Dye Adsorbent Materials Based on Porous Ceramics from Glass Fiber-Reinforced Plastic and Clay
Abstract
1. Introduction
2. Materials and Methods
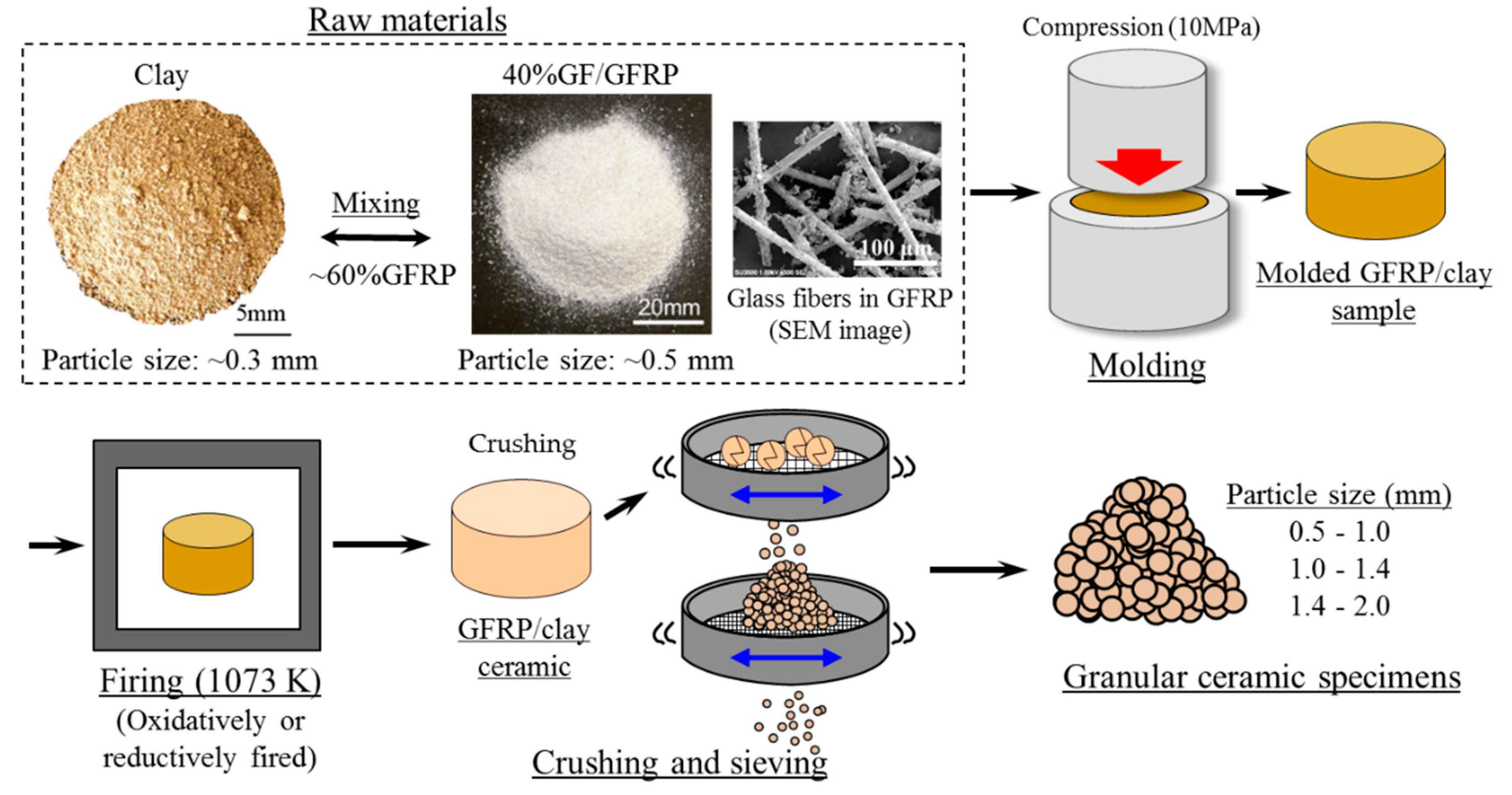
2.1. GFRP/Clay Ceramic Specimens Used for MB Dye Adsorption Tests
- (1)
- Clay was crushed using a rotary mill (New Power Mill ABS-W, Osaka Chemical Co., Ltd., Osaka, Japan) and then sifted using a 0.3-mm mesh screen.
- (2)
- GFRP was also crushed in the rotary mill and then sifted with a 0.5 mm-mesh screen. Here, the maximum length of the glass fibers in GFRP, which were sifted with the 0.5 mm-mesh screen, was approximately 150 µm [7].
- (3)
- The crushed GFRP was mixed with the clay in the mass ratios listed in Table 2.
- (4)
- The GFRP and clay mixture was solidified by pressing into a mold at 10 MPa. The molded samples had a diameter of 74 mm and a thickness of 50–60 mm.
- (5)
- The molded samples were heated in an oxidizing or reducing atmosphere at a firing temperature of 1073 K in an electric furnace (KY-4N, Kyoei Electric Kilns Co., Ltd., Tajimi, Japan). The samples were then held at the firing temperature for 1 h and allowed to cool to room temperature in the furnace. For oxidative firing, samples were heated at 100 K h−1 to the firing temperature. In reductive firing, the samples were heated at 400 K h−1.
- (6)
- The produced GFRP/clay ceramics were then crushed with a hammer and selected to yield a predetermined particle size, as shown in Table 2.
2.2. Measurements of Apparent Porosity, Pore-Size Distribution, Specific Surface Area, and Carbide Content of the GFRP/Clay Ceramic
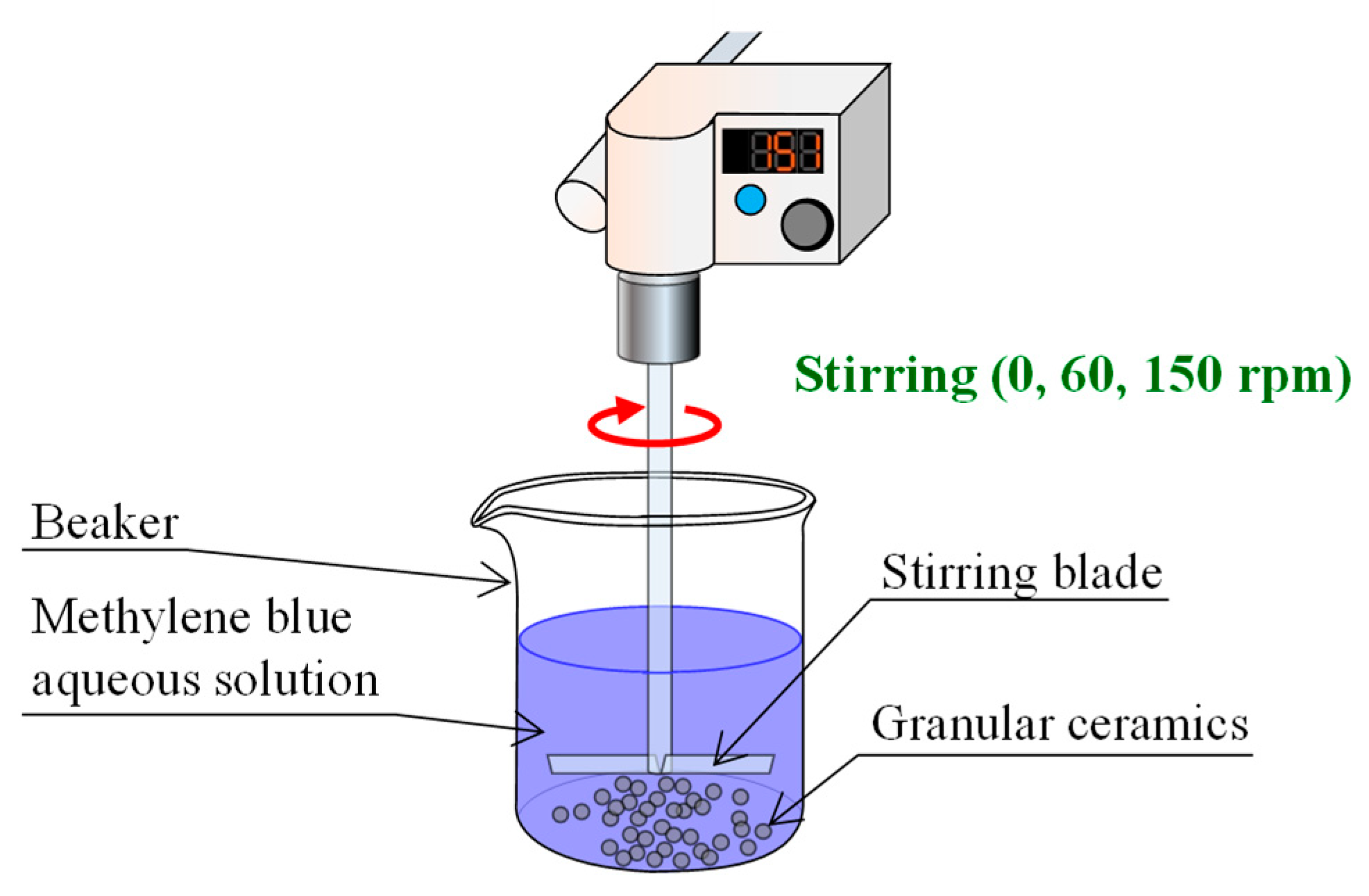
2.3. MB Dye Adsorption Tests
- (1)
- Ceramic specimens were washed with distilled water, and were dried in an electric furnace at 378 K for over 24 h before MB dye adsorption test.
- (2)
- MB dye powder (molecular formula: C16H18N3SCl, Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) was dissolved in distilled water to produce an MB solution with a concentration of 1 × 10−4 mol/L (32 ppm).
- (3)
- A 1-g portion of the granular ceramic specimen was placed into a beaker containing 50 mL of MB solution and the MB solution was stirred with a stirring device (EYLA ZZ-1010, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) at speeds of 0, 60, and 150 rpm.
- (4)
- The MB dye concentration and pH value in the aqueous solution were measured after 1, 10, 30, 60, and 120 min.
3. Results and Discussion
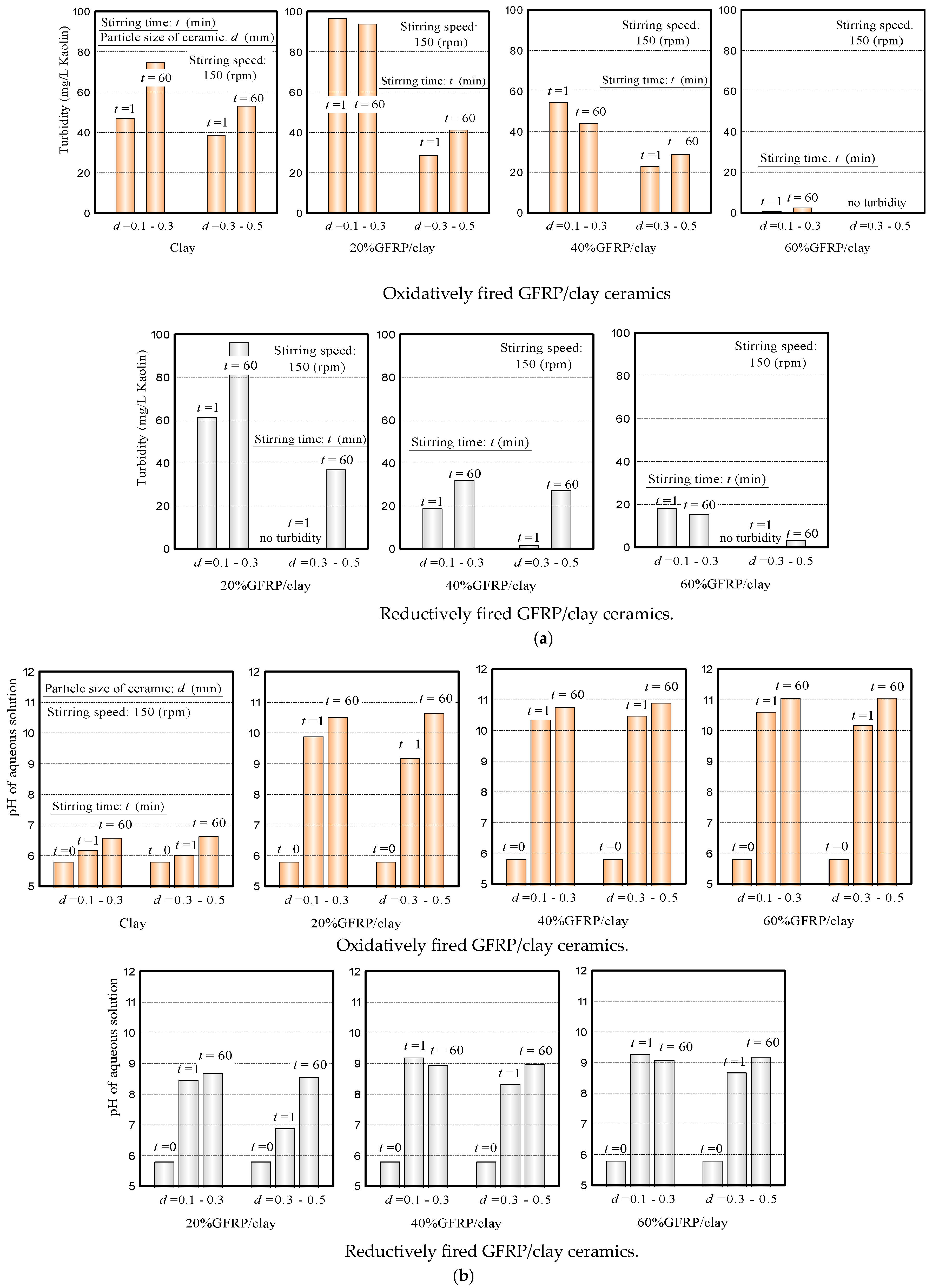
3.1. Preliminary Experiments
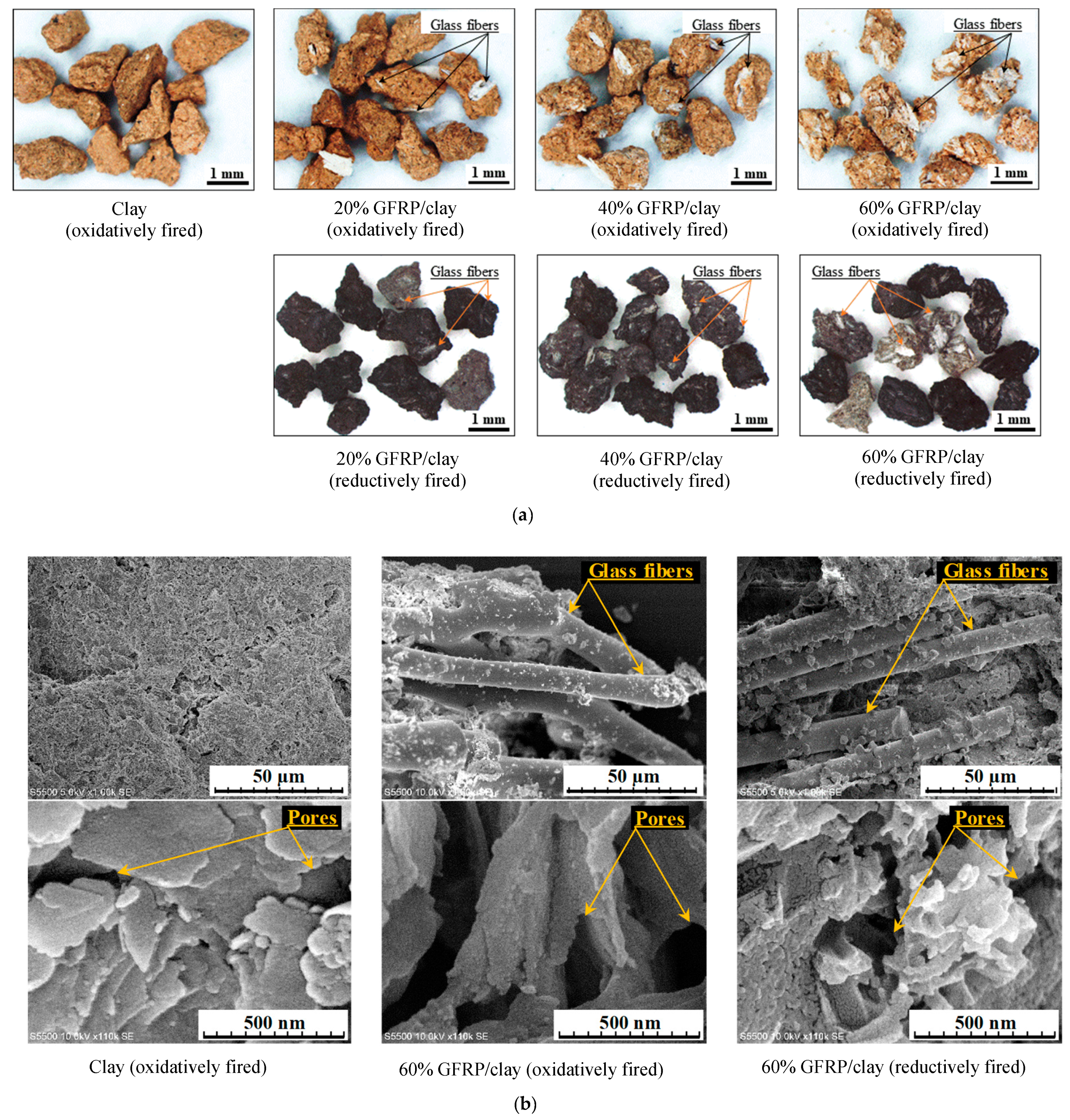
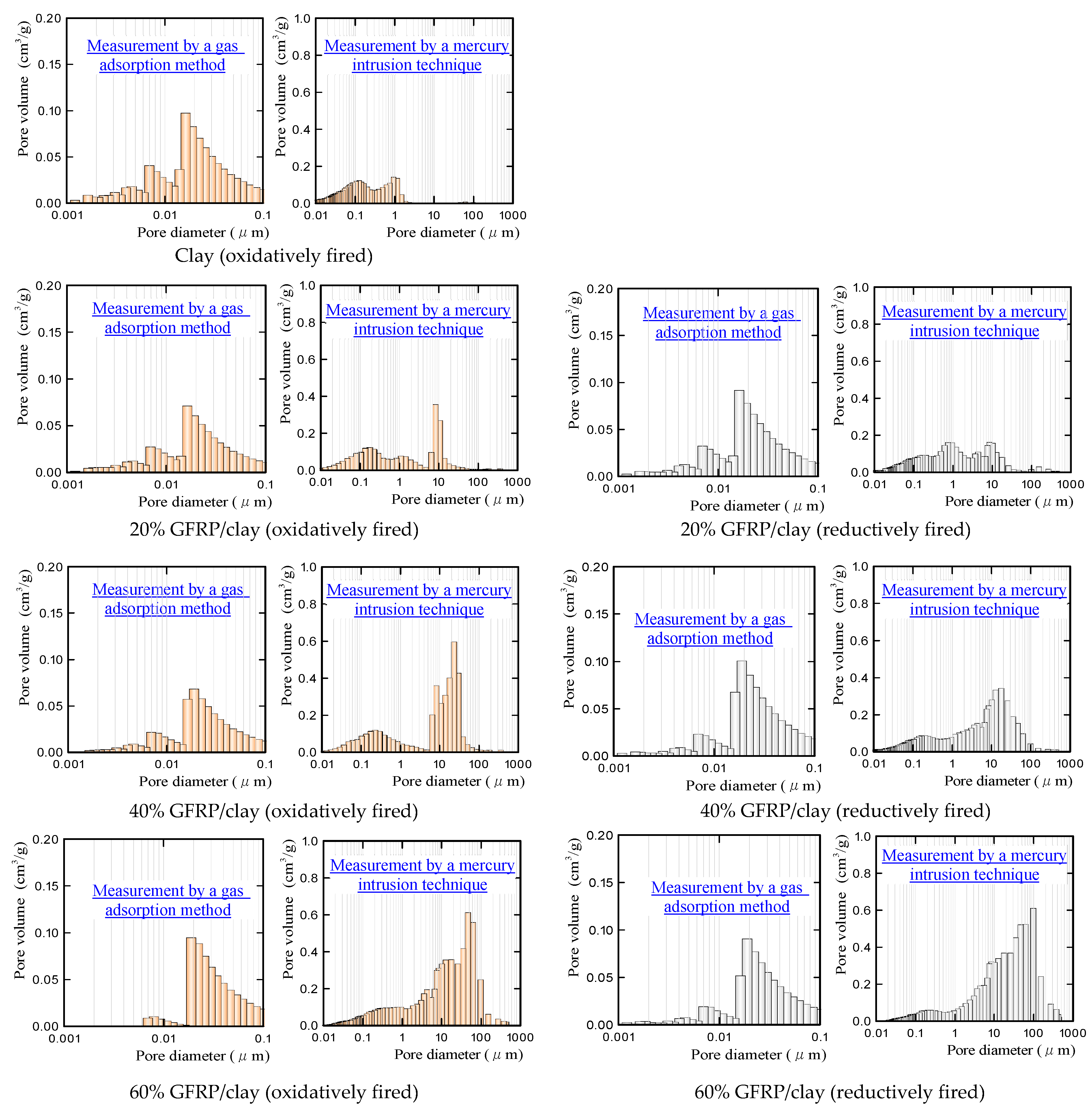
3.2. Apparent Porosities, Specific Surface Areas, and Carbide Contents of the GFRP/Clay Ceramic Specimens
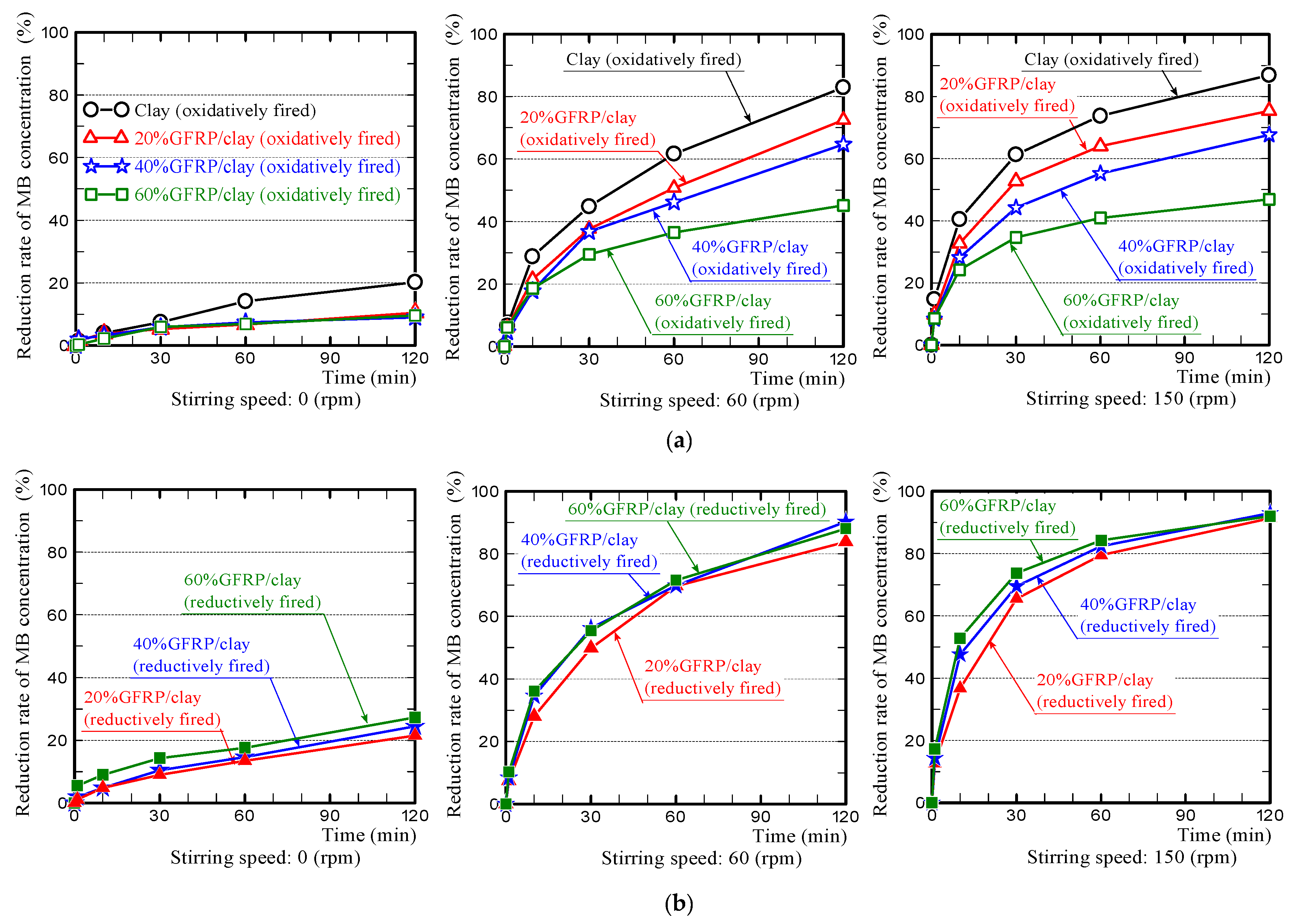
3.3. MB Dye Adsorption Ability of the GFRP/Clay Ceramics
- (1)
- The MB dye adsorption ability of the oxidatively fired 60% GFRP/clay ceramic was the lowest due to it having the largest glass fiber content, which had no ability to adsorb MB dye. In addition, this ceramic possessed the smallest specific surface area, as shown in Table 3.
- (2)
- In contrast, the MB dye adsorption ability of the reductively fired 60% GFRP/clay ceramic was the highest. We attribute this result to the ceramic because it contains a larger carbide proportion, a comparatively high specific surface area, and high porosity; however, this ceramic also contained the joint-largest glass fiber content.
- (1)
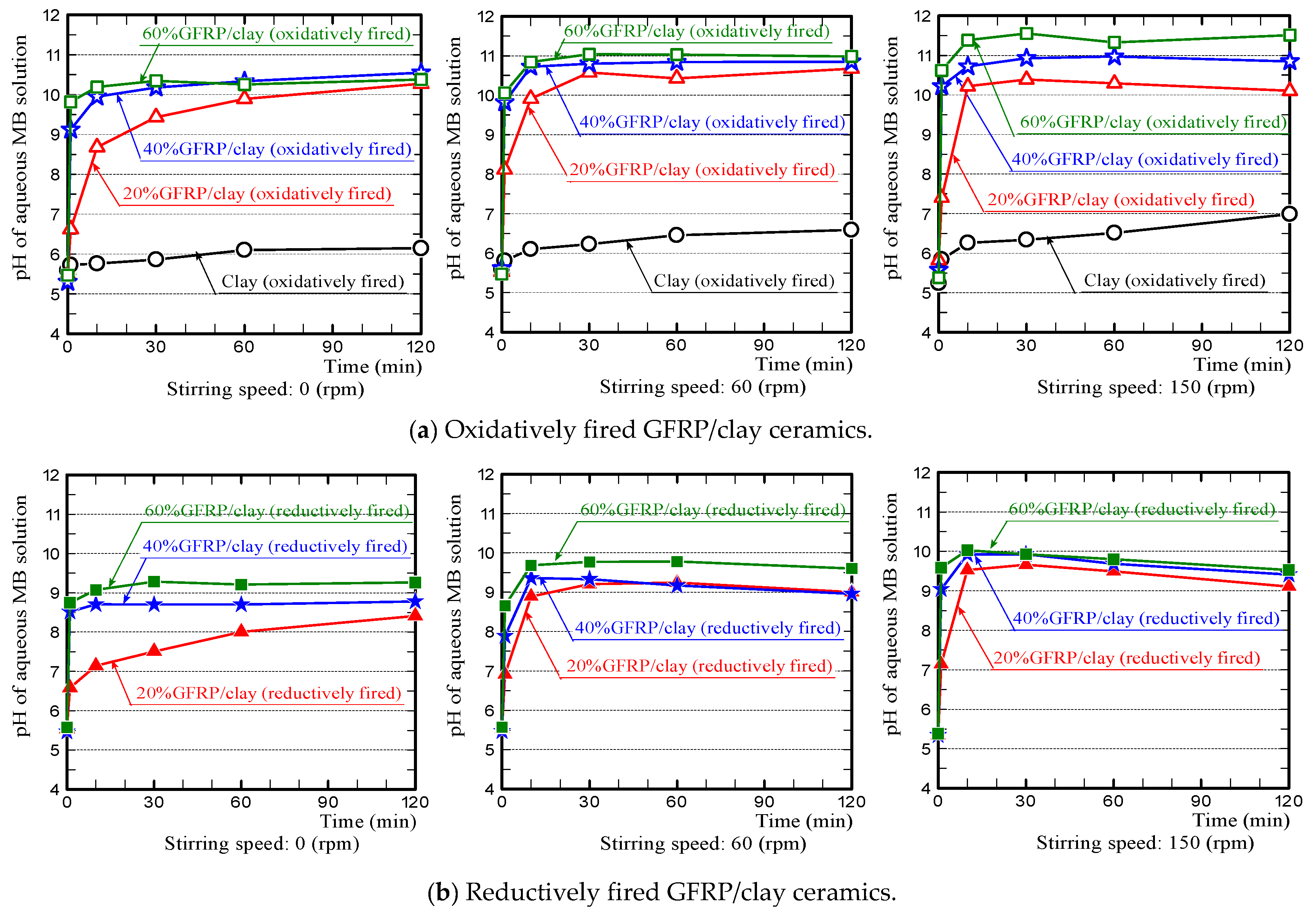
- The pH value of the MB solution, in which the GFRP/clay ceramic was soaked, initially increased sharply because the glass fibers in the ceramic structure released calcium ions. This phenomenon is similar to the results of our preliminary experiments (see Figure 6b in Section 3.1).
- (2)
- For the phenomenon where the pH value of the MB solution, in which the reductively fired GFRP/clay ceramic was soaked, decreased gradually after an initial sharp rise. We assume the following: The color of MB solution turns from blue to colorless when the cations of MB dye in the aqueous solution are reduced. Therefore, MB dye cations were reduced by adsorption to the plastic carbide in the ceramic, such that there was a decrease in the hydroxide ion concentration in the aqueous solution.
- (3)
- The pH value of the MB solution, in which the clay ceramic without glass fibers was soaked, increased gradually over time and eventually changed from weakly acidic to nearly neutral. Since the MB dye cations exchanged with other cations in the aqueous solution, this gradually neutralized the aqueous solution.
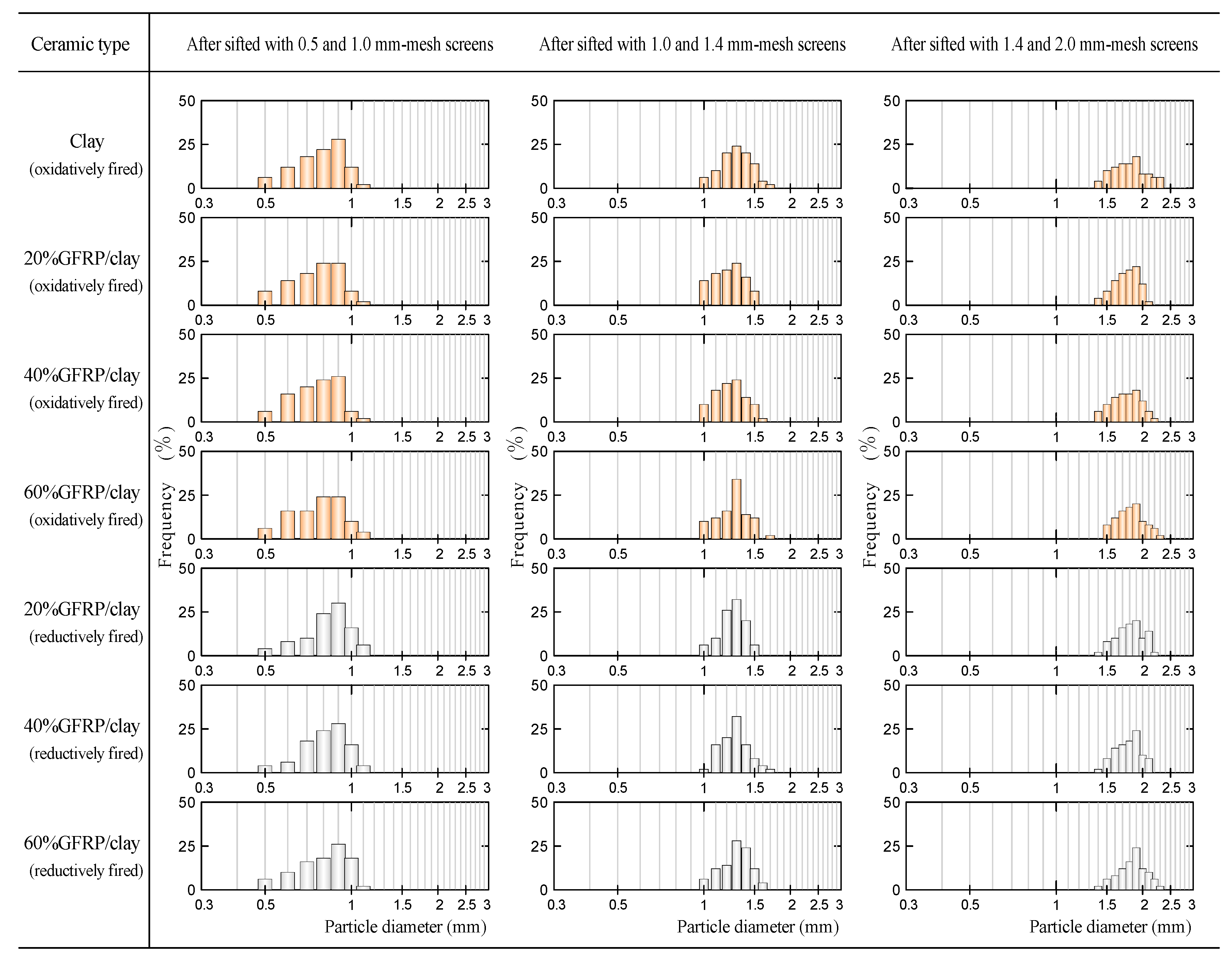
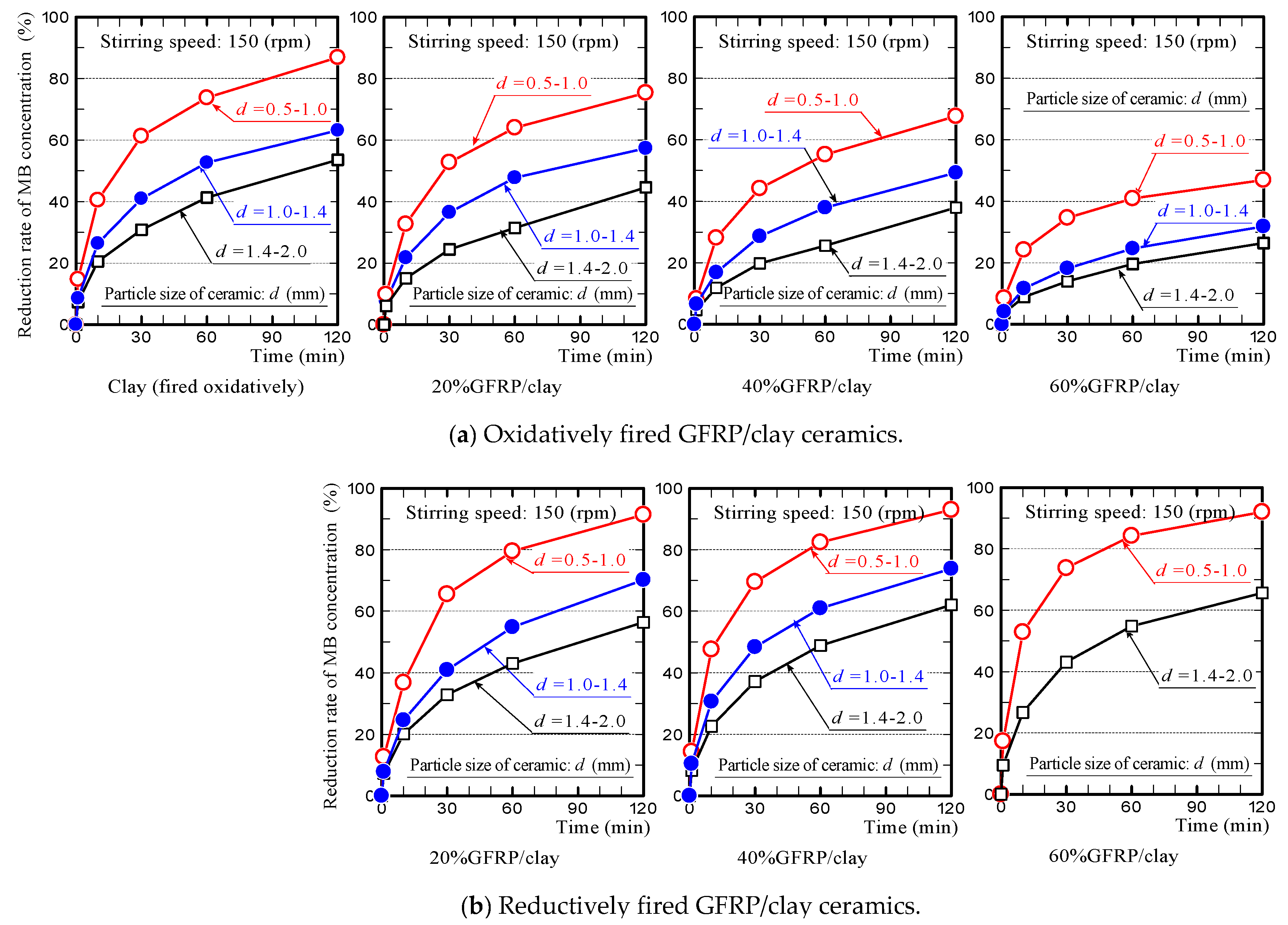
3.4. Influence of Particle Size on the MB Dye Adsorption Ability of GFRP/Clay Ceramic
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagaoka, T. Value-Added Recycling of Disposal Plastics. J. Jpn. Soc. Technol. Plast. 2008, 49, 175–179. [Google Scholar] [CrossRef]
- Yang, Y.; Boom, R.; Irion, B.; Heerden, D.; Kuiper, P.; Wit, H. Recycling of Composite Materials. Chem. Eng. Process. 2012, 51, 53–68. [Google Scholar] [CrossRef]
- Materials Science Society of Japan. Global Environment and Materials; Shokabo Co., Ltd.: Tokyo, Japan, 1999; pp. 61–74. [Google Scholar]
- Kinoshita, H.; Kaizu, K.; Hasegawa, S.; Ando, T.; Kawamura, R.; Ikeda, K.; Kobayashi, T.; Fukuyama, H. Production and Material Properties of Ceramic From Waste Glass Fiber Reinforced Plastic. J. Environ. Eng. 2013, 8, 27–40. [Google Scholar] [CrossRef][Green Version]
- Yasuda, Y.; Kinoshita, H.; Yasui, K.; Yuji, T.; Okamura, Y.; Sezaki, M.; Kawamura, R. Ceramics utilizing glass fiber-reinforced plastic as civil engineering materials to counteract the heat island phenomenon. Mech. Eng. J. 2016, 3, 16-00078. [Google Scholar] [CrossRef]
- Yasui, K.; Goto, S.; Kinoshita, H.; Kamiunten, S.; Yuji, T.; Okamura, Y.; Mungkung, N.; Sezaki, M. Ceramic waste glass fiber-reinforced plastic-containing filtering materials for turbid water treatment. Environ. Earth Sci. 2016, 75, 1135. [Google Scholar] [CrossRef]
- Yasuda, Y.; Iwasaki, H.; Yasui, K.; Tanaka, A.; Kinoshita, H. Development of walkway blocks with high water permeability using waste glass fiber-reinforced plastic. AIMS Energy 2018, 6, 1032–1049. [Google Scholar] [CrossRef]
- Fatima, M.; Farooq, R.; Lindström, R.W.; Saeed, M. A review on biocatalytic decomposition of azo dyes and electrons recovery. J. Mol. Liq. 2017, 246, 275–281. [Google Scholar] [CrossRef]
- Herrera-González, A.M.; Caldera-Villalobos, M.; Peláez-Cid, A.-A. Adsorption of textile dyes using an activated carbon and crosslinked polyvinyl phosphonic acid composite. J. Environ. Manag. 2019, 234, 237–244. [Google Scholar] [CrossRef]
- Lucas, M.; Dias, A.A.; Sampaio, A.; Amaral, C.; Peres, J.A. Degradation of a textile reactive Azo dye by a combined chemical–biological process: Fenton’s reagent-yeast. Water Res. 2007, 41, 1103–1109. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Sato, T. Characteristics and Applications of clays. Clay Sci. 2001, 41, 26–33. (In Japanese) [Google Scholar]
- Iimura, K. Adsorption of Mechyren blue by various clays and zeorite. Clay Sci. 1966, 6, 14–21. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Liu, L.; Tan, S.J.; Horikawa, T.; Do, D.D.; Nicholson, D.; Liu, J. Water adsorption on carbon—A review. Adv. Colloid Interface Sci. 2017, 250, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Kamemoto, T.; Yano, M.; Harano, Y. Phenol Adsorption on Active Carbon in Liquid Phase. Kagaku Kogaku Ronbunshu 1977, 3, 289–295. (In Japanese) [Google Scholar] [CrossRef]


| Component | Raw Materials | |
|---|---|---|
| Clay (Mass %) | 40% GF/GFRP (Mass %) | |
| SiO2 | 65.8 | 54.9 |
| Al2O3 | 21.9 | 16.3 |
| Fe2O3 | 4.79 | 0.77 |
| K2O | 3.37 | 0.15 |
| MgO | 1.67 | - |
| CaO | 1.31 | 26.7 |
| TiO2 | 0.87 | 0.56 |
| Others | 0.29 | 0.62 |
| No. | Samples | Mixing Ratio of GFRP (Mass %) | Firing Conditions | Particle Size of Ceramic Specimens (mm) |
|---|---|---|---|---|
| 1 | Clay | 0 | Oxidatively fired at 1073 K | 0.5–1.0 1.0–1.4 1.4–2.0 |
| 2 | 20% GFRP/clay | 20 | ||
| 3 | 40% GFRP/clay | 40 | ||
| 4 | 60% GFRP/clay | 60 | ||
| 5 | 20% GFRP/clay | 20 | Reductively fired at 1073 K | |
| 6 | 40% GFRP/clay | 40 | ||
| 7 | 60% GFRP/clay | 60 | ||
| Clay particle size: ~0.3 mm, GFRP particle size: ~0.5 mm | ||||
| Component | Oxidatively Fired | Reductively Fired | |||||
|---|---|---|---|---|---|---|---|
| Clay | 20% GFRP/Clay | 40% GFRP/Clay | 60% GFRP/Clay | 20% GFRP/Clay | 40% GFRP/Clay | 60% GFRP/Clay | |
| SiO2 | 63.5 | 62.6 | 59.1 | 50.0 | 62.2 | 61.2 | 56.2 |
| Al2O3 | 23.9 | 22.1 | 20.7 | 17.7 | 18.5 | 9.13 | 4.79 |
| Fe2O3 | 5.13 | 4.87 | 4.16 | 4.09 | 6.13 | 7.56 | 7.34 |
| K2O | 3.76 | 3.26 | 2.91 | 2.00 | 3.73 | 3.77 | 3.11 |
| MgO | 1.79 | 1.66 | 1.75 | 1.51 | 2.24 | 2.43 | 2.14 |
| CaO | 0.51 | 4.02 | 9.93 | 23.2 | 5.34 | 12.9 | 22.7 |
| TiO2 | 1.11 | 0.86 | 0.80 | 1.03 | 1.21 | 1.56 | 1.49 |
| Others | 0.27 | 0.58 | 0.71 | 0.45 | 0.65 | 1.46 | 2.22 |
| No. | Samples | Apparent Porosity (%) | Specific Surface Area (m2/g) | Carbon Content (%) |
|---|---|---|---|---|
| 1 | Clay (Oxidatively fired) | 31.9 | 11.0 | 0.06 |
| 2 | 20% GFRP/clay (Oxidatively fired) | 38.2 | 7.05 | 0.24 |
| 3 | 40% GFRP/clay (Oxidatively fired) | 52.7 | 5.74 | 0.25 |
| 4 | 60% GFRP/clay (Oxidatively fired) | 62.9 | 2.83 | 0.26 |
| 5 | 20% GFRP/clay (Reductively fired) | 43.1 | 14.9 | 0.85 |
| 6 | 40% GFRP/clay (Reductively fired) | 53.8 | 14.2 | 0.99 |
| 7 | 60% GFRP/clay (Reductively fired) | 66.2 | 11.3 | 1.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasui, K.; Sasaki, K.; Ikeda, N.; Kinoshita, H. Dye Adsorbent Materials Based on Porous Ceramics from Glass Fiber-Reinforced Plastic and Clay. Appl. Sci. 2019, 9, 1574. https://doi.org/10.3390/app9081574
Yasui K, Sasaki K, Ikeda N, Kinoshita H. Dye Adsorbent Materials Based on Porous Ceramics from Glass Fiber-Reinforced Plastic and Clay. Applied Sciences. 2019; 9(8):1574. https://doi.org/10.3390/app9081574
Chicago/Turabian StyleYasui, Kentaro, Koya Sasaki, Naoya Ikeda, and Hiroyuki Kinoshita. 2019. "Dye Adsorbent Materials Based on Porous Ceramics from Glass Fiber-Reinforced Plastic and Clay" Applied Sciences 9, no. 8: 1574. https://doi.org/10.3390/app9081574
APA StyleYasui, K., Sasaki, K., Ikeda, N., & Kinoshita, H. (2019). Dye Adsorbent Materials Based on Porous Ceramics from Glass Fiber-Reinforced Plastic and Clay. Applied Sciences, 9(8), 1574. https://doi.org/10.3390/app9081574





