dorsal horn after injury or stimulation of the sciatic nerve of rats. Brain Res. Bull. 1993, 30, 453–459. [Google Scholar] [CrossRef]
- De Novellis, V.; Vita, D.; Gatta, L.; Luongo, L.; Bellini, G.; De Chiaro, M.; Marabese, I.; Siniscalco, D.; Boccella, S.; Piscitelli, F.; et al. The blockade of the transient receptor potential vanilloid type 1 and fatty acid amide hydrolase decreases symptoms and central sequelae in the medial prefrontal cortex of neuropathic rats. Mol. Pain. 2011, 7, 7–25. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lu, C.W.; Wu, C.C.; Huang, S.K.; Wang, S.J. Palmitoylethanolamide inhibits glutamate release in rat cerebrocortical nerve terminals. Int. J. Mol. Sci. 2015, 16, 5555–5571. [Google Scholar] [CrossRef]
- Marcello, L.; Cavaliere, C.; Colangelo, A.M.; Bianco, M.R.; Cirillo, G.; Alberghina, L.; Papa, M. Remodelling of supraspinal neuroglial network in neuropathic pain is featured by a reactive gliosis of the nociceptive amygdala. Eur. J. Pain 2013, 17, 799–810. [Google Scholar] [CrossRef]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef]
- Rinne, P.; Guillamat-Prats, R.; Rami, M.; Bindila, L.; Ring, L.; Lyytikäinen, L.P.; Raitoharju, E.; Oksala, N.; Lehtimäki, T.; Weber, C.; et al. Palmitoylethanolamide Promotes a Proresolving Macrophage Phenotype and Attenuates Atherosclerotic Plaque Formation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2562–2575. [Google Scholar] [CrossRef] [PubMed]
- Ativie, F.; Albayram, O.; Bach, K.; Pradier, B.; Zimmer, A.; Bilkei-Gorzo, A. Enhanced microglial activity in FAAH (-/-) animals. Life Sci. 2015, 138, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Cuzzocrea, S.; Crupi, R. An Update of Palmitoylethanolamide and Luteolin Effects in Preclinical and Clinical Studies of Neuroinflammatory Events. Antioxidants 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.I.; Kölliker-Frers, R.; Barreto, G.; Blanco, E.; Capani, F. Glial Modulation by N-acylethanolamides in Brain Injury and Neurodegeneration. Front. Aging Neurosci. 2016, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Barbierato, M.; Zusso, M.; Bruschetta, G.; Impellizzeri, D.; Cuzzocrea, S.; Giusti, P. N-Palmitoylethanolamine and Neuroinflammation: A Novel Therapeutic Strategy of Resolution. Mol. Neurobiol. 2015, 52, 1034–1042. [Google Scholar] [CrossRef]
- Scuderi, C.; Bronzuoli, M.R.; Facchinetti, R.; Pace, L.; Ferraro, L.; Broad, K.D.; Serviddio, G.; Bellanti, F.; Palombelli, G.; Carpinelli, G.; et al. Ultramicronized palmitoylethanolamide rescues learning and memory impairments in a triple transgenic mouse model of Alzheimer’s disease by exerting anti-inflammatory and neuroprotective effects. Transl. Psychiatry 2018, 8, 32. [Google Scholar] [CrossRef]
- Cristiano, C.; Pirozzi, C.; Coretti, L.; Cavaliere, G.; Lama, A.; Russo, R.; Lembo, F.; Mollica, M.P.; Meli, R.; Calignano, A.; et al. Palmitoylethanolamide counteracts autistic-like behaviours in BTBR T+tf/J mice: Contribution of central and peripheral mechanisms. Brain Behav. Immun. 2018, 74, 166–175. [Google Scholar] [CrossRef]
- Lo Verme, J.; Fu, J.; Starita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef]
- Sasso, O.; La Rana, G.; Itiello, S.; Russo, R.; D’Agostino, G.; Iacono, A.; Russo, E.; Citraro, R.; Cuzzocrea, S.; Piazza, P.V.; et al. Palmitoylethanolamide modulates pentobarbital-evoked hypnotic effect in mice: Involvement of allopregnanolone biosynthesis. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2010, 20, 195–206. [Google Scholar] [CrossRef]
- Moreno, S.; Farioli-Vecchioli, S.; Cerù, M.P. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience 2004, 123, 131–145. [Google Scholar] [CrossRef]
- Zolezzi, J.M.; Santos, M.J.; Bastías-Candia, S.; Pinto, C.; Godoy, J.A.; Inestrosa, N.C. PPARs in the central nervous system: Roles in neurodegeneration and neuroinflammation. Biol. Rev. Camb. Philos. Soc. 2019, 92, 2046–2069. [Google Scholar] [CrossRef] [PubMed]
- Nisbett, K.E.; Pinna, G. Emerging Therapeutic Role of PPAR-α in Cognition and Emotions. Front. Pharmacol. 2018, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, G.; La Rana, G.; Russo, R.; Sasso, O.; Iacono, A.; Esposito, E.; Mattace Raso, G.; Cuzzocrea, S.; Loverme, J.; Piomelli, D.; et al. Central administration of palmitoylethanolamide reduces hyperalgesia in mice via inhibition of NF-kappaB nuclear signalling in dorsal root ganglia. Eur. J. Pharmacol. 2009, 613, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Dlugos, A.; Childs, E.; Stuhr, K.L.; Hillard, C.J.; de Wit, H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology 2018, 37, 2416–2427. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, J.; Urigüen, L.; Rubio, G.; Palomo, T. Role of endocannabinoid system in mental diseases. Neurotox. Res. 2004, 6, 213–224. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Chiurchiù, V.; Díaz-Alonso, J.; Bari, M.; Guzmán, M.; Maccarrone, M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res. 2013, 52, 633–650. [Google Scholar] [CrossRef]
- Lerner, R.; Pascual Cuadrado, D.; Post, J.M.; Lutz, B.; Bindila, L. Broad Lipidomic and Transcriptional Changes of Prophylactic PEA Administration in Adult Mice. Front. Neurosci. 2013, 13, 527. [Google Scholar] [CrossRef]
- Keppel Hesselink, J.M.; de Boer, T.; Witkamp, R.F. Palmitoylethanolamide: A Natural Body-Own Anti-Inflammatory Agent, Effective and Safe against Influenza and Common Cold. Int. J. Inflamm. 2013, 151028. [Google Scholar] [CrossRef]
- Artukoglu, B.B.; Beyer, C.; Zuloff-Shani, A.; Brener, E.; Bloch, M.H. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain Physician 2017, 20, 353–362. [Google Scholar]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016, 62, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Facci, L.; Dal Toso, R.; Romanello, S.; Buriani, A.; Skaper, S.D.; Leon, A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. USA 1995, 92, 3376–3380. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Melck, D.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; De Petrocellis, L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001, 358, 249–255. [Google Scholar] [CrossRef]
- Ghonghadze, M.; Pachkoria, K.; Okujava, M.; Antelava, N.; Gongadze, N. Endocannabinoids receptors mediated central and peripheral effects (Review). Georgian Med. News 2020, 298, 137–143. [Google Scholar]
- Wu, W.F.; Tan, X.J.; Dai, Y.B.; Krishnan, V.; Warner, M.; Gustafsson, J.Å. Targeting estrogen receptor β in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2013, 110, 3543–3548. [Google Scholar] [CrossRef]
- Beggiato, S.; Tomasini, M.C.; Cassano, T.; Ferraro, L. Chronic Oral Palmitoylethanolamide Administration Rescues Cognitive Deficit and Reduces Neuroinflammation, Oxidative Stress, and Glutamate Levels in A Transgenic Murine Model of Alzheimer’s Disease. J. Clin. Med. 2020, 9, 428. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef]
- Grillo, S.L.; Keereetaweep, J.; Grillo, M.A.; Chapman, K.D.; Koulen, P. N-Palmitoylethanolamine depot injection increased its tissue levels and those of other acylethanolamide lipids. Drug Des. Dev. Ther. 2013, 7, 747–752. [Google Scholar] [CrossRef]
- Molinari, C.; Morsanuto, V.; Ghirlanda, S.; Ruga, S.; Notte, F.; Gaetano, L.; Uberti, F. Role of Combined Lipoic Acid and Vitamin D3 on Astrocytes as a Way to Prevent Brain Ageing by Induced Oxidative Stress and Iron Accumulation. Oxidative Med. Cell. Longev. 2019, 2843121. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Ruga, S.; Stoppa, I.; Galla, R.; Notte, F.; Molinari, C. Effect of Mixed Lipoic Acid, Vitamin D, Phosphatidylserine and Homotaurine to Obtain a New Formulation for Brain Ageing Prevention. EC Neurol. 2019, 11.5, 302–312. [Google Scholar]
- Nwachukwu, J.C.; Li, W.; Pineda-Torra, I.; Huang, H.Y.; Ruoff, R.; Shapiro, E.; Taneja, S.S.; Logan, S.K.; Garabedian, M.J. Transcriptional regulation of the androgen receptor cofactor androgen receptor trapped clone-27. Mol. Endocrinol. 2007, 21, 2864–2876. [Google Scholar] [CrossRef]
- Nwachukwu, J.C.; Southern, M.R.; Kiefer, J.R.; Afonine, P.V.; Adams, P.D.; Terwilliger, T.C.; Nettles, K.W. Improved crystallographic structures using extensive combinatorial refinement. Structure 2003, 21, 1923–1930. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle 2008, 7, 1020–1035. [Google Scholar] [CrossRef] [PubMed]
- Thill, M.; Fischer, D.; Kelling, K.; Hoellen, F.; Dittmer, C.; Hornemann, A.; Salehin, D.; Diedrich, K.; Friedrich, M.; Becker, S. Expression of vitamin D receptor (VDR), cyclooxygenase-2 (COX-2) and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in benign and malignant ovarian tissue and 25-hydroxycholecalciferol (25(OH2)D3) and prostaglandin E2 (PGE2) serum level in ovarian cancer patients. J. Steroid. Biochem. Mol. Biol. 2010, 121, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Morsanuto, V.; Lattuada, D.; Colciaghi, B.; Cochis, A.; Bulfoni, A.; Colombo, P.; Bolis, G.; Molinari, C. Protective effects of vitamin D3 on fimbrial cells exposed to catalytic iron damage. J. Ovarian Res. 2016, 9, 34. [Google Scholar] [CrossRef]
- Dampf Stone, A.; Batie, S.F.; Sabir, M.S.; Jacobs, E.T.; Lee, J.H.; Whitfield, G.K.; Haussler, M.R.; Jurutka, P.W. Resveratrol potentiates vitamin D and nuclear receptor signaling. J. Cell. Biochem. 2015, 116, 1130–1143. [Google Scholar] [CrossRef]
- Bartik, L.; Whitfield, G.K.; Kaczmarska, M.; Lowmiller, C.L.; Moffet, E.W.; Furmick, J.K.; Hernandez, Z.; Haussler, C.A.; Haussler, M.R.; Jurutka, P.W. Curcumin: A novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J. Nutr. Biochem. 2010, 21, 1153–1161. [Google Scholar] [CrossRef]
- Davis, C.D.; Milner, J.A. Nutrigenomics, vitamin D and cancer prevention. J. Nutr. Nutr. 2011, 4, 1–11. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Aprile, S.; Ghirlanda, S.; Stoppa, I.; Cochis, A.; Grosa, G.; Rimondini, L.; Molinari, C. Biological effects of combined resveratrol and vitamin D3 on ovarian tissue. J. Ovarian Res. 2017, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Grasso, S.; Bramanti, V.; Tomassoni, D.; Bronzi, D.; Malfa, G.; Traini, E.; Napoli, M.; Renis, M.; Amenta, F.; Avola, R. Effect of lipoic acid and α-glyceryl-phosphoryl-choline on astroglial cell proliferation and differentiation in primary culture. J. Neurosci. Res. 2014, 92, 86–94. [Google Scholar] [CrossRef]
- Arivazhagan, P.; Ramanathan, K.; Panneerselvam, C. Effect of DL-alpha-lipoic acid on mitochondrial enzymes in aged rats. Chem. Biol. Interact. 2001, 138, 189–198. [Google Scholar] [CrossRef]
- Liu, J. The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: An overview. Neurochem. Res. 2008, 33, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Beggiato, S.; Borelli, A.C.; Ferraro, L.; Tanganelli, S.; Antonelli, T.; Tomasini, M.C. Palmitoylethanolamide Blunts Amyloid-β42-Induced Astrocyte Activation and Improves Neuronal Survival in Primary Mouse Cortical Astrocyte-NeuronCo-Cultures. J. Alzheimers Dis. 2018, 61, 389–399. [Google Scholar] [CrossRef]
- Zolese, G.; Bacchetti, T.; Ambrosini, A.; Wozniak, M.; Bertoli, E.; Ferretti, G. Increased plasma concentrations of palmitoylethanolamide, an endogenous fatty acid amide, affect oxidative damage of human low-density lipoproteins: An in vitro study. Atherosclerosis 2005, 182, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Uberti, F.; Caimmi, P.P.; Pietronave, S.; Mary, D.A.; Dianzani, C.; Micalizzi, E.; Melensi, M.; Boldorini, R.; Nicosia, G.; et al. Different expression and function of the endocannabinoid system in human epicardial adipose tissue in relation to heart disease. Can. J. Cardiol. 2013, 29, 499–509. [Google Scholar] [CrossRef]
- Strokin, M.; Sergeeva, M.; Reiser, G. Proinflammatory treatment of astrocytes with lipopolysaccharide results in augmented Ca2+ signaling through increased expression of via phospholipase A2 (iPLA2). Am. J. Physiol. Cell Physiol. 2011, 300, C542–C549. [Google Scholar] [CrossRef] [PubMed]
- Schildge, S.; Bohrer, C.; Beck, K.; Schachtrup, C. Isolation and culture of mouse cortical astrocytes. J. Vis. Exp. JoVE 2013, 71, 50079. [Google Scholar] [CrossRef]
- Lange, S.C.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A.; Norenberg, M.D. Primary cultures of astrocytes: Their value in understanding astrocytes in health and disease. Neurochem. Res. 2012, 37, 2569–2588. [Google Scholar] [CrossRef]
- Fossati, L.; Dechaume, R.; Hardillier, E.; Chevillon, D.; Prevost, C.; Bolze, S.; Maubon, N. Use of simulated intestinal fluid for Caco-2 permeability assay of lipophilic drugs. Int. J. Pharm. 2008, 360, 148–155. [Google Scholar] [CrossRef]
- DiMarco, R.L.; Hunt, D.R.; Dewi, R.E.; Heilshorn, S.C. Improvement of paracellular transport in the Caco-2 drug screening model using protein-engineered substrates. Biomaterials 2017, 129, 152–162. [Google Scholar] [CrossRef]
- Christides, T.; Ganis, J.C.; Sharp, P.A. In vitro assessment of iron availability from commercial Young Child Formulae supplemented with prebiotics. Eur. J. Nut. 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; D’Onofrio, M.; Brandi, R.; Arisi, I.; Zucco, F.; Felsani, A. Cell growing density affects the structural and functional properties of Caco-2 differentiated monolayer. J. Cell. Physiol. 2011, 226, 1531–1543. [Google Scholar] [CrossRef]
- Obringer, C.; Manwaring, J.; Goebel, C.; Hewitt, N.J.; Rothe, H. Suitability of the in vitro Caco-2 assay to predict the oral absorption of aromatic amine hair dyes. Toxicol. In Vitro 2016, 32, 1–7. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines. Nutrients 2017, 9, 1008. [Google Scholar] [CrossRef]
- Konishi, Y.; Hagiwara, K.; Shimizu, M. Transepithelial transport of fluorescein in Caco-2 cell monolayers and use of such transport in in vitro evaluation of phenolic acid availability. Biosci. Biotechnol. Biochem. 2002, 66, 2449–2457. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Bardelli, C.; Molinari, C. Protective effects of 1α,25-Dihydroxyvitamin D3 on cultured neural cells exposed to catalytic iron. Physiol. Rep. 2016, 4, e12769. [Google Scholar] [CrossRef]
- Molinari, C.; Morsanuto, V.; Polli, S.; Uberti, F. Cooperative Effects of Q10, Vitamin D3, and L-Arginine on Cardiac and Endothelial Cells. J. Vasc. Res. 2018, 55, 47–60. [Google Scholar] [CrossRef]
- Langley, P.; Müller-Schwefe, G.; Nicolaou, A.; Liedgens, H.; Pergolizzi, J.; Varrassi, G. The societal impact of pain in the European Union: Health-related quality of life and healthcare resource utilization. J. Med. Econ. 2010, 13, 571–581. [Google Scholar] [CrossRef]
- Paladini, A.; Fusco, M.; Cenacchi, T.; Schievano, C.; Piroli, A.; Varrassi, G. Palmitoylethanolamide, a Special Food for Medical Purposes, in the Treatment of Chronic Pain: A Pooled Data Meta-analysis. Pain Physician 2016, 19, 11–24. [Google Scholar]
- Beggiato, S.; Tomasini, M.C.; Ferraro, L. Palmitoylethanolamide (PEA) as a Potential Therapeutic Agent in Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 821. [Google Scholar] [CrossRef]
- Peritore, A.F.; Siracusa, R.; Crupi, R.; Cuzzocrea, S. Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients 2019, 11, 2175. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J. Neuroinflamm. 2014, 11, 136. [Google Scholar] [CrossRef]
- Smart, D.; Jonsson, K.O.; Vandevoorde, S.; Lambert, D.M.; Fowler, C.J. ‘Entourage’ effects of N-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Br. J. Pharmacol. 2002, 136, 452–458. [Google Scholar] [CrossRef]
- Starowicz, K.; Makuch, W.; Osikowicz, M.; Piscitelli, F.; Petrosino, S.; Di Marzo, V.; Przewlocka, B. Spinal anandamide produces analgesia in neuropathic rats: Possible CB(1)- and TRPV1-mediated mechanisms. Neuropharmacology 2012, 62, 1746–1755. [Google Scholar] [CrossRef]
- Mize, A.L.; Shapiro, R.A.; Dorsa, D.M. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology 2003, 144, 306–312. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsanuto, V.; Galla, R.; Molinari, C.; Uberti, F. A New Palmitoylethanolamide Form Combined with Antioxidant Molecules to Improve Its Effectivess on Neuronal Aging. Brain Sci. 2020, 10, 457. https://doi.org/10.3390/brainsci10070457
Morsanuto V, Galla R, Molinari C, Uberti F. A New Palmitoylethanolamide Form Combined with Antioxidant Molecules to Improve Its Effectivess on Neuronal Aging. Brain Sciences. 2020; 10(7):457. https://doi.org/10.3390/brainsci10070457
Chicago/Turabian StyleMorsanuto, Vera, Rebecca Galla, Claudio Molinari, and Francesca Uberti. 2020. "A New Palmitoylethanolamide Form Combined with Antioxidant Molecules to Improve Its Effectivess on Neuronal Aging" Brain Sciences 10, no. 7: 457. https://doi.org/10.3390/brainsci10070457
APA StyleMorsanuto, V., Galla, R., Molinari, C., & Uberti, F. (2020). A New Palmitoylethanolamide Form Combined with Antioxidant Molecules to Improve Its Effectivess on Neuronal Aging. Brain Sciences, 10(7), 457. https://doi.org/10.3390/brainsci10070457





