Auditory and Somatosensory P3 Are Complementary for the Assessment of Patients with Disorders of Consciousness
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
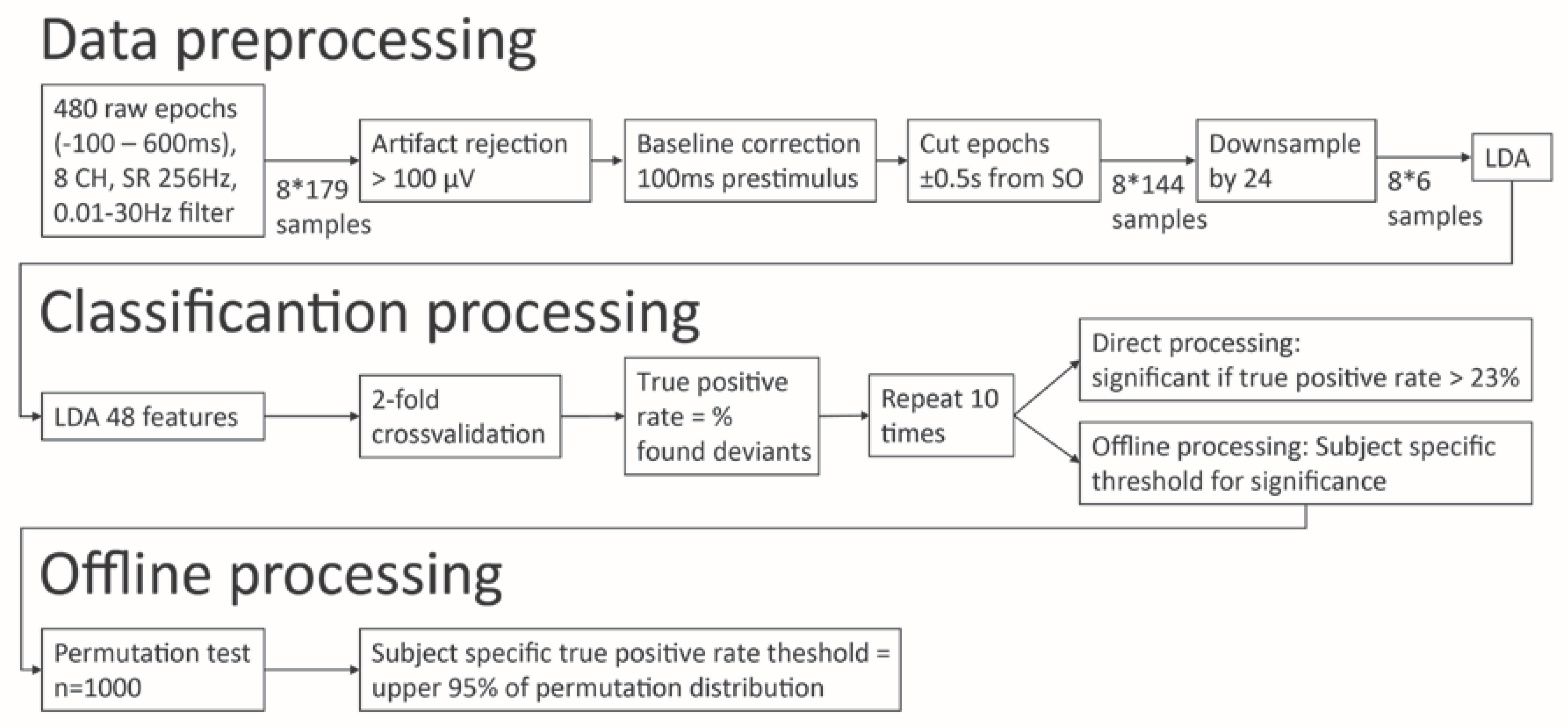
2.2. P3 Assessment and Data Processing
2.3. Statistics
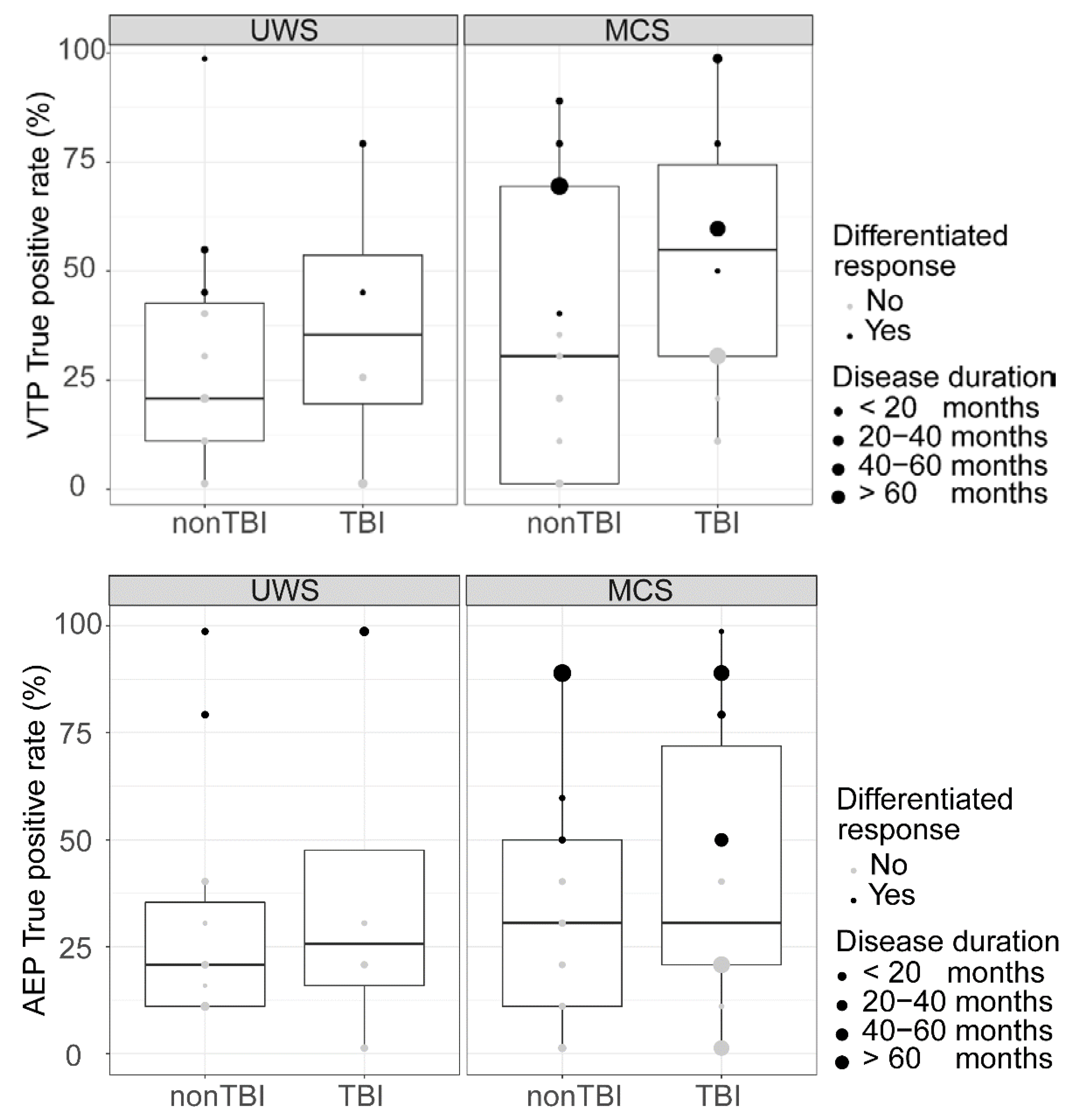
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; Von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef]
- Monti, M.; Laureys, S.; Owen, A.M. The vegetative state. Br. Med. J. 2010, 341, 292–296. [Google Scholar] [CrossRef]
- Fins, J.J.; Master, M.G.; Gerber, L.M.; Giacino, J.T. The Minimally Conscious State: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar]
- Giacino, J.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef]
- Wannez, S.; Eheine, L.; Ethonnard, M.; Gosseries, O.; Laureys, S.; Coma Science Group Collaborators. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann. Neurol. 2017, 81, 883–889. [Google Scholar] [CrossRef]
- Thibaut, A.; Chatelle, C.; Wannez, S.; Deltombe, T.; Stender, J.; Schnakers, C.; Laureys, S.; Gosseries, O. Spasticity in disorders of consciousness: A behavioral study. Eur. J. Phys. Rehabil. 2015, 51, 389–397. [Google Scholar]
- Fossati, M.C.B.; Bejor, M.; Chatelle, C.; Martens, G.; Laureys, S.; Thibaut, A. Spasticity and pain in patients with disorders of consciousness. Ann. Phys. Rehabil. Med. 2018, 61, e267–e268. [Google Scholar] [CrossRef]
- Andrews, K.; Murphy, L.; Munday, R.; Littlewood, C. Misdiagnosis of the vegetative state: Retrospective study in a rehabilitation unit. Br. Med. J. 1996, 313, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Stender, J.; Gosseries, O.; Bruno, M.-A.; Charland-Verville, V.; Vanhaudenhuyse, A.; Demertzi, A.; Chatelle, C.; Thonnard, M.; Thibaut, A.; Heine, L.; et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: A clinical validation study. Lancet 2014, 6736, 8–16. [Google Scholar] [CrossRef]
- Edlow, B.L.; Chatelle, C.; Spencer, C.A.; Chu, C.J.; Bodien, Y.G.; O’Connor, K.L.; Hirschberg, R.E.; Hochberg, L.R.; Giacino, J.T.; Rosenthal, E.S.; et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017, 140, 2399–2414. [Google Scholar] [CrossRef]
- Coleman, M.R.; Rodd, J.M.; Davis, M.H.; Johnsrude, I.S.; Menon, D.K.; Pickard, J.D.; Owen, A.M. Do vegetative patients retain aspects of language comprehension? Evidence from fMRI. Brain 2007, 130, 2494–2507. [Google Scholar] [CrossRef] [PubMed]
- Gosseries, O.; Zasler, N.D.; Laureys, S. Recent advances in disorders of consciousness: Focus on the diagnosis. Brain Inj. 2014, 28, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Schiff, N.D. Cognitive Motor Dissociation Following Severe Brain Injuries. JAMA Neurol. 2015, 72, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Di Perri, C.; Thibaut, A.; Heine, L.; Annen, J.; Laureys, S. Towards new methods of diagnosis in disorders of consciousness—Authors’ reply. Lancet Neurol. 2016, 15, 1115–1116. [Google Scholar] [CrossRef]
- Owen, A.M.; Coleman, M.R.; Boly, M.; Davis, M.H.; Laureys, S.; Pickard, J.D. Detecting awareness in the vegetative state. Science 2006, 313, 1402. [Google Scholar] [CrossRef]
- Monti, M.M.; Vanhaudenhuyse, A.; Coleman, M.R.; Boly, M.; Pickard, J.D.; Tshibanda, L.; Owen, A.M.; Laureys, S. Willful Modulation of Brain Activity in Disorders of Consciousness. N. Engl. J. Med. 2010, 362, 579–589. [Google Scholar] [CrossRef]
- Lulé, R.; Noirhomme, Q.; Kleih, S.C.; Chatelle, C.; Halder, S.; Demertzi, A.; Bruno, M.-A.; Gosseries, O.; Vanhaudenhuyse, A.; Schnakers, C.; et al. Probing command following in patients with disorders of consciousness using a brain-computer interface. Clin. Neurophysiol. 2013, 124, 101–106. [Google Scholar] [CrossRef]
- Chennu, S.; Finoia, P.; Kamau, E.; Monti, M.M.; Allanson, J.; Pickard, J.D.; Owen, A.M.; Bekinschtein, T.A. Dissociable endogenous and exogenous attention in disorders of consciousness. NeuroImage Clin. 2013, 3, 450–461. [Google Scholar] [CrossRef]
- Pokorny, C.; Klobassa, D.S.; Pichler, G.; Erlbeck, H.; Real, R.G.L.; Kübler, A.; Lesenfants, D.; Habbal, D.; Noirhomme, Q.; Risetti, M.; et al. The auditory P300-based single-switch brain-computer interface: Paradigm transition from healthy subjects to minimally conscious patients. Artif. Intell. Med. 2013, 59, 81–90. [Google Scholar] [CrossRef]
- Kotchoubey, B.; Lang, S.; Mezger, G.; Schmalohr, D.; Schneck, M.; Semmler, A.; Bostanov, V.; Birbaumer, N. Information processing in severe disorders of consciousness: Vegetative state and minimally conscious state. Clin. Neurophysiol. 2005, 116, 2441–2453. [Google Scholar] [CrossRef]
- Perrin, F.; Schnakers, C.; Schabus, M.; Degueldre, C.; Goldman, S.; Brédart, S.; Faymonville, M.-E.; Lamy, M.; Moonen, G.; Luxen, A.; et al. Brain Response to One’s Own Name in Vegetative State, Minimally Conscious State, and Locked-in Syndrome. Arch. Neurol. 2006, 63, 562–569. [Google Scholar] [CrossRef]
- Chennu, S.; Bekinschtein, T.A. Arousal Modulates Auditory Attention and Awareness: Insights from Sleep, Sedation, and Disorders of Consciousness. Front. Psychol. 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2009, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, T.A.; Dehaene, S.; Rohaut, B.; Tadel, F.; Cohen, L.; Naccache, L. Neural signature of the conscious processing of auditory regularities. Proc. Natl. Acad. Sci. USA 2009, 106, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Faugeras, F.; Rohaut, B.; Weiss, N.; Bekinschtein, T.A.; Galanaud, D.; Puybasset, L.; Bolgert, F.; Sergent, C.; Cohen, L.; Dehaene, S.; et al. Event related potentials elicited by violations of auditory regularities in patients with impaired consciousness. Neuropsychologia 2012, 50, 403–418. [Google Scholar] [CrossRef]
- Schnakers, C.; Perrin, F.; Schabus, M.; Majerus, S.; LeDoux, D.; Damas, P.; Boly, M.; Vanhaudenhuyse, A.; Bruno, M.-A.; Moonen, G.; et al. Voluntary brain processing in disorders of consciousness. Neurology 2008, 71, 1614–1620. [Google Scholar] [CrossRef]
- Lugo, Z.R.; Rodriguez, J.; Lechner, A.; Ortner, R.; Gantner, I.S.; Laureys, S.; Noirhomme, Q.; Eguger, C. A Vibrotactile P300-Based Brain–Computer Interface for Consciousness Detection and Communication. Clin. EEG Neurosci. 2014, 45, 14–21. [Google Scholar] [CrossRef]
- Pan, J.; Xie, Q.; He, Y.; Wang, F.; Di, H.; Laureys, S.; Yu, R.; Li, Y. Detecting awareness in patients with disorders of consciousness using a hybrid brain–computer interface. J. Neural Eng. 2014, 11, 56007. [Google Scholar] [CrossRef]
- Chatelle, C.; Spencer, C.A.; Halgren, E.; Hochberg, L.R.; Edlow, B.L. Feasibility of an EEG-based brain-computer interface in the intensive care unit. Clin. Neurophysiol. 2018, 129, 1519–1525. [Google Scholar] [CrossRef]
- Eguger, C.; Spataro, R.; Allison, B.Z.; Heilinger, A.; Ortner, R.; Cho, W.; La Bella, V. Complete Locked-in and Locked-in Patients: Command Following Assessment and Communication with Vibro-Tactile P300 and Motor Imagery Brain-Computer Interface Tools. Front. Neurosci. 2017, 11, 251. [Google Scholar]
- Guger, C.; Spataro, R.; Pellas, F.; Allison, B.Z.; Heilinger, A.; Ortner, R.; Cho, W.; Xu, R.; La Bella, V.; Edlinger, G.; et al. Assessing Command-Following and Communication with Vibro-Tactile P300 Brain-Computer Interface Tools in Patients With Unresponsive Wakefulness Syndrome. Front. Neurosci. 2018, 12, 423. [Google Scholar] [CrossRef]
- Nichols, T.E.; Holmes, A.P. Nonparametric Permutation Tests for Functional Neuroimaging. Hum. Brain Mapp. 2001, 25, 887–910. [Google Scholar]
- Noirhomme, Q.; Lesenfants, D.; Gomez, F.; Soddu, A.; Schrouff, J.; Garraux, G.; Luxen, A.; Phillips, C.; Laureys, S. Biased binomial assessment of cross-validated estimation of classification accuracies illustrated in diagnosis predictions. NeuroImage Clin. 2014, 4, 687–694. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Jox, R.J.; Bernat, J.L.; Laureys, S.; Racine, E. Disorders of consciousness: Responding to requests for novel diagnostic and therapeutic interventions. Lancet Neurol. 2012, 11, 732–738. [Google Scholar] [CrossRef]
- Schnakers, C.; Perrin, F.; Schabus, M.; Hustinx, R.; Majerus, S.; Moonen, G.; Boly, M.; Vanhaudenhuyse, A.; Bruno, M.-A.; Laureys, S. Detecting consciousness in a total locked-in syndrome: An active event-related paradigm. Neurocase 2009, 15, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Tzovara, A.; Rossetti, A.O.; Juan, E.; Suys, T.; Viceic, D.; Rusca, M.; Oddo, M.; De Lucia, M. Prediction of awakening from hypothermic post anoxic coma based on auditory discrimination. Ann. Neurol. 2016, 79, 748–757. [Google Scholar] [CrossRef]
- Sitt, J.D.; King, J.-R.; El Karoui, I.; Rohaut, B.; Faugeras, F.; Gramfort, A.; Cohen, L.; Sigman, M.; Dehaene, S.; Naccache, L. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014, 137, 2258–2270. [Google Scholar] [CrossRef]
- Annen, J.; Blandiaux, S.; Lejeune, N.; Bahri, M.A.; Thibaut, A.; Cho, W.; Guger, C.; Chatelle, C.; Laureys, S. BCI Performance and Brain Metabolism Profile in Severely Brain-Injured Patients Without Response to Command at Bedside. Front. Neurosci. 2018, 12, 370. [Google Scholar] [CrossRef]
- Cruse, D.; Chennu, S.; Chatelle, C.; Bekinschtein, T.A.; Fernández-Espejo, D.; Pickard, J.D.; Laureys, S.; Owen, A.M. Bedside detection of awareness in the vegetative state: A cohort study. Lancet 2011, 378, 2088–2094. [Google Scholar] [CrossRef]
- Cruse, D.; Chennu, S.; Chatelle, C.; Fernández-Espejo, D.; Bekinschtein, T.A.; Pickard, J.D.; Laureys, S.; Owen, A.M. Relationship between etiology and covert cognition in the minimally conscious state. Neurology 2012, 78, 816–822. [Google Scholar] [CrossRef]
- Curley, W.H.; Forgacs, P.B.; Voss, H.U.; Conte, M.M.; Schiff, N.D. Characterization of EEG signals revealing covert cognition in the injured brain. Brain 2018, 141, 1404–1421. [Google Scholar] [CrossRef]
- Kalmar, K.; Giacino, J.T. The JFK coma recovery scale-revised. Neuropsychol. Rehabil. 2005, 15, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Kotchoubey, B.; Pavlov, Y.G. Approaches to sleep in severely brain damaged patients: Opposite or complementary? Reply to “Sleep and Circadian Rhythms in Severely Brain-Injured Patients—A Comment”. Clin. Neurophysiol. 2018, 129, 1785–1787. [Google Scholar]
- Piarulli, A.; Bergamasco, M.; Thibaut, A.; Cologan, V.; Gosseries, O.; Laureys, S. EEG ultradian rhythmicity differences in disorders of consciousness during wakefulness. J. Neurol. 2016, 263, 1746–1760. [Google Scholar] [CrossRef] [PubMed]
- Kempny, A.M.; James, L.; Yelden, K.; Duport, S.; Farmer, S.F.; Playford, E.D.; Leff, A.P. NeuroImage: Clinical Patients with a severe prolonged Disorder of Consciousness can show classical EEG responses to their own name compared with others’ names. NeuroImage Clin. 2018, 19, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Wangshan, H.; Di, H.; Hu, X.; Jing, S.; Thibaut, A.; Di Perri, C.; Huang, W.; Nie, Y.; Schnakers, C.; Laureys, S. Cerebral response to subject’s own name showed high prognostic value in traumatic vegetative state. BMC Med. 2015, 13, 83. [Google Scholar]
- Langner, R.; Kellermann, T.; Eickhoff, S.B.; Boers, F.; Chatterjee, A.; Willmes, K.; Sturm, W. Staying responsive to the world: Modality-specific and -nonspecific contributions to speeded auditory, tactile, and visual stimulus detection. Hum. Brain Mapp. 2012, 33, 398–418. [Google Scholar] [CrossRef]
- Yin, E.; Zeyl, T.; Saab, R.; Hu, D.; Zhou, Z.; Chau, T.T. An Auditory-Tactile Visual Saccade-Independent P300 Brain–Computer Interface. Int. J. Neural Syst. 2016, 26, 1650001. [Google Scholar] [CrossRef]


| Direct | Offline | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Gender | Age | Time Since Injury | Aetiology | CRS-R Subscores | AEP | VTP | AEP | VTP |
| HC | F | 28 | nonTBI | 100 | 100 | 100 | 100 | ||
| HC | F | 23 | nonTBI | 100 | 100 | 100 | 100 | ||
| HC | M | 23 | nonTBI | 90 | 100 | 100 | 100 | ||
| HC | F | 21 | nonTBI | 100 | 100 | 100 | 100 | ||
| HC | F | 24 | nonTBI | 100 | 80 | 100 | 90 | ||
| HC | F | 22 | nonTBI | 100 | 100 | 90 | 100 | ||
| HC | F | 27 | nonTBI | 100 | 100 | 100 | 100 | ||
| HC | M | 24 | nonTBI | 100 | 100 | 80 | 100 | ||
| HC | F | 25 | nonTBI | 100 | 100 | 100 | 70 | ||
| HC | F | 24 | nonTBI | 100 | 20 | 90 | 100 | ||
| HC | M | nonTBI | 100 | 100 | 90 | 100 | |||
| HC | M | 48 | nonTBI | 100 | 100 | 100 | 100 | ||
| EMCS | M | 24 | nonTBI | 1,3,2,1,0,2 | 20 | 60 | 0 | 40 | |
| EMCS | M | 26 | 3.9 | TBI | 3,5,4,1,0,2 | 70 | 90 | 90 | 80 |
| MCS | M | 66 | 4.5 | TBI | 2,3,5,3,1,2 | 10 | 70 | 20 | 10 |
| MCS | F | 43 | 2.6 | nonTBI | 2,3,6,2,1,2 | 30 | 20 | 60 | 10 |
| MCS | F | 19 | 4.2 | nonTBI | 1,0,5,1,0,1 | 20 | 20 | 40 | 0 |
| MCS | M | 25 | 4.8 | nonTBI | 1,0,5,1,0,2 | 20 | 70 | 50 | 80 |
| MCS | F | 51 | 5.1 | nonTBI | 2,3,2,2,1,2 | 60 | 55 | 30 | 90 |
| MCS | M | 63 | 2.1 | nonTBI | 2,2,0,1,0,1 | 10 | 10 | 0 | 40 |
| MCS | M | 47 | 3.5 | TBI | 2,3,2,1,0,2 | 60 | 100 | 40 | 80 |
| MCS | M | 58 | 2.4 | nonTBI | 3,1,5,2,1,2 | 0 | 30 | 50 | 35 |
| MCS | M | 53 | 3.0 | nonTBI | 2,3,2,2,0,2 | 0 | 40 | 60 | 90 |
| MCS | M | 61 | 4.3 | nonTBI | 2,3,2,1,0,1 | 100 | 0 | 10 | 0 |
| MCS | M | 56 | 5.0 | nonTBI | 2,1,5,2,1,1 | 20 | 20 | 0 | 20 |
| MCS | M | 34 | 1.7 | TBI | 4,5,6,1,1,2 | 20 | 10 | 100 | 20 |
| MCS | M | 52 | 1.9 | TBI | 3,3,5,1,1,1 | 0 | 20 | 10 | 50 |
| MCS | M | 64 | 55.9 | TBI | 3,5,6,2,1,2 | 0 | 90 | 0 | 60 |
| MCS | M | 18 | 7.8 | TBI | 3,3,5,2,0,1 | 20 | 100 | 80 | 100 |
| MCS | M | 55 | 68.7 | TBI | 2,0,5,1,0,1 | 30 | 10 | 20 | 30 |
| MCS | M | 22 | 39.2 | TBI | 1,3,1,1,0,1 | 50 | 20 | 50 | 30 |
| MCS | F | 41 | 79.0 | nonTBI | 3,0,1,1,0,1 | 40 | 60 | 90 | 70 |
| MCS | M | 20 | 13.7 | TBI | 3,3,5,2,0,2 | 10 | 100 | 20 | 100 |
| MCS | M | 57 | 7.8 | nonTBI | 1,1,1,1,0,1 | 20 | 25 | 0 | 0 |
| MCS | F | 49 | 3.9 | nonTBI | 2,1,1,2,0,2 | 30 | 0 | 30 | 0 |
| MCS | F | 47 | 3.9 | nonTBI | 1,3,5,1,0,1 | 20 | 10 | 20 | 30 |
| MCS | F | 40 | 58.9 | TBI | 0,3,2,1,0,2 | 60 | 100 | 90 | 60 |
| UWS | F | 33 | 10.5 | nonTBI | 2,1,1,1,0,2 | 30 | 40 | 10 | 20 |
| UWS | M | 46 | 4.9 | TBI | 1,0,1,1,0,2 | 0 | 10 | 20 | 80 |
| UWS | F | 27 | 2.2 | TBI | 2,1,1,1,0,2 | 10 | 40 | 30 | 45 |
| UWS | M | 59 | 2.0 | nonTBI | 2,0,2,1,0,2 | 10 | 0 | 10 | 10 |
| UWS | M | 65 | 5.0 | nonTBI | 1,0,2,1,0,2 | 10 | 0 | 40 | 40 |
| UWS | F | 54 | 4.3 | nonTBI | 1,0,1,1,0,2 | 0 | 50 | 10 | 45 |
| UWS | M | 50 | 5.6 | TBI | 2,2,2,1,0,2 | 10 | 20 | 0 | 25 |
| UWS | M | 57 | 3.7 | nonTBI | 0,0,5,1,0,0 | 30 | 10 | 20 | 30 |
| UWS | M | 57 | 1.5 | nonTBI | 2,1,2,1,0,1 | 80 | 0 | 30 | 100 |
| UWS | M | 65 | 4.9 | nonTBI | 1,1,1,1,0,1 | 100 | 0 | 100 | 10 |
| UWS | M | 56 | 5.9 | nonTBI | 1,1,1,1,0,1 | 10 | 75 | 20 | 55 |
| UWS | F | 46 | 5.9 | nonTBI | 0,0,1,1,0,2 | 70 | 0 | 80 | 0 |
| UWS | F | 71 | 1.3 | nonTBI | 0,0,1,1,0,1 | 10 | 10 | 15 | 0 |
| UWS | F | 32 | 12.7 | TBI | 1,0,1,1,0,2 | 60 | 10 | 100 | 0 |
| UWS | M | 59 | 2.0 | nonTBI | 2,1,1,1,0,2 | 20 | 0 | 10 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annen, J.; Mertel, I.; Xu, R.; Chatelle, C.; Lesenfants, D.; Ortner, R.; Bonin, E.A.C.; Guger, C.; Laureys, S.; Müller, F. Auditory and Somatosensory P3 Are Complementary for the Assessment of Patients with Disorders of Consciousness. Brain Sci. 2020, 10, 748. https://doi.org/10.3390/brainsci10100748
Annen J, Mertel I, Xu R, Chatelle C, Lesenfants D, Ortner R, Bonin EAC, Guger C, Laureys S, Müller F. Auditory and Somatosensory P3 Are Complementary for the Assessment of Patients with Disorders of Consciousness. Brain Sciences. 2020; 10(10):748. https://doi.org/10.3390/brainsci10100748
Chicago/Turabian StyleAnnen, Jitka, Isabella Mertel, Ren Xu, Camille Chatelle, Damien Lesenfants, Rupert Ortner, Estelle A.C. Bonin, Christoph Guger, Steven Laureys, and Friedemann Müller. 2020. "Auditory and Somatosensory P3 Are Complementary for the Assessment of Patients with Disorders of Consciousness" Brain Sciences 10, no. 10: 748. https://doi.org/10.3390/brainsci10100748
APA StyleAnnen, J., Mertel, I., Xu, R., Chatelle, C., Lesenfants, D., Ortner, R., Bonin, E. A. C., Guger, C., Laureys, S., & Müller, F. (2020). Auditory and Somatosensory P3 Are Complementary for the Assessment of Patients with Disorders of Consciousness. Brain Sciences, 10(10), 748. https://doi.org/10.3390/brainsci10100748






