Pump-Probe Time-Resolved Serial Femtosecond Crystallography at SACLA: Current Status and Data Collection Strategies
Abstract
1. Introduction
2. Pump-Probe Time-Resolved SFX Experiments
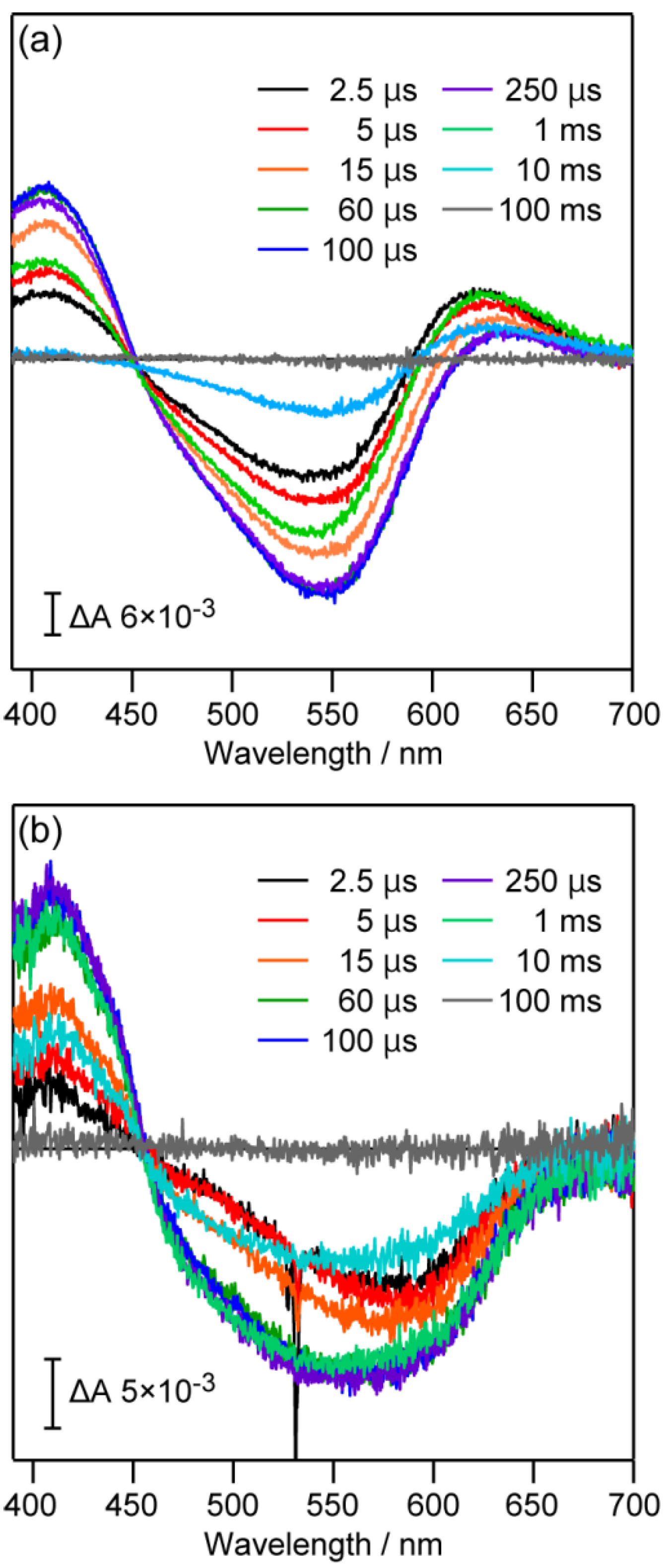
3. In Crystallo TR Ultraviolet-Visible Spectroscopy
4. Data Collection Strategy for Pump-Probe TR-SFX Using a Highly Viscous Sample
- The data processing pipeline [33], which was based on Cheetah [34], was used for real-time feedback during the beamtime, hit finding, and sorting of “Light” and “Dark” images. In the pump-laser setup, a portion of the pump beam (~5%) was picked off and its intensity signal that was detected by a photodiode was used to tag the image as “Light”.
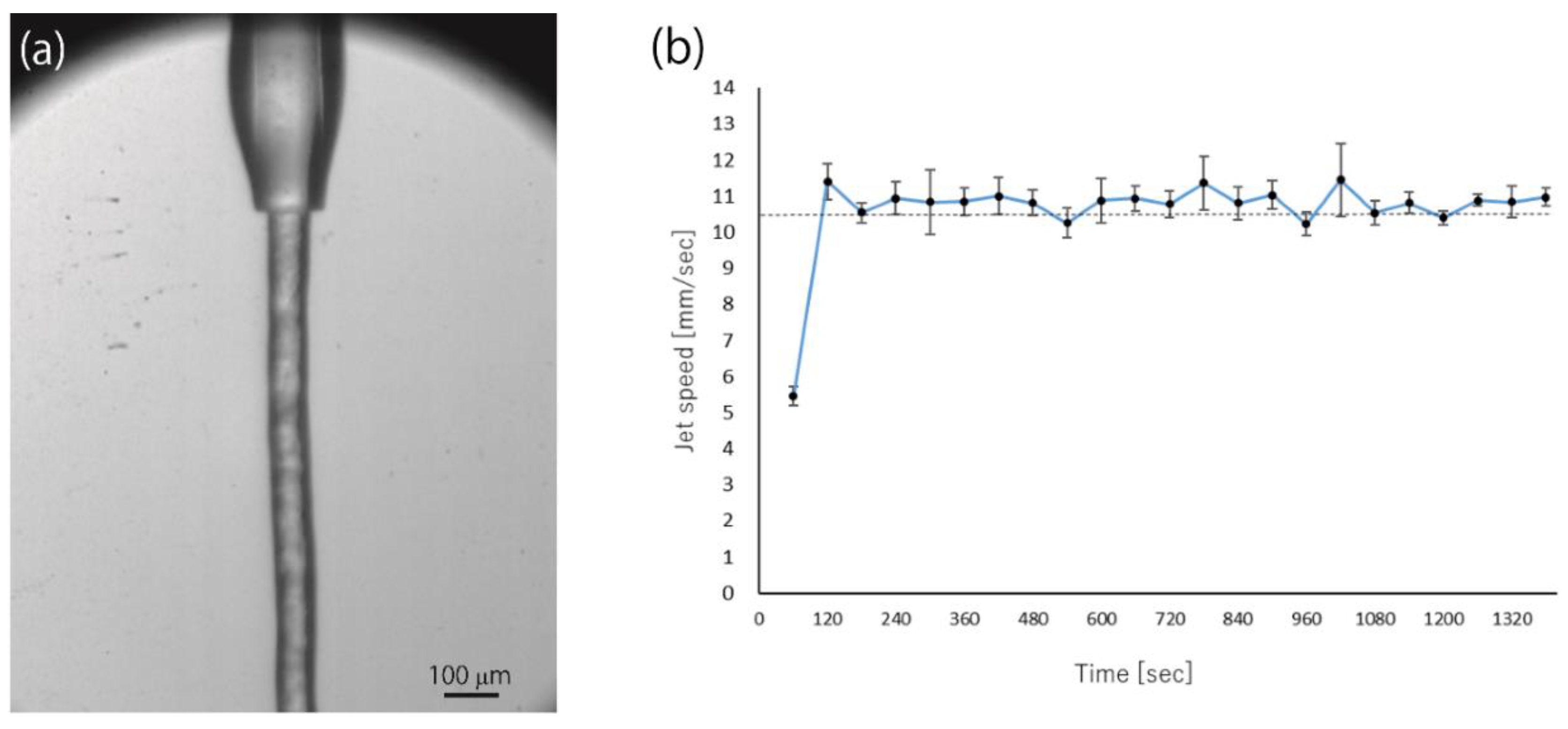
- Protein microcrystals that were embedded in the carrier media were injected to the X-ray beam interaction point while using the high-viscosity sample injector [21]. The stability of the sample streams was essential for pump-probe TR-SFX experiments, because the pump-illuminated crystals must be exchanged with the fresh ones before the “Dark” data collection.
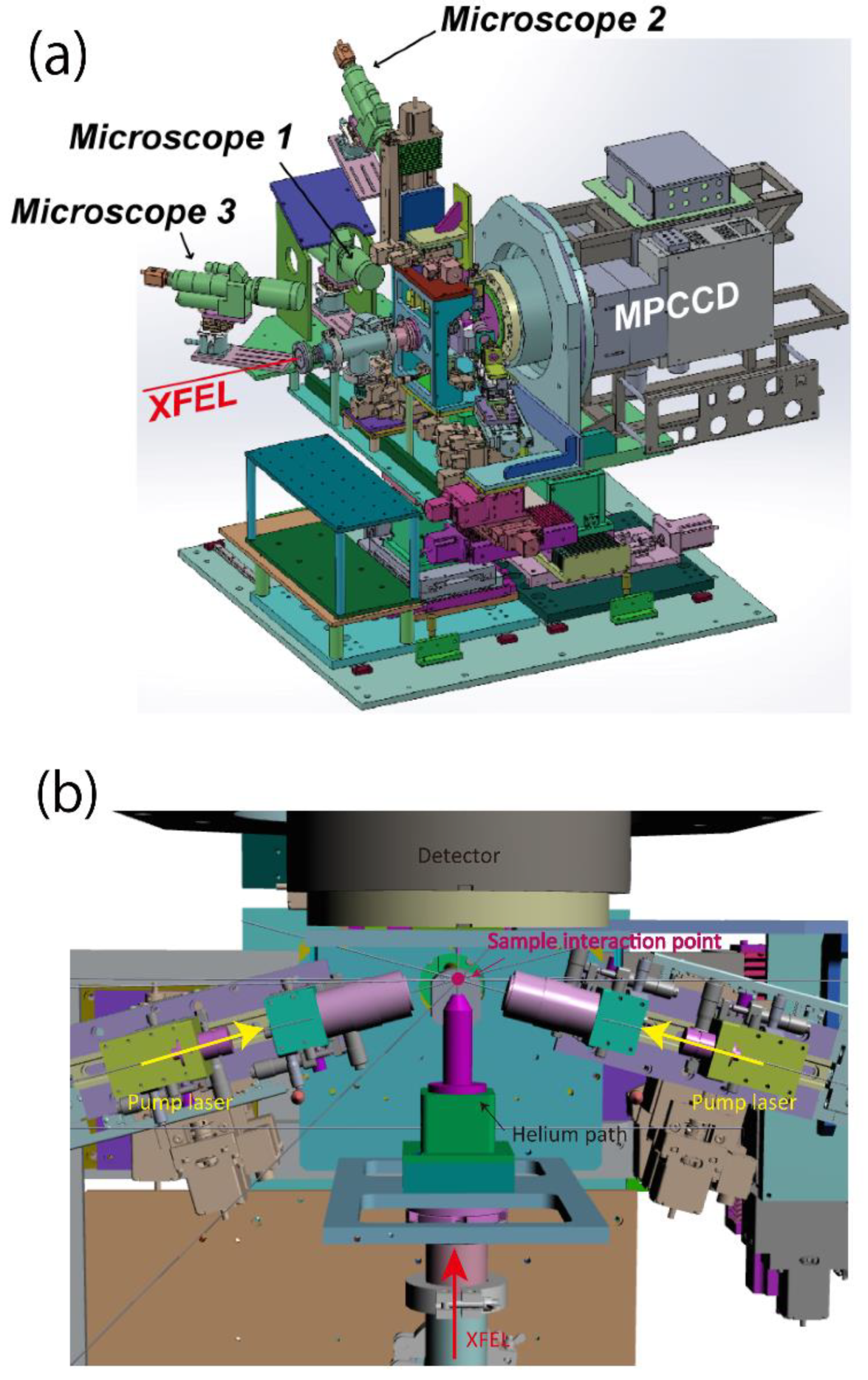
- The injector height was aligned by using the sample stage (Z direction), so that the injector bottom was distant to the XFEL focal point by 300 µm. The monitor image of Microscope 1 was helpful (Figure 1) for the alignment.
- The sample stream was aligned to the XFEL focal point by using the sample stage (X direction). The monitor image of Microscope 1 was also helpful for this alignment. The hit rate was maximized in the best alignment.
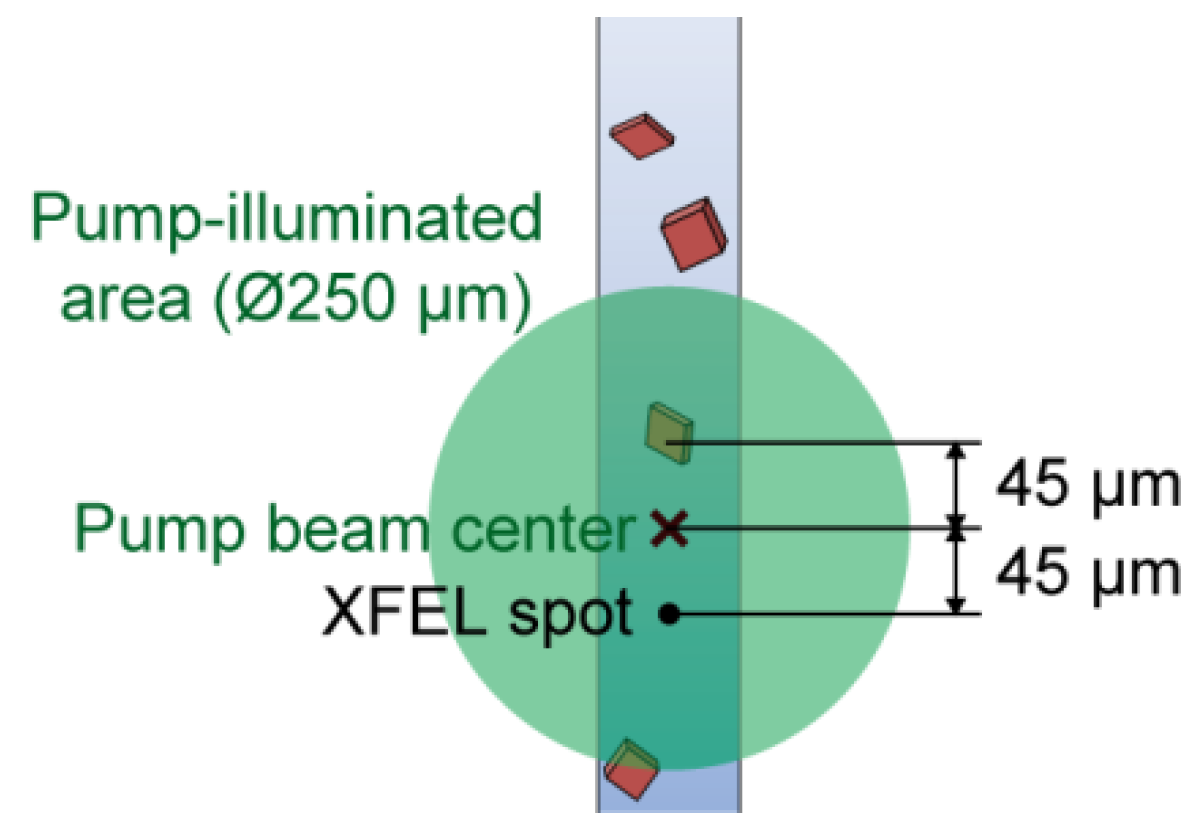
- The sample stream was aligned to the pump focal area by using the sample stage (Y direction). The monitor image of Microscope 2 was helpful in this alignment (Figure 1). In the best alignment, the pump-light scattering from the sample stream was maximized.
- The delay time (Δt) between the pump and XFEL pulses, which is controlled by a pulse generator (DG645, Stanford Research Systems), was set. The timing jitter for Δt < 16.7 ms was <1 ns, but it was approximately 0.1 ms for Δt > 16.7 ms at SACLA.
- Diffraction images were collected: typically, the hit rate of highly viscous samples is 20–30% by adjusting the crystal density to avoid multiple hits. 10,000–15,000 diffraction patterns are collected as hit “Light” images in an hour in the case of the 15-Hz pump laser condition. Given the typical indexing rate of 60–70%, 6000–10,000 indexed images are obtained in an hour. Because ~20,000 indexed images are needed to clearly visualize the differences between “Light” and “Dark”, one dataset takes 3–4 h, including the time for sample replenishment.
- Measurement was repeated by using different values of Δt to afford a molecular movie.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moffat, K. Time-resolved macromolecular crystallography. Annu. Rev. Biophys. Biophys. Chem. 1989, 18, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Neutze, R.; Wouts, R.; van der Spoel, D.; Weckert, E.; Hajdu, J. Potential for biomolecular imaging with femtosecond X-ray pulses. Nature 2000, 406, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.N.; Fromme, P.; Barty, A.; White, T.A.; Kirian, R.A.; Aquila, A.; Hunter, M.S.; Schulz, J.; DePonte, D.P.; Weierstall, U.; et al. Femtosecond X-ray protein nanocrystallography. Nature 2011, 470, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Boutet, S.; Lomb, L.; Williams, G.J.; Barends, T.R.; Aquila, A.; Doak, R.B.; Weierstall, U.; DePonte, D.P.; Steinbrener, J.; Shoeman, R.L.; et al. High-resolution protein structure determination by serial femtosecond crystallography. Science 2012, 337, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Aoyagi, H.; Asaka, T.; Asano, Y.; Azumi, N.; Bizen, T.; Ego, H.; Fukami, K.; Fukui, T.; Furukawa, Y.; et al. A compact X-ray free-electron laser emitting in the sub-angstrom region. Nat. Photonics 2012, 6, 540–544. [Google Scholar] [CrossRef]
- Emma, P.; Akre, R.; Arthur, J.; Bionta, R.; Bostedt, C.; Bozek, J.; Brachmann, A.; Bucksbaum, P.; Coffee, R.; Decker, F.J.; et al. First lasing and operation of an angstrom-wavelength free-electron laser. Nat. Photonics 2010, 4, 641–647. [Google Scholar] [CrossRef]
- Nango, E.; Royant, A.; Kubo, M.; Nakane, T.; Wickstrand, C.; Kimura, T.; Tanaka, T.; Tono, K.; Song, C.; Tanaka, R.; et al. A three-dimensional movie of structural changes in bacteriorhodopsin. Science 2016, 354, 1552–1557. [Google Scholar] [CrossRef]
- Suga, M.; Akita, F.; Sugahara, M.; Kubo, M.; Nakajima, Y.; Nakane, T.; Yamashita, K.; Umena, Y.; Nakabayashi, M.; Yamane, T.; et al. Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 2017, 543, 131–135. [Google Scholar] [CrossRef]
- Tosha, T.; Nomura, T.; Nishida, T.; Saeki, N.; Okubayashi, K.; Yamagiwa, R.; Sugahara, M.; Nakane, T.; Yamashita, K.; Hirata, K.; et al. Capturing an initial intermediate during the P450nor enzymatic reaction using time-resolved XFEL crystallography and caged-substrate. Nat. Commun. 2017, 8, 1585. [Google Scholar] [CrossRef]
- Levantino, M.; Yorke, B.A.; Monteiro, D.C.; Cammarata, M.; Pearson, A.R. Using synchrotrons and XFELs for time-resolved X-ray crystallography and solution scattering experiments on biomolecules. Curr. Opin. Struct. Biol. 2015, 35, 41–48. [Google Scholar] [CrossRef]
- Aquila, A.; Hunter, M.S.; Doak, R.B.; Kirian, R.A.; Fromme, P.; White, T.A.; Andreasson, J.; Arnlund, D.; Bajt, S.; Barends, T.R.; et al. Time-resolved protein nanocrystallography using an X-ray free-electron laser. Opt. Express 2012, 20, 2706–2716. [Google Scholar] [CrossRef] [PubMed]
- Tenboer, J.; Basu, S.; Zatsepin, N.; Pande, K.; Milathianaki, D.; Frank, M.; Hunter, M.; Boutet, S.; Williams, G.J.; Koglin, J.E.; et al. Time-resolved serial crystallography captures high-resolution intermediates of photoactive yellow protein. Science 2014, 346, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Pande, K.; Hutchison, C.D.; Groenhof, G.; Aquila, A.; Robinson, J.S.; Tenboer, J.; Basu, S.; Boutet, S.; DePonte, D.P.; Liang, M.; et al. Femtosecond structural dynamics drives the trans/cis isomerization in photoactive yellow protein. Science 2016, 352, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Barends, T.R.; Foucar, L.; Ardevol, A.; Nass, K.; Aquila, A.; Botha, S.; Doak, R.B.; Falahati, K.; Hartmann, E.; Hilpert, M.; et al. Direct observation of ultrafast collective motions in CO myoglobin upon ligand dissociation. Science 2015, 350, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Williams, G.J.; Messerschmidt, M.; Seibert, M.M.; Montanez, P.A.; Hayes, M.; Milathianaki, D.; Aquila, A.; Hunter, M.S.; Koglin, J.E.; et al. The Coherent X-ray Imaging instrument at the Linac Coherent Light Source. J. Synchrotron Radiat. 2015, 22, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Tono, K.; Nango, E.; Sugahara, M.; Song, C.Y.; Park, J.; Tanaka, T.; Tanaka, R.; Joti, Y.; Kameshima, T.; Ono, S.; et al. Diverse application platform for hard X-ray diffraction in SACLA (DAPHNIS): Application to serial protein crystallography using an X-ray free-electron laser. J. Synchrotron Radiat. 2015, 22, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Sierra, R.G.; Batyuk, A.; Sun, Z.; Aquila, A.; Hunter, M.S.; Lane, T.J.; Liang, M.; Yoon, C.H.; Alonso-Mori, R.; Armenta, R.; et al. The Macromolecular Femtosecond Crystallography Instrument at the Linac Coherent Light Source. J. Synchrotron Radiat. 2019, 26, 346–357. [Google Scholar] [CrossRef]
- Kubo, M.; Nango, E.; Tono, K.; Kimura, T.; Owada, S.; Song, C.; Mafune, F.; Miyajima, K.; Takeda, Y.; Kohno, J.Y.; et al. Nanosecond pump-probe device for time-resolved serial femtosecond crystallography developed at SACLA. J. Synchrotron Radiat. 2017, 24, 1086–1091. [Google Scholar] [CrossRef]
- Kameshima, T.; Ono, S.; Kudo, T.; Ozaki, K.; Kirihara, Y.; Kobayashi, K.; Inubushi, Y.; Yabashi, M.; Horigome, T.; Holland, A.; et al. Development of an X-ray pixel detector with multi-port charge-coupled device for X-ray free-electron laser experiments. Rev. Sci. Instrum. 2014, 85. [Google Scholar] [CrossRef]
- Weierstall, U.; James, D.; Wang, C.; White, T.A.; Wang, D.; Liu, W.; Spence, J.C.; Bruce Doak, R.; Nelson, G.; Fromme, P.; et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat. Commun. 2014, 5, 3309. [Google Scholar] [CrossRef]
- Shimazu, Y.; Tono, K.; Tanaka, T.; Yamanaka, Y.; Nakane, T.; Mori, C.; Terakado Kimura, K.; Fujiwara, T.; Sugahara, M.; Tanaka, R.; et al. High-viscosity sample-injection device for serial femtosecond crystallography at atmospheric pressure. J. Appl. Crystallogr. 2019, 52. [Google Scholar] [CrossRef] [PubMed]
- Landau, E.M.; Rosenbusch, J.P. Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 14532–14535. [Google Scholar] [CrossRef] [PubMed]
- DePonte, D.P.; Weierstall, U.; Schmidt, K.; Warner, J.; Starodub, D.; Spence, J.C.H.; Doak, R.B. Gas dynamic virtual nozzle for generation of microscopic droplet streams. J. Phys. D Appl. Phys. 2008, 41, 19. [Google Scholar] [CrossRef]
- Nogly, P.; Panneels, V.; Nelson, G.; Gati, C.; Kimura, T.; Milne, C.; Milathianaki, D.; Kubo, M.; Wu, W.; Conrad, C.; et al. Lipidic cubic phase injector is a viable crystal delivery system for time-resolved serial crystallography. Nat. Commun. 2016, 7, 12314. [Google Scholar] [CrossRef]
- Sugahara, M.; Mizohata, E.; Nango, E.; Suzuki, M.; Tanaka, T.; Masuda, T.; Tanaka, R.; Shimamura, T.; Tanaka, Y.; Suno, C.; et al. Grease matrix as a versatile carrier of proteins for serial crystallography. Nat. Methods 2015, 12, 61–63. [Google Scholar] [CrossRef]
- Sugahara, M.; Nakane, T.; Masuda, T.; Suzuki, M.; Inoue, S.; Song, C.; Tanaka, R.; Nakatsu, T.; Mizohata, E.; Yumoto, F.; et al. Hydroxyethyl cellulose matrix applied to serial crystallography. Sci. Rep. 2017, 7, 703. [Google Scholar] [CrossRef]
- Togashi, T.; Sato, T.; Ogawa, K.; Katayama, T.; Owada, S.; Inubushi, Y.; Tono, K.; Yabashi, M. Arrival-timing diagnostics for pump-probe experiments in SACLA, using X-ray-induced optical transparency in GaAs. In Proceedings of the 19th International Conference on Ultrafast Phenomena, Okinawa, Japan, 7–11 July 2014. [Google Scholar] [CrossRef]
- Mafune, F.; Miyajima, K.; Tono, K.; Takeda, Y.; Kohno, J.Y.; Miyauchi, N.; Kobayashi, J.; Joti, Y.; Nango, E.; Iwata, S.; et al. Microcrystal delivery by pulsed liquid droplet for serial femtosecond crystallography. Acta Crystallogr. D Struct. Biol. 2016, 72, 520–523. [Google Scholar] [CrossRef]
- Zimanyi, L.; Keszthelyi, L.; Lanyi, J.K. Transient spectroscopy of bacterial rhodopsins with an optical multichannel analyzer. 1. Comparison of the photocycles of bacteriorhodopsin and halorhodopsin. Biochemistry 1989, 28, 5165–5172. [Google Scholar] [CrossRef]
- Kouyama, T.; Nasuda-Kouyama, A.; Ikegami, A.; Mathew, M.K.; Stoeckenius, W. Bacteriorhodopsin photoreaction: Identification of a long-lived intermediate N (P, R350) at high pH and its M-like photoproduct. Biochemistry 1988, 27, 5855–5863. [Google Scholar] [CrossRef]
- Fuller, F.D.; Gul, S.; Chatterjee, R.; Burgie, E.S.; Young, I.D.; Lebrette, H.; Srinivas, V.; Brewster, A.S.; Michels-Clark, T.; Clinger, J.A.; et al. Drop-on-demand sample delivery for studying biocatalysts in action at X-ray free-electron lasers. Nat. Methods 2017, 14, 443–449. [Google Scholar] [CrossRef]
- Shimada, A.; Kubo, M.; Baba, S.; Yamashita, K.; Hirata, K.; Ueno, G.; Nomura, T.; Kimura, T.; Shinzawa-Itoh, K.; Baba, J.; et al. A nanosecond time-resolved XFEL analysis of structural changes associated with CO release from cytochrome c oxidase. Sci. Adv. 2017, 3, e1603042. [Google Scholar] [CrossRef] [PubMed]
- Nakane, T.; Joti, Y.; Tono, K.; Yabashi, M.; Nango, E.; Iwata, S.; Ishitani, R.; Nureki, O. Data processing pipeline for serial femtosecond crystallography at SACLA. J. Appl. Crystallogr. 2016, 49, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Barty, A.; Kirian, R.A.; Maia, F.R.; Hantke, M.; Yoon, C.H.; White, T.A.; Chapman, H. Cheetah: Software for high-throughput reduction and analysis of serial femtosecond X-ray diffraction data. J. Appl. Crystallogr. 2014, 47, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nango, E.; Kubo, M.; Tono, K.; Iwata, S. Pump-Probe Time-Resolved Serial Femtosecond Crystallography at SACLA: Current Status and Data Collection Strategies. Appl. Sci. 2019, 9, 5505. https://doi.org/10.3390/app9245505
Nango E, Kubo M, Tono K, Iwata S. Pump-Probe Time-Resolved Serial Femtosecond Crystallography at SACLA: Current Status and Data Collection Strategies. Applied Sciences. 2019; 9(24):5505. https://doi.org/10.3390/app9245505
Chicago/Turabian StyleNango, Eriko, Minoru Kubo, Kensuke Tono, and So Iwata. 2019. "Pump-Probe Time-Resolved Serial Femtosecond Crystallography at SACLA: Current Status and Data Collection Strategies" Applied Sciences 9, no. 24: 5505. https://doi.org/10.3390/app9245505
APA StyleNango, E., Kubo, M., Tono, K., & Iwata, S. (2019). Pump-Probe Time-Resolved Serial Femtosecond Crystallography at SACLA: Current Status and Data Collection Strategies. Applied Sciences, 9(24), 5505. https://doi.org/10.3390/app9245505




