Extraction Efficiency of a Commercial Espresso Machine Compared to a Stainless-Steel Column Pressurized Hot Water Extraction (PHWE) System for the Determination of 23 Pharmaceuticals, Antibiotics and Hormones in Sewage Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
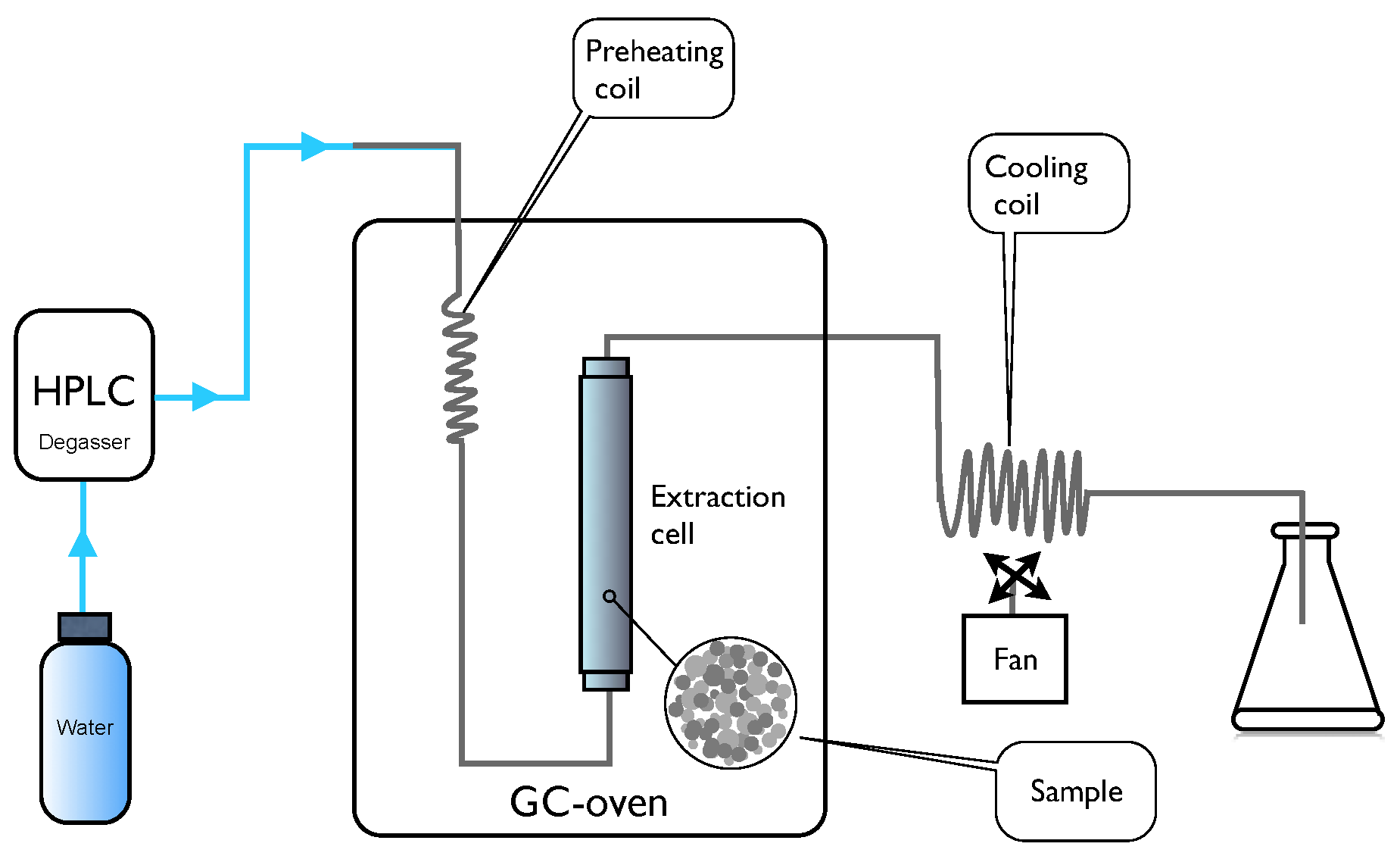
2.2. PHWE
2.3. Espresso Machine Extraction (Espresso Method)
2.4. Solid-phase Extraction (SPE Method)
2.5. Analytical Separation and Detection (UPLC-MS/MS Method)
2.6. Calculations
2.6.1. Absolute Recoveries of Isotopic Labelled Compounds
2.6.2. Quantification of APIs in Sludge
2.6.3. Statistical Analysis
3. Results and Discussion
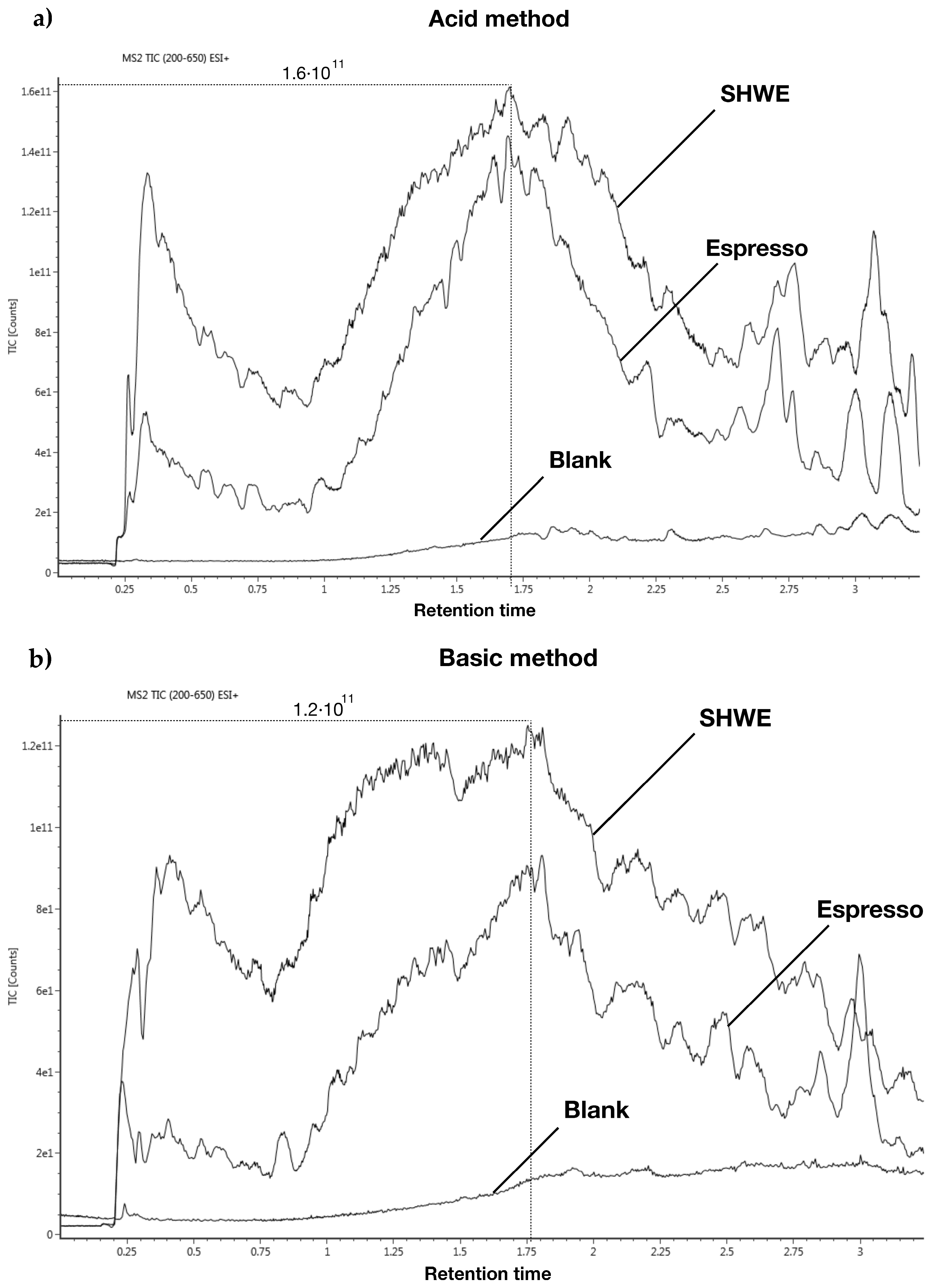
3.1. Absolute Recoveries of Spiked Isotope Labelled Compounds and Ion Suppression
3.2. Pharmaceuticals Extracted from Sludge
3.3. Comparison of Extraction Results with Effluent and Influent Data
3.4. Comparison of Extraction Results with Literature Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wahlberg, C.; Björlenius, B.; Ek, M.; Paxéus, N.; Gårdstam, L. Avloppsreningsverkens Förmåga att ta Hand om Läkemedelsrester och Andra farliga Ämnen; Naturvårdsverket: Stockholm, Sweden, 2008; pp. 1–162. [Google Scholar]
- Halling-Sørensen, B.; Nielsen, S.N.; Lanzky, P.F.; Ingerslev, F.; Lützhøft, H.H.; Jørgensen, S. Occurrence, fate and effects of pharmaceutical substances in the environment-A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Pérez-Carrera, E.; Hansen, M.; León, V.M.; Björklund, E.; Krogh, K.A.; Halling-Sørensen, B.; González-Mazo, E. Multiresidue method for the determination of 32 human and veterinary pharmaceuticals in soil and sediment by pressurized-liquid extraction and LC-MS/MS. Anal. Bioanal. Chem. 2010, 398, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Navas, C.; Björklund, E.; Bak, S.A.; Hansen, M.; Krogh, K.A.; Maya, F.; Cerdà, V. Pollution pathways of pharmaceutical residues in the aquatic environment on the island of Mallorca, Spain. Arch. Environ. Contam. Toxicol. 2013, 65, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- Osorio, V.; Larrañaga, A.; Aceña, J.; Pérez, S.; Barceló, D. Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian Rivers. Sci. Total Environ. 2016, 540, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Paíga, P.; Santos, L.H.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of pharmaceuticals in the Lis river (Portugal): Sources, fate and seasonal variation. Sci. Total Environ. 2016, 573, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Kay, P.; Hughes, S.R.; Ault, J.R.; Ashcroft, A.E.; Brown, L.E. Widespread, routine occurrence of pharmaceuticals in sewage effluent, combined sewer overflows and receiving waters. Environ. Pollut. 2017, 220, 1447–1455. [Google Scholar] [CrossRef]
- Björklund, E.; Svahn, O.; Bak, S.; Bekoe, S.O.; Hansen, M. Pharmaceutical residues affecting the UNESCO biosphere reserve Kristianstads Vattenrike wetlands: Sources and sinks. Arch. Environ. Contam. Toxicol. 2016, 71, 423–436. [Google Scholar] [CrossRef]
- Verlicchi, P.; Zambello, E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: Occurrence and environmental risk in the case of application on soil—A critical review. Sci. Total Environ. 2015, 538, 750–767. [Google Scholar] [CrossRef]
- Jederlund, L. Frågor och svar, REVAQ, 160108. 2016. [Google Scholar]
- Hansson, E.; Johansson, M. Rapport Avlopp på våra åkrar—En Rapport om Miljögifter i slam; Naturskyddsföreningen: Stockholm, Sweden, 2012; pp. 1–26. [Google Scholar]
- Petrie, B.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Multi-residue analysis of 90 emerging contaminants in liquid and solid environmental matrices by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1431, 64–78. [Google Scholar] [CrossRef]
- Barron, L.; Tobin, J.; Paull, B. Multi-residue determination of pharmaceuticals in sludge and sludge enriched soils using pressurized liquid extraction, solid phase extraction and liquid chromatography with tandem mass spectrometry. J. Environ. Monit. 2008, 10, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Ekpeghere, K.I.; Lee, J.W.; Kim, H.Y.; Shin, S.K.; Oh, J.E. Determination and characterization of pharmaceuticals in sludge from municipal and livestock wastewater treatment plants. Chemosphere 2017, 168, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, R.H.; Olofsson, U.; Rendahl, P.; Johansson, M.I.; Tysklind, M.; Andersson, B.A. Behavior of fluoroquinolones and trimethoprim during mechanical, chemical, and active sludge treatment of sewage water and digestion of sludge. Environ. Sci. Technol. 2006, 40, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Alder, A.C.; Giger, W.; Theiß, N.; Ternes, T.A. Extraction and determination of sulfonamides, macrolides, and trimethoprim in sewage sludge. J. Chromatogr. A 2005, 1085, 179–189. [Google Scholar] [CrossRef]
- Radjenović, J.; Jelić, A.; Petrović, M.; Barceló, D. Determination of pharmaceuticals in sewage sludge by pressurized liquid extraction (PLE) coupled to liquid chromatography-tandem mass spectrometry (LC-MS/MS). Anal. Bioanal. Chem. 2009, 393, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Runnqvist, H.; Bak, S.A.; Hansen, M.; Styrishave, B.; Halling-Sørensen, B.; Björklund, E. Determination of pharmaceuticals in environmental and biological matrices using pressurised liquid extraction—Are we developing sound extraction methods? J. Chromatogr. A 2010, 1217, 2447–2470. [Google Scholar] [CrossRef] [PubMed]
- Luque-Munoz, A.; Vílchez, J.L.; Zafra-Gómez, A. Multiclass method for the determination of pharmaceuticals and personal care products in compost from sewage sludge using ultrasound and salt-assisted liquid–liquid extraction followed by ultrahigh performance liquid chromatography-tandem mass spectrometry analysis. J. Chromatogr. A 2017, 1507, 72–83. [Google Scholar] [CrossRef]
- Clark, J.H. Green chemistry: Challenges and opportunities. Green Chem. 1999, 1, 1–8. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Kronholm, J.; Hartonen, K.; Riekkola, M.L. Analytical extractions with water at elevated temperatures and pressures. Trac Trends Anal. Chem. 2007, 26, 396–412. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Yang, Y.; Miller, D.J. Extraction of organic pollutants from environmental solids with sub-and supercritical water. Anal. Chem. 1994, 66, 2912–2920. [Google Scholar] [CrossRef]
- Stoob, K.; Singer, H.P.; Stettler, S.; Hartmann, N.; Mueller, S.R.; Stamm, C.H. Exhaustive extraction of sulfonamide antibiotics from aged agricultural soils using pressurized liquid extraction. J. Chromatogr. A 2006, 1128, 1–9. [Google Scholar] [CrossRef]
- Svahn, O.; Björklund, E. Thermal stability assessment of antibiotics in moderate temperature and subcriticalwater using a pressurized dynamic flow-through system. Int. J. Innov. Appl. Stud. 2015, 11, 872–880. [Google Scholar]
- Sánchez-López, J.A.; Zimmermann, R.; Yeretzian, C. Insight into the time-resolved extraction of aroma compounds during espresso coffee preparation: Online monitoring by PTR-ToF-MS. Anal. Chem. 2014, 86, 11696–11704. [Google Scholar] [CrossRef]
- Mestdagh, F.; Davidek, T.; Chaumonteuil, M.; Folmer, B.; Blank, I. The kinetics of coffee aroma extraction. Food Res. Int. 2014, 63, 271–274. [Google Scholar] [CrossRef]
- Severini, C.; Ricci, I.; Marone, M.; Derossi, A.; De Pilli, T. Changes in the aromatic profile of espresso coffee as a function of the grinding grade and extraction time: A study by the electronic nose system. J. Agric. Food Chem. 2015, 63, 2321–2327. [Google Scholar] [CrossRef]
- Salamanca, C.A.; Fiol, N.; González, C.; Saez, M.; Villaescusa, I. Extraction of espresso coffee by using gradient of temperature. Effect on physicochemical and sensorial characteristics of espresso. Food Chem. 2017, 214, 622–630. [Google Scholar] [CrossRef]
- Armenta, S.; de la Guardia, M.; Esteve-Turrillas, F.A. Hard cap espresso machines in analytical chemistry: What else? Anal. Chem. 2016, 88, 6570–6576. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Coscollà, C.; Yusà, V.; Armenta, S.; de la Guardia, M.; Esteve-Turrillas, F.A. Comprehensive analysis of airborne pesticides using hard cap espresso extraction-liquid chromatography-high-resolution mass spectrometry. J. Chromatogr. A 2017, 1506, 27–36. [Google Scholar]
- Gallart-Mateu, D.; Pastor, A.; de la Guardia, M.; Armenta, S.; Esteve-Turrillas, F.A. Hard cap espresso extraction-stir bar preconcentration of polychlorinated biphenyls in soil and sediments. Anal. Chim. Acta 2017, 952, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Svahn, O. Tillämpad Miljöanalytisk kemi för Monitorering och Åtgärder av Antibiotika-och Läkemedelsrester i Vattenriket. Doctoral Dissertation, Lund University, Lund, Sweden, 2016. [Google Scholar]
- Svahn, O.; Björklund, E. Increased electrospray ionization intensities and expanded chromatographic possibilities for emerging contaminants using mobile phases of different pH. J. Chromatogr. B 2016, 1033, 128–137. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef]
- Kondo, T.; Yang, Y. Comparison of elution strength, column efficiency, and peak symmetry in subcritical water chromatography and traditional reversed-phase liquid chromatography. Anal. Chim. Acta 2003, 494, 157–166. [Google Scholar] [CrossRef]
- Fick, J.; Lindberg, R.H.; Brorström-Lundén, E. Results from the Swedish National Screening Programme 2010; IVL, Swedish Environmental Research Institute Ltd.: Stockholm, Sweden, 2011; pp. 1–56. [Google Scholar]
- Nieto, A.; Borrull, F.; Pocurull, E.; Marcé, R.M. Pressurized liquid extraction: A useful technique to extract pharmaceuticals and personal-care products from sewage sludge. Trac Trends Anal. Chem. 2010, 29, 752–764. [Google Scholar] [CrossRef]
- Svahn, O.; Björklund, E. Describing sorption of pharmaceuticals to lake and river sediments, and sewage sludge from UNESCO Biosphere Reserve Kristianstads Vattenrike by chromatographic asymmetry factors and recovery measurements. J. Chromatogr. A 2015, 1415, 73–82. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.F.; Jelic, A.; López-Serna, R.; Mozeto, A.A.; Petrovic, M.; Barceló, D. Occurrence and distribution of pharmaceuticals in surface water, suspended solids and sediments of the Ebro river basin, Spain. Chemosphere 2011, 85, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Golet, E.M.; Strehler, A.; Alder, A.C.; Giger, W. Determination of fluoroquinolone antibacterial agents in sewage sludge and sludge-treated soil using accelerated solvent extraction followed by solid-phase extraction. Anal. Chem. 2002, 74, 5455–5462. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Smyth, S.A.; Barclay, K.; Sauvé, S.; Gagnon, C. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in Canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; Hansen, M.; Kjølholt, J.; Stuer-Lauridsen, F.; Ternes, T.; Halling-Sørensen, B. Assessment of the importance of sorption for steroid estrogens removal during activated sludge treatment. Chemosphere 2005, 61, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Östman, M.; Lindberg, R.H.; Fick, J.; Björn, E.; Tysklind, M. Screening of biocides, metals and antibiotics in Swedish sewage sludge and wastewater. Water Res. 2017, 115, 318–328. [Google Scholar] [CrossRef]
- Ramil, M.; El Aref, T.; Fink, G.; Scheurer, M.; Ternes, T.A. Fate of beta blockers in aquatic-sediment systems: Sorption and biotransformation. Environ. Sci. Technol. 2009, 44, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Malmborg, J.; Magnér, J. Pharmaceutical residues in sewage sludge: Effect of sanitization and anaerobic digestion. J. Environ. Manag. 2015, 153, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: Removal and ecotoxicological impact of wastewater discharges and sludge disposal. J. Hazard. Mater. 2012, 239, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Peysson, W.; Vulliet, E. Determination of 136 pharmaceuticals and hormones in sewage sludge using quick, easy, cheap, effective, rugged and safe extraction followed by analysis with liquid chromatography–time-of-flight-mass spectrometry. J. Chromatogr. A 2013, 1290, 46–61. [Google Scholar] [CrossRef] [PubMed]
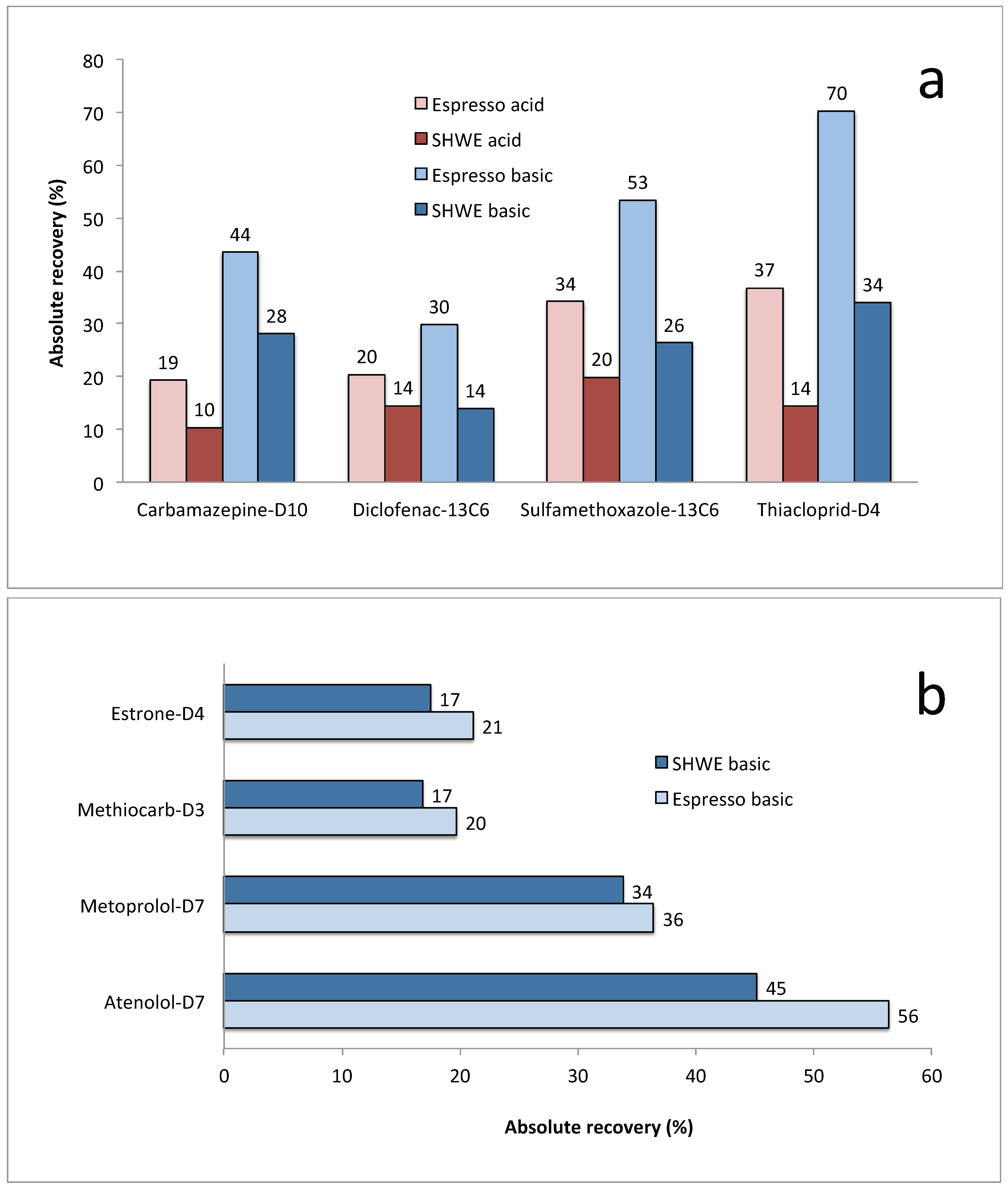
| Compound | UPLC Condition | Espresso (n = 3) (%) | RSD (%) | PHWE (n = 3) (%) | RSD (%) |
|---|---|---|---|---|---|
| Thiacloprid-d4a | Basic | 70 | 17 | 34 | 17 |
| Carbamazepine-D10 | Basic | 44 | 18 | 28 | 4 |
| Diclofenac-13C6 | Basic | 30 | 18 | 14 | 24 |
| Sulfamethoxazole-13C6 | Basic | 53 | 7 | 26 | 19 |
| Atenolol-d7 | Basic | 56 | 5 | 45 | 10 |
| Methiocarb-d3 | Basic | 20 | 12 | 17 | 5 |
| Metoprolol-d7 | Basic | 36 | 15 | 34 | 9 |
| Estrone-d4 | Basic | 21 | 29 | 17 | 2 |
| Average recovery 7 compounds | Basic | 37 | 24 | ||
| Thiacloprid-d4a | Acid | 37 | 22 | 14 | 18 |
| Carbamazepine-d10 | Acid | 19 | 31 | 10 | 7 |
| Diclofenac-13C6 | Acid | 20 | 11 | 14 | 12 |
| Sulfamethoxazole-13C6 | Acid | 34 | 18 | 20 | 13 |
| Average recovery 3 compounds | Acid | 24 | 16 |
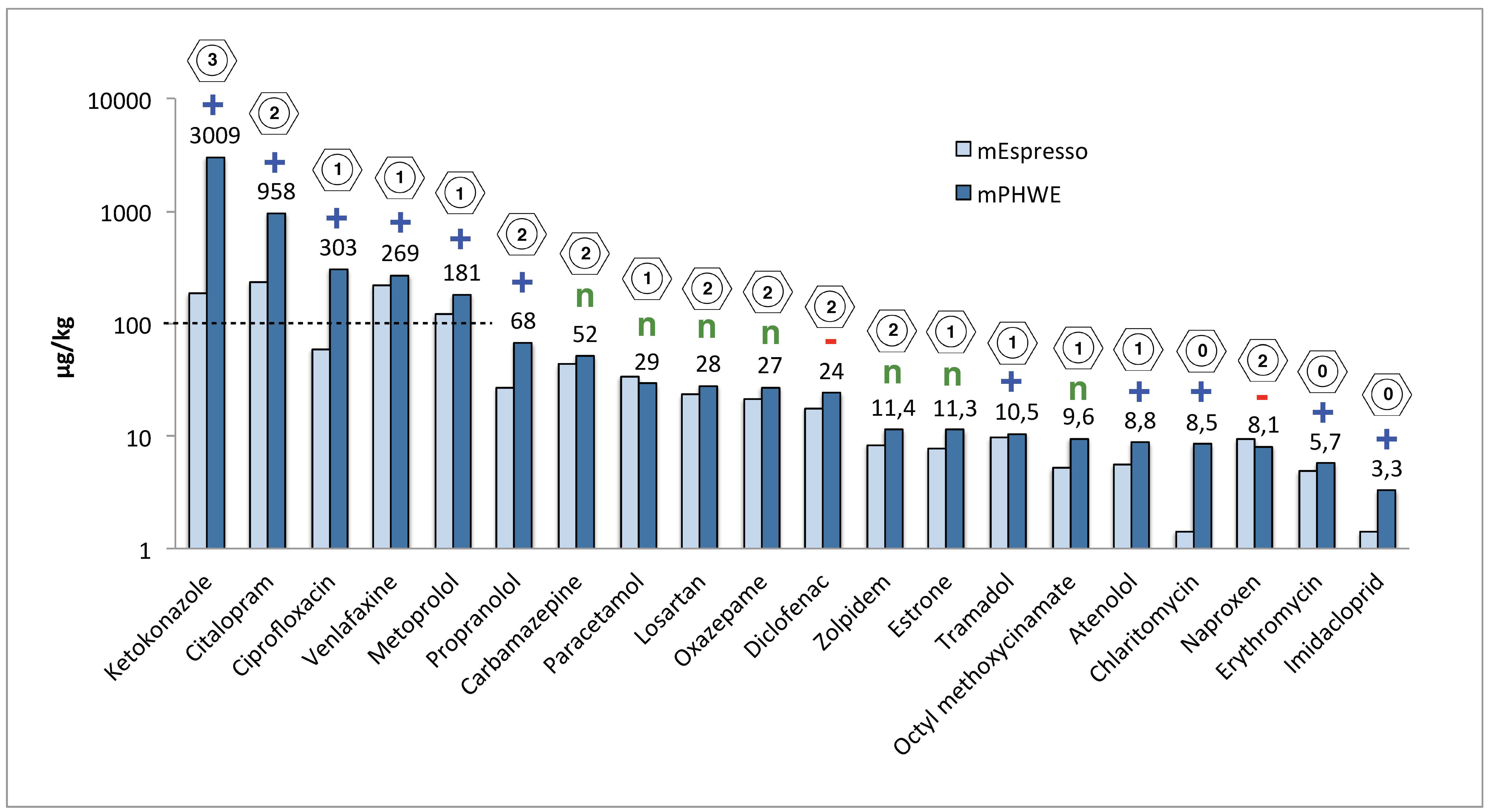
| Substance | pKa | Charge (pH 7) | Log D (pH 7) | log Kow | Espresso (n = 3) μg/kg | RSD (%) | PHWE (n = 3) μg/kg | RSD (%) | Water Inlet ng/L | Water Outlet ng/L | Skövde Sludge μg/kg | Stockholm Sludge μg/kg | Umeå Sludge μg/kg | Scientific Paper μg/kg | [REF] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atenolol | 9.67 | + | −2.14 | 0.16 | 5.6 | 15 | 8.8 | 3 | 1348 | 215 | 13 | 9 | 12 | 6;22 | [14] |
| Carbamazepine | - | n | 2.28 | 2.45 | 43 | 26 | 52 | 10 | 1031 | 547 | 190 | 200 | 120 | 18;32 | [41] |
| Clarithromycin | 8.99 | + | 1.84 | 3.16 | 1.4 | 34 | 8.5 | 22 | 131 | 22 | 13 | 1.4 | 4.5 | 24 | [17] |
| Ciprofloxacin | 6.38 | (+:–) | −0.81 | 0.28 | 60 | 38 | 303 | 40 | 58 | 46 | 450 | 250 | 170 | 2420 | [42] |
| Citalopram | 9.78 | + | 1.27 | 2.51 | 233 | 31 | 958 | 8 | 155 | 32 | 760 | 570 | 630 | 725 | [43] |
| Diclofenac | 4.00 | – | 1.37 | 4.06 | 18 | 16 | 24 | 11 | 713 | 577 | 59 | 31 | 10 | 192 | [18] |
| Erythromycin | 8.80 | + | 1.2 | 2.50 | 4.8 | 30 | 5.7 | 8 | 385 | 267 | 1000 | 150 | 120 | 62 | [18] |
| Estrone | 10.30 | n | 4.31 | 3.13 | 7.9 | 6 | 11 | 9 | 49 | 3 | 33 | 36 | 2 | 23;28 | [44] |
| Fluconazole | 2.56 | n | 0.56 | 0.25 | 0.5 | 15 | 0.4 | 12 | 51 | 105 | 3.5 | 13 | 47 | <LOQ, n.d | [45] |
| Imidacloprid | 5.28 | pH6 + | 1.09 | 0.57 | 1.4 | 38 | 3.3 | 24 | 8 | 14 | n.a | n.a | n.a | ||
| Ketokonazole | 3.96, 6.75 | + | 4.06 | 4.30 | 186 | 36 | 3009 | 12 | 69 | 0 | 510 | 1200 | 1100 | 910 | [45] |
| Losartan | 4.12 | n | 4.94 | 4.01 | 23 | 7 | 28 | 22 | 326 | 205 | n.a | n.a | n.a | ||
| Metoprolol | 9.60 | + | −0.81 | 1.88 | 123 | 21 | 181 | 7 | 999 | 533 | 180 | 410 | 210 | 29:92 | [46] |
| Naproxen | 4.19 | – | 0.45 | 3.18 | 9.3 | 25 | 8.1 | 13 | 2421 | 290 | <LOQ | <LOQ | <LOQ | 4 | [47] |
| Octylmethoxycinamate | - | n | 5.38 | 6.10 | 5.2 | 5 | 10 | 31 | 64 | 31 | n.a | n.a | n.a | ||
| Oxazepame | - | n | 2.06 | 2.31 | 22 | 33 | 27 | 14 | 374 | 403 | 43 | 18 | 12 | ||
| Paracetamol | 9.46 | n | 0.74 | 1.08 | 34 | 29 | 29 | 33 | 22528 | 0 | 73 | 11 | <LOQ | ||
| Propranolol | 9.42 | + | 1.15 | 3.48 | 27 | 22 | 68 | 9 | 47 | 16 | n.a | n.a | n.a | <1.26 | [48] |
| Sulfamethoxazole | 6.16 | – | 0.14 | 0.89 | n.d | n.d | 476 | 118 | n.d | [49] | |||||
| Tramadol | 9.23 | + | 0.24 | 2.51 | 10 | 12 | 11 | 10 | 168 | 153 | <LOQ | 68 | <LOQ | 43 | [49] |
| Trimethoprim | 7.20 | + | 0.92 | 0.91 | 0.2 | 33 | 1.2 | 19 | 77 | 17 | 27 | 2.2 | 2.5 | 5:13 | [49] |
| Venlafaxine | 8.91 | + | 0.84 | 3.28 | 219 | 7 | 269 | 20 | 174 | 88 | 86 | 310 | 150 | 318 | [43] |
| Zolpidem | 5.65 | n | 3 | 3.85 | 8.4 | 9 | 11 | 26 | 12 | 4 | 7.7 | 8.3 | 3.2 | 38 | [49] |
| Sum concentration | 1042 | 5027 | 31664 | 3686 | 3448 | 3288 | 2593 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svahn, O.; Björklund, E. Extraction Efficiency of a Commercial Espresso Machine Compared to a Stainless-Steel Column Pressurized Hot Water Extraction (PHWE) System for the Determination of 23 Pharmaceuticals, Antibiotics and Hormones in Sewage Sludge. Appl. Sci. 2019, 9, 1509. https://doi.org/10.3390/app9071509
Svahn O, Björklund E. Extraction Efficiency of a Commercial Espresso Machine Compared to a Stainless-Steel Column Pressurized Hot Water Extraction (PHWE) System for the Determination of 23 Pharmaceuticals, Antibiotics and Hormones in Sewage Sludge. Applied Sciences. 2019; 9(7):1509. https://doi.org/10.3390/app9071509
Chicago/Turabian StyleSvahn, Ola, and Erland Björklund. 2019. "Extraction Efficiency of a Commercial Espresso Machine Compared to a Stainless-Steel Column Pressurized Hot Water Extraction (PHWE) System for the Determination of 23 Pharmaceuticals, Antibiotics and Hormones in Sewage Sludge" Applied Sciences 9, no. 7: 1509. https://doi.org/10.3390/app9071509
APA StyleSvahn, O., & Björklund, E. (2019). Extraction Efficiency of a Commercial Espresso Machine Compared to a Stainless-Steel Column Pressurized Hot Water Extraction (PHWE) System for the Determination of 23 Pharmaceuticals, Antibiotics and Hormones in Sewage Sludge. Applied Sciences, 9(7), 1509. https://doi.org/10.3390/app9071509





