Iridescent Techniques in Ceramics: Physico-Chemical Analysis and Colorimetric Characterization of the Headquarters of the Botín Foundation in Santander
Abstract
Featured Application
Abstract
1. Introduction
2. Iridescent and Pearl Ceramic Techniques
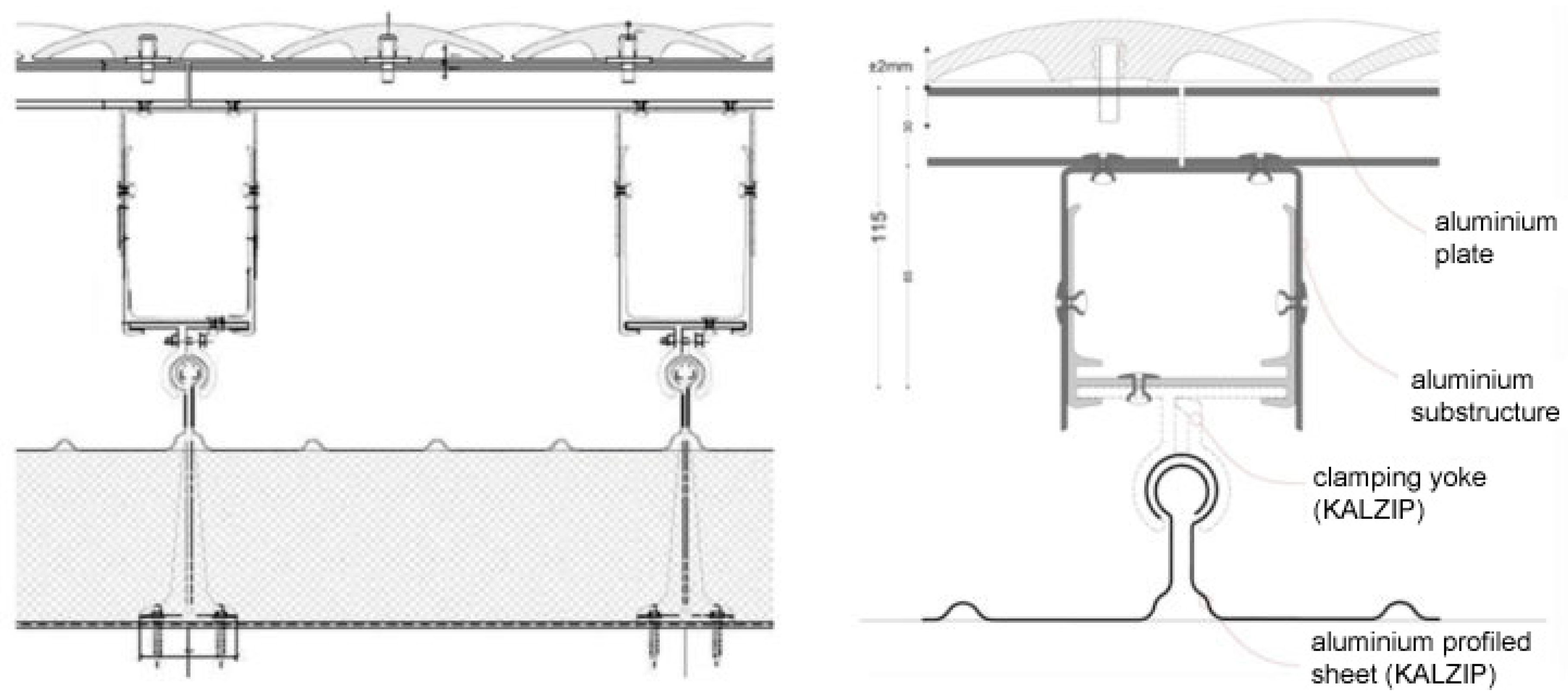
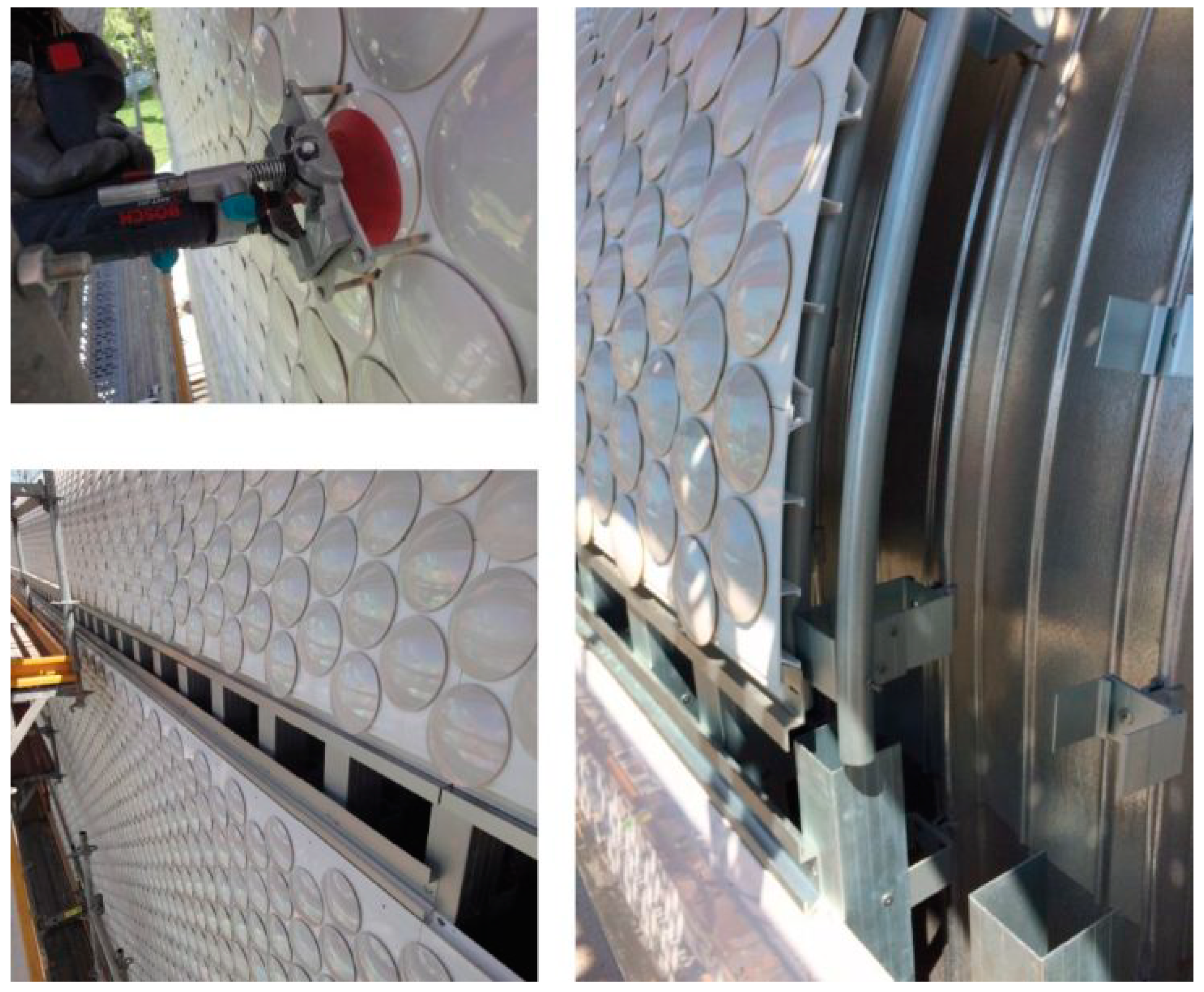
3. Botín Foundation Headquarters in Santander. Ceramic and Light Reflection
- -
- Anodized aluminum clamps: mechanical fixing and leveling system.
- -
- Metallic substructure of anodized aluminum profiles which are rectangular-shaped in the straight sections and circular-shaped in the curved sections.
- -
- Continuous lining of aluminum ribbed sheets, produced by extrusion, with a seaside marine environment coating, mechanized using a thermal-friction system based on computer-aided design and computer aided manufacturing (CAD-CAM) system parameters, designed and programmed by Disset for this enclosure.
- -
- Circular ceramic tiles, 156 mm in diameter, produced by Cumella Ceramics, mechanized using an aluminum anchor threaded into the aluminum sheets by means of a drill system with a suction cup and a calibrated tightening torque.
- -
- Special aluminum tiles with a seaside coating for expansion joints, deck registers, evacuation as well as rainwater collection channels and roof anchors.
- -
- Profiled aluminum sheet finishing with seaside coating, around the enclosure’s perimeter.
4. Description of the Ceramic Tile Manufacturing Process
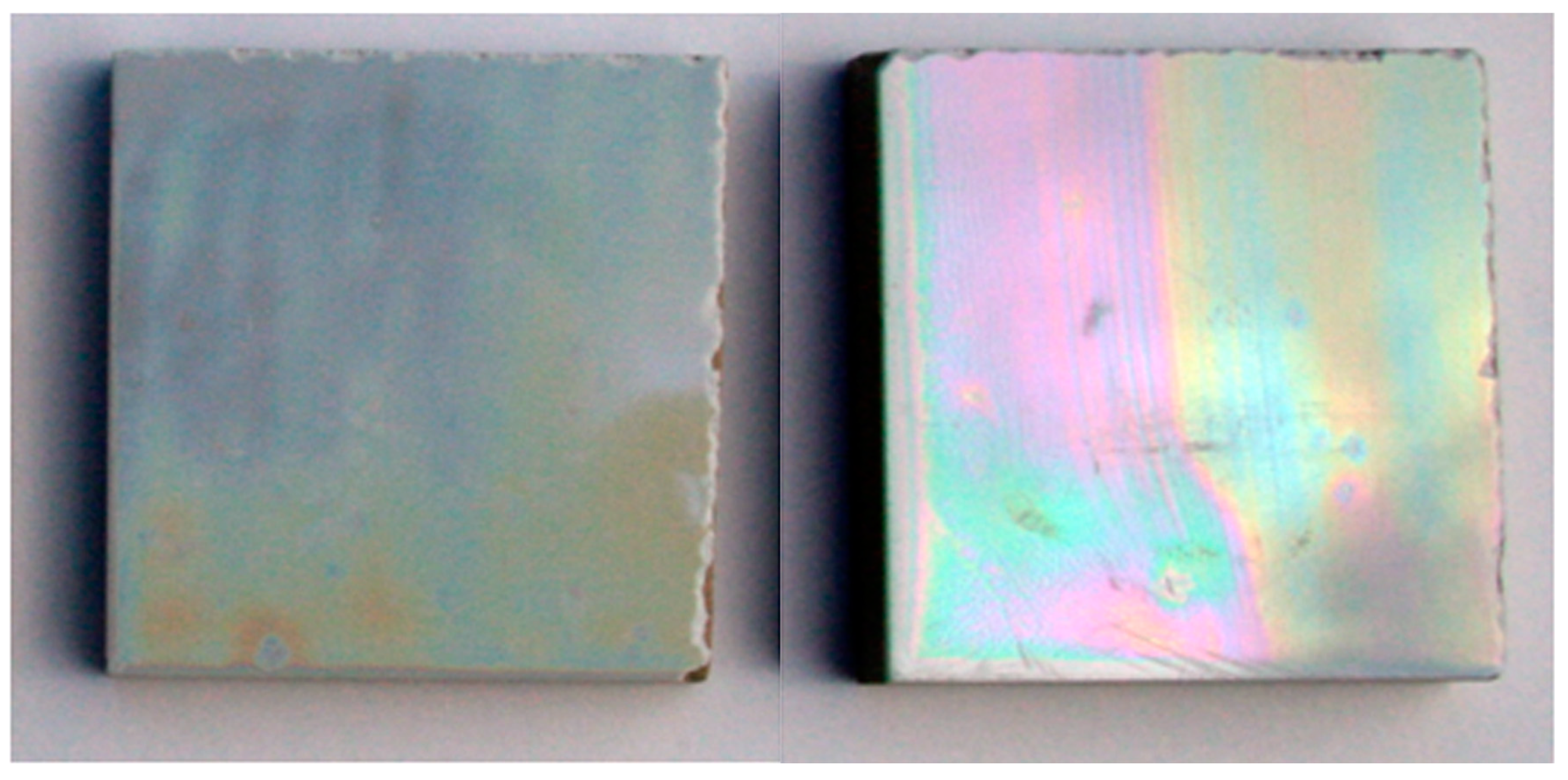
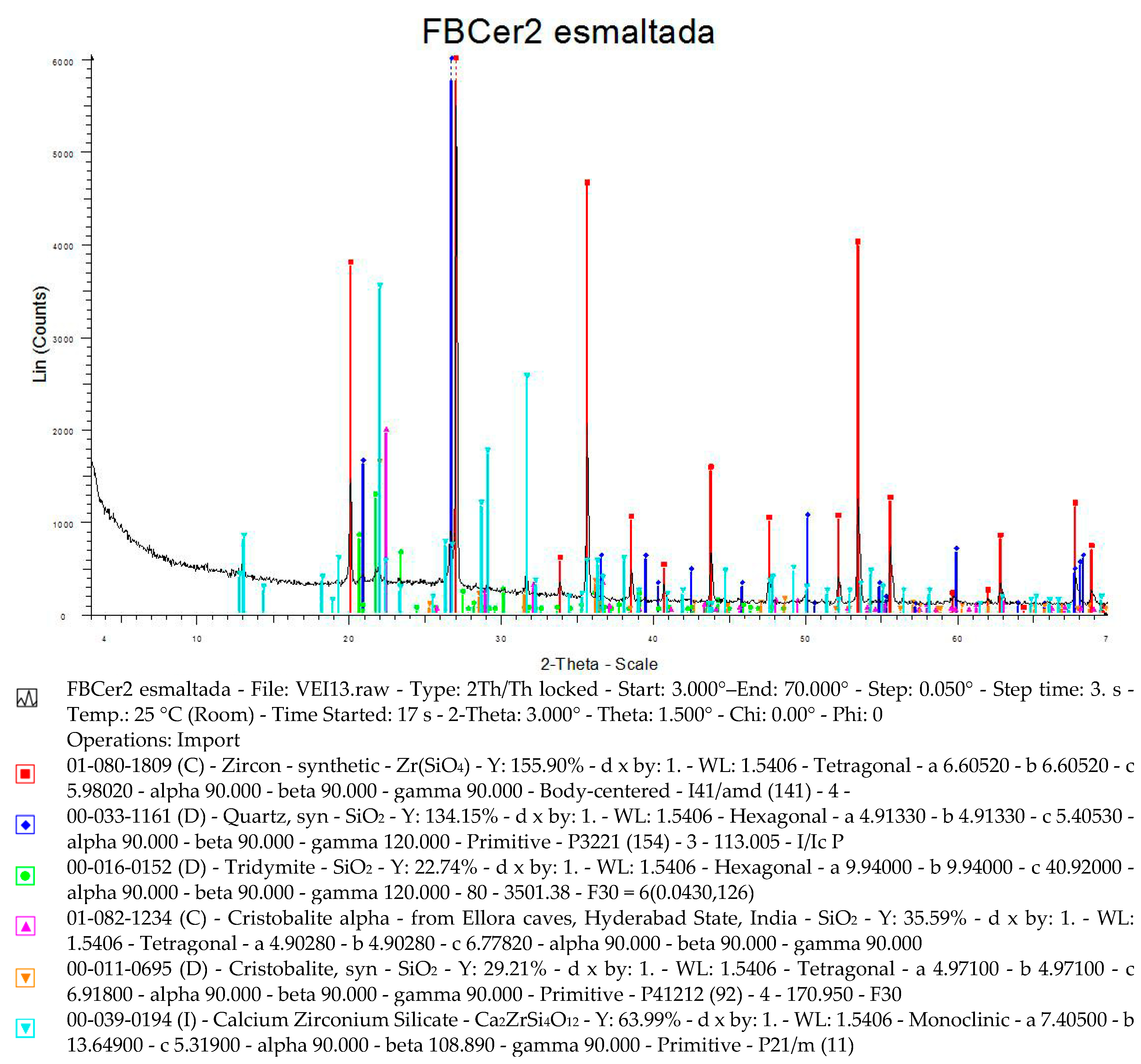
5. Physical-Chemical Analysis of the Ceramic Tiles
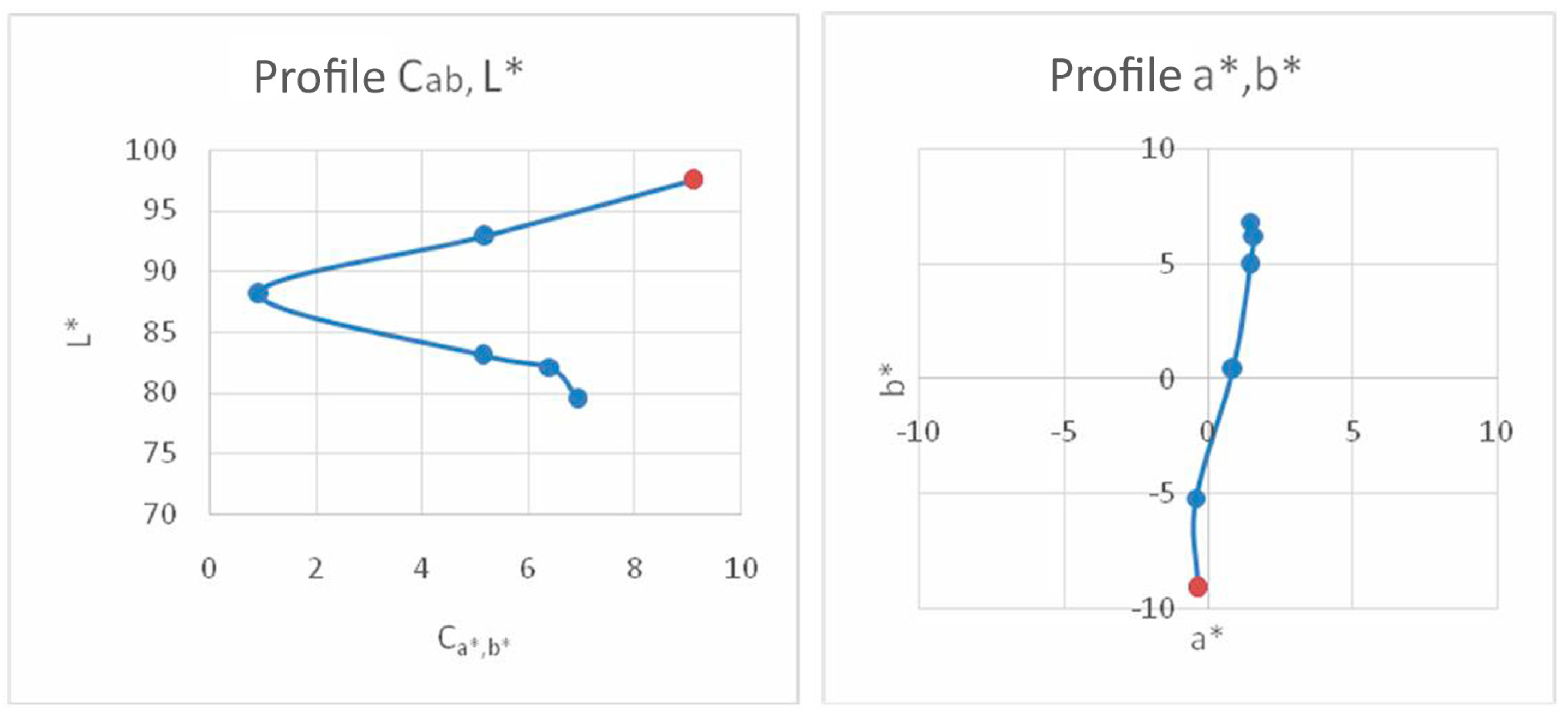
6. Colorimetric Study
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salazar, J.; Sakamoto, T. (Eds.) Rhythms, Cycles, Performances-Ceramics in Architecture; ASCER; ACTAR Publishers: Barcelona, Spain, 2010; ISBN 978-8461394050. [Google Scholar]
- Delbene, G. Public Private Ephemeral: Ceramics in Architecture; ASCER; ACTAR Publishers: Barcelona, Spain, 2008; ISBN 978-8461215096. [Google Scholar]
- Norberg-Schulz, Ch. Arquitectura Occidental; Gustavo Gili: Barcelona, Spain, 1973; ISBN 8425218055. [Google Scholar]
- Benevolo, L. Storia Dell’architettura Moderna; Editori Laterza: Bari, Italy, 1981; ISBN 9788842086222. [Google Scholar]
- Ceramic Floor and Wall Tiles. Innovation, Avant-Garde and Sustainability in Public and Private Spaces. Report. Available online: http://www.promateriales.com/pdf/pm2209.pdf (accessed on 14 December 2018).
- Cumella, A. Cerámics for Architecture, Granollers. Available online: http://www.cumella.cat/projectes/contemporanis/ (accessed on 16 December 2018).
- Derby, B. Inkjet printing ceramics: From drops to solid. J. Eur. Ceram. Soc. 2011, 31, 2543–2550. [Google Scholar] [CrossRef]
- Grespania. Technical Solutions. H&C Tiles. TiO2 Coating That Reduces Environmental Pollution. Available online: https://www.grespania.com/baldosas-autolimpiables-anticontaminaci%C3%B3n-h&c-tiles/ref640010es (accessed on 9 January 2019).
- Pérez-Monserrat, E.M.; Fort, R.; Lopez-Arce, P.; Alvarez de Buergo, M.; Varas-Muriel, M.J. Contribution of analytical techniques to determine the technologies used in the ceramic materials from the Former Workers Hospital of Maudes, Madrid (Spain). J. Eur. Ceram. Soc. 2013, 33, 479–491. [Google Scholar] [CrossRef][Green Version]
- Casasola, R.; Rincón, J.M.; Romero, M. Glass–ceramic glazes for ceramic tiles: A review. J. Mater. Sci. 2012, 47, 553–582. [Google Scholar] [CrossRef]
- Pradell, T.; Pavlov, R.S.; Gutiérrez, P.C.; Climent-Font, A.; Molera, J. Composition, nanostructure, and optical properties of silver and silvercopper lusters. J. Appl. Phys. 2012, 112, 054307. [Google Scholar] [CrossRef]
- Bobin, O.; Schvoerer, M.; Miane, J.L.; Fabre, J.F. Colored metallic shine associated to luster decoration of glazed ceramics: A theoretical analysis of the optical properties. J. Non-Cryst. Solids 2003, 332, 28–34. [Google Scholar] [CrossRef]
- Ding, H.-Y.; Li, H.; Wang, G.-Q.; Liu, T.; Zhou, G.-H. Bio-Corrosion Behavior of Ceramic Coatings Containing Hydroxyapatite on Mg-Zn-Ca Magnesium Alloy. Appl. Sci. 2018, 8, 569. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Lin, B.; Liu, J.-J.; Song, X.-F.; Key, J. An Experimental Study on Mechanical Modeling of Ceramics Based on Microstructure. Appl. Sci. 2015, 5, 1337–1349. [Google Scholar] [CrossRef]
- Llusar, M.; Rodrigues, C.; Labrincha, J.; Flores, M.; Monrós, G. Reinforcement of single-firing ceramic glazes with the addition of polycrystalline tetragonal zirconia (3Y–TZP) or zircon. J. Eur. Ceram. Soc. 2002, 22, 639–652. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Fan, Y. Solar spectral optical properties of rutile TiO2 coated mica-titania pigments. Dyes Pigments 2014, 109, 90–95. [Google Scholar] [CrossRef]
- Yuan, L.; Han, A.; Ye, M.; Chen, X.; Ding, C.; Yao, L. Synthesis and characterization of novel non-toxic BiFe1−xAlxO3/mica-titania pigments with high NIR reflectance. Ceram. Int. 2017, 43, 16488–16494. [Google Scholar] [CrossRef]
- Kaya, S.Y.; Karasu, B. Process parameters determination of phosphorescent pigment added, frit-based wall tiles vetrosa decorations. Ceram. Int. 2012, 38, 2757–2766. [Google Scholar] [CrossRef]
- Chen, T.; Zha, J.; Zhang, X.; Hu, X.; Jiang, W.; Xie, Z.; Jiang, W. Synthesis and characterization of PrxZr1-xSiO4 (x = 0–0.08) yellow pigments via non-hydrolytic sol-gel method. J. Eur. Ceram. Soc. 2018, 38, 4568–4575. [Google Scholar] [CrossRef]
- Snyders, E.; Potgieter, J.H.; Nel, J.T. The effect of milling and percentage dissociation of plasma dissociated zircon on the colour of Pr-yellow and V-blue zircon pigments. J. Eur. Ceram. Soc. 2006, 26, 1599–1603. [Google Scholar] [CrossRef]
- Gao, Y.F.; Zhao, F.; Liu, Y.; Luo, H.J. Synthesis and characterization of ZrO2 capsules and crystalline ZrO2 thin layers on Fe2O3 powders. CrystEngComm 2011, 13, 3511–3514. [Google Scholar] [CrossRef]
- Cavalcante, P.; Dondi, M.; Guarini, G.; Raimondo, M.; Baldi, G. Colour performance of ceramic nano-pigments. Dyes Pigments 2009, 80, 226–232. [Google Scholar] [CrossRef]
- Jovaní, M.; Domingo, M.; Machado, T.R.; Longo, E.; Beltrán-Mir, H.; Cordoncillo, E. Pigments based on Cr and Sb doped TiO2 prepared by microemulsionmediated solvothermal synthesis for inkjet printing on ceramics. Dyes Pigments 2015, 116, 106–113. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, X.; Chen, T.; Liu, J.; Zhang, X. Preparation and chromatic properties of C@ZrSiO4 inclusion pigment via non-hydrolytic sol-gel method. Dyes Pigments 2015, 114, 55–59. [Google Scholar] [CrossRef]
- Badenes, J.A.; Llusar, M.; Tena, M.A.; Calbo, J.; Monrós, G. Praseodymium-doped cubic Ca–ZrO2 ceramic stain. J. Eur. Ceram. Soc. 2020, 22, 1981–1990. [Google Scholar] [CrossRef]
- Ozel, E.; Turan, S. Production of coloured zircon pigments from zircon. J. Eur. Ceram. Soc. 2007, 27, 1751–1757. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Jiang, W.; Liu, J.; Jiang, W.; Xie, Z. Synthesis and application of C@ZrSiO4 inclusion ceramic pigment from cotton cellulose as a colorant. J. Eur. Ceram. Soc. 2016, 36, 1811–1820. [Google Scholar] [CrossRef]
- Colomban, P. The use of metal nanoparticles to produce yellow, red and iridescent colour, from bronze age to present times in lustre pottery and glass: Solid state chemistry, spectroscopy and nanostructure. J. Nano Res. 2009, 8, 109–132. [Google Scholar] [CrossRef]
- Fermo, P.; Padeletti, G. The Use of Nano-Particles to Produce Iridescent Metallic Effects on Ancient Ceramic Objects. J. Nanosci. Nanotechnol. 2012, 12, 1–6. [Google Scholar] [CrossRef]
- Barbera, G.; Barone, G.; Crupi, V.; Longo, F.; Majolino, D.; Mazzoleni, P.; Sabatino, G.; Tanasi, D.; Venuti, V. Study of Late Roman and Byzantine glass by the combined use of analytical techniques. J. Non-Cryst. Solids 2012, 358, 1554–1561. [Google Scholar] [CrossRef]
- Garofano, I.; Robador, M.D.; Perez-Rodriguez, J.L.; Castaing, J.; Pacheco, C.; Duran, A. Ceramics from the Alcazar Palace in Seville (Spain) dated between the 11th and 15th centuries: Compositions, technological features and degradation processes. J. Eur. Ceram. Soc. 2015, 35, 4307–4319. [Google Scholar] [CrossRef]
- Holakooei, P.; Senna, C.A.; Vasconcelos, T.L.; Archanjo, B.S.; Achete, C.A.; Abed-Esfahani, A.; Molera, J. Flashed copper and silver luster nano-structures: Characterization and technology. Ceram. Int. 2016, 42, 7757–7766. [Google Scholar] [CrossRef]
- Roque, J.; Molera, J.; Sciau, P.; Pantos, E.; Vendrell-Saz, M. Copper and silver nanocrystals in lustre lead glazes: Development and optical properties. J. Eur. Ceram. Soc. 2006, 26, 3813–3824. [Google Scholar] [CrossRef]
- Pradell, T.; Fernandes, R.; Molina, G.; Smith, A.D.; Molera, J.; Climent-Font, A.; Tite, M.S. Technology of production of Syrian lustre (11th to 13th century). J. Eur. Ceram. Soc. 2018, 38, 2716–2727. [Google Scholar] [CrossRef]
- Hainschwang, T.; Notari, F. The cause of iridescence in rainbow andradite from Nara: Japan. Gems Gemol. 2006, 42, 248–258. [Google Scholar] [CrossRef]
- Fu, J.; Peng, Y.; Tian, X. Pigments Having Angle Dependence of the Interference Colors and Its Production Process. U.S. Patent US8066811B2, 29 November 2011. [Google Scholar]
- Tena, M.A.; Meseguer, S.; Gargori, C.; Forés, A.; Badenes, J.A.; Monrós, G. Study of Cr-SnO2 ceramic pigment and of Ti/Sn ratio on formation and coloration of these materials. J. Eur. Ceram. Soc. 2007, 27, 215–221. [Google Scholar] [CrossRef]
- Cannio, M.; Bondioli, F. Mechanical activation of raw materials in the synthesis of Fe2O3–ZrSiO4 inclusion pigment. J. Eur. Ceram. Soc. 2012, 32, 643–647. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, L.; Xu, X.; Zhao, J.; Eriksson, M.; Zhong, Y.; Adolfsson, E.; Liu, Y.; Kocjan, A. Fractography of self-glazed zirconia with improved reliability. J. Eur. Ceram. Soc. 2017, 37, 4339–4345. [Google Scholar] [CrossRef]
- Cabrera, M.J.; Montins, V.; Solsona, D.; Sala, J.M. Obtención de efectos físico-ópticos para la decoración de baldosas cerámicas. Bol. Soc. Española Cerám. Vidrio 2012, 51, IX–XVI. [Google Scholar] [CrossRef]
- Liu, C.; Yen, M.; Han, A.; Li, J. Structural analysis and characterization of doped spinel Co2_xMxTiO4 (M = Mg2+, Mn2+, Ni2+, Cu2+ and Zn2+) coated mica composite pigments. Ceram. Int. 2015, 41, 5537–5546. [Google Scholar] [CrossRef]
- Jing, Ch.; Xiaobo, S.; Bing, H. The preparation and characteristics of cobalt blue colored mica titania pearlescent pigments by microemulsions. Dyes Pigments 2007, 75, 766–769. [Google Scholar] [CrossRef]
- Bayat, N.; Baghshahi, S.; Alizadeh, P. Synthesis of white pearlescent pigments using the surface response method of statistical analysis. Ceram. Int. 2008, 34, 2029–2035. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Kim, T.G.; Seo, G.S.; Park, J.H.; Suh, C.S.; Park, S.S.; Hong, S.S.; Lee, G.D. Effect of substrate on the phase transformation of TiO2 in pearlescent pigment. J. Ind. Eng. Chem. 2008, 14, 213–218. [Google Scholar] [CrossRef]
- Tenório Cavalcante, P.M.; Dondi, M.; Guarini, G.; Barros, F.M.; Da Luz, A.B. Ceramic application of mica titania pearlescent pigments. Dyes Pigments 2007, 74, 1–7. [Google Scholar] [CrossRef][Green Version]
- Bertaux, S.; Reynders, P.; Heintz, J.M. Sintering of nanocrystalline Ta2O5 and ZrO2 films compared to that of TiO2 films. J. Eur. Ceram. Soc. 2006, 26, 923–932. [Google Scholar] [CrossRef]
- Siligardi, C.; Montecchi, M.; Montorsi, M.; Pasquali, L. Ceria-Containing Frit for Luster in Modern Ceramic Glaze. J. Am. Ceram. Soc. 2010, 93, 2545–2550. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Canovi, L.; Viani, A.; Bertocchi, P.; Corradini, C.; Lassinantti Gualtieri, M.; Gazzadi, G.C.; Zapparoli, M.; Berthierf, S. Mechanism of lustre formation in scheelite-based glazes. J. Eur. Ceram. Soc. 2013, 33, 2055–2064. [Google Scholar] [CrossRef]
- Dondi, M.; Zanelli, C.; Ardit, M.; Cruciani, G.; Mantovani, L.; Tribaudino, M.; Andreozzi, G.B. Ni-free, black ceramic pigments based on Co—Cr—Fe—Mn spinels: A reappraisal of crystal structure, colour and technological behaviour. Ceram. Int. 2013, 39, 9533–9547. [Google Scholar] [CrossRef]
- He, X.; Wang, F.; Liu, H.; Niu, L.; Wang, X. Synthesis and color properties of the TiO2@CoAl2O4 blue pigments with low cobalt content applied in ceramic glaze. J. Am. Ceram. Soc. 2018, 101, 2578–2588. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, M.; Lang, Y.; Wei, H.; Wang, C. Synthesis and chromatic properties of zircon encapsulated ceramic black pigment with carbon sphere as carbon source. J. Eur. Ceram. Soc. 2018, 38, 2218–2227. [Google Scholar] [CrossRef]
- Romero, M.; Rincón, J.M. Surface and Bulk Crystallization of Glass-Ceramic in the Na2O–CaO–ZnO–PbO–Fe2O3–Al2O3–SiO2 System Derived from a Goethite Waste. J. Am. Ceram. Soc. 1999, 82, 1313–1317. [Google Scholar] [CrossRef]
- Colomban, P. Secrets retrouvés du Lustre Abbasside. Rev. Céram. Verre 2004, 139, 13–19. [Google Scholar]
- Nebot-Díaz, I. Nuevas Tecnologías para el Sector Cerámico de Castellón: Desarrollo de Esmaltes Vitrocristalinos y Vitrocerámicos; Universitat Jaume I: Castellón, Spain, 2001. [Google Scholar]
- Pastor, J.Y.; Poza, P.; LLorca, J.; Peña, J.I.; Merino, R.I.; Orera, V.M. Mechanical properties of directionally solidified Al2O3–ZrO2(Y2O3) eutectics. Mater. Sci. Eng. 2001, 308, 241–249. [Google Scholar] [CrossRef]
- Peña, J.I.; Merino, R.I.; de la Fuente, G.F.; Orera, V.M. Aligned ZrO2(c)-CaZrO3 eutectics grown by the laser floating zone method: Electrical and optical properties. Adv. Mater. 1996, 8, 909–912. [Google Scholar] [CrossRef]
- Merino, R.I. Cerámicas eutécticas solidificadas direccionalmente para fotónica y electrocerámica. Rev. Real Acad. Cienc. 2006, 61, 47–86. [Google Scholar]
- Montins Nebot, V.; Cabrera Ibáñez, M.J.; Solsona Monzonis, D.; Sala Iniesta, J.M. Petitioner: Vidres S.A. Coating for Ceramic Bodies. Patent Application nº. 201230096, 29 July 2013. [Google Scholar]
- Vielhaber, L. Tecnología de los Esmaltes; Editorial Reverté: Barcelona, Spain, 1961. [Google Scholar]
- Echarri Iribarren, V.; González Avilés, A.B.; Ródenas, M.; Olivares, J. Cerámica y vibración de la luz. Nuevas técnicas de nacarado e irisado y caracterización colorimétrica. Inform. Constr. 2016, 68, 5–67. [Google Scholar] [CrossRef]
- AENOR-CEN. UNE-EN ISO 10545-3 <0.5%. Baldosas Cerámicas. Parte 3: Determinación de la Absorción de Agua, de la Porosidad Abierta, de la Densidad Relativa Aparente, y de la Densidad Aparente; ISO 10545-3:1995, Incluye CORRIGENDUM Técnico 1:1997; Asociación Española de Normalización (AENOR): Madrid, Spain, 1997. [Google Scholar]
- AENOR-CEN. UNE-EN ISO 10545-4. Baldosas Cerámicas. Parte 4: Determinación de la Resistencia a la Flexión y de la Fuerza de Rotura; ISO 10545-4:2004; Asociación Española de Normalización (AENOR): Madrid, Spain, 2009. [Google Scholar]
- AENOR-CEN. UNE-EN ISO 10545-7. Baldosas Cerámicas. Parte 7: Determinación de la Resistencia a la Abrasión Superficial de las Baldosas Esmaltadas; ISO 10545-7:1996; Asociación Española de Normalización (AENOR): Madrid, Spain, 1999. [Google Scholar]
- AENOR-CEN. UNE-EN ISO 10545-12. Baldosas Cerámicas. Parte 12: DETERMINACIÓN de la Resistencia a la Helada; ISO 10545-12:1995, Incluye Corrigendum Técnico 1:1997; Asociación Española de Normalización (AENOR): Madrid, Spain, 1997. [Google Scholar]
- AENOR-CEN. UNE-EN ISO 10545-13. Baldosas Cerámicas. Parte 13: Determinación de la Resistencia Química; ISO 10545-13:1995; Asociación Española de Normalización (AENOR): Madrid, Spain, 1998. [Google Scholar]
- AENOR-CEN. ISO 10545-9. Baldosas Cerámicas. Parte 9: Determinación de la Resistencia al Choque Térmico; ISO 10545-9:1994; Asociación Española de Normalización (AENOR): Madrid, Spain, 1997. [Google Scholar]
- ASTM E2194-14, Standard Test Method for Multiangle Color Measurement of Metal Flake Pigmented Materials; ASTM International: West Conshohocken, PA, USA, 2014.
- Klein, G.A. Industrial Color Physics; Springer: New York, NY, USA, 2010. [Google Scholar]
- Chorro, E.; Perales, E.; Burgos, F.J.; Gómez, O.; Vilaseca, M.; Viqueira, V.; Pujol, J.; Martínez-Verdú, F.M. The minimum number of measurements for colour, sparkle, and graininess characterisation in gonio-apparent panels. Color. Technol. 2015, 131, 303–309. [Google Scholar] [CrossRef]
- Ferrero, A.; Perales, E.; Rabal, A.; Campos, J.; Martínez-Verdú, F.M.; Chorro, E.; Pons, A. Color representation and interpretation of special effect coatings. J. Opt. Soc. Am. 2014, 32, 436–447. [Google Scholar] [CrossRef]




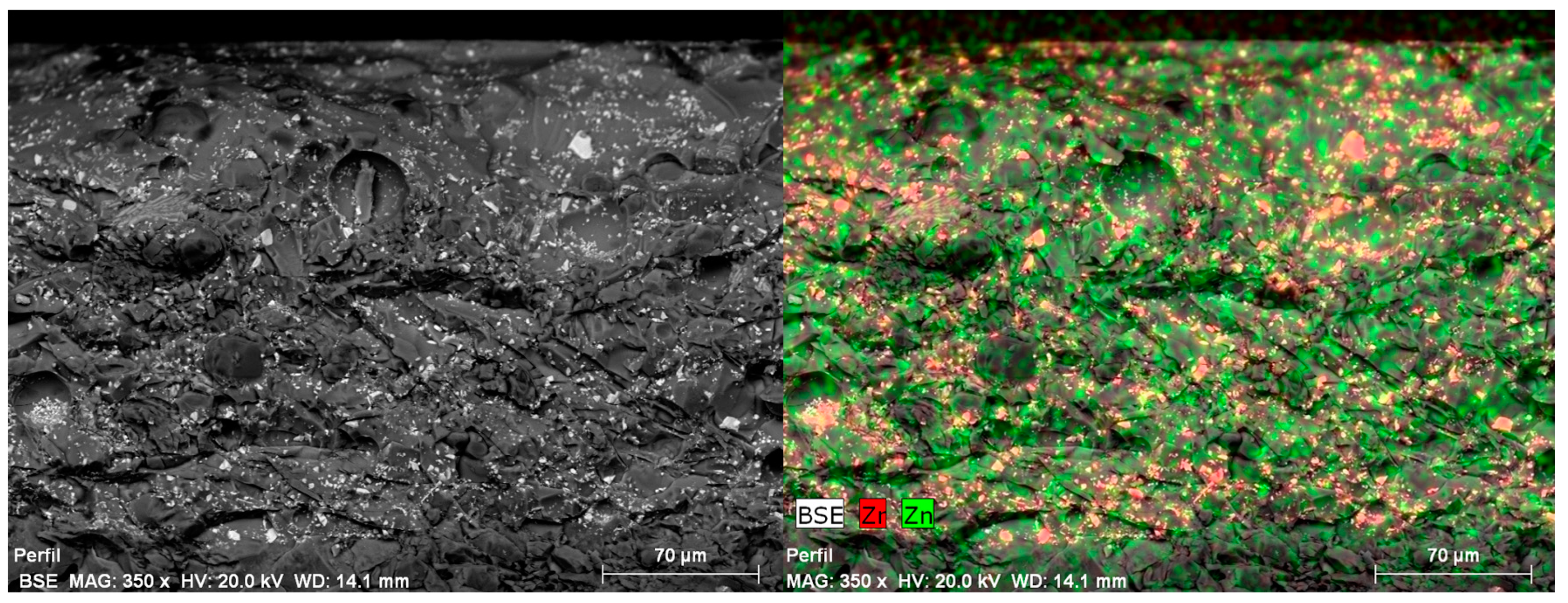
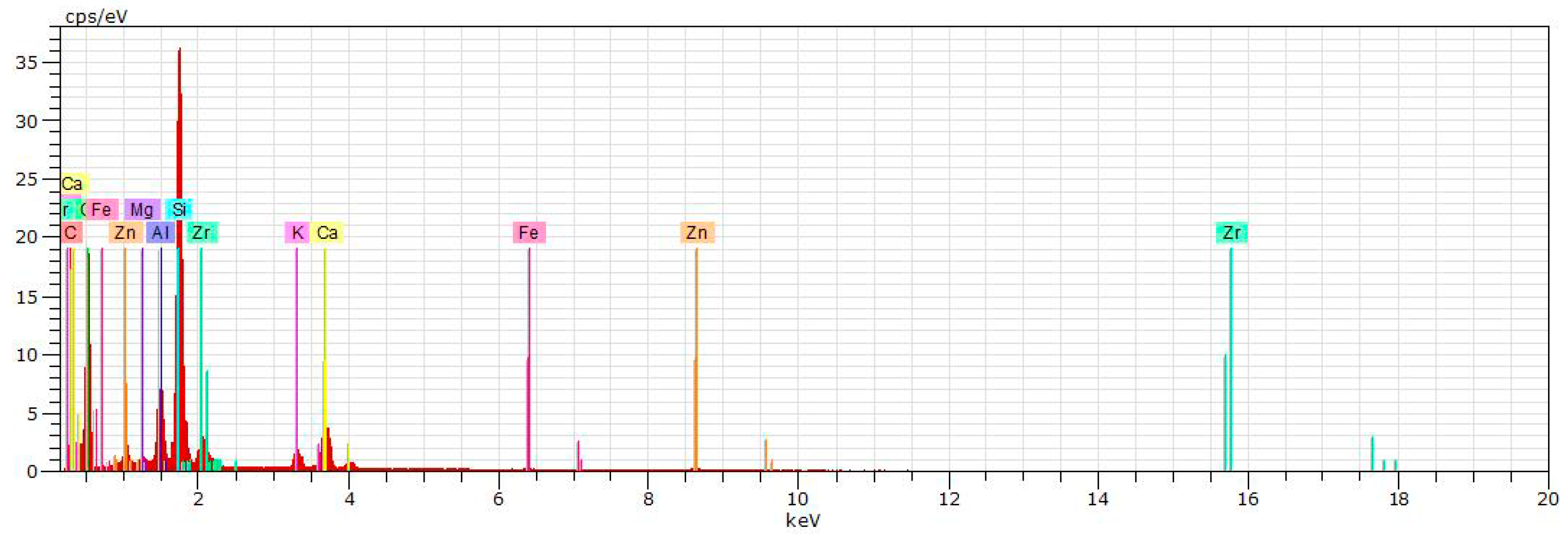
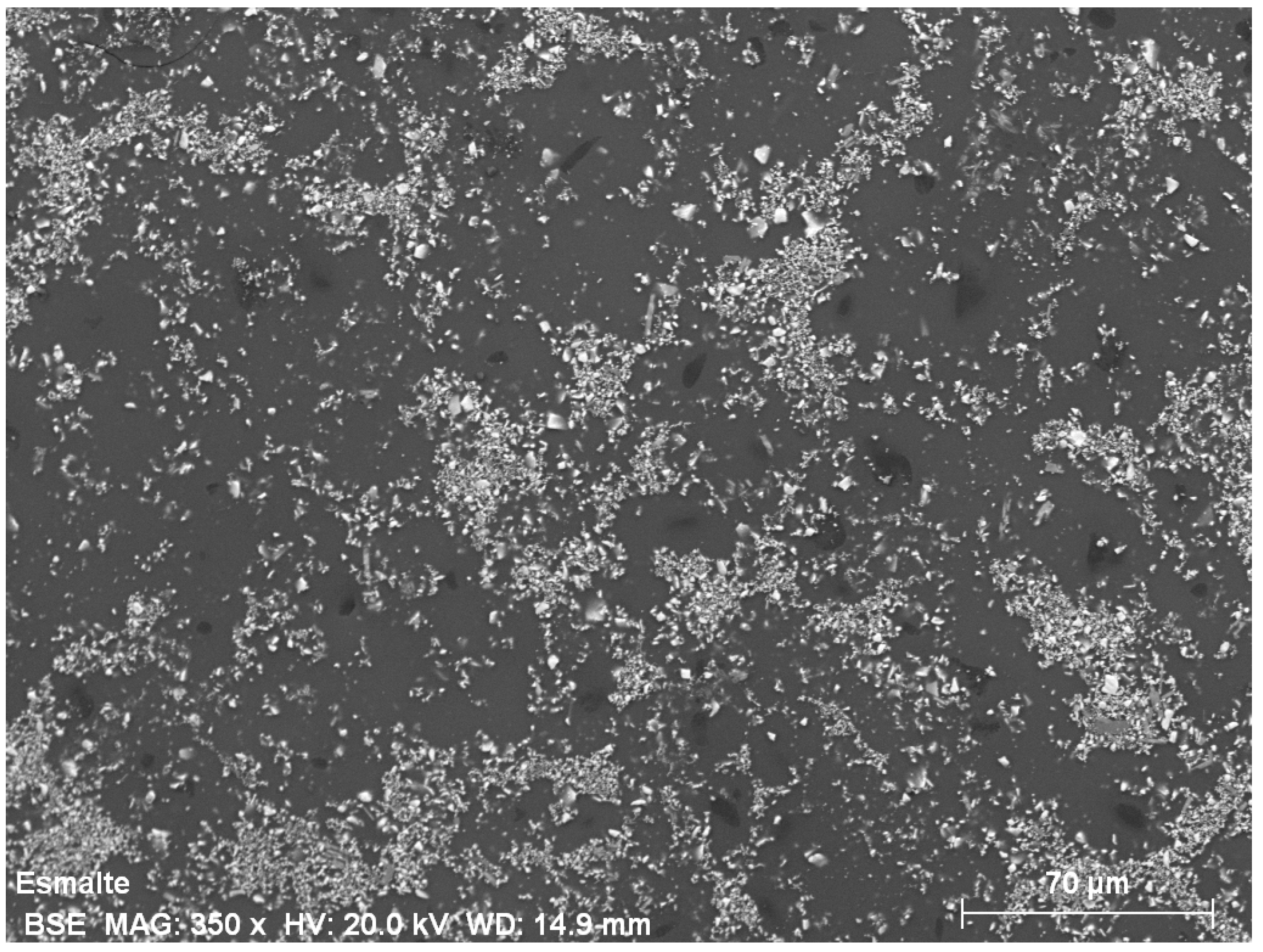
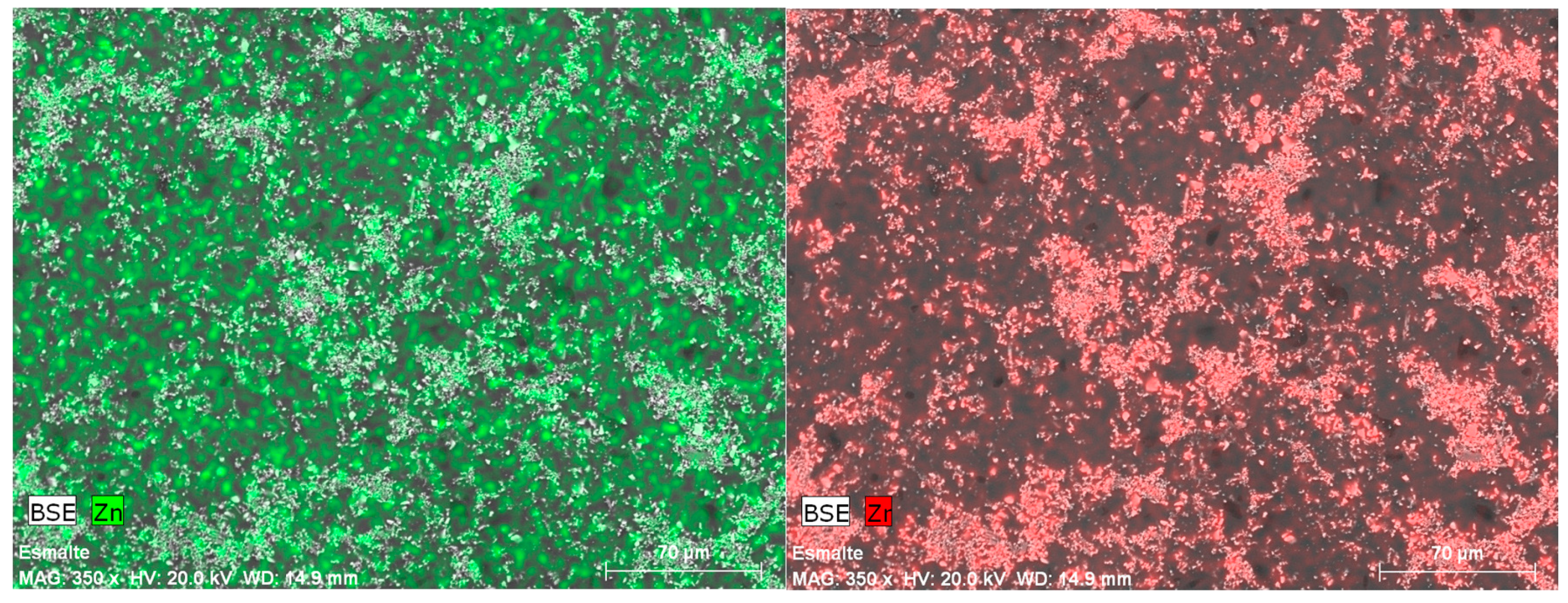
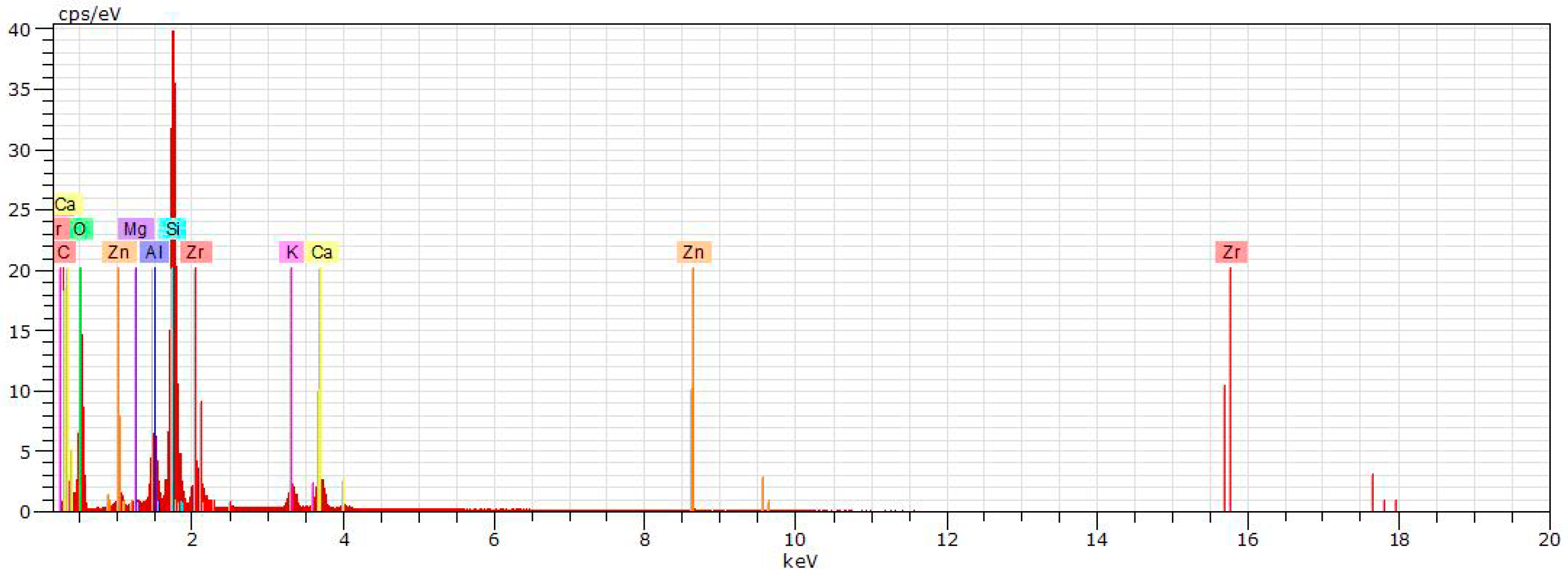

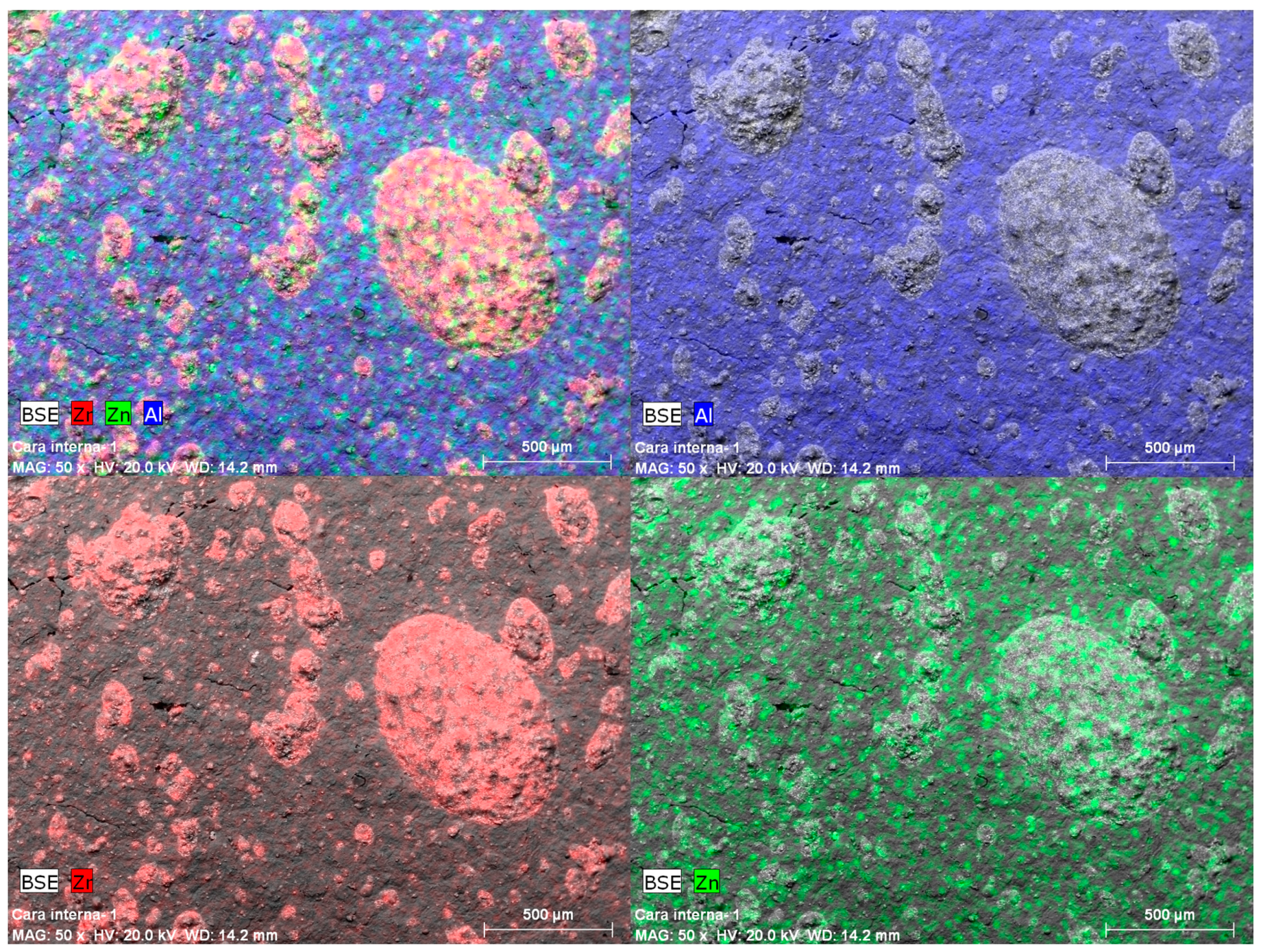
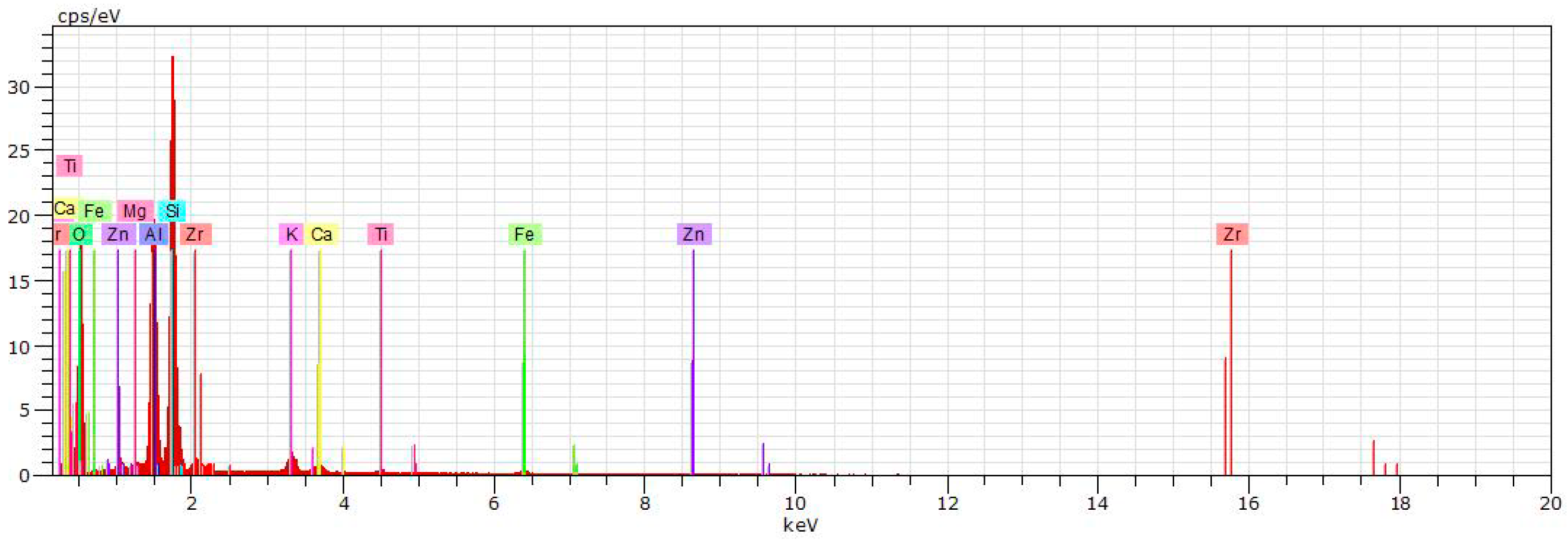
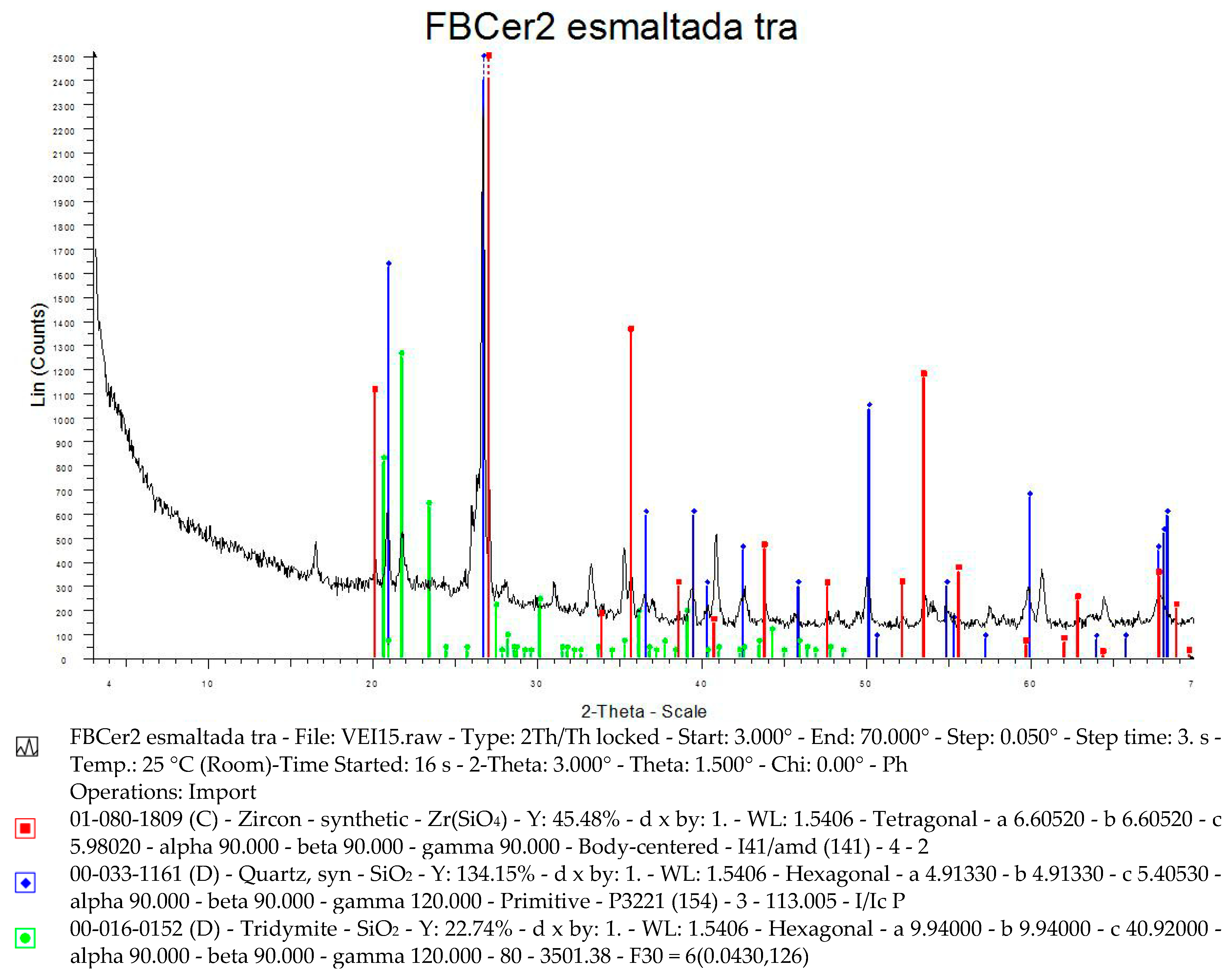
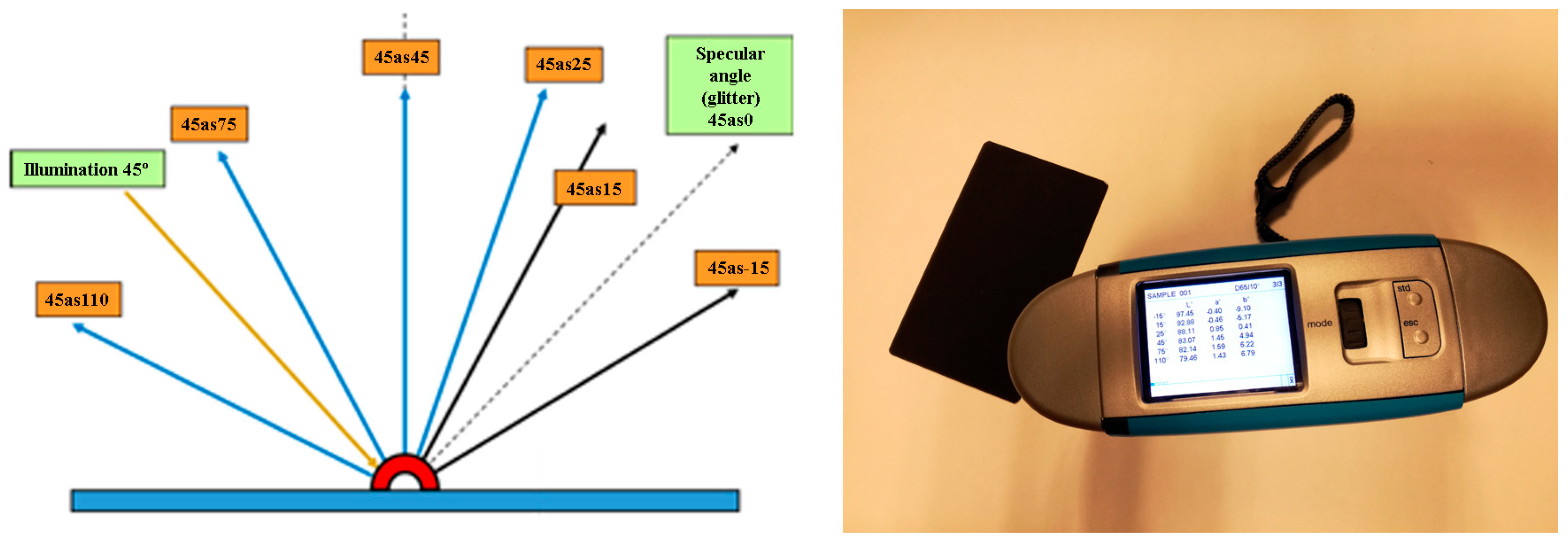
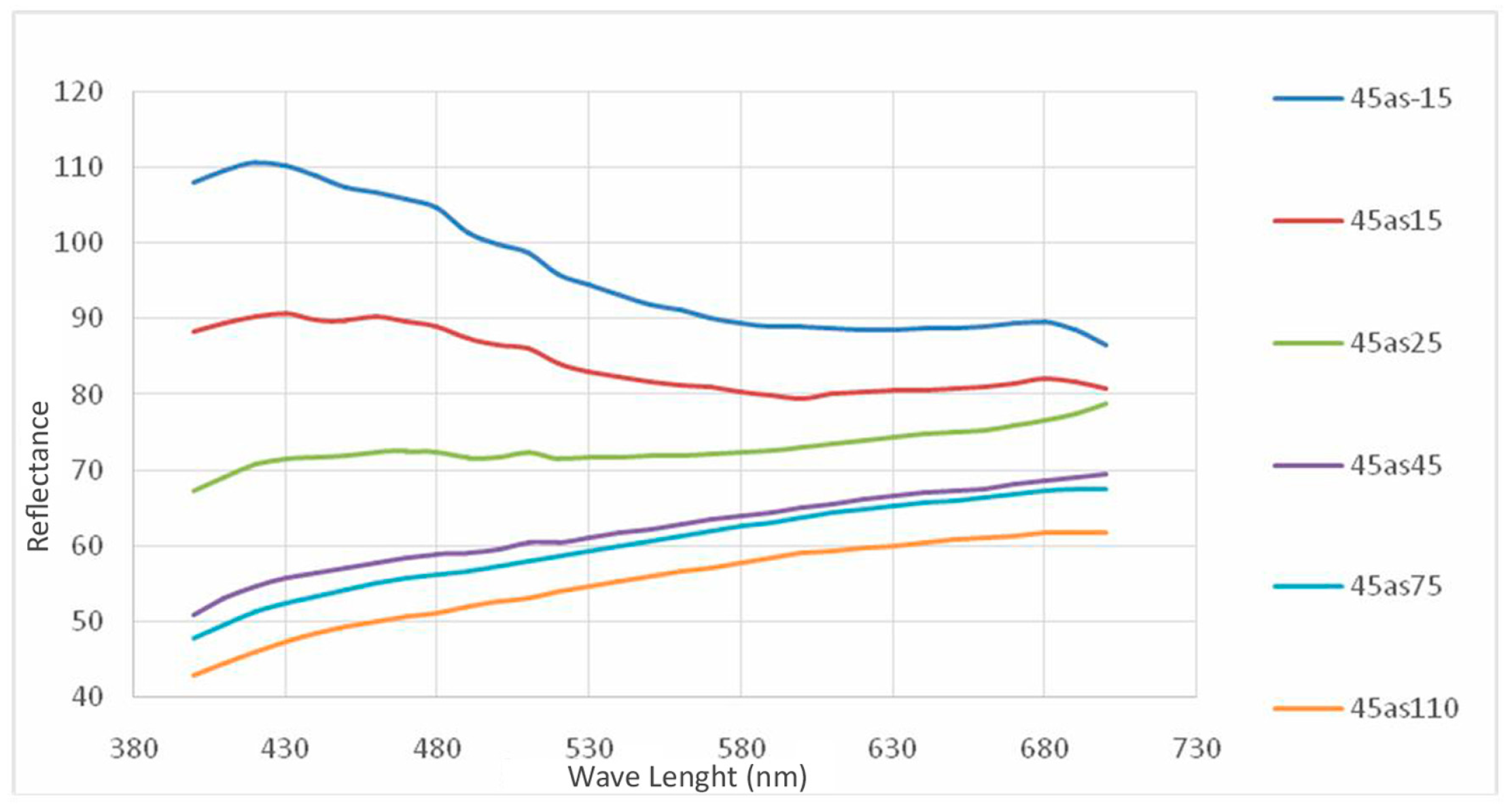
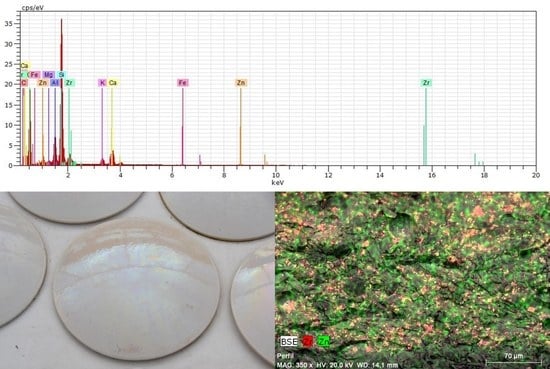
| Norm. C [wt.%] | |||||
|---|---|---|---|---|---|
| SAMPLE 1 Profile | SAMPLE 2 Glaze | SAMPLE 3 Hidden Face (A) | SAMPLE 3 Hidden Face (B) | SAMPLE 4 Interior | |
| C | 14.18 | 4.55 | |||
| O | 52.55 | 47.32 | 52.16 | 50.09 | 51.04 |
| F | 0.41 | ||||
| Mg | 0.63 | 0.30 | 0.55 | 0.56 | 0.99 |
| Al | 3.83 | 4.48 | 15.21 | 12.90 | 16.85 |
| Si | 16.79 | 25.37 | 24.77 | 25.86 | 27.34 |
| K | 1.36 | 2.73 | 1.99 | 2.11 | 1.74 |
| Ca | 4.79 | 4.37 | 0.76 | 1.41 | 0.25 |
| Ti | 0.50 | 0.40 | 0.64 | ||
| Fe | 0.97 | 0.88 | 1.15 | ||
| Zn | 1.21 | 1.02 | 0.13 | 0.38 | |
| Zr | 4.66 | 9.15 | 2.97 | 5.01 | |
| TOTAL | 100% | 100% | 100% | 100% | 100% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echarri-Iribarren, V.; González-Avilés, Á.B.; Viqueira-Pérez, V. Iridescent Techniques in Ceramics: Physico-Chemical Analysis and Colorimetric Characterization of the Headquarters of the Botín Foundation in Santander. Appl. Sci. 2019, 9, 1521. https://doi.org/10.3390/app9081521
Echarri-Iribarren V, González-Avilés ÁB, Viqueira-Pérez V. Iridescent Techniques in Ceramics: Physico-Chemical Analysis and Colorimetric Characterization of the Headquarters of the Botín Foundation in Santander. Applied Sciences. 2019; 9(8):1521. https://doi.org/10.3390/app9081521
Chicago/Turabian StyleEcharri-Iribarren, Víctor, Ángel B. González-Avilés, and Valentín Viqueira-Pérez. 2019. "Iridescent Techniques in Ceramics: Physico-Chemical Analysis and Colorimetric Characterization of the Headquarters of the Botín Foundation in Santander" Applied Sciences 9, no. 8: 1521. https://doi.org/10.3390/app9081521
APA StyleEcharri-Iribarren, V., González-Avilés, Á. B., & Viqueira-Pérez, V. (2019). Iridescent Techniques in Ceramics: Physico-Chemical Analysis and Colorimetric Characterization of the Headquarters of the Botín Foundation in Santander. Applied Sciences, 9(8), 1521. https://doi.org/10.3390/app9081521