Environmental Pollutants Impair Transcriptional Regulation of the Vitellogenin Gene in the Burrowing Mud Crab (Macrophthalmus Japonicus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisms
2.2. Exposure Experiments
2.3. Characterization of the M. Japonicus Vitellogenin (VTG) Genes and Phylogenetic Analysis
2.4. RNA Isolation and mRNA Expression Analysis
2.5. Statistical Analysis
3. Results
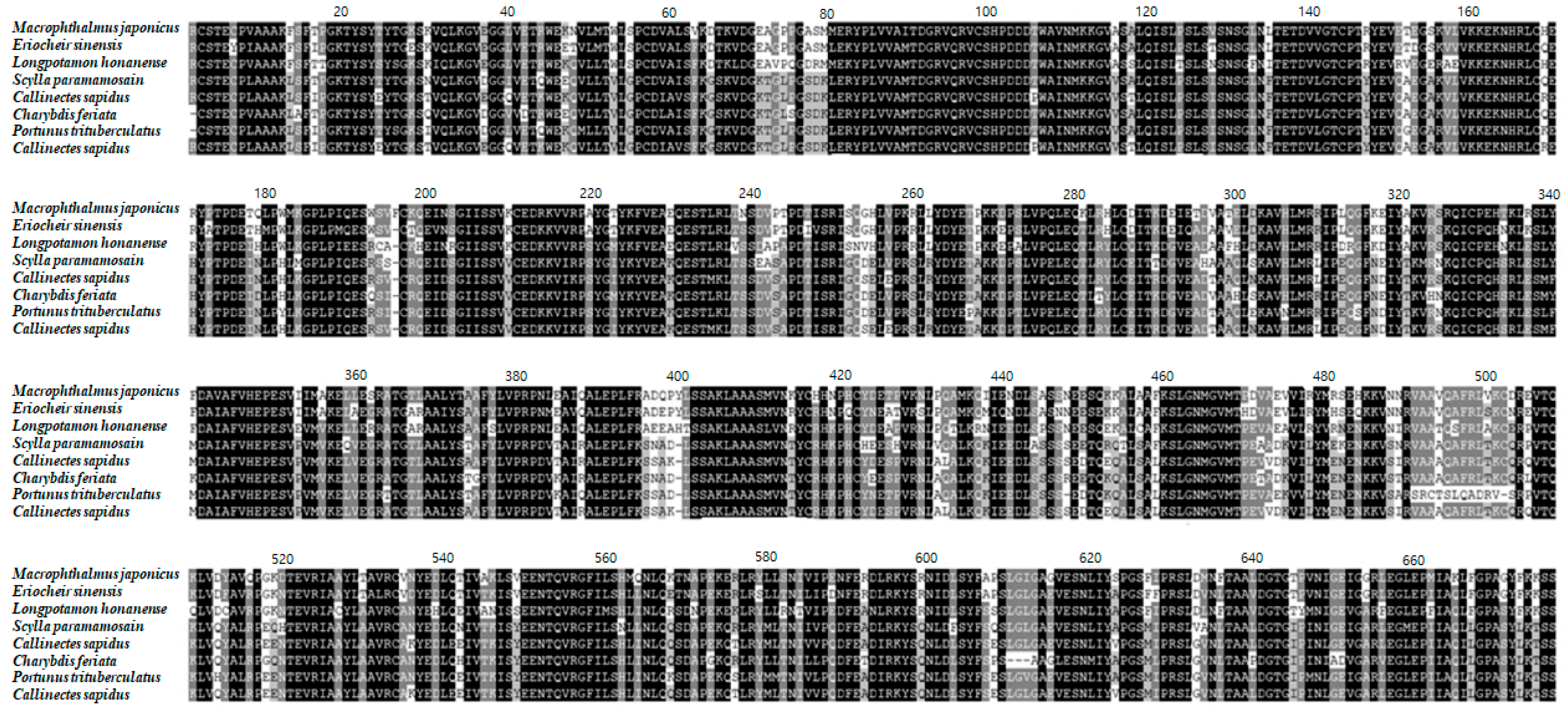
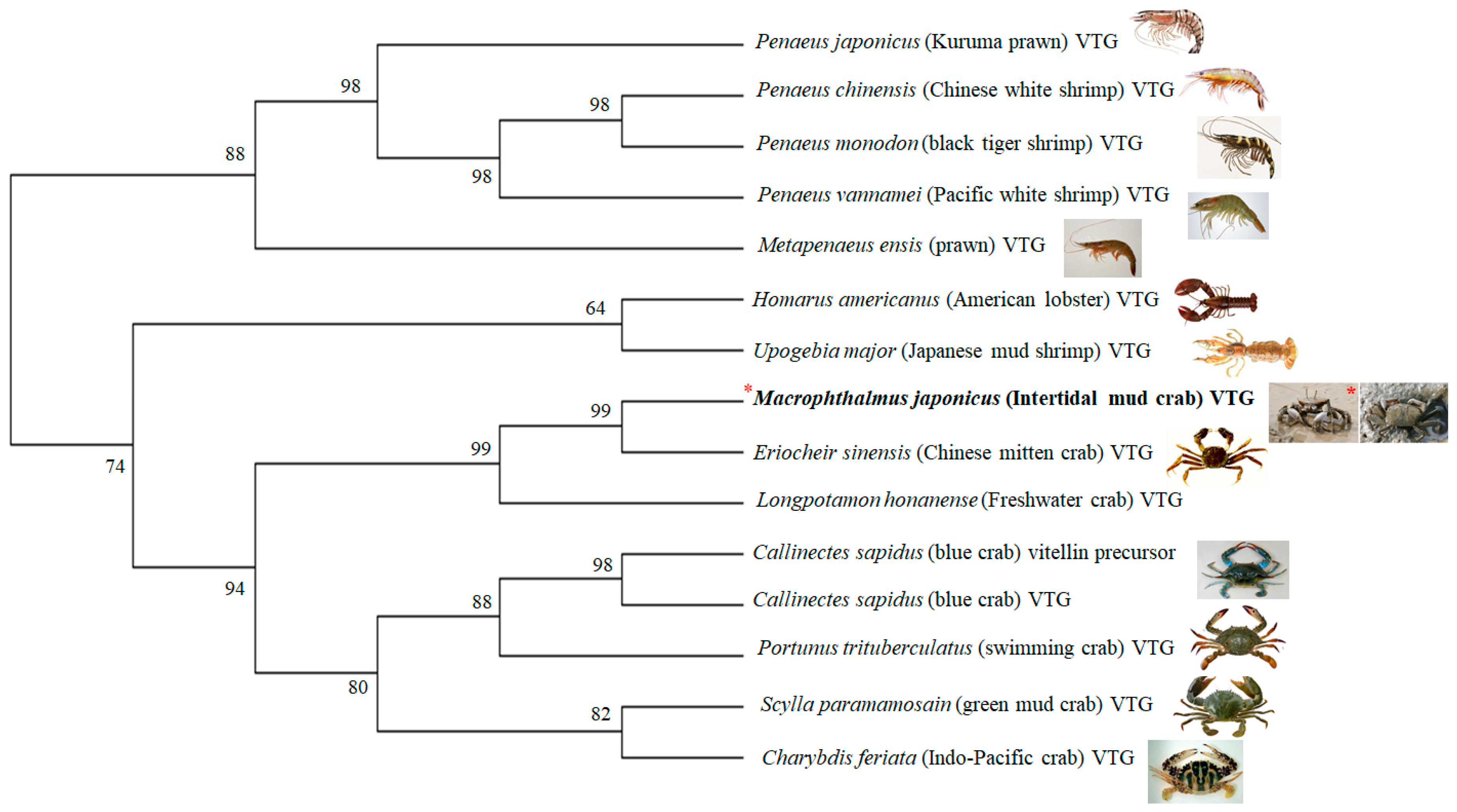
3.1. Characterization of the M. Japonicus VTG Gene
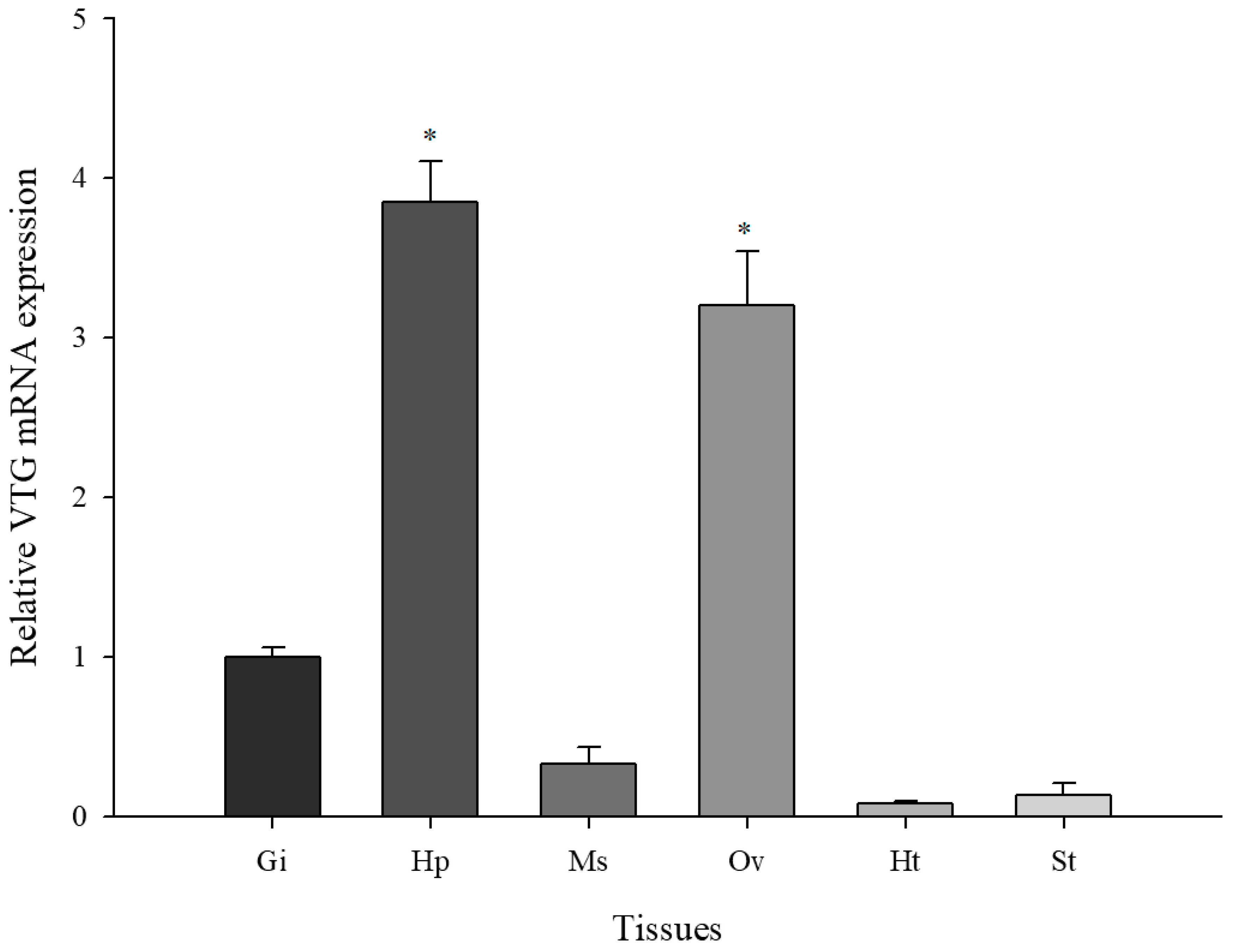
3.2. Basal Levels of M. Japonicus VTG Expression in Multiple Tissues
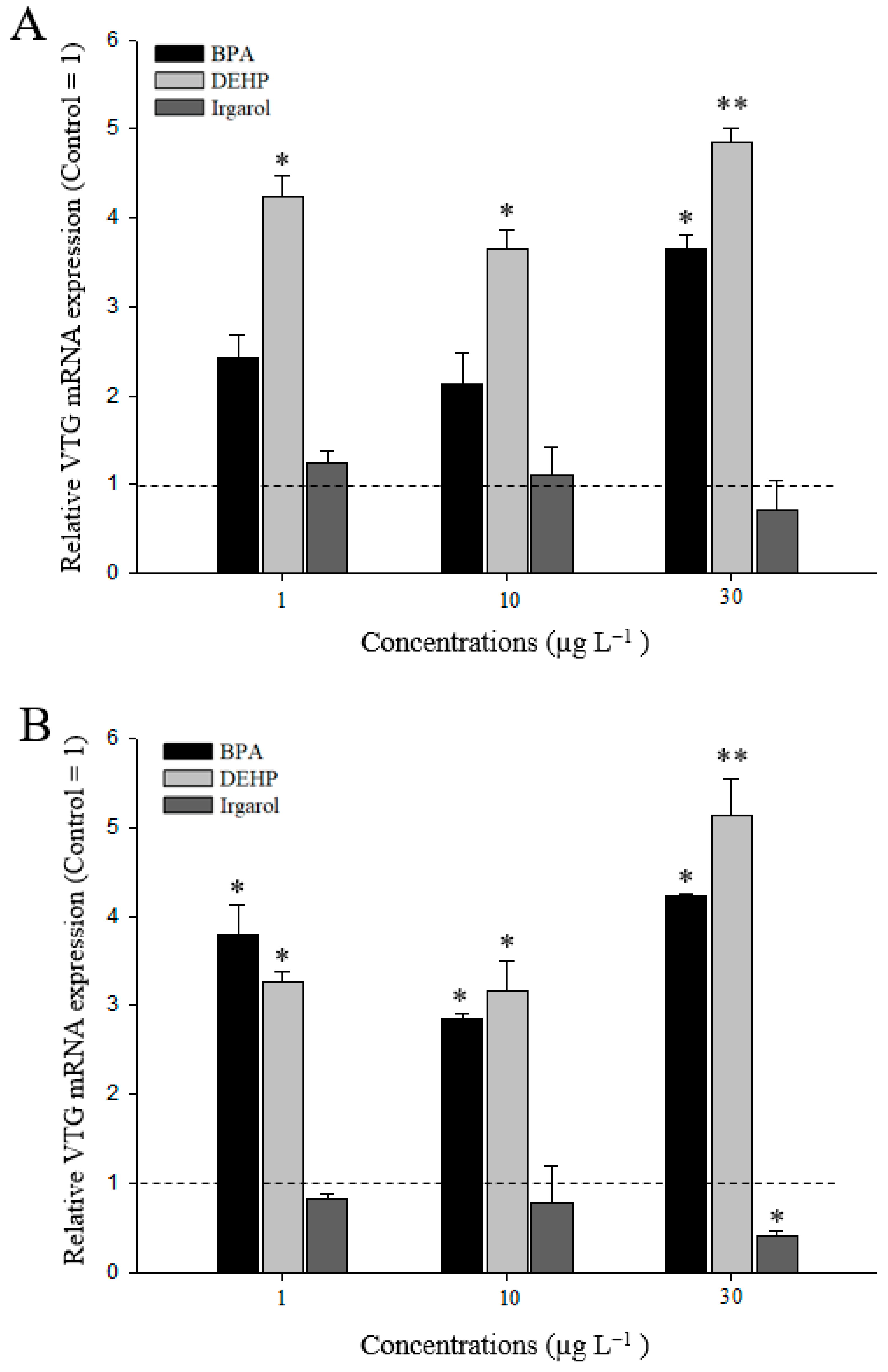
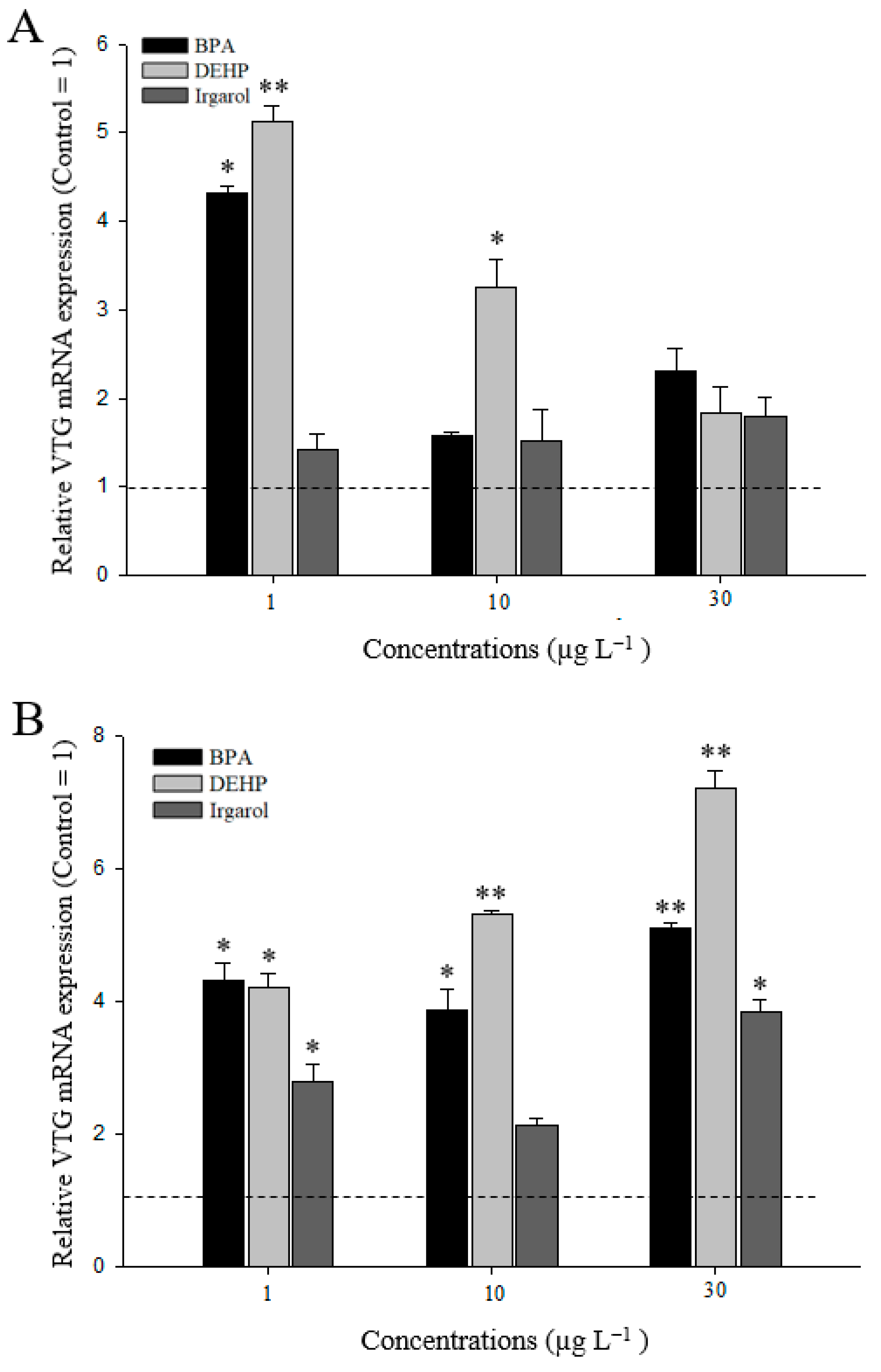
3.3. VTG Gene Expressions in M. Japonicus Ovaries and Hepatopancreas after DEHP, BPA, or Irgarol Exposures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tsukimura, B.; Waddy, S.L.; Vogel, J.M.; Linder, C.J.; Bauer, D.K.; Borst, D.W. Characterization and quantification of yolk proteins in the lobster, Homarus americanus. J. Exp. Zool. 2002, 292, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, H.T.; Dai, Z.M.; Yang, W.J. Molecular characterization and expression analysis of vitellogenin in the marine crab Portunus trituberculatus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 142, 456–464. [Google Scholar] [CrossRef]
- Okumura, T.; Yamano, K.; Sakiyama, K. Vitellogenin gene expression and hemolymph vitellogenin during vitellogenesis, final maturation, and oviposition in female kuruma prawn, Marsupenaeus japonicus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Subramoniam, T. Mechanisms and control of vitellogenesis in crustaceans. Fish. Sci. 2011, 77, 1–21. [Google Scholar] [CrossRef]
- Jia, X.; Chen, Y.; Zou, Z.; Lin, P.; Wang, Y.; Zhang, Z. Characterization and expression profile of Vitellogenin gene from Scylla paramamosain. Gene 2013, 520, 119–130. [Google Scholar] [CrossRef]
- Thongda, W.; Chung, J.S.; Tsutsui, N.; Zmora, N.; Katenta, A. Seasonal variations in reproductive activity of the blue crab, Callinectes sapidus: Vitellogenin expression and levels of vitellogenin in the hemolymph during ovarian development. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 179, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V.; Gagne, F.; Marin, M.G.; Ricciardi, F.; Blaise, C. Vitellogenin as a biomarker of exposure to estrogenic compounds in aquatic invertebrates: A review. Environ. Int. 2008, 34, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kwak, T.S.; Kwak, I.S. Vitellogenin Gene Characterization and Expression of Asian Paddle Crabs (Charybdis japonica) following Endocrine Disrupting Chemicals. Ocean Sci. J. 2014, 49, 127–135. [Google Scholar] [CrossRef]
- Jeon, J.M.; Lee, S.O.; Kim, K.S.; Baek, H.J.; Kim, S.; Kim, I.K.; Mykles, D.L.; Kim, H.W. Characterization of two vitellogenin cDNAs from a Pandalus shrimp (Pandalopsis japonica): Expression in hepatopancreas is down-regulated by endosulfan exposure. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 157, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Neris, J.B.; Olivares, D.M.M.; Velasco, F.G.; Luzardo, F.H.M.; Correia, L.O.; González, L.N. HHRISK: A code for assessment of human health risk due to environmental chemical pollution. Ecotoxicol. Environ. Saf. 2018, 170, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Shafei, A.; Ramzy, M.M.; Hegazy, A.I.; Husseny, A.K.; El-Hadary, U.G.; Taha, M.M.; Mosa, A.A. The molecular mechanisms of action of the endocrine disrupting chemical bisphenol A in the development of cancer. Gene 2018, 647, 235–243. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.E.; Michael, T.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S.streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xiang-Li, L.I.; Liang, R.J. Bisphenol A pollution of surface water and its environmental factors. J. Ecol. Rural Environ. 2009, 25, 94–97. [Google Scholar]
- Park, K.; Kwak, I.S. Characterize and Gene Expression of Heat Shock Protein 90 in Marine Crab Charybdis japonica following Bisphenol A and 4-Nonylphenol Exposures. Environ. Health Toxicol. 2014, 29, e2014002. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Gu, M.B.; Sang, B.I.; Kwack, S.J.; Kim, K.B.; Lee, B.M. Risk reduction of adverse effects due to di-(2-ethylhexyl) phthalate (DEHP) by utilizing microbial degradation. J. Toxicol. Environ. Health. A 2009, 72, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Zhang, L.; Ma, Y.; Han, J.; Yang, L.; Zhou, B. Endocrine disruption by di-(2-ethylhexyl)-phthalate in Chinese rare minnow (Gobiocypris rarus). Environ. Toxicol. Chem. 2013, 32, 1846–1854. [Google Scholar] [CrossRef]
- Adeogun, A.O.; Ibor, O.R.; Imiuwa, M.E.; Omogbemi, E.D.; Chukwuka, A.V.; Omiwole, R.A.; Arukwe, A. Endocrine disruptor responses in African sharptooth catfish (Clarias gariepinus) exposed to di-(2-ethylhexyl)-phthalate. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 213, 7–18. [Google Scholar] [CrossRef]
- Li, X.; Yin, P.; Zhao, L. Phthalate esters in water and surface sediments of the Pearl River Estuary: Distribution, ecological, and human health risks. Environ. Sci. Pollut. Res. Int. 2016, 23, 19341–19349. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Wirth, E.; Schiff, K.; Fulton, M. Antifouling biocides in water and sediments from California marinas. Mar. Pollut. Bull. 2013, 69, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.B.; Diamante, G.; Giroux, M.; Xu, E.G.; Abessa, D.M.S.; Schlenk, D. Changes in thyroid status of Menidia beryllina exposed to the antifouling booster irgarol: Impacts of temperature and salinity. Chemosphere 2018, 209, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H.; Minamide, S.; Takeuchi, I. Identification and characterization of heat shock protein 90 (HSP90) in the hard coral Acropora tenuis in response to Irgarol 1051. Mar. Pollut. Bull. 2018, 133, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: A review. Environ. Int. 2004, 30, 235–248. [Google Scholar] [CrossRef]
- Li, L.; Li, X.J.; Wu, Y.M.; Yang, L.; Li, W.; Wang, Q. Vitellogenin regulates antimicrobial responses in Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2017, 69, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, J.; Nishida, M.; Wada, K. Genetic and behavioral diversity in the Macrophthalmus japonicus species complex (Crustacea: Brachyura: Ocypodidae). Mar. Biol. 2002, 140, 1–8. [Google Scholar]
- Tanaka, Y.; Aoki, S.; Okamoto, K. Effects of the bioturbating crab Macrophthalmus japonicus on abiotic and biotic tidal mudflat characteristics in the Tama River, Tokyo Bay, Japan. Plankton. Benthos. Res. 2017, 12, 34–43. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Kim, W.S.; Park, K.; Kim, J.; Lee, M.O.; Kwak, I.S. Chitinase gene responses and tissue sensitivity in an intertidal mud crab (Macrophthalmus japonicus) following low or high salinity stress. Cell Stress Chaperones 2015, 20, 517–526. [Google Scholar] [CrossRef]
- Park, K.; Kwak, T.S.; Kim, W.S.; Kwak, I.S. Changes in exoskeleton surface roughness and expression of chitinase genes in mud crab Macrophthalmus japonicus following heavy metal differences of estuary. Mar. Pollut. Bull. 2019, 138, 11–18. [Google Scholar] [CrossRef]
- Park, K.; Nikapitiya, C.; Kwak, T.S.; Kwak, I.S. Antioxidative-related Genes Expression Following Perfluorooctane Sulfonate (PFOS) Exposure in the Intertidal Mud Crab, Macrophthalmus japonicus. Ocean Sci. J. 2015, 50, 547–556. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Kim, W.S.; Park, K.; Kwak, I.S. Identification of potential markers and sensitive tissues for low or high salinity stress in an intertidal mud crab (Macrophthalmus japonicus). Fish Shellfish Immunol. 2014, 41, 407–416. [Google Scholar] [CrossRef]
- Park, K.; Nikapitiya, C.; Kim, W.S.; Kwak, T.S.; Kwak, I.S. Changes of exoskeleton surface roughness and expression of crucial participation genes for chitin formation and digestion in the mud crab (Macrophthalmus japonicus) following the antifouling biocide irgarol. Ecotoxicol. Environ. Saf. 2016, 132, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Nikapitiya, C.; Kwak, I.S. Identification and expression of proteolysis response genes for Macrophthalmus japonicus exposure to irgarol toxicity. Ann. Limnol. Int. J. Limnol. 2016, 52, 65–74. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2_ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tiu, S.H.; Hui, H.L.; Tsukimura, B.; Tobe, S.S.; He, J.G.; Chan, S.M. Cloning and expression study of the lobster (Homarus americanus) vitellogenin: Conservation in gene structure among decapods. Gen. Comp. Endocrinol. 2009, 160, 36–46. [Google Scholar] [CrossRef]
- Anderson, T.A.; Levitt, D.G.; Banaszak, L.J. The structural basis of lipid interactions in lipovitellin, a soluble lipoprotein. Structure 1998, 6, 895–909. [Google Scholar] [CrossRef]
- Yang, J.; Sun, H.; Qian, Y.; Yang, J. Impairments of cadmium on vitellogenin accumulation in the hepatopancreas of freshwater crab Sinopotamon henanense. Environ. Sci. Pollut. Res. Int. 2017, 24, 18160–18167. [Google Scholar] [CrossRef]
- Okuno, A.; Yang, W.J.; Jayasankar, V.; Saido-Sakanaka, H.; Huong, D.T.; Jasmani, S.; Atmomarsono, M.; Subramoniam, T.; Tsutsui, N.; Ohira, T.; et al. Deduced primary structure of vitellogenin in the giant freshwater prawn, Macrobrachium rosenbergii, and yolk processing during ovarian maturation. J. Exp. Zool. 2002, 292, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Abdu, U.; Davis, C.; Khalaila, I.; Sagi, A. The vitellogenin cDNA of Cherax quadricarinatus encodes a lipoprotein with calcium binding ability, and its expression is induced following the removal of the androgenic gland in a sexually plastic system. Gen. Comp. Endocrinol. 2002, 127, 263–272. [Google Scholar] [CrossRef]
- Tsutsui, N.; Saido-Sakanaka, H.; Yang, W.J.; Jayasankar, V.; Jasmani, S.; Okuno, A.; Ohira, T.; Okumura, T.; Aida, K.; Wilder, M.N. Molecular characterization of a cDNA encoding vitellogenin in the coonstriped shrimp, Pandalus hypsinotus and site of vitellogenin mRNA expression. J. Exp. Zool. A Ecol. Genet. Physiol. 2004, 301, 802–814. [Google Scholar] [CrossRef]
- Zmora, N.; Trant, J.; Chan, S.M.; Chung, J.S. Vitellogenin and its messenger RNA during ovarian development in the female blue crab, Callinectes sapidus: Gene expression, synthesis, transport, and cleavage. Biol. Reprod. 2007, 77, 138–146. [Google Scholar] [CrossRef]
- Kang, B.J.; Nanri, T.; Lee, J.M.; Saito, H.; Han, C.H.; Hatakeyama, M.; Saigusa, M. Vitellogenesis in both sexes of gonochoristic mud shrimp, Upogebia major (Crustacea): Analyses of vitellogenin gene expression and vitellogenin processing. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 149, 589–598. [Google Scholar] [CrossRef]
- Ding, X.; Nagaraju, G.P.C.; Novotney, D.; Lovett, D.L.; Borst, D.W. Yolk protein expression in the green crab, Carcinus maenas. Aquaculture 2010, 298, 325–331. [Google Scholar] [CrossRef]
- Allner, B.; Hennies, M.; Lerche, C.F.; Schmidt, T.; Schneider, K.; Willner, M.; Stahlschmidt-Allner, P. Kinetic determination of vitellogenin induction in the epidermis of cyprinid and perciform fishes: Evaluation of sensitive enzyme-linked immunosorbent assays. Environ. Toxicol. Chem. 2016, 35, 2916–2930. [Google Scholar] [CrossRef]
- Maradonna, F.; Nozzi, V.; Dalla Valle, L.; Traversi, I.; Gioacchini, G.; Benato, F.; Colletti, E.; Gallo, P.; Di Marco Pisciottano, I.; Mita, D.G.; et al. A developmental hepatotoxicity study of dietary bisphenol A in Sparus aurata juveniles. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 166, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, J.; Xu, P.; Yuan, C.; Qin, F.; Liu, S.; Zheng, Y.; Yang, Y.; Wang, Z. Low-dose bisphenol A disrupts gonad development and steroidogenic genes expression in adult female rare minnow Gobiocypris rarus. Chemosphere 2014, 112, 435–442. [Google Scholar] [CrossRef]
- Heindler, F.M.; Alajmi, F.; Huerlimann, R.; Zeng, C.; Newman, S.J.; Vamvounis, G.; van Herwerden, L. Toxic effects of polyethylene terephthalate microparticles and Di(2-ethylhexyl)phthalate on the calanoid copepod, Parvocalanus crassirostris. Ecotoxicol. Environ. Saf. 2017, 141, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, Y.; Gao, Y.; Wang, X.; Zhou, B. The impact of long term exposure to phthalic acid esters on reproduction in Chinese rare minnow (Gobiocypris rarus). Environ. Pollut. 2015, 203, 130–136. [Google Scholar] [CrossRef]
- Ye, T.; Kang, M.; Huang, Q.; Fang, C.; Chen, Y.; Shen, H.; Dong, S. Exposure to DEHP and MEHP from hatching to adulthood causes reproductive dysfunction and endocrine disruption in marine medaka (Oryzias melastigma). Aquat. Toxicol. 2014, 146, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Bao, V.W.W.; Leung, K.M.Y.; Lui, G.C.S.; Lam, M.H.W. Acute and chronic toxicities of Irgarol alone and in combination with copper to the marine copepod Tigriopus japonicus. Chemosphere 2013, 90, 1140–1148. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Hou, X.; Yue, W.; Huang, S.; Wang, C. Tissue expression profiles unveil the gene interaction of hepatopancreas, eyestalk, and ovary in the orecocious female Chinese mitten crab, Eriocheir sinensis. BMC Genet. 2019, 20, 12. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Jo, H.; Kim, D.-K.; Kwak, I.-S. Environmental Pollutants Impair Transcriptional Regulation of the Vitellogenin Gene in the Burrowing Mud Crab (Macrophthalmus Japonicus). Appl. Sci. 2019, 9, 1401. https://doi.org/10.3390/app9071401
Park K, Jo H, Kim D-K, Kwak I-S. Environmental Pollutants Impair Transcriptional Regulation of the Vitellogenin Gene in the Burrowing Mud Crab (Macrophthalmus Japonicus). Applied Sciences. 2019; 9(7):1401. https://doi.org/10.3390/app9071401
Chicago/Turabian StylePark, Kiyun, Hyunbin Jo, Dong-Kyun Kim, and Ihn-Sil Kwak. 2019. "Environmental Pollutants Impair Transcriptional Regulation of the Vitellogenin Gene in the Burrowing Mud Crab (Macrophthalmus Japonicus)" Applied Sciences 9, no. 7: 1401. https://doi.org/10.3390/app9071401
APA StylePark, K., Jo, H., Kim, D.-K., & Kwak, I.-S. (2019). Environmental Pollutants Impair Transcriptional Regulation of the Vitellogenin Gene in the Burrowing Mud Crab (Macrophthalmus Japonicus). Applied Sciences, 9(7), 1401. https://doi.org/10.3390/app9071401






