Autologous Tooth Graft after Endodontical Treated Used for Socket Preservation: A Multicenter Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Preoperative Work-Up
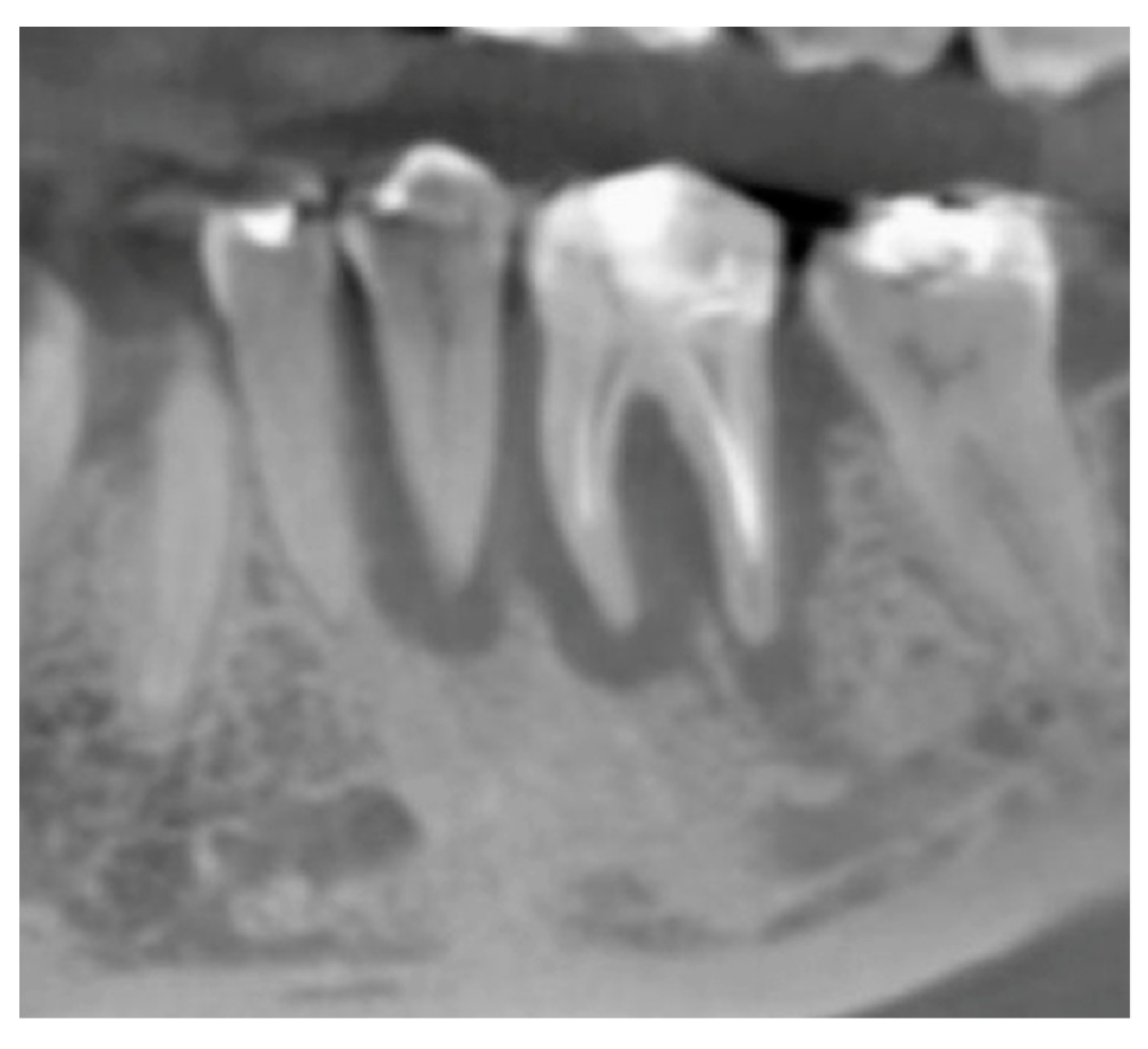
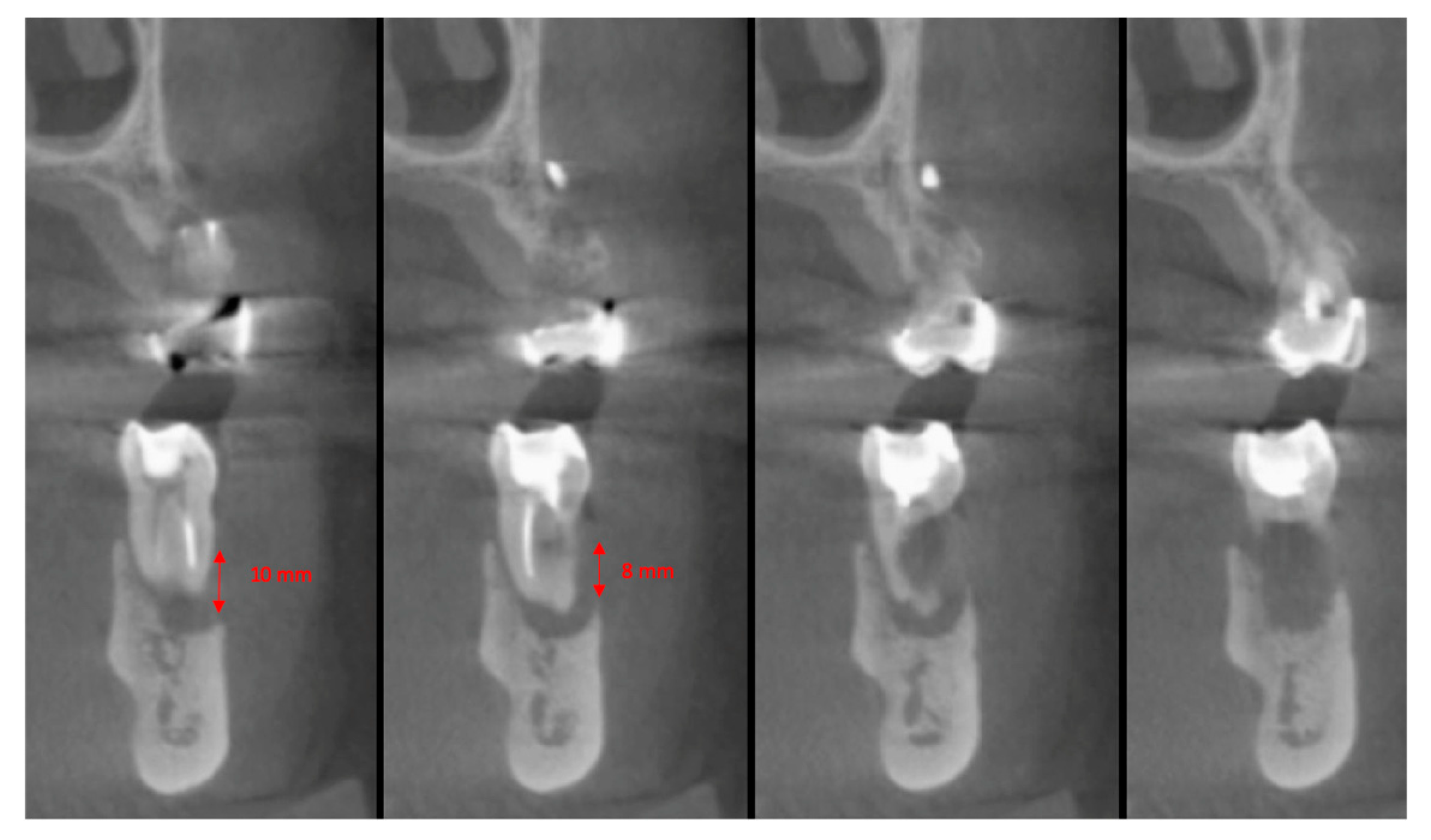
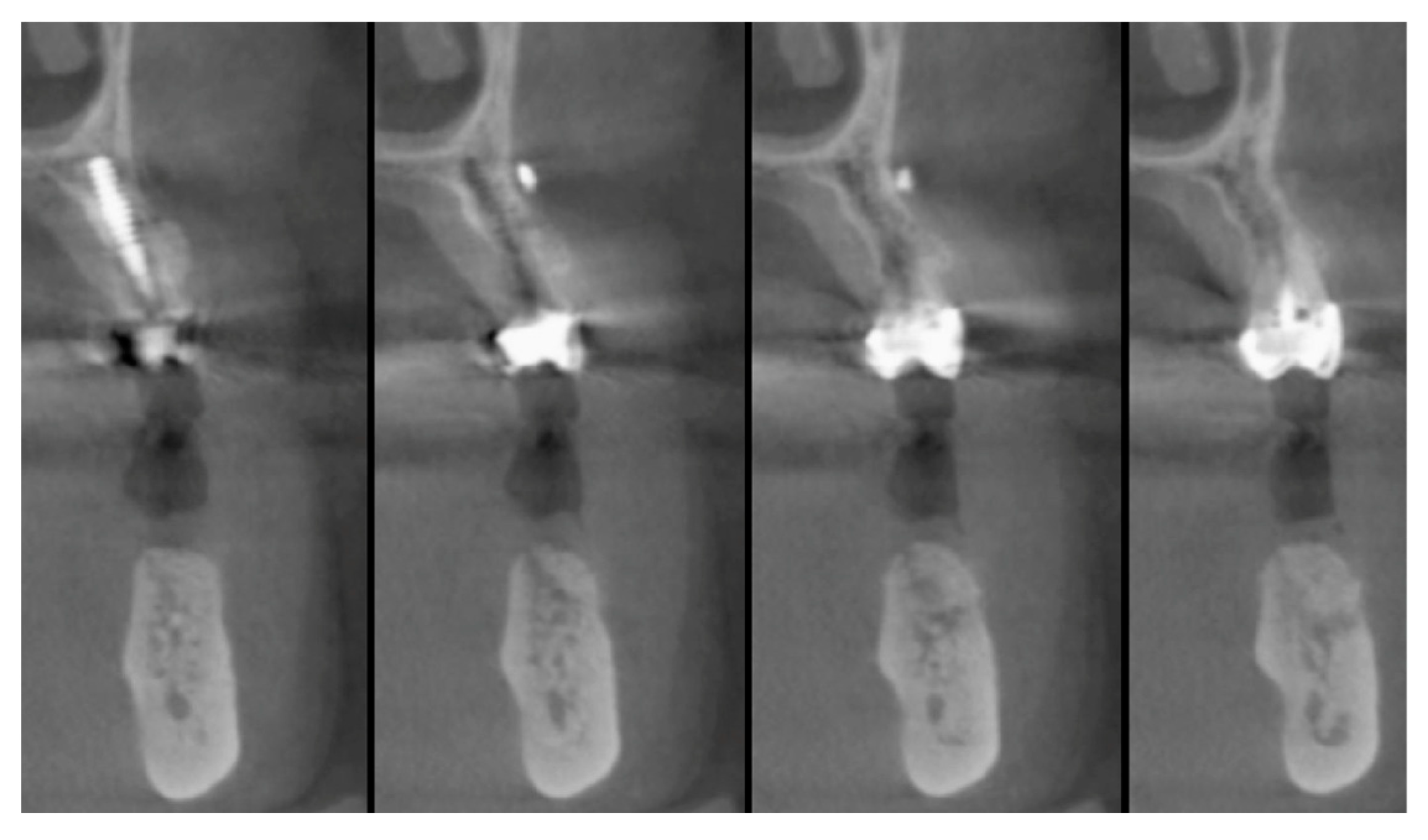
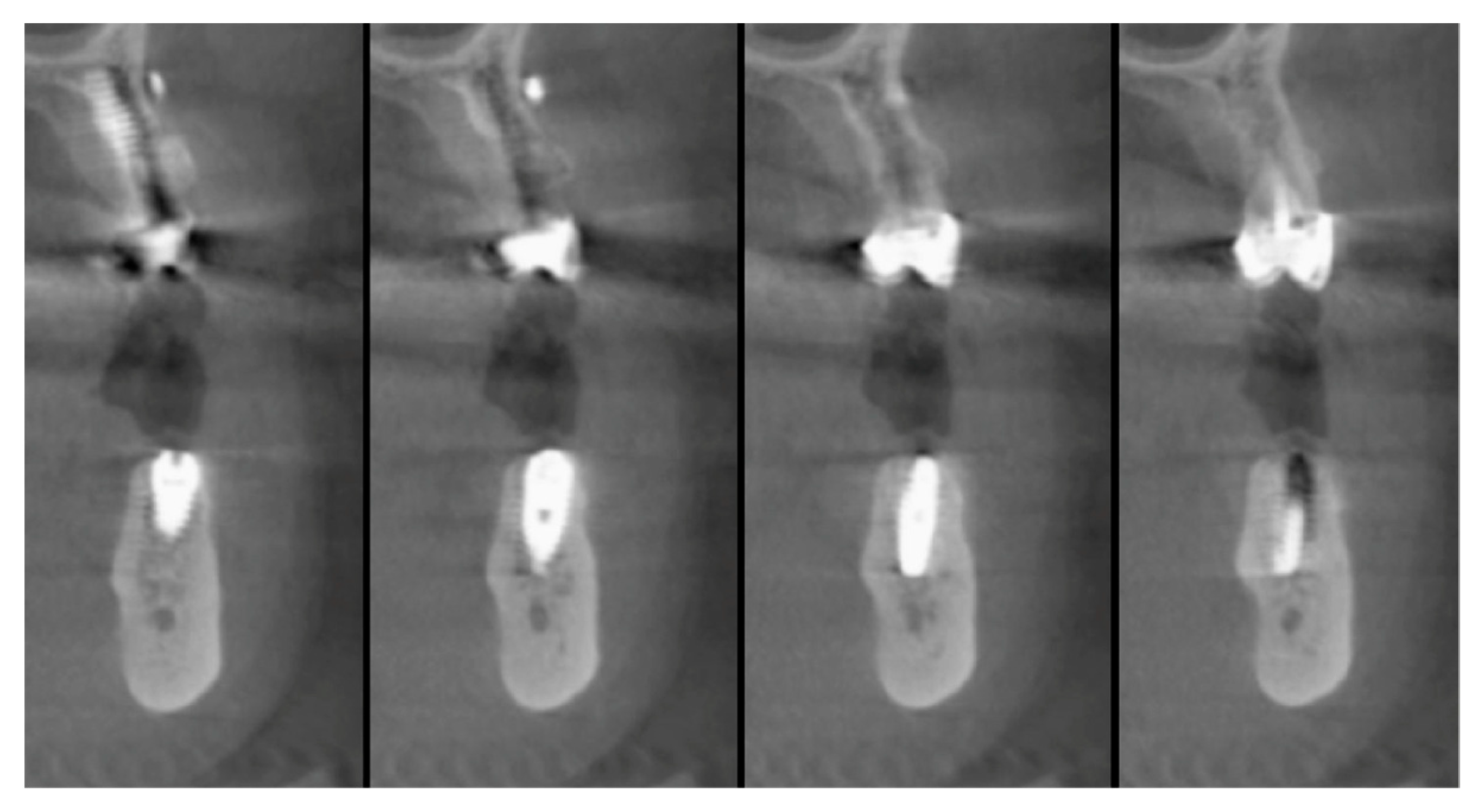
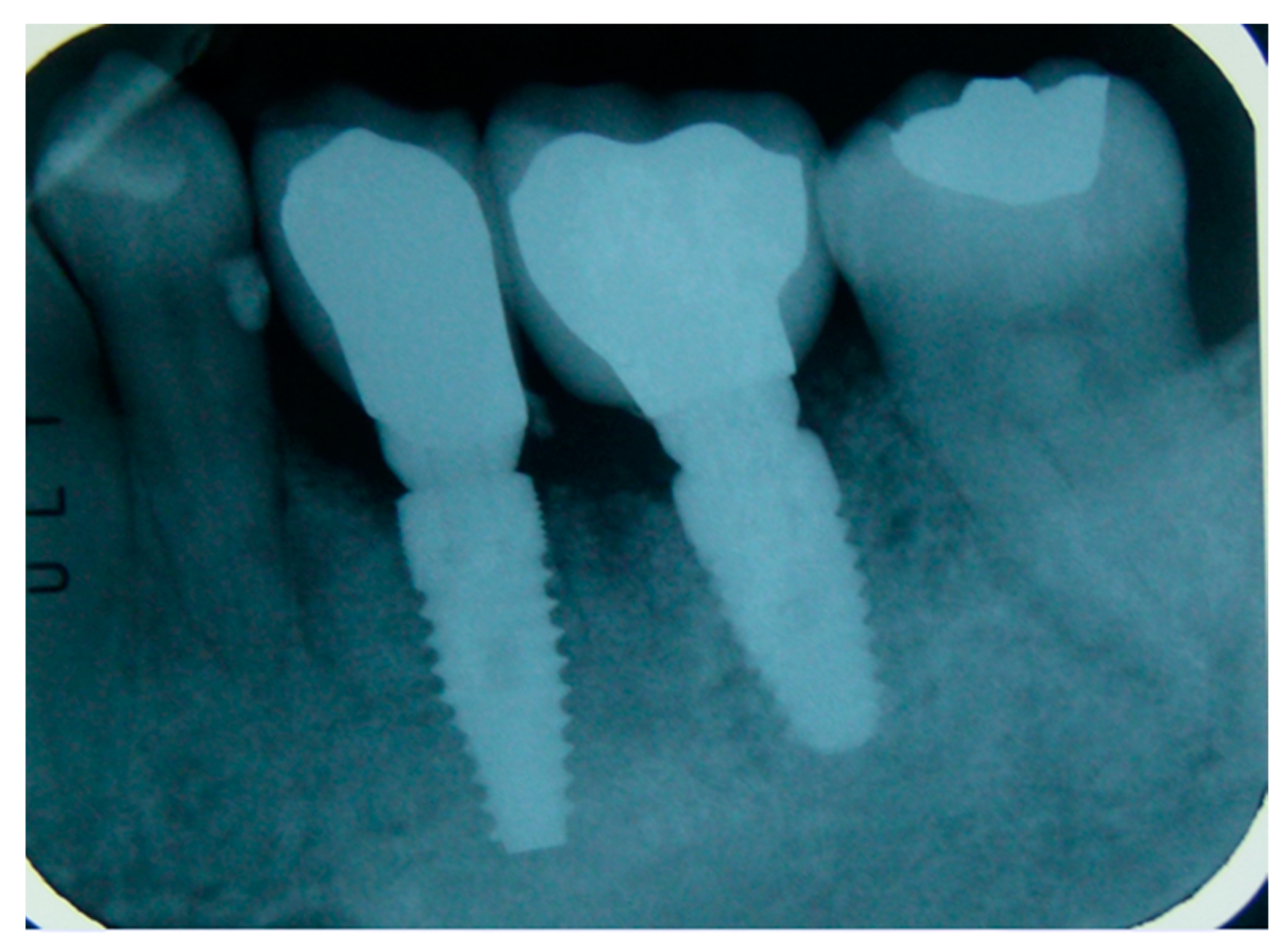
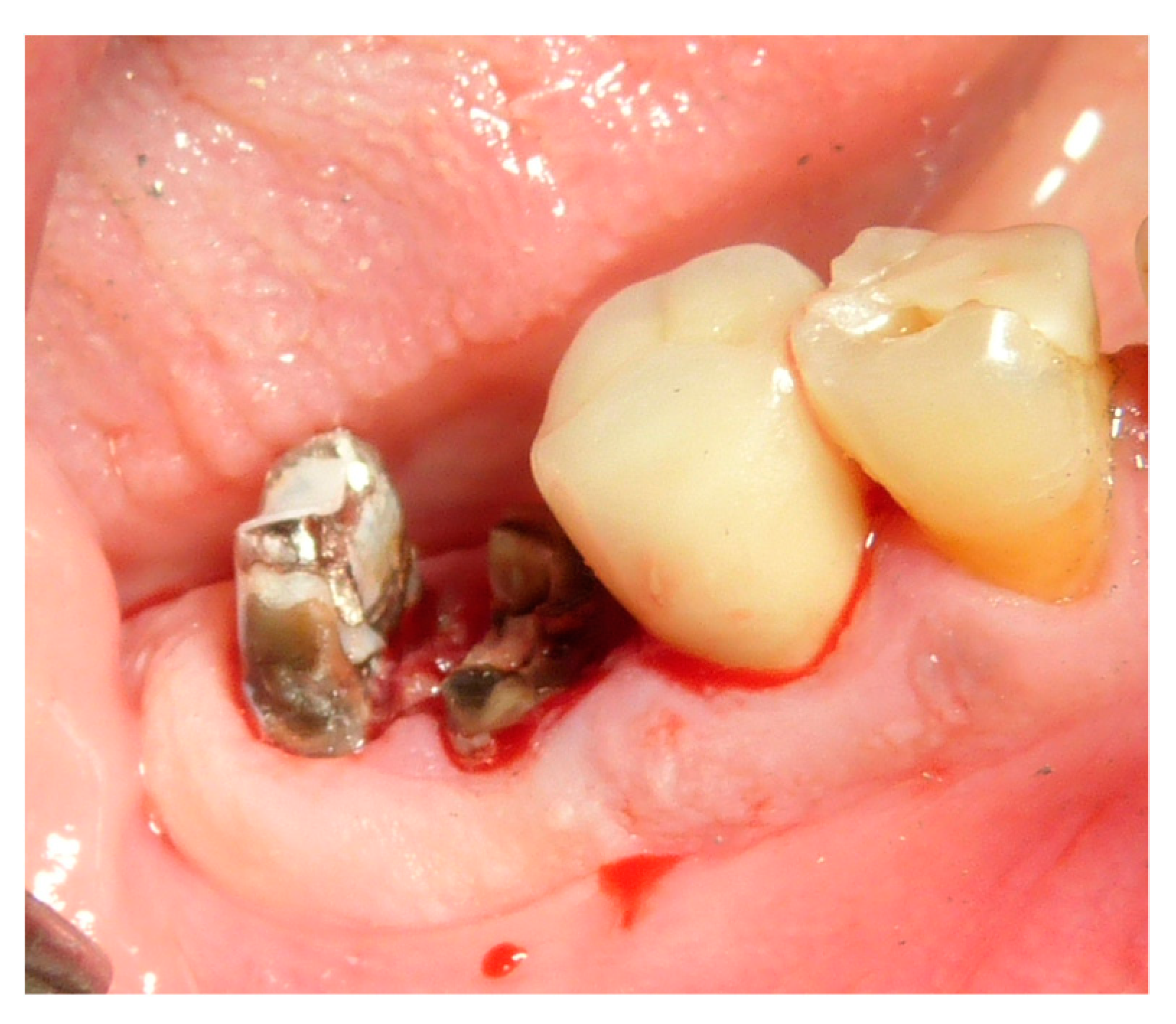
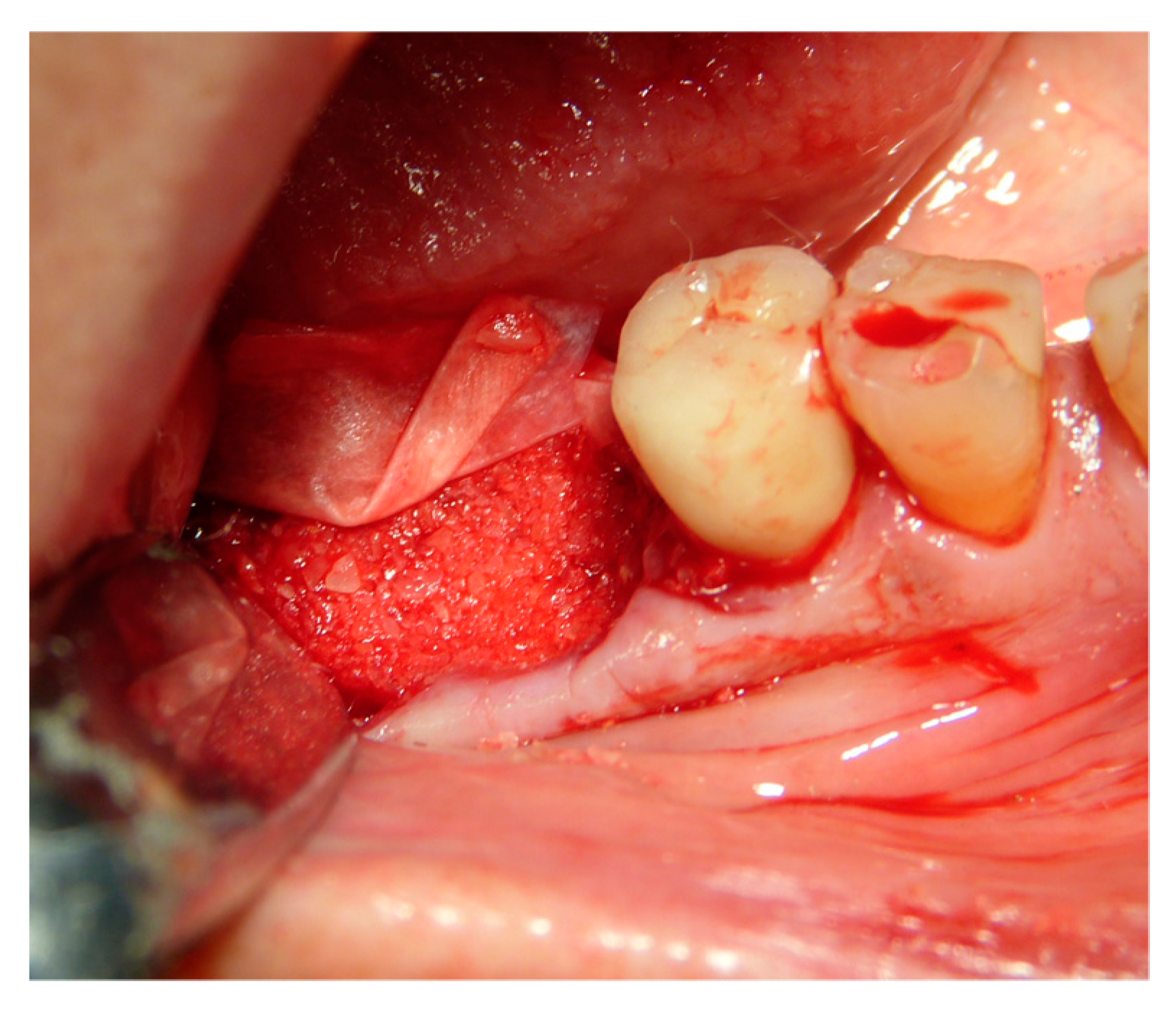
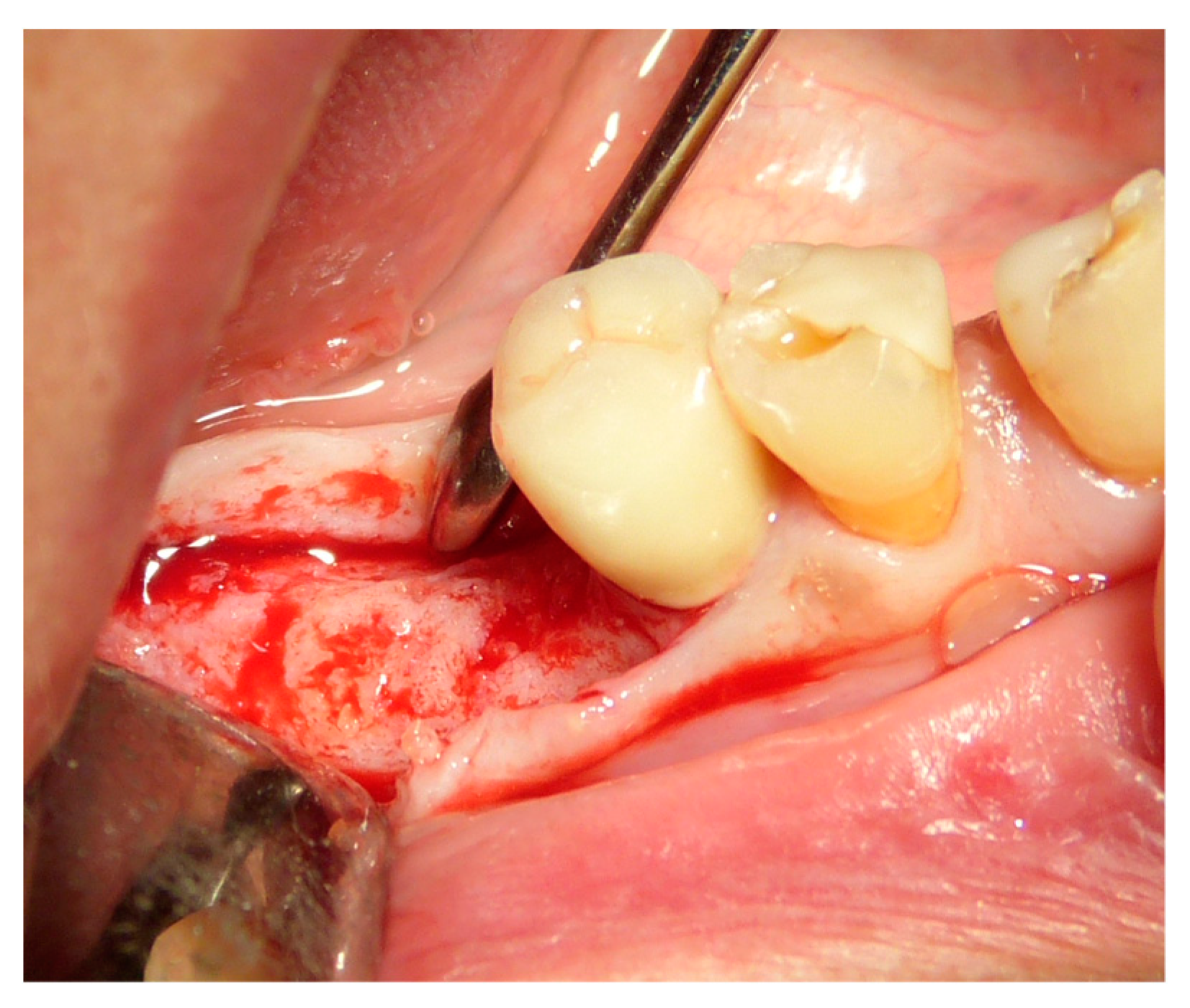
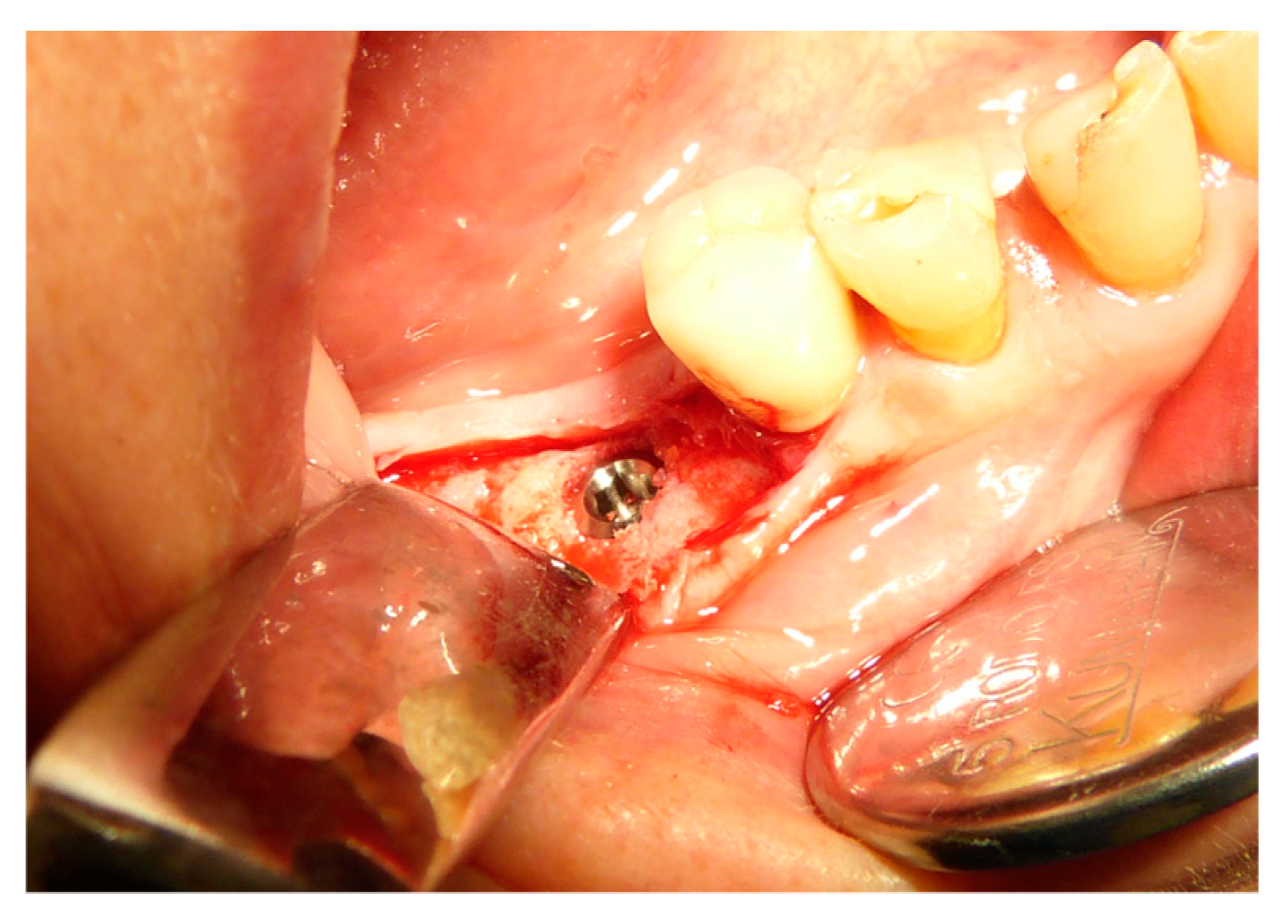
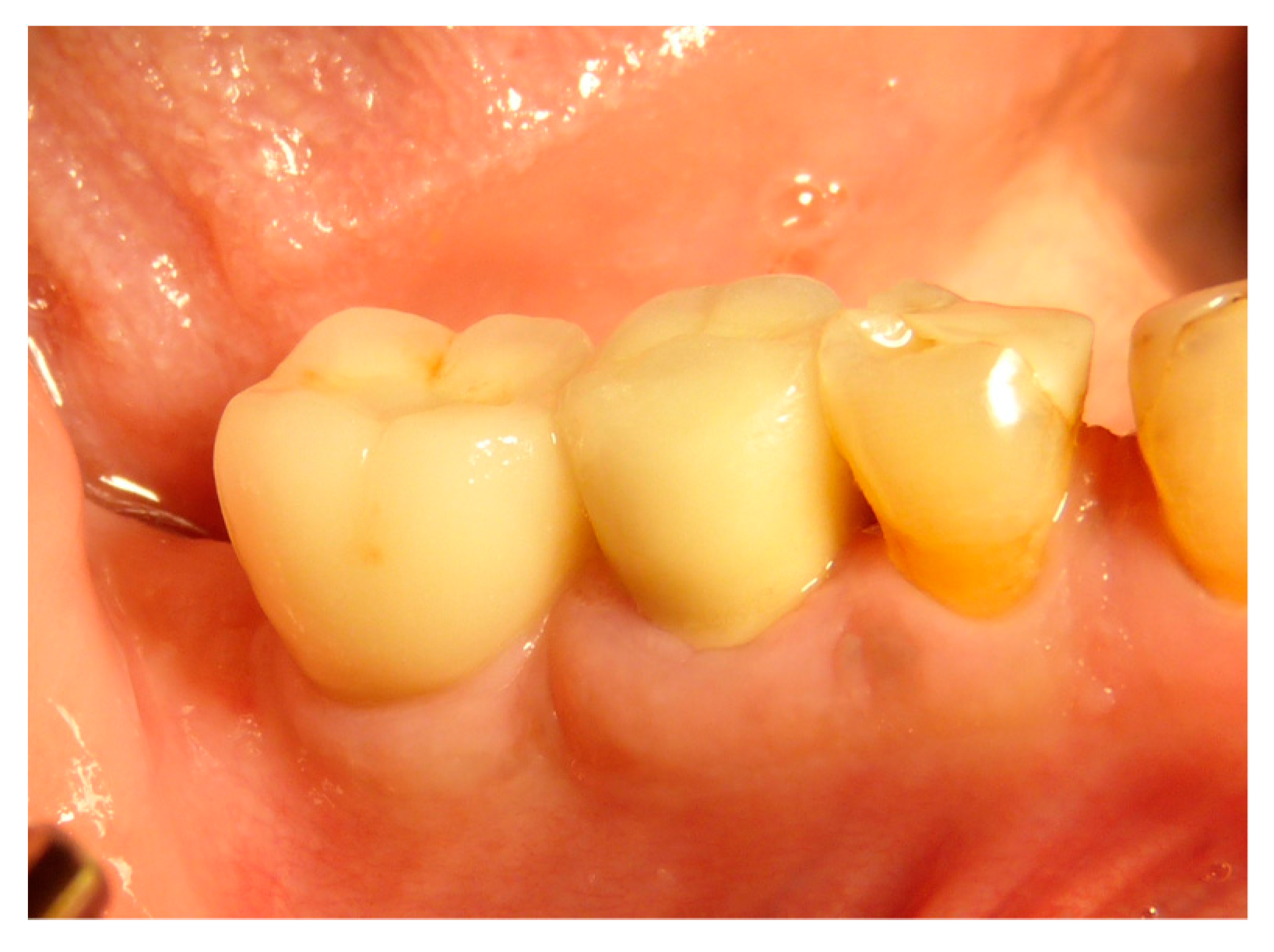
2.5. Surgical Procedures and Follow-Up
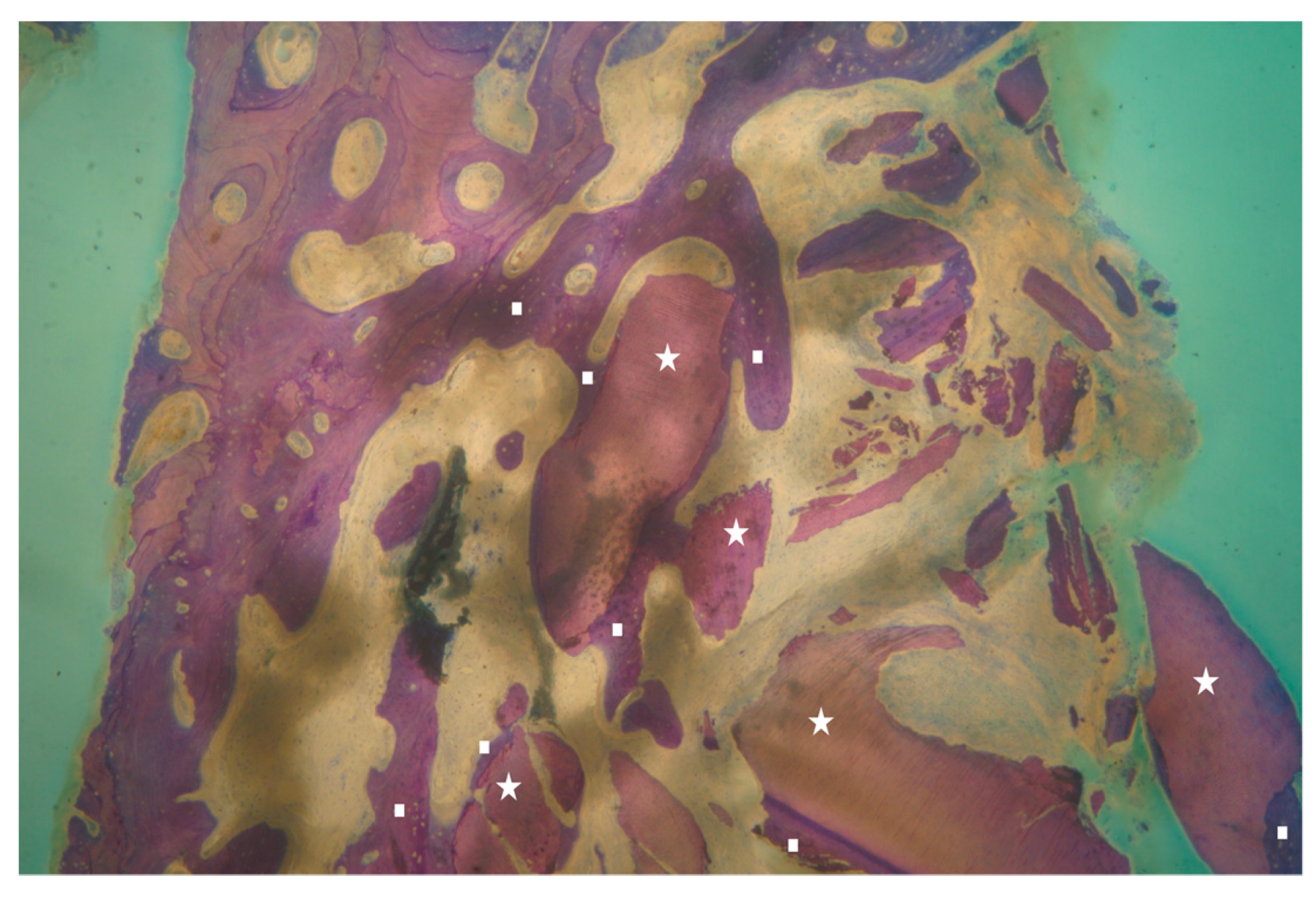
2.6. Performing Biopsies for Histological Evaluation
2.7. Collection and Statistical Analysis of Data
2.8. Histological Technique
3. Results
4. Discussion
5. Conclusions
6. Approval
Author Contributions
Funding
Conflicts of Interest
References
- Atieh, M.A.; Alsabeeha, N.H.; Payne, A.G.; Duncan, W.; Faggion, C.M.; Esposito, M. Interventions for replacing missing teeth: Alveolar ridge preservation techniques for dental implant site development. Cochrane Database Syst. Rev. 2015, 5, CD010176. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.W. Effect of alveolare ridge preservation after tooth exytraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Vittorini Orgeas, G.; Clementini, M.; De Risi, V.; De Sanctis, M. Surgical techniques for alveolar socket preservation: A systematic review. Int. J. Oral Maxillofac. Implant. 2013, 28, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, J.D.; Urist, M.R. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch. Oral Biol. 1967, 12, 999–1008. [Google Scholar] [CrossRef]
- Bang, G.; Urist, M.R. Bone induction in excavation chambers in matrix of decalcified dentin. Arch. Surg. 1967, 94, 781–789. [Google Scholar] [CrossRef]
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human dentin-matrix-derived bone morphogenetic protein. J. Dent. Res. 1991, 70, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M. Bone Morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine Growth Factor Rev. 2005, 16, 369–376. [Google Scholar] [CrossRef]
- Rijal, G.; Shin, H.I. Human tooth-derived biomaterial as a graft substitute for hard tissue regeneration. Regen. Med. 2017, 12, 263–273. [Google Scholar] [CrossRef]
- Boyne, P.J. Experimental evaluation of the osteogenic potential of bone graft materials. Annu. Meet. Am. Inst. Oral Biol. 1969, 13–21. [Google Scholar] [PubMed]
- Mellonig, J.T.; Bowers, G.M.; Cotton, W.R. Comparison of bone graft materials. Part II. New bone formation with autografts and allografts: A histological evaluation. J. Periodontol. 1981, 52, 297–302. [Google Scholar] [CrossRef]
- Colnot, C.; Romero, D.M.; Huang, S.; Helms, J.A. Mechanisms of action of demineralized bone matrix in the repair of cortical bone defects. Clin. Orthop. Relat. Res. 2005, 435, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Sonohara, M.; Hayacibara, R.; Cardaropoli, G.; Lindhe, J. Lateral ridge augmentation by the use of grafts comprised of autologous bone or a biomaterial. An experiment in the dog. J. Clin. Periodontol. 2002, 29, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Minamizato, T.; Koga, T.; Nakatani, Y.; Umebayashi, M.; Sumita, Y.; Ikeda, T.; Asahina, I. Clinical application of autogenous partially demineralized dentin matrix prepared immediately after extraction for alveolar bone regeneration in implant dentistry: A pilot study. Int. J. Oral Maxillofac. Surg. 2018, 47, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, Y.K.; Park, Y.H.; Park, J.C.; Ku, J.K.; Um, I.W.; Kim, J.Y. Evaluation of the Healing Potential of Demineralized Dentin Matrix Fixed with Recombinant Human Bone Morphogenetic Protein-2 in Bone Grafts. Materials (Basel) 2017, 10, 1049. [Google Scholar] [CrossRef]
- Y Baena, R.R.; Lupi, S.M.; Pastorino, R.; Maiorana, C.; Lucchese, A.; Rizzo, S. Radiographic evaluation of regenerated bone following poly (lactic-co-glycolic) acid/hydroxyapatite and deproteinized bovine bone graft in sinus lifting. J. Craniofac. Surg. 2013, 24, 845–848. [Google Scholar] [CrossRef]
- Bono, N.; Tarsini, P.; Candiani, G. Demineralized dentin and enamel matrices as suitable substrates for tooth regeneration: Thorough characterization and in vitro. J. Appl. Biol. Func. Mater. 2017, 15, e236–e243. [Google Scholar]
- Rizzo, S.; Zampetti, P.; Rodriguez, Y.; Baena, R.; Svanosio, D.; Lupi, S.M. Retrospective analysis of 521 endosseous implants placed under antibiotic prophylaxis and review of literature. Minerva Stomatol. 2010, 59, 75–88. [Google Scholar]
- Minetti, E.; Berardini, M.; Trisi, P. A new tooth processing apparatous allowing to obtain dentin grafts for bone augmentation: The tooth transformer. Open Dent. J. 2019, 13, 6–14. [Google Scholar] [CrossRef]
- Eliasson, A.L.; Blomqvist, F.; Wennerberg, A.; Johansson, A. A retrospective analysis of early and delayed loading of full-arch mandibular prostheses using three different implant systems: Clinical results with up to 5 years of loading. Clin. Implant. Dent. Relat. Res. 2009, 11, 134–148. [Google Scholar] [CrossRef]
- Dierens, M.; Vandeweghe, S.; Kisch, J.; Nilner, K.; De Bruyn, H. Long-term follow-up of turned single implants placed in periodontally healthy patients after 16–22 years: Radiographic and peri-implant outcome. Clin. Oral Impl. Res. 2012, 23, 197–204. [Google Scholar] [CrossRef]
- Eghbali, A.; De Bruyn, H.; De Rouck, T.; Cleymaet, R.; Wyn, I.; Cosyn, J. Single implant treatment in healing versus healed sites of the anterior maxilla: A clinical and radiographic evaluation. Clin. Implant. Dent. Relat. Res. 2012, 14, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Creugers, N.H.; Kreulen, C.M.; Snoek, P.A.; De Kanter, R.J. A systematic review of single-tooth restorations supported by implants. J. Dent. 2000, 28, 209–217. [Google Scholar] [CrossRef]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rate of implant-supported single crowns. Clin. Oral Implant. Res. 2008, 19, 119–130. [Google Scholar] [CrossRef] [PubMed]
| Surgeons | 10 |
|---|---|
| Extracted teeth | 119 |
| incisive 26 | |
| canine 8 | |
| premolars 36 | |
| molars 49 | |
| Extraction reasons | Crown trauma |
| external root resorption | |
| Periodontitis | |
| Missing tooth | |
| Root fracture | |
| Infected root | |
| Socket sites treated | 106 |
| mandible 60 | |
| maxillae 59 | |
| Implants | 106 (Certain—3i Biomet) |
| 3,25–4–5 mm diameter | |
| 10–11,5–13 mm lenght | |
| Implant failure | 1 |
| Percentage of alveolar socket defects | 1 walls 12% | 2 walls 3% | 3 walls 43% | 4 walls 42% |
| Histomorphometric Analysis | Percentage | Standard Deviation |
|---|---|---|
| Vital Bone/Total Bone average (VB%) | 21.89 | ±9.72 |
| Residual Graft/Total Volume average (Graft%) | 16.60 | ±7.09 |
| Bone Volume/Total Volume average (BV%) | 41.47 | ±11.51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minetti, E.; Palermo, A.; Ferrante, F.; Schmitz, J.H.; Lung Ho, H.K.; Dih Hann, S.N.; Giacometti, E.; Gambardella, U.; Contessi, M.; Celko, M.; et al. Autologous Tooth Graft after Endodontical Treated Used for Socket Preservation: A Multicenter Clinical Study. Appl. Sci. 2019, 9, 5396. https://doi.org/10.3390/app9245396
Minetti E, Palermo A, Ferrante F, Schmitz JH, Lung Ho HK, Dih Hann SN, Giacometti E, Gambardella U, Contessi M, Celko M, et al. Autologous Tooth Graft after Endodontical Treated Used for Socket Preservation: A Multicenter Clinical Study. Applied Sciences. 2019; 9(24):5396. https://doi.org/10.3390/app9245396
Chicago/Turabian StyleMinetti, Elio, Andrea Palermo, Franco Ferrante, Johannes H. Schmitz, Henry Kim Lung Ho, Simon Ng. Dih Hann, Edoardo Giacometti, Ugo Gambardella, Marcello Contessi, Martin Celko, and et al. 2019. "Autologous Tooth Graft after Endodontical Treated Used for Socket Preservation: A Multicenter Clinical Study" Applied Sciences 9, no. 24: 5396. https://doi.org/10.3390/app9245396
APA StyleMinetti, E., Palermo, A., Ferrante, F., Schmitz, J. H., Lung Ho, H. K., Dih Hann, S. N., Giacometti, E., Gambardella, U., Contessi, M., Celko, M., Ballini, A., Mortellaro, C., Trisi, P., & Mastrangelo, F. (2019). Autologous Tooth Graft after Endodontical Treated Used for Socket Preservation: A Multicenter Clinical Study. Applied Sciences, 9(24), 5396. https://doi.org/10.3390/app9245396






