1. Introduction
Germanium is considered as a critical raw material by the European Union [
1]. It is important to many different industrial sectors like optic fibers, infrared optics, electronics, photovoltaics or catalysts for polyethylene terephthalate (PET) production [
2]. Ge minerals are not present in economically mineable aggregates, but they are scattered in their surroundings. Therefore, Ge is obtained as an accompanying element during the processing of other raw materials—mainly from coal ash and by-products of zinc production. About 30% of Ge production comes from recycling [
2].
One of the ways to process Ge is leaching in aqueous solutions of different acids (usually sulfuric, hydrochloric or oxalic). Dissolved Ge may be later precipitated from sulfuric solutions using hydrogen sulfide [
3] or tannic acid, alternatively using ion-exchange resins [
4,
5], membranes [
6], or solvent extraction [
7]. Precipitation of tannin complex is more Ge-selective than precipitation by pH adjustment. It was found that tannic acid gave better results than the application of sodium hydroxide or ammonia [
8]. In aqueous solutions, tannic acid forms a water insoluble complex with Ge, firstly described in 1938 by Davies and Morgan [
9,
10,
11]. The Ge-tannin complex in aqueous solutions is formed according to the following equation [
12]:
where T is tannin. Precipitation with tannic acid allows to recover 99% of the Ge present in the solution and to achieve final Ge concentration below 0.5 mg/dm
3 [
12].
Tannic acid is not a single compound. Generally, its chemical formula is described as C
76H
52O
46 and molecular weight 1701.2 g/mol. However, it is a mixture of gallic acid, gallotannins, and their derivatives [
13]. The application of tannins in non-ferrous metallurgy is not limited only to Ge. It is used as a depressant in flotation processes for ore treatment or Au recovery [
14].
The application of tannin for selective precipitation of Ge was investigated during the processing of industrial materials from Yunnan Chihong Zinc and Germanium (China) [
12], Çinkur Plant (Kayseri, Turkey) [
15], or Puertollano IGCC power plant (Spain) [
16]. The composition of the solution used for the precipitation of Ge-tannin complex is different and depends on the source of initial material. Leachates from processing of coal fly ash or zinc refining by-products besides germanium may contain Zn as well as Ni, V, Sb, As, or Fe [
8,
12,
16,
17]. However, there is no information concerning processing of industrial solutions containing Ge, Zn, In, and Sn.
In this study, the precipitation of Ge from sulfate solution containing Sn, In, and Zn using technical and pure tannic acid was analyzed. The detinning operation, which was an essential part of the entire process, was also described. The initial solution was obtained after the leaching of the dross coming from thermal oxidation of by-product alloy from New Jersey process, described in the previous study [
18,
19].
2. Materials and Methods
Solutions used for precipitation of Ge-tannin complex were prepared according to the procedure developed in the previous publications [
19]. The following conditions of GeIn dross leaching were applied: concentration of sulfuric acid solution—10%, temperature—80 °C, process time—2 h, and dross-to-solution ratio (S/L)—0.1 kg/dm
3. In the first part of the investigation, freshly prepared leachates were used. In the second part, detinned solutions were tested.
Two kinds of tannic acid were investigated: pure (99%, Stanlab, Lublin, Poland) and technical (80%, Roeper, Hamburg, Germany). They were used in the original powder form as well as dissolved in water. A solution of NaOH (30%, PCC Rokita, Brzeg Dolny, Poland) was applied for pH adjustment, H2O2 (30%, Avantor, Gliwice, Poland) was used for tin removal, while Magnafloc 338 (BASF, Bradford, UK) was used as a flocculant.
The original solutions obtained after the leaching of GeIn dross, which contained tin, were used for initial tests. However, it was found that tin was also precipitated by tannic acid. Therefore, the solution was detinned using 30% H2O2 solution in the amount of 6 cm3 per 1 dm3 of the original solutions while mixing. Then, the resulting solution was heated to 70 °C and mixed for 1 h to remove excess H2O2. Finally, 2 cm3 of 0.1% flocculant solution was added, mixing was stopped and the suspension was left overnight to settle. The clarified part of the suspension was collected, while the suspension was filtered—the filtrate was combined with clarified solution and used for further investigation, whereas the solid phase was analyzed.
Ge was removed from the solution according to the following methodology. Firstly, the pH of the solution was adjusted to 2.0 using 30% NaOH. Then, the solution was heated to the desired temperature and the appropriate amount of tannic acid in the form of powder or solution (20%) was added while mixing. The suspension was mixed for a set period of time and filtered. For several tests tannic acid was added in portions. In this case, the suspension was mixed for 1 h before sample collection and the addition of the next portion. The mixing speed for all tests was 500 rpm.
Solutions and solids were analyzed at the IMN’s Department of Analytical Chemistry according to the previously applied procedure [
18]. The composition of solutions and solids was determined using ICP OES (Inductively Coupled Plasma—Optical Emission Spectrometry; OPTIMA 5300V, PerkinElmer, Waltham, MA, USA). Solutions were diluted and acidified using HCl (35%, Avantor, Gliwice, Poland) prior to analysis. Samples of Ge-tannin precipitates were wetted and dissolved in the mixture of concentrated HNO
3 (65%, Avantor, Gliwice, Poland) and HF (40%, VWR, Lutterworth, UK). In the case of Sn precipitate, an initial pretreatment was performed by melting with Na
2O
2 (p.a., Avantor, Gliwice, Poland) at 600 °C for 30 min in a corundum crucible before dissolution in acid mixture.
3. Results
3.1. Ge Precipitation from Solutions Containing Sn
Firstly, the precipitation of Ge-tannin complex from the original solutions obtained after leaching of GeIn dross was investigated.
Table 1 and
Figure 1A present the temperature influence on composition of solutions and precipitates as well as precipitation yields of Ge, In, and Sn. During these tests 100 g pure tannic acid was added to 1 dm
3 of the solution. Results of pure tannic acid addition in 20 g portions to 1 dm
3 of the solutions are shown in
Table 2 and
Figure 1B.
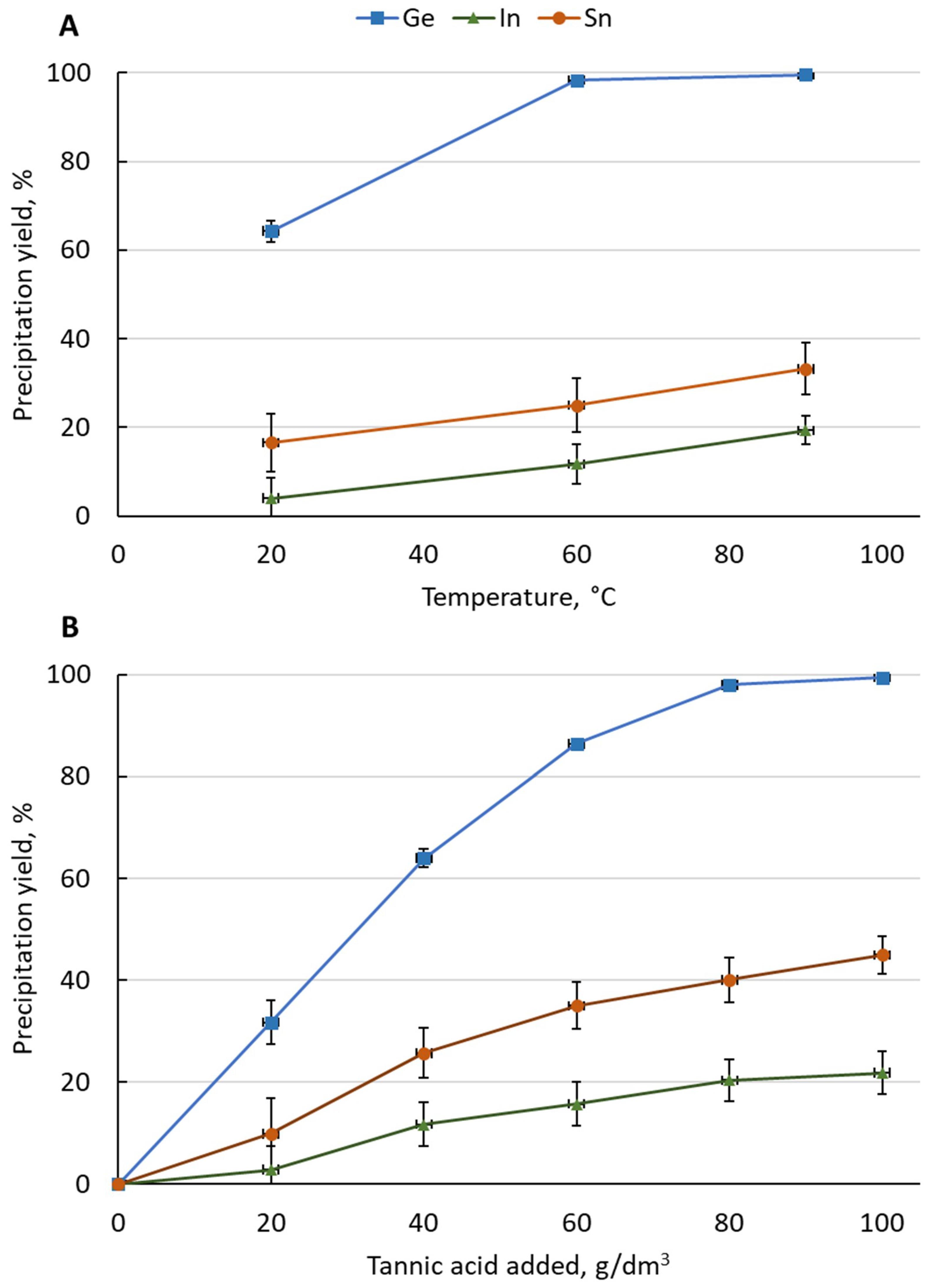
It was noticed that after the addition of solid tannic acid it was not dissolved in the solution, but formed a suspension. Ge concentration in solution after addition of tannic acid decreased from 6.0 g/dm3 down to 0.03 g/dm3. The lowest concentration was noticed for the highest applied temperature. Moreover, the concentration of Ge in the precipitate increased from 3.67% for 20 °C to 5.48% at 90 °C. It corresponded to a change in Ge precipitation yield from 64% at 20 °C to >99% at 90 °C. The concentration of Sn also significantly decreased from 4.4 g/dm3 in the original solution down to 3.0 g/dm3. Sn precipitation yield changed from 16% at 20 °C to 33% at 90 °C. It was accompanied by the increase of tin content in the precipitate—from 0.77% at 20°C to 1.96% at 90°. The change in In concentration was small—it decreased from 1.7 to 1.4 g/dm3. In concentration in the precipitate was also low—between 0.03% at 20 °C and 0.13% at 90 °C. The precipitation yield of In varied from 4% at 20 °C to 19% at 90 °C.
In the case of tannic acid addition in portions, it was observed that Ge concentration in the solution declined during the process. The decrease was highest for first portion added—from 7.8 g/dm3 in the initial solution to 5.2 g/dm3 (32% precipitation yield). For the last added portion, the decrease was the lowest—from 0.15 g/dm3 to 0.04 g/dm3—total Ge precipitation yield increased from ca. 98% to >99%. The concentration of Sn also significantly decreased during the course of the process—from 5.4 g/dm3 in the initial solution to 2.8 g/dm3 after the fifth portion of tannic acid was added. Sn precipitation yield after the last portion was found at the level of 45%. A small decrease of In concentration was also noticeable—from 2.1 gm/dm3 to 1.6 g/dm3 (22% precipitation yield). A change of Ge content in the precipitate was also observed. After the first added portion it contained 9.37% Ge, while after the fifth portion Ge content was 6.55%. The concentration of Sn and In in the precipitate was stable and in the range between 1.65% and 1.73% and 0.12% and 0.14%, respectively.
3.2. Sn Removal
The investigation of the solution containing Sn was associated with significant drawbacks. The original solutions were not stable, due to solution turbidity caused by Sn oxidation. Additionally, the obtained tannin precipitates contained undesirable Sn. Therefore, Sn was removed from the original solutions using H
2O
2. Solution composition before and after detinning is shown in
Table 3.
It was noticed that the addition of H2O2 followed by heating to 70 °C for 1 h allowed the removal of >99% of the Sn present in the original solution. Additionally, part of the Ge and the In (ca. 15%) were also precipitated. Zn was the only major component of the solution, and its concentration in the liquid did not significantly change.
3.3. Ge Precipitation from Detinned Solution Using Solid and Dissolved Tannic Acid
The comparison of two ways of tannic acid addition (as a solid and dissolved in water) was investigated. Firstly, it was done using tannic acid powder and the freshly prepared 20% aqueous solution. In both cases three 20 g portions of the acid per 1 dm
3 of the solution were added. The results of the tests are shown in
Table 4 and
Figure 2A. Secondly, the time influence on the composition of the solution after tannic acid addition was determined. Changes in the compositions and precipitation yields are shown in
Table 5 and
Figure 2B.
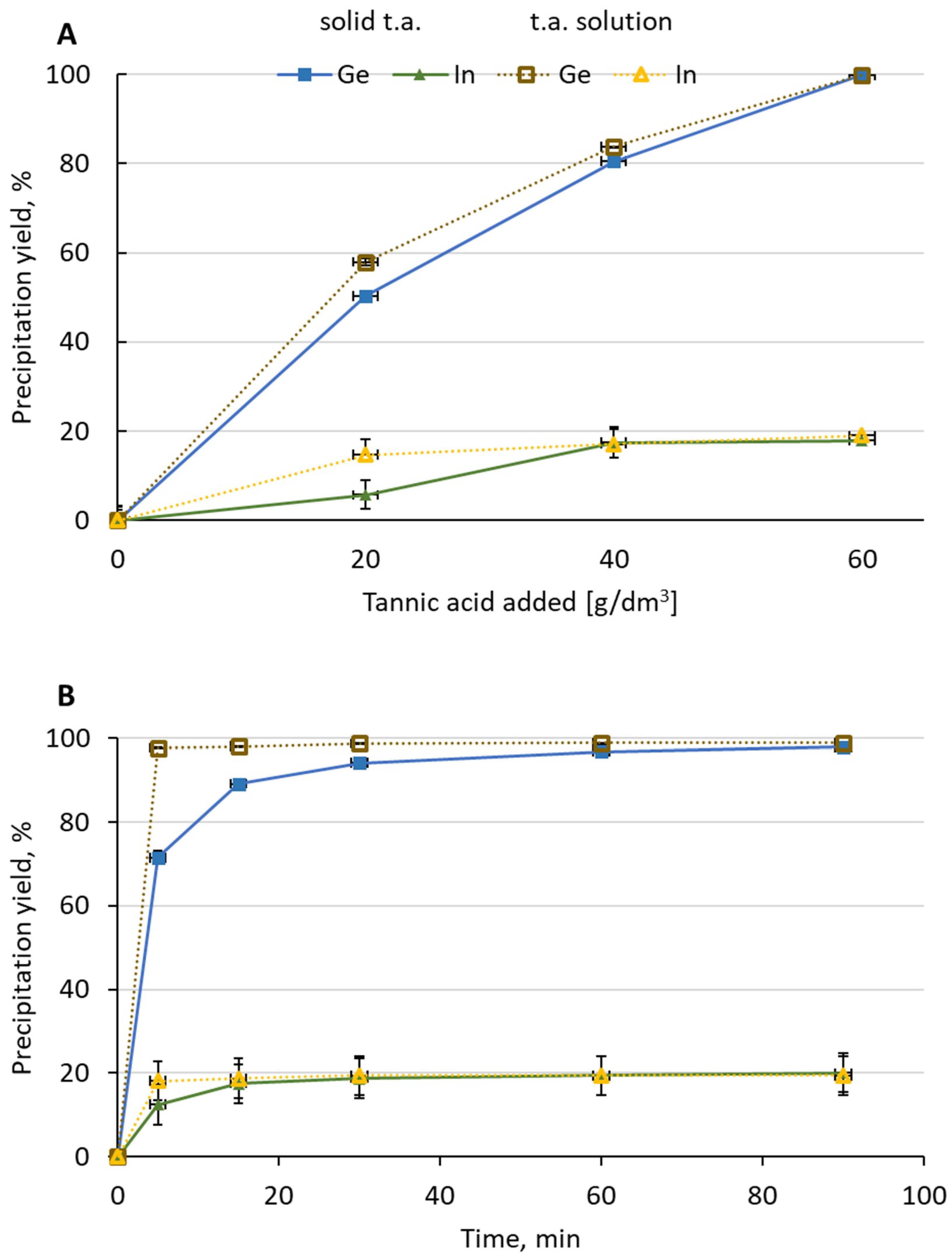
There was no significant influence of the tannic acid form on the process efficiency. A small difference between Ge concentrations in the solution after the first added portion was noticed—2.4 g/dm3 for the powder form and 1.9 g/dm3 for the dissolved tannic acid. After the addition of the next portions, the difference became less significant—0.91 g/dm3 vs. 0.89 g/dm3 after the second portion and 0.010 g/dm3 vs. 0.0057 g/dm3 after the third portion. A slightly higher Ge precipitation yield after first (58%–50%) and second (84%–80%) portions was noticed for tannic acid solution. After the addition of the third portion, Ge precipitation yield in both cases exceeded 99%. The concentration of In in the solution slightly decreased from 1.6 g/dm3 in the initial solution to 1.1–1.3 g/dm3. The final In precipitation yield in both cases was ca. 19%. The form of tannic acid added had no influence on In concentration. The concentration of Ge in the precipitate decreased as portions of the acid were added—for powder it declined from 7.09% after the first portion to 6.09% after the third portion, while for the solution it changed from 7.45% to 5.96%. On the other hand, the concentration of In in the precipitate increased with the amount of tannic acid added—from 0.14% to 0.21% for powder form and from 0.19% to 0.26% for the solution. It was noticed that more pure precipitate was obtained when solid tannic acid was applied—after the third portion added it contained more Ge and less In than the precipitate obtained after addition of tannic acid solution.
The form of the added tannic acid had an influence on the Ge precipitation process rate. The highest difference in Ge concentrations was noticed at the beginning of the process. After 5 min the solution contained 1.6 g/dm3 Ge for powder form and 0.13 g/dm3 for solution. During the course of the process the difference became less significant—after 30 min it was 0.33 g/dm3 and 0.074 g/dm3, while after 90 min it was 0.12 g/dm3 and 0.065 g/dm3, respectively. Ge precipitation yield for tannic acid solution exceeded 97% after 5 min. In the case of solid tannic acid, a similar result was achieved after 60 min. In the case of In concentration, there was no significant difference. The final In precipitation yield was ca. 20% for both forms of tannic acid.
3.4. Ge Precipitation from Detinned Solution Using Tannic Acid of Different Purity
The influence of tannic acid purity on the Ge precipitation process was investigated. Analytical (99%) and technical (80%) purity grades were analyzed. In all cases three 20 g portions of the acid per 1 dm
3 of the solution were added. The results of the tests are presented in
Table 6 and
Figure 3.
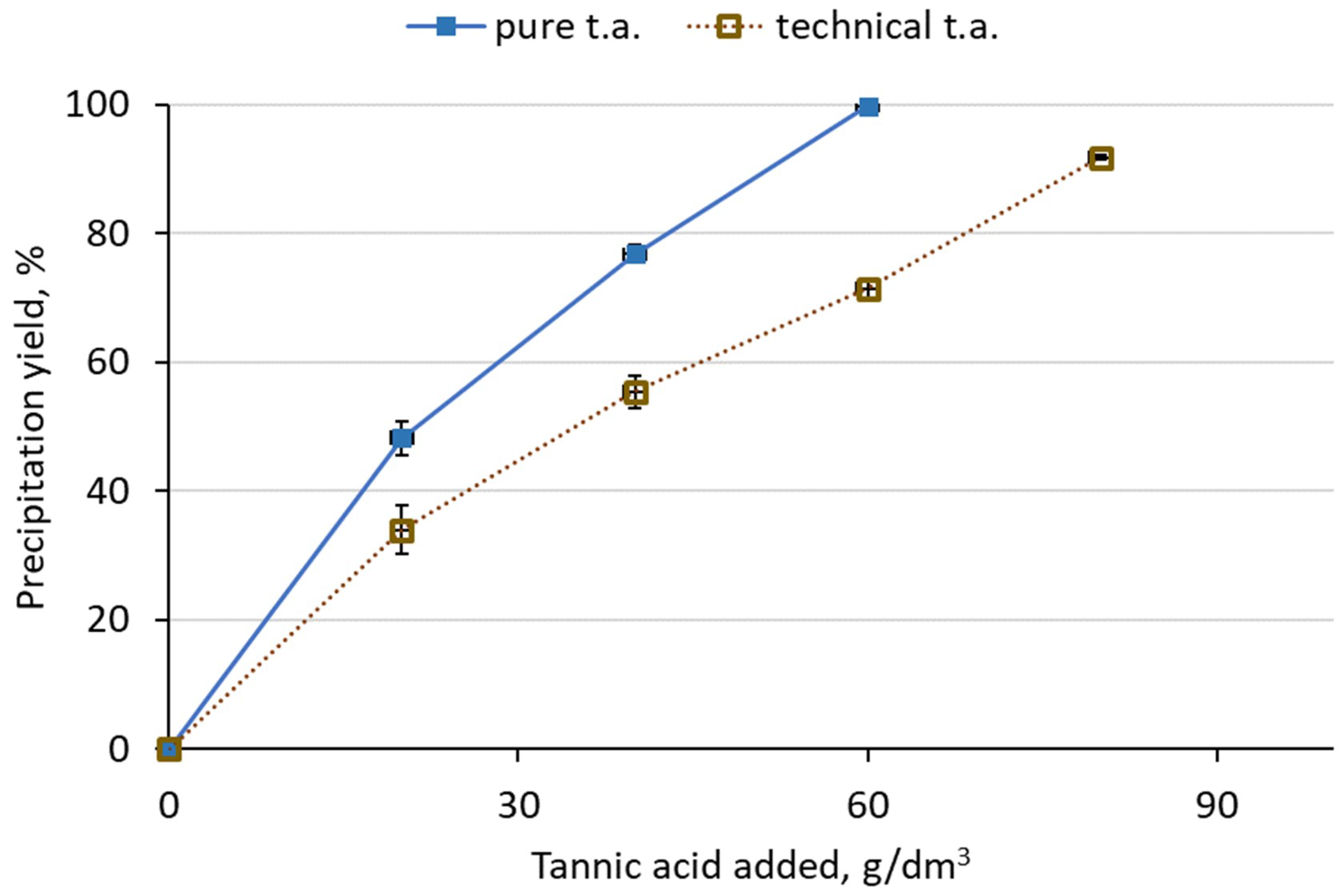
The purity of tannic acid had some influence on the Ge content in the solution. A higher decrease of Ge concentration was achieved for the pure form. It was noticed that for 60 g/dm3 active tannic acid added in pure form, Ge concentration was 0.013 g/dm3, while precipitation yield exceeded 99%. For technical tannic acid, Ge concentration was 0.46 g/dm3, while precipitation yield was 92%.
4. Discussion
During the tests on the precipitation of Ge-tannin complex from the solution containing Sn, it was found that Ge was almost completely precipitated. However, Sn and In were also present in the precipitate. Sn was present in the solution in divalent form, because its tetravalent form is not soluble in weak sulfuric acid. Divalent Sn was partially precipitated by tannic acid. In is not precipitated by tannic acid [
20]. Therefore, its presence may be explained by adsorption of In on precipitate surface. The highest Ge precipitation yield was at 90 °C. It may be explained by an increase of tannic acid solubility and reaction rate.
Detinning of the solution with H2O2 allowed almost complete removal of Sn from the solution. Sn was oxidized to its tetravalent state, which precipitated from weakly acidic sulfate solution. During precipitation, parts of Ge and In were coprecipitated on the surface of the tiny SnO2 precipitate.
In the case of detinned solution, it was noticed that less tannic acid was consumed to precipitate >99% Ge. It was due to the lower Ge and Sn content in the detinned solution. Original leachates contained 6.3–7.8 g/dm
3 Ge, while the detinned one contained 5.6 g/dm
3. Part of the tannin was also used for Sn precipitation. Therefore, in the first case, tannic acid consumption was 100 g/dm
3, whereas in the second one, it was 60 g/dm
3. To achieve >99% Ge precipitation yield and Ge concentration <0.05 g/dm
3, ca. 11–13 g of pure tannic acid should be used per each 1 g of dissolved Ge. This level of tannic acid consumption was achieved for Ge concentration level >5 g/dm
3. For lower Ge concentrations more tannin should be used—e.g., it was reported that for 0.06 g/dm
3, 10 g of tannic acid was needed to precipitate 0.02811 g Ge [
12].
The form of tannic acid had an influence mainly on the process kinetics. When 60 g of tannic acid solution was added at once, over 95% of Ge was precipitated after just 5 min. For the powder form, more than 60 min was required to achieve a similar result. It is connected with the availability of tannic acid molecules. When the dissolved form is used, the reaction takes place almost immediately, whereas the powder form is first dissolving and then dissolved molecules react with Ge. Slight differences in Ge precipitation yields were observed, when smaller amounts of tannic acid were applied. However, the differences decrease as more acid was added. The purity of tannic acid also had an influence on the precipitation yield of Ge. Technical tannic acid contained 80% of active compounds. It was noticed that if 60 g/dm3 tannic acid was added to the pure one, >99% Ge was precipitated, while for the technical one it was 71%.
The presence of In in the precipitate may be explained by the high concentration of the precipitate in the suspension and high moisture (ca. 55%) of the filtered solid. It was not possible to remove whole In during post-filtration washing.
Based on the results of the tests, the following procedure for leachate processing may be proposed. Firstly, solution is detinned using H2O2 and heated to 70 °C to remove any H2O2 remaining in the liquid and filtered. Then tannic acid is added to the solution at 80–90 °C while mixing. The process is carried out for at least 90 min. The selection of the tannic acid form should depend on several factors. The solution may be used to reduce process time; however, the amount of generated effluents would increase. Technical tannic acid may be also used to reduce process costs; however the final Ge-tannin precipitate will contain less Ge than one precipitated with pure tannic acid.
The main product, i.e., Ge-tannin precipitate, may be later used for Ge recovery either by incineration or another technique, which may allow the reuse of tannic acid. The precipitate from detinning may be later re-leached with dross to recover at least part of the Ge and In. The resulting solution contains >1.3 g/dm3 In, and therefore it may be used for In recovery by, e.g., precipitation.
5. Conclusions
The precipitation of Ge from leachate containing mainly Zn, Ge, In, and Sn with tannic acid was investigated. Precipitation yields of the elements were determined. It was found that detinning by H2O2 is a necessary step. It allows the removal of Sn, which after the addition of tannic acid co-precipitates with Ge. Precipitation yield of Ge increases with temperature, process time and amount of added tannic acid. The preferred process conditions for Ge precipitation using tannic acid powder are 80–90 °C, 90 min, while for 20 wt% solution, >98% Ge is precipitated after 15 min. Tannic acid solution allows a faster precipitation process, but it also increases the volume of generated effluents. The precipitation of Ge with tannin is quite selective—Ge precipitation yield is >99%, while In is <20%.
Author Contributions
Conceptualization, M.D.; methodology, M.D. and G.B.; investigation, M.D., M.K., and P.K.; resources, K.L.-S.; writing the original draft preparation, M.D.; writing, reviewing and editing, A.C., K.L.-S., and M.C.; visualization, M.D. and M.C.; supervision, M.D. and A.C.; funding acquisition, A.C.
Funding
This activity has received funding from the European Institute of Innovation and Technology (EIT) under grant agreement No [EIT/EIT Raw Materials/SGA2018]. This European body receives support from the Horizon 2020 research and innovation program.
Acknowledgments
We would like to thank IMN Department of Analytical Chemistry for analysis of the samples.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- European Commision. Communication from the Comission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the 2017 list of Critical Raw Materials for the EU; European Commision: Brussels, Belgium, 2017. [Google Scholar]
- U.S. Geological Survey. Mineral Commodities Summaries 2018: Germanium; U.S. Geological Survey: Reston, VA, USA, 2018.
- Torma, A.E.; Jian, H. Extraction Processes for Gallium and Germanium. Miner. Process. Extr. Metall. Rev. 1991, 7, 235–258. [Google Scholar] [CrossRef]
- Virolainen, S.; Heinonen, J.; Paatero, E. Selective recovery of germanium with N-methylglucamine functional resin from sulfate solutions. Sep. Purif. Technol. 2013, 104, 193–199. [Google Scholar] [CrossRef]
- Nusen, S.; Zhu, Z.; Chairuangsri, T.; Cheng, C.Y. Recovery of germanium from synthetic leach solution of zinc refinery residues by synergistic solvent extraction using LIX 63 and Ionquest 801. Hydrometallurgy 2015, 151, 122–132. [Google Scholar] [CrossRef]
- Nozoe, A.; Ohto, K.; Kawakita, H. Germanium Recovery using Catechol Complexation and Permeation through an Anion-Exchange Membrane. Sep. Sci. Technol. 2012, 47, 62–65. [Google Scholar] [CrossRef]
- Harbuck, D.D.; Judd, J.C.; Behunin, D.V. Germanium Solvent Extraction from Sulfuric Acid Solutions (and Co-Extraction of Germanium and Gallium). Solvent Extr. Ion Exch. 1991, 9, 383–401. [Google Scholar] [CrossRef]
- Bayat, S.; Aghazadeh, S.; Noaparast, M.; Gharabaghi, M.; Taheri, B. Germanium separation and purification by leaching and precipitation. J. Cent. South Univ. 2016, 23, 2214–2222. [Google Scholar] [CrossRef]
- Davies, G.R.; Morgan, G. The gravimetric determination of germanium. Analyst 1938, 63, 388–397. [Google Scholar] [CrossRef]
- Holness, H. The precipitation of germanium by tannin. Anal. Chim. Acta 1948, 2, 254–260. [Google Scholar] [CrossRef]
- Slabbert, N. Complexation of condensed tannins with metal-ions. In Plant Polyphenols; Springer: Boston, MA, USA, 1992; Volume 59, pp. 421–436. [Google Scholar]
- Liang, D.Q.; Wang, J.K.; Wang, Y.H.; Wang, F.; Jiang, J.B. Behavior of tannins in germanium recovery by tannin process. Hydrometallurgy 2008, 93, 140–142. [Google Scholar] [CrossRef]
- Zhang, L.L.; Liu, Y.C.; Hu, X.Y.; Wang, Y.M.; Xu, M. Binding and Precipitation of Germanium(IV) by Penta-O-galloyl-beta-D-glucose. J. Agric. Food Chem. 2018, 66, 11000–11007. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, J.; Anderson, C.G. Tannins in Mineral Processing and Extractive Metallurgy. Metals 2015, 5, 1520–1542. [Google Scholar] [CrossRef] [Green Version]
- Kul, A.; Topkaya, Y. Recovery of germanium and other valuable metals from zinc plant residues. Hydrometallurgy 2008, 92, 87–94. [Google Scholar] [CrossRef]
- Arroyo Torralvo, F.; Fernández-Pereira, C.; García Villard, E.; Luna, Y.; Leiva, C.; Vilches, L.; Villegas, R. Low environmental impact process for germanium recovery from an industrial residue. Miner. Eng. 2018, 128, 106–114. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; Lopez-Soler, A.; Chimenos, J.M.; Fernandez, A.I.; Burgos, S.; Pena, F.G. Ge extraction from gasification fly ash. Fuel 2005, 84, 1384–1392. [Google Scholar] [CrossRef]
- Kulawik, S.; Prajsnar, R.; Chmielarz, A.; Cybulski, A.; Michalski, R.; Klejnowska, K.; Drzazga, M.; Krawiec, G. Thermal Oxidation of Indium, Germanium, and Tin form Lead-Bearing Alloys Generaterd in Zinc Refinement. Metals 2019, 9, 166. [Google Scholar] [CrossRef]
- Drzazga, M.; Prajsnar, R.; Chmielarz, A.; Benke, G.; Leszczyńska-Sejda, K.; Ciszewski, M.; Bilewska, K.; Krawiec, G. Germanium and Indium Recovery from Zinc Metallurgy by-Products—Dross Leaching in Sulphuric and Oxalic Acids. Metals 2018, 8, 1041. [Google Scholar] [CrossRef]
- Busev, A.I. The Analytical Chemistry of Indium; Pergamon Press: Oxford, UK, 1962. [Google Scholar]
Figure 1.
Influence of (A) temperature and (B) tannic acid amount on precipitation yield of Ge, In, and Sn from the solution ((A)—2 h, 100 g/dm3 pure tannic acid, 1 portion; (B)—90 °C, pure tannic acid added in portions, 1 h between portions).
Figure 1.
Influence of (A) temperature and (B) tannic acid amount on precipitation yield of Ge, In, and Sn from the solution ((A)—2 h, 100 g/dm3 pure tannic acid, 1 portion; (B)—90 °C, pure tannic acid added in portions, 1 h between portions).
Figure 2.
Influence of (A) tannic acid added in powder form and as 20% solution and (B) time on the precipitation yield of Ge and In from detinned solution ((A)—90 °C, tannic acid added in portions, 1 h between portions; (B)—90 °C, 60 g/dm3 tannic acid added in 1 portion).
Figure 2.
Influence of (A) tannic acid added in powder form and as 20% solution and (B) time on the precipitation yield of Ge and In from detinned solution ((A)—90 °C, tannic acid added in portions, 1 h between portions; (B)—90 °C, 60 g/dm3 tannic acid added in 1 portion).
Figure 3.
Influence of tannic acid purity on Ge precipitation yield in the solution (90 °C, tannic acid added in portions, 1 h between portions).
Figure 3.
Influence of tannic acid purity on Ge precipitation yield in the solution (90 °C, tannic acid added in portions, 1 h between portions).
Table 1.
Composition of the solution and precipitate after precipitation with tannic acid (2 h, 100 g/dm3 pure tannic acid, 1 portion).
Table 1.
Composition of the solution and precipitate after precipitation with tannic acid (2 h, 100 g/dm3 pure tannic acid, 1 portion).
| Temperature °C | Csolution g/dm3 | pHsolution | Cprecipitate % |
|---|
| Ge | In | Sn | Ge | In | Sn |
|---|
| initial | 6.0 | 1.7 | 4.4 | 2.0 | - | - | - |
| 20 | 2.1 | 1.6 | 3.6 | 1.6 | 3.67 | 0.03 | 0.77 |
| 60 | 0.1 | 1.5 | 3.3 | 1.4 | 5.48 | 0.07 | 1.04 |
| 90 | 0.03 | 1.4 | 3.0 | 1.3 | 5.34 | 0.13 | 1.96 |
Table 2.
Composition of the solution and precipitate after precipitation with tannic acid (90 °C, pure tannic acid added in portions, 1 h between portions).
Table 2.
Composition of the solution and precipitate after precipitation with tannic acid (90 °C, pure tannic acid added in portions, 1 h between portions).
| Tannic Acid g/dm3 | Csolution g/dm3 | pHsolution | Cprecipitate % |
|---|
| Ge | pHsolution | Sn | Ge | In | Sn |
|---|
| initial | 7.8 | 2.1 | 5.4 | 2.0 | - | - | - |
| 20 | 5.2 | 2.0 | 4.7 | 1.8 | 9.37 | 0.12 | 1.69 |
| 40 | 2.7 | 1.8 | 3.8 | 1.6 | 8.81 | 0.12 | 1.73 |
| 60 | 1.0 | 1.7 | 3.3 | 1.4 | 8.21 | 0.13 | 1.71 |
| 80 | 0.15 | 1.6 | 3.1 | 1.3 | 7.23 | 0.14 | 1.70 |
| 100 | 0.04 | 1.6 | 2.8 | 1.3 | 6.55 | 0.13 | 1.65 |
Table 3.
Composition of the solution and precipitate before and after tin removal (70 °C, 1 h, 6 cm3/dm3 H2O2).
Table 3.
Composition of the solution and precipitate before and after tin removal (70 °C, 1 h, 6 cm3/dm3 H2O2).
| Step | Csolution g/dm3 | pHsolution | Cprecipitate % |
|---|
| Ge | In | Sn | Zn | Ge | In | Sn | Zn |
|---|
| initial | 6.3 | 1.8 | 4.6 | 5.7 | 0.1 | - | - | - | - |
| detinned | 5.6 | 1.6 | 0.024 | 5.7 | 0.0 | 7.97 | 2.74 | 43.9 | 0.53 |
Table 4.
Composition of the solution and precipitate after precipitation with tannic acid powder and 20 wt% solution (90 °C, tannic acid added in portions, 1 h between portions).
Table 4.
Composition of the solution and precipitate after precipitation with tannic acid powder and 20 wt% solution (90 °C, tannic acid added in portions, 1 h between portions).
| Tannic Acid Form | Tannic Acid g/dm3 | Csolution g/dm3 | pHsolution | Cprecipitate % |
|---|
| Ge | In | Ge | In |
|---|
| - | initial | 5.6 | 1.6 | 2.0 | - | - |
| powder | 20 | 2.4 | 1.3 | 1.6 | 7.09 | 0.14 |
| 40 | 0.9 | 1.2 | 1.3 | 6.57 | 0.17 |
| 60 | 0.01 | 1.1 | 1.3 | 6.09 | 0.21 |
| 20 wt% solution | 20 | 1.9 | 1.3 | 1.5 | 7.45 | 0.19 |
| 40 | 0.9 | 1.2 | 1.3 | 6.74 | 0.23 |
| 60 | 0.006 | 1.1 | 1.2 | 5.96 | 0.26 |
Table 5.
Composition of the solution after precipitation with tannic acid powder and 20 wt% solution (90 °C, 60 g/dm3 active tannic added in 1 portion).
Table 5.
Composition of the solution after precipitation with tannic acid powder and 20 wt% solution (90 °C, 60 g/dm3 active tannic added in 1 portion).
| Time min | Csolution g/dm3 |
|---|
| Powder | 20 wt% Solution |
|---|
| Ge | In | Ge | In |
|---|
| initial | 5.6 | 1.6 | 5.6 | 1.6 |
| 15 | 1.6 | 1.4 | 0.13 | 1.3 |
| 30 | 0.61 | 1.3 | 0.11 | 1.3 |
| 60 | 0.33 | 1.3 | 0.074 | 1.3 |
| 90 | 0.12 | 1.3 | 0.066 | 1.3 |
Table 6.
Concentration of germanium after precipitation with pure and technical tannic acid powder (90 °C, tannic acid added in portions, 1 h between portions).
Table 6.
Concentration of germanium after precipitation with pure and technical tannic acid powder (90 °C, tannic acid added in portions, 1 h between portions).
| Tannic Acid g/dm3 | CGe,solution g/dm3 |
|---|
| Pure (99%) | Technical (80%) |
|---|
| initial | 5.6 | 5.6 |
| 20 | 2.9 | 3.7 |
| 40 | 1.3 | 2.5 |
| 60 | 0.013 | 1.6 |
| 80 | - | 0.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).