1. Introduction
Fixed implant-supported prostheses are considered a successful and predictable treatment for the rehabilitation of edentulous patients in the presence of an adequate volume of available bone. In edentulous atrophic jaws, bone deficiencies can prevent implants placement in the ideal prosthetic position with impairment of function and aesthetics. To overcome these limitations, augmentation procedures for improving bone dimensions are needed. To date, bony reconstruction approaches of advanced maxillary horizontal atrophy involve the use of guided bone regeneration, ‘‘split’’ ridge osteotomy, and block grafts with different types of materials [
1,
2,
3].
Guided bone regeneration (GBR) is a well-documented surgical procedure that uses particulate autogenous bone or bone substitutes in conjunction with occlusive membranes to prevent undesirable competing migration of non-osteogenic cells into the bony defect. The stability of the graft and barrier membrane and the tension-free flap closure are essential to secure successful outcomes [
4]. There is strong evidence for the effectiveness and predictability of GBR in promoting lateral bone augmentation for limited to moderate bony deficiencies [
5]. Nevertheless, to achieve ridge augmentations in severe and extensive defects, the technique, requiring non-resorbable membranes or titanium mesh and a long-term healing period, is more prone to soft tissue dehiscence, with subsequent infection and possible failure of regeneration procedure [
6,
7,
8,
9].
The ridge splitting/expansion procedure consists of a sagittal osteotomy and a controlled greenstick out-fracture of the buccal cortical plate, labially displaced to create the space that is filled with an interpositional particulate graft and covered with a membrane [
3,
10]. This technique has proved to be a predictable and effective to correct alveolar width deficiencies of limited edentulous areas with high implant survival rate, gain in horizontal alveolar ridge width, and few biological and technical complications [
11]. In severe and extensive bony deficiencies, with the horizontal width less than 3 mm, treatment outcomes could be jeopardized since the alveolar ridge expansion predisposes to fractures of the buccal or palatal cortical plate and hampers a sufficient blood supply during the healing phase [
3,
10,
11].
Autogenous bone block grafting is the best scientifically documented technique for the treatment of advanced horizontal atrophy in edentulous jaws [
1,
2]. However, this is a complex intervention and requires the need for general anesthesia, operation theater, and hospitalization because, in extensive and severe bone resorptions, block grafts are often harvested from the iliac crest or calvaria. Furthermore, the procedure is an operator-sensitive technique, which requires surgical skills in trimming and adapting blocks to the native bone, in achieving grafts immobilization, filling residual gaps, covering the grafted area with resorbable membranes, and suturing the flap tension-free [
2]. The main drawbacks for the patients are the increased surgery invasiveness, longer surgical times, and more severe postoperative morbidity, mainly due to the extraoral donor sites.
In the augmentation procedures, a variety of bone restorative materials with different characteristics may be used, including autogenous bone, allografts, xenografts, and alloplasts, as well as different barrier membranes or osteosynthesis materials [
12,
13,
14,
15].
Autogenous bone, harvested from intra- or extraoral donor sites, is still considered the gold standard among the different biomaterials for bone thickness reconstruction due to its osteogenic, osteoinductive, and osteoconductive properties. Further advantages are a physical and chemical structure identical to that of the host site, a better vascularization potential, and the absence of immunogenicity and disease transmission capacity. Nevertheless, the use of autogenous bone presents some drawbacks, such as two different surgical sites, morbidity and complications of the donor site, lengthy surgical procedure, and unpredictable resorption [
14].
Homologous, heterologous, or alloplastic grafts, instead, are only osteoconductive, promoting the proliferation of new vessels and guiding the growth and proliferation of osteoblasts onto their surface. Bone substitute materials provide biological support during healing, and their low and gradual replacement with the newly formed bone maintains the augmented volume and prevents soft tissue collapse. Furthermore, when compared to autogenous bone, they have the advantage of unlimited availability and less biologic costs to patients since the additional surgery for harvesting is not required [
12,
14].
The choice between different augmentation techniques and different grafting materials is related to the bony defect location and extension, morphological features of the site, hard and soft tissue characteristics, patient’s individual needs and expectations, and, lastly, the surgeon’s preferences/skill.
New possibilities in the reconstruct maxillary bony structures have been introduced over the past decade, with recent advancements in three-dimensional (3D) imaging acquisition with computed tomography (CT) and in computer-aided design/computer-aided manufacturing (CAD/CAM) technologies, which made it possible to directly produce customized biocompatible scaffold based on individual patient-specific anatomical data [
16,
17,
18].
The present short communication reported a new therapeutic approach for the reconstruction of the horizontal severely resorbed edentulous maxilla with custom-made bovine-derived xeno-hybrid composite bone blocks, fabricated using CT and CAD/CAM technology.
2. Materials and Methods
No Ethical Committee approval was required as, according to the Italian national legislation and ordinance of the local inspection authority, it is not needed for short communications reporting the description of a surgical protocol. Nevertheless, the patient’s informed consent related to the therapeutic plan and associated risks and the use of the images was obtained.
The protocol for horizontal bone-augmentation in the edentulous atrophic maxilla with the deproteinized bovine-bone composite scaffold, custom-made using CAD/CAM technologies, is indicated for patients requiring full-arch fixed prosthesis. In the horizontal severely resorbed edentulous maxilla (class IV according to the Cawood and Howell classification), the knife-edge edentulous ridge, sufficient in height but very deficient in thickness, prevents the placement of implants in the ideal prosthetic position for supporting a full-arch fixed rehabilitation [
19].
2.1. Diagnosis and Treatment Planning
All patients were subjected to a complete examination of the oral hard and soft tissues and radiographic assessment by cone-beam computed tomography (CBCT). The pre-existing removable prosthesis was evaluated. If this denture was not adequate, a new provisional diagnostic denture with teeth in the ideal position to satisfy functional, phonetic, and aesthetic requisites was fabricated. The pre-existing or new denture was used both as a radiographic template, to transfer prosthetic information during the radiographic exam for a correct treatment planning, and as a temporary prosthesis during the healing phase.
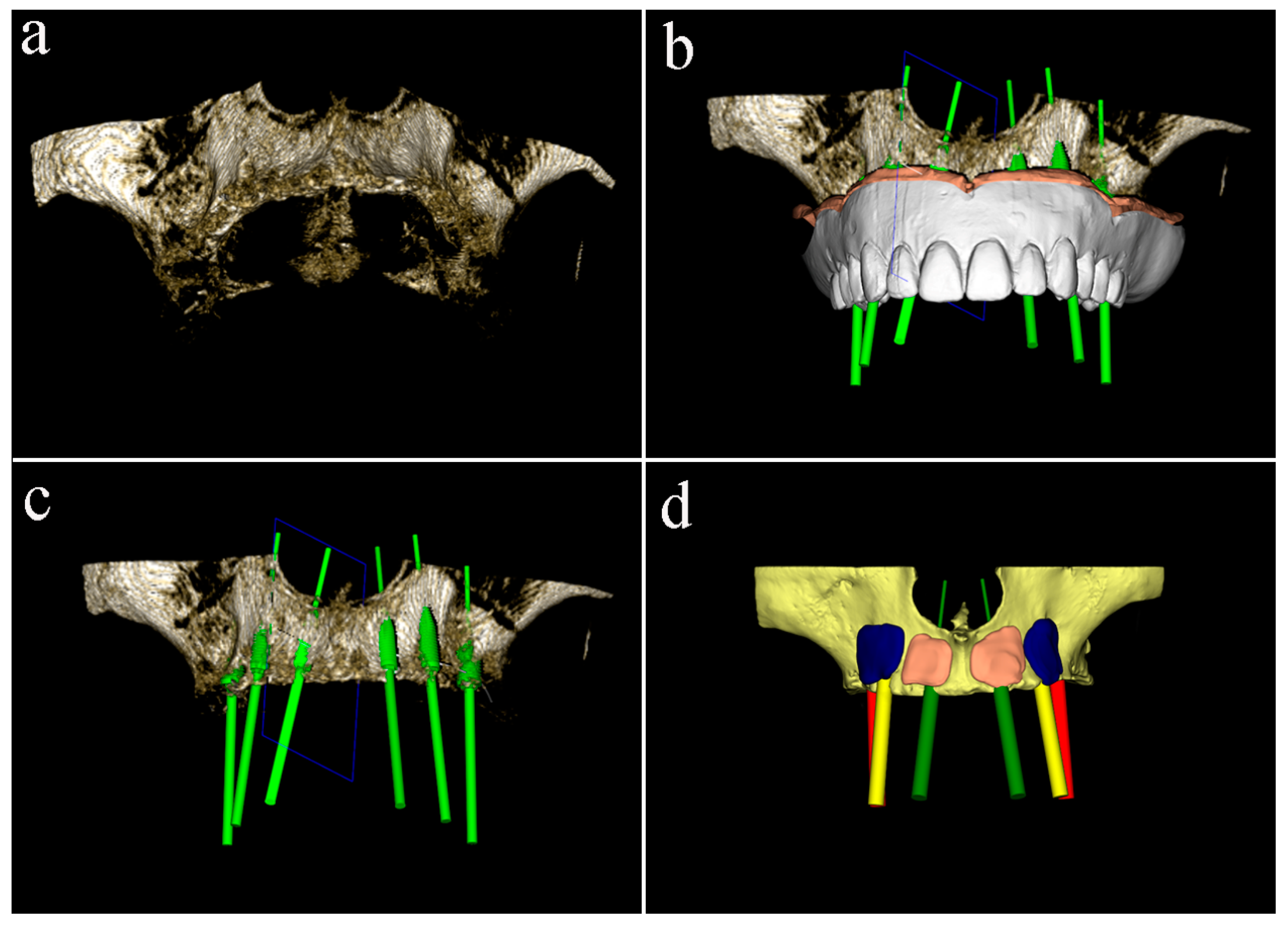
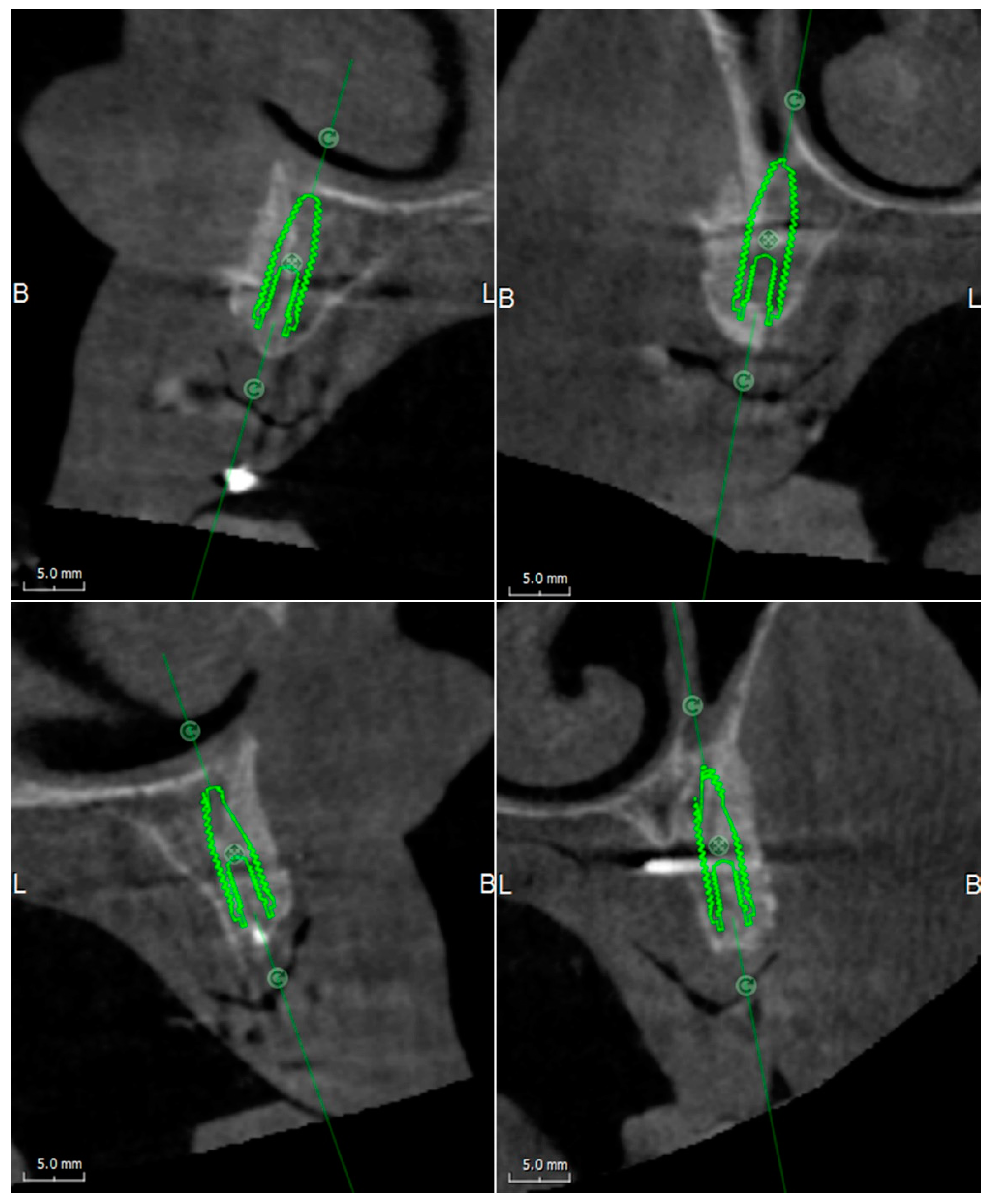
Cone-beam computed tomography (CBCT) of the patient, wearing the radiographic template and a bite index with an extra-oral volume transfer element, was carried out (Evobite, 3DIEMME, Cantù, Italy, 2014). The CT scans were saved in digital imaging and communication in medicine (DICOM) format, and anatomic and prosthetic data were imported into a dedicated diagnostic and medical imaging software (3Diagnosys 4.2, 3DIEMME). The software, superimposing the digital model of the denture on the three-dimensional computer images, allowed to virtually plan the prosthetic-driven position of the implants, and the consequent ridge augmentation with graft blocks perfectly adapted to the residual bone structure (
Figure 1).
2.2. Manufacturing of Customized Graft Blocks
The 3D planning virtual models were saved in a stereolithographic (STL) file and mailed to the IBI SA - Industrie Biomediche Insubri SA (Switzerland). The customized blocks in the exact 3D model shape were manufactured using the 5axes CAD-CAM process to machine-mill the bovine-derived mineral matrix (SmartBone® on Demand graft. IBI S.A. Industrie Biomediche Insubri S.A. via Cantonale 67, Switzerland). After the physical-chemical reinforcement process with biodegradable aliphatic polymers and bioactive agents, the customized blocks were packaged, sterilized, and sent to the surgeon, ready for clinical use.
2.3. Surgical and Prosthetic Procedure
The surgical procedure was performed by the same experienced surgeon (G.L.M.) in the outpatient setting and aseptic conditions. Immediately prior to surgery, patients rinsed with a 0.2% chlorhexidine digluconate solution (Corsodyl, GlaxoSmithKline Consumer Healthcare, Genval, Belgium) for 2 min. The intervention was carried out under oral sedation with diazepam 0.25 mg/kg (Valium-2, Roche S.p.A., Italy) and local anesthesia with 2% mepivacaine and 1:100,000 epinephrine (Carbocaine, AstraZeneca, Milan, Italy).
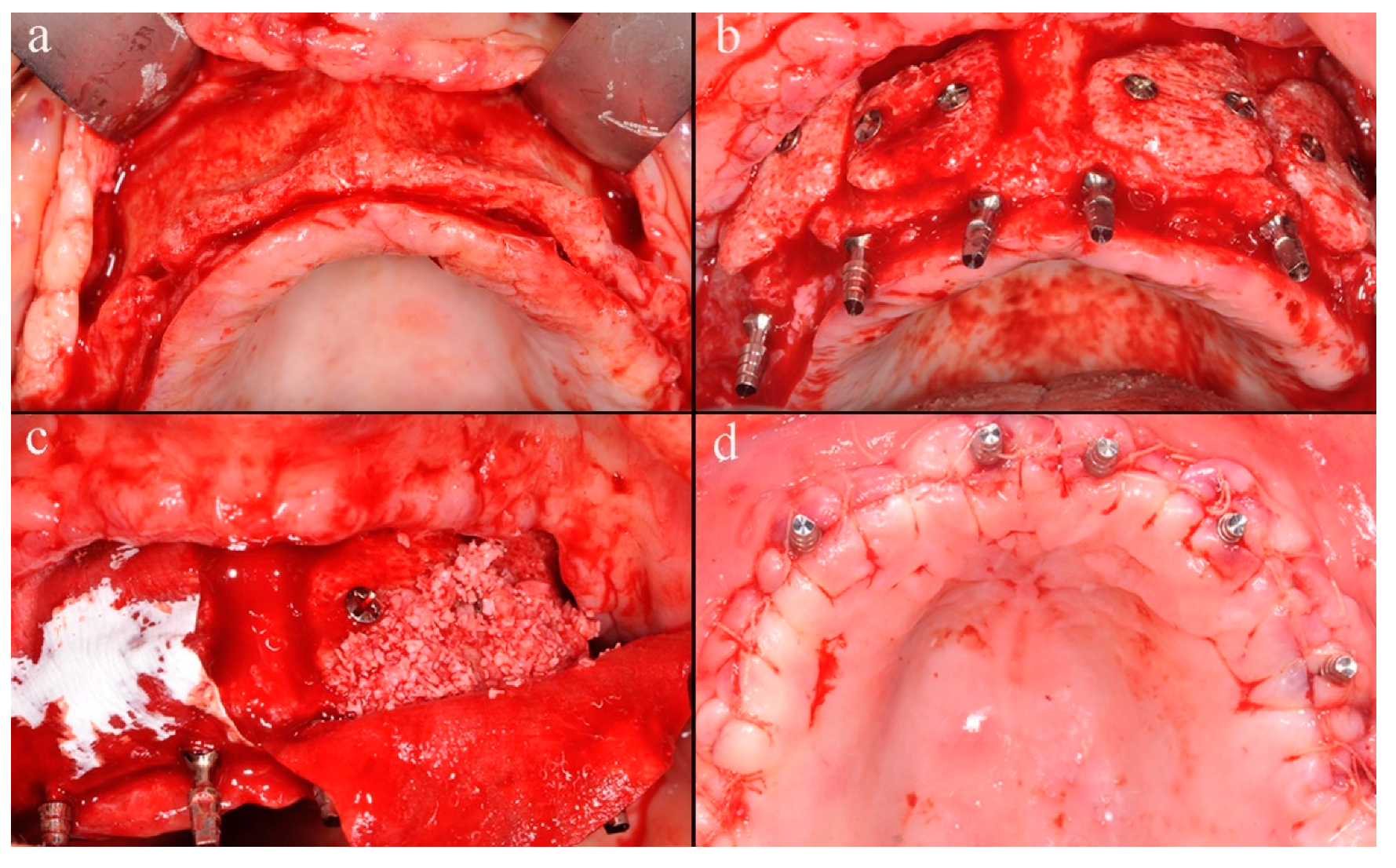
A midcrestal incision from the maxillary tuberosity on one side to the opposite one was performed, and a mucoperiosteal flap was raised to obtain the full exposition of the maxillary bone (
Figure 2a).
After the flap elevation, the cortical plate was perforated with a small round bur to expose the marrow spaces for increasing the migration of osteogenic cells and accelerating revascularization of the graft. The custom-made blocks were placed into planned positions on the maxillary buccal surface and rigidly fixed with titanium screws with caution to not break the grafts. Six immediate provisional implants were inserted to support the temporary denture during the healing period (
Figure 2b). Two resorbable porcine collagen membranes (Creos ™ Matricel GmbH, Herzogenrath, Germany) were fixed on the palatal plate using titanium screws to avoid connective tissue ingrowth, which might compromise the integration of the grafts. Blocks and remaining spaces of the recipient site were filled with a mix of particulate deproteinized bovine bone mineral (Creos ™ Matricel GmbH, Herzogenrath, Germany) and autogenous bone chips, previously collected with bone-scraper (
Figure 2c). Membranes were stabilized over the augmented area with additional buccal titanium self-tapping screws, to protect and prevent the partial resorption of grafted materials. The flap was coronally advanced through periosteal releasing incisions, adapted, and sutured to achieve tension-free primary closure (
Figure 2d). At last, the temporary denture was delivered, fittingly modified, for avoiding any harmful compression on the grafted area, which might jeopardize the integration process.
Medication protocol included administering 875 mg of amoxicillin plus 125 mg of clavulanic acid (Augmentin, GlaxoSmithKline, London, UK) twice daily for 7 days, starting 1 h before surgery. Analgesia was achieved with 400 mg of ketoprofen (Ibifen, Aprilia, Latina, Italy) for a maximum of three times daily according to individual needs. During the first month postoperatively, patients were instructed to gently clean the surgical area with a soft toothbrush and rinse with 0.12% chlorhexidine digluconate (Corsodyl, GlaxoSmithKline Consumer Healthcare S.p.A., Baranzate (MI), Italy) three times daily.
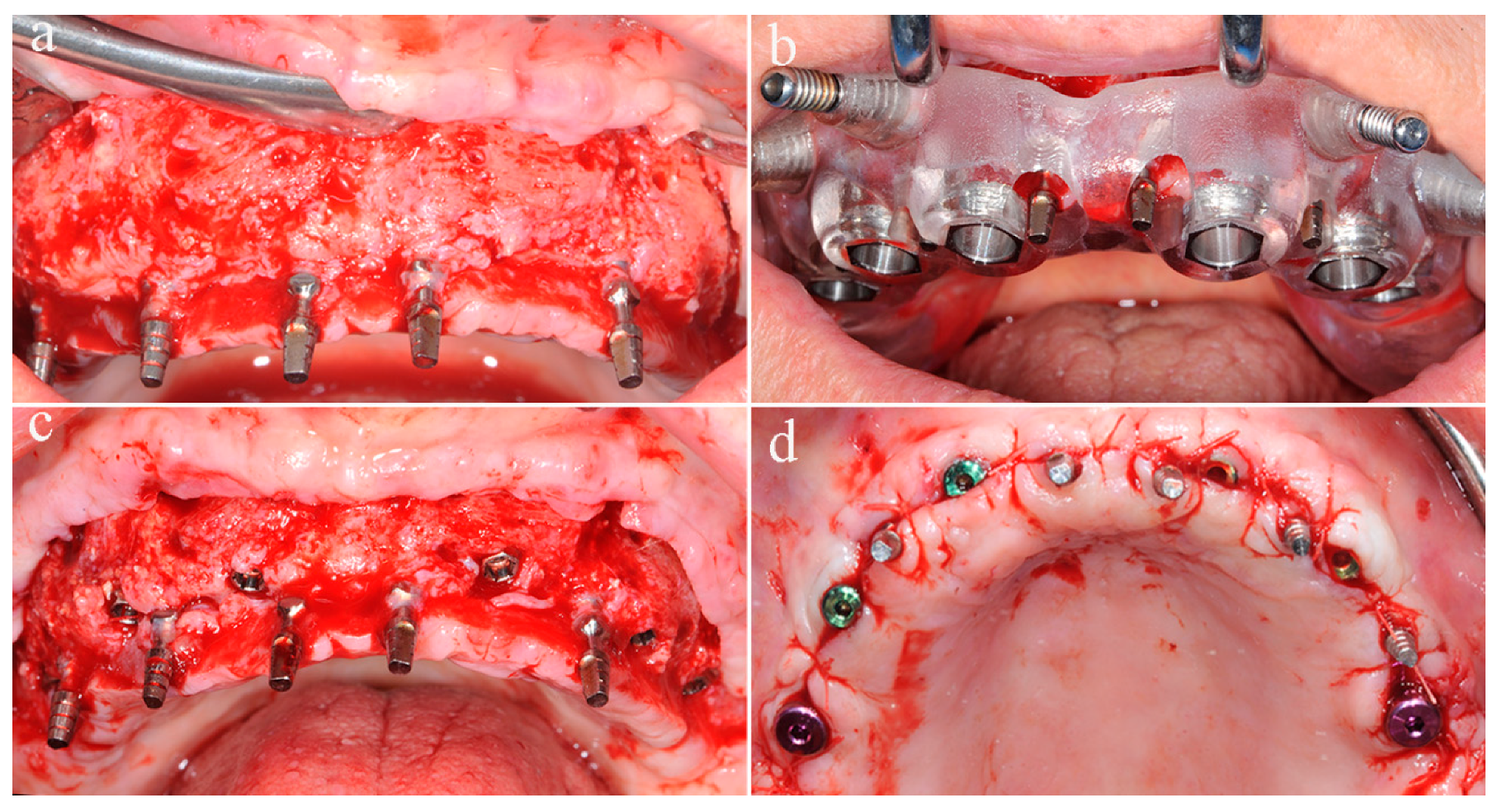
After six months of uneventful healing, a CBCT was carried out to assess the integration of the grafts, and the second intervention was scheduled for removing titanium screws and positioning implants. In addition, a stereolithographic surgical template was produced.
A full-thickness flap was performed along the same incisions of the reconstructive surgery, the fixation screws were removed (
Figure 3a), and bone biopsies were harvested at grafted sites with a trephine bur used from the buccal to palatal aspect.
Six implants were placed at planning sites with the surgical template, and good primary stability was achieved, the healing abutments were connected, for avoiding the uncovering intervention, and the flap was repositioned and sutured (
Figure 3b–d). During the osseointegration phase, before implants loading, the patient wore the temporary denture supported by immediate provisional implants.
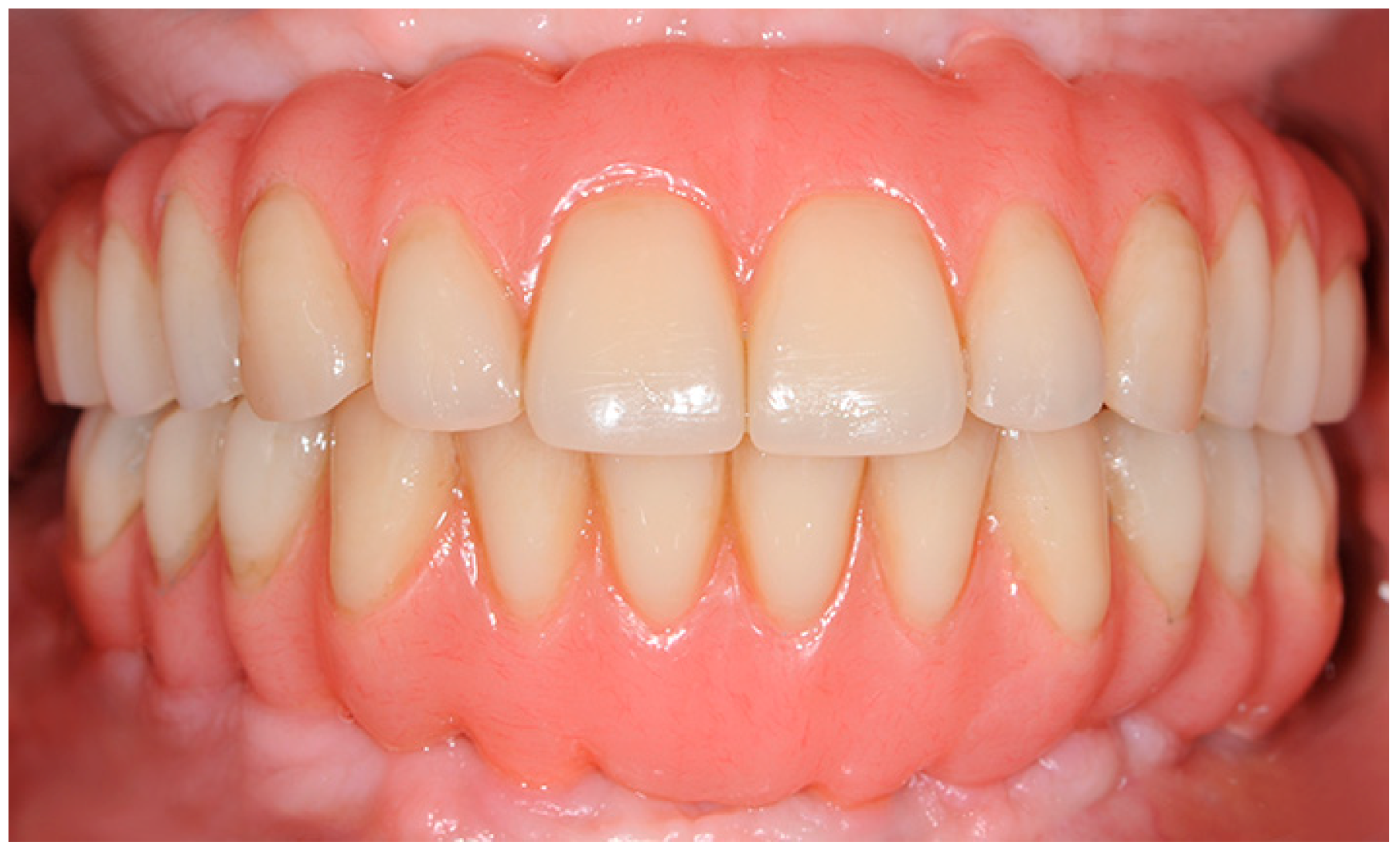
After 6–8 months, the six immediate provisional implants were removed, and a new full-arch screw-retained temporary prosthesis was delivered. After soft tissue maturation, the definitive prosthetic rehabilitation was performed (
Figure 4).
3. Results
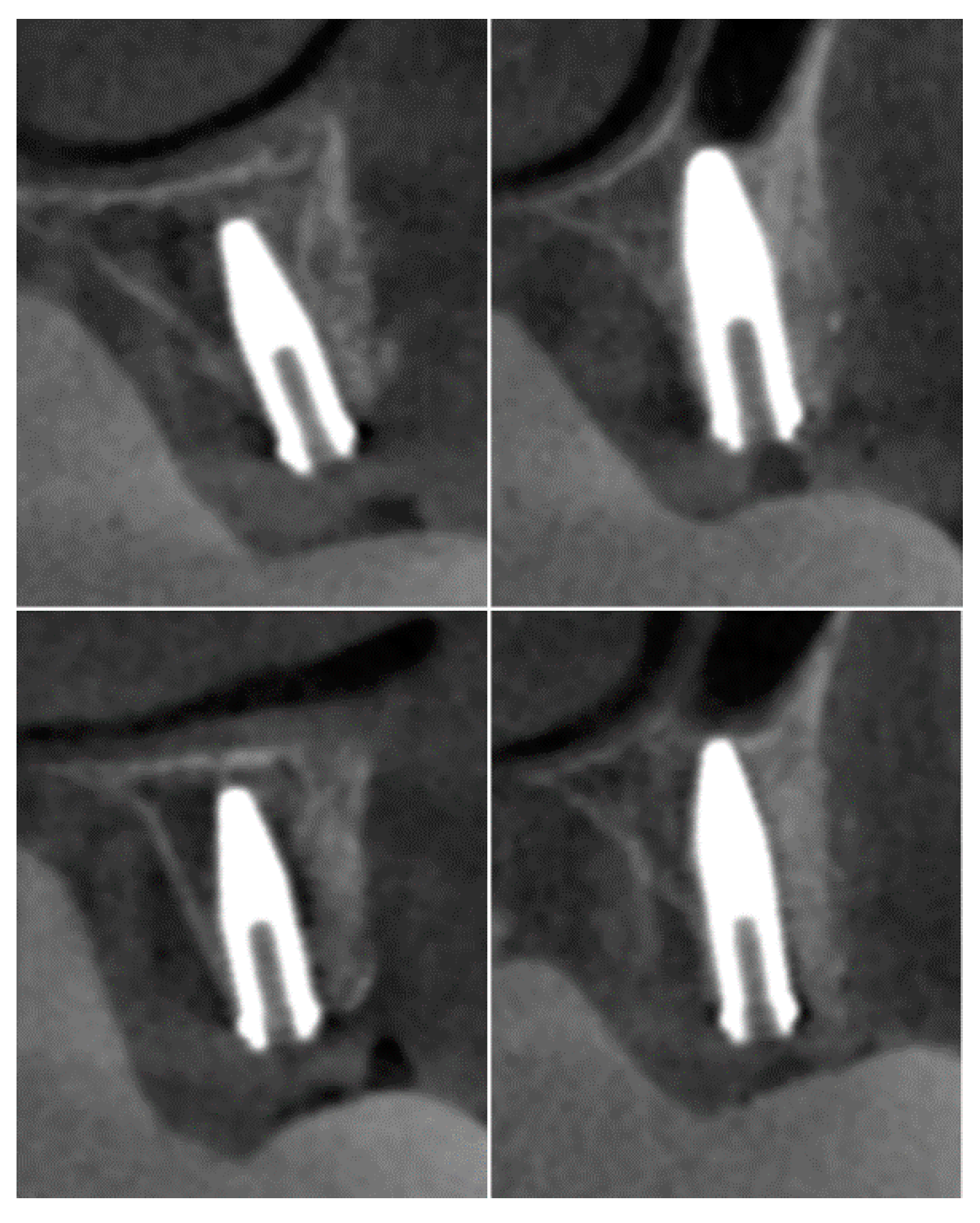
The CBCT performed at six months after the first intervention of the bone reconstruction showed the integration between the graft and the recipient site and a three-dimensional volume of the alveolar ridge adequate for the placement of the implants (
Figure 5).
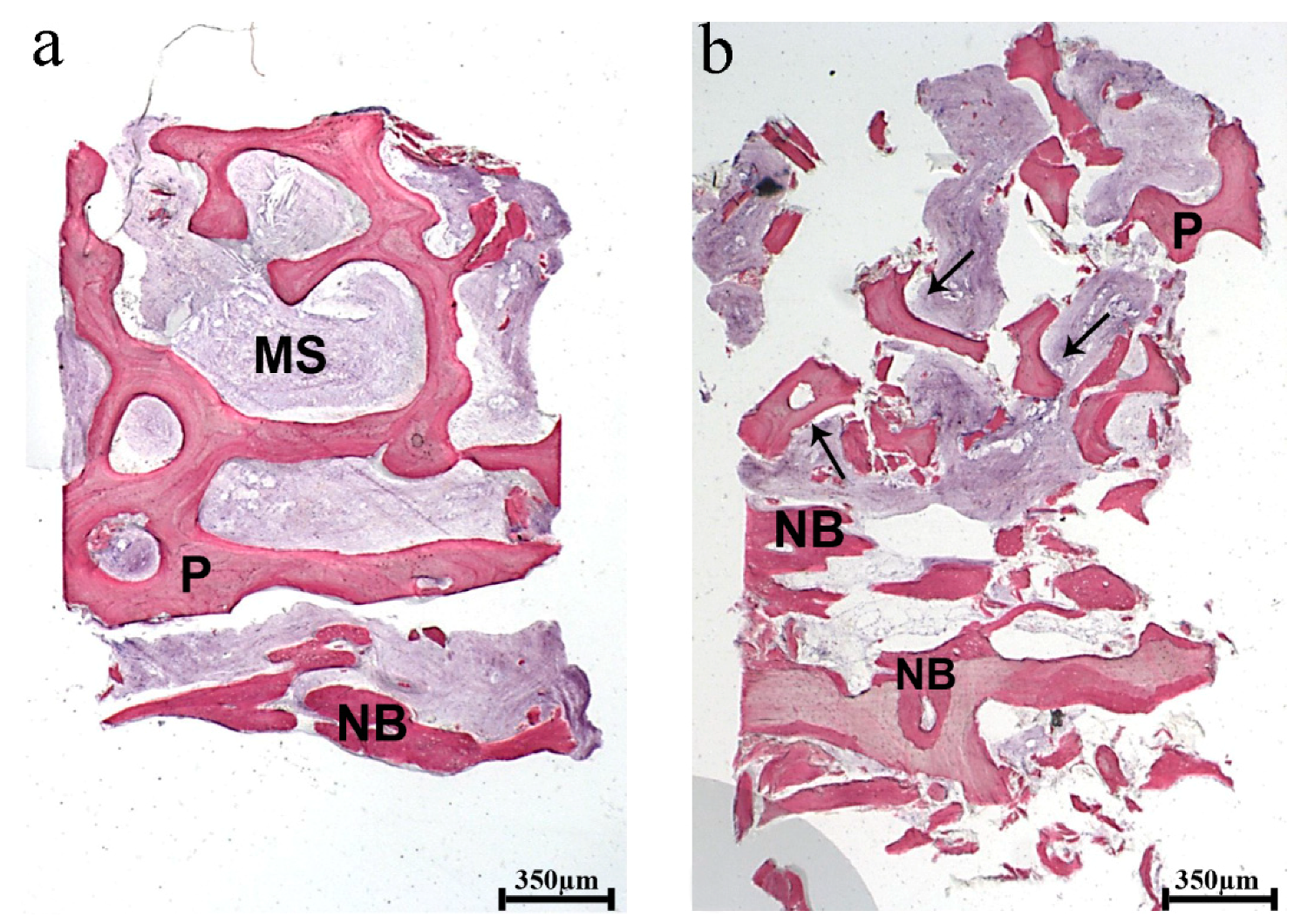
At low magnification, the histological analysis showed the specimens consisting of biomaterial block, recognizable by the shape and the regular arrangement of the trabeculae with large marrow spaces representing about 25% of the bone structure. In the marginal portion of the biopsies, only a newly formed bone was detected (
Figure 6).
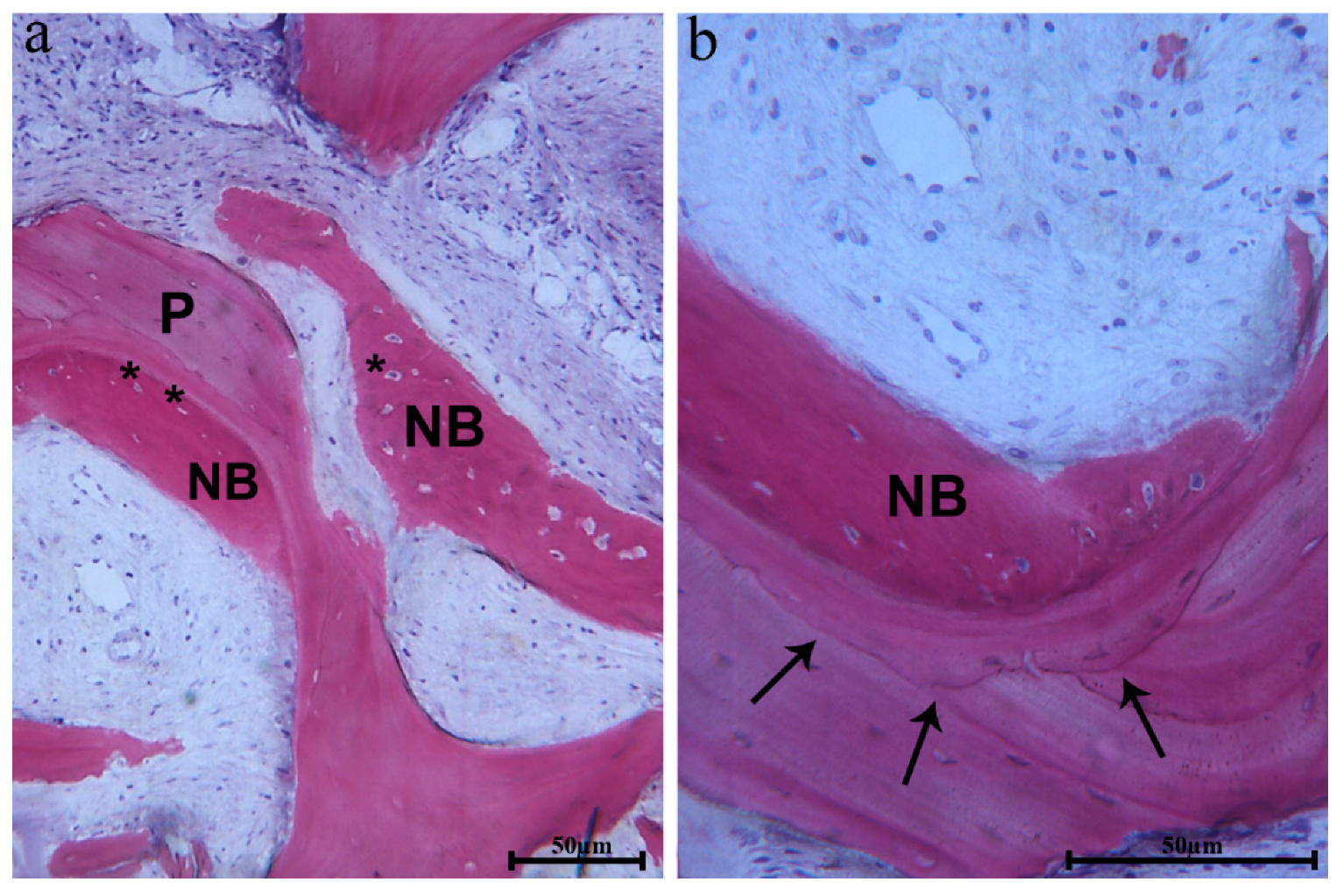
In many fields, biomaterial trabeculae were lined by newly formed bone, showing large osteocytes lacunae embedded into the newly formed bone, which was close to the biomaterial surface (
Figure 7a).
The newly formed bone also had a high affinity for dyes and was positive for acid fuchsin. Moreover, in some fields, the biomaterial interface showed a similar affinity when it was close to the newly formed bone (
Figure 7b). In these areas, the osteocyte lacunae of biomaterials were colonized by cells (
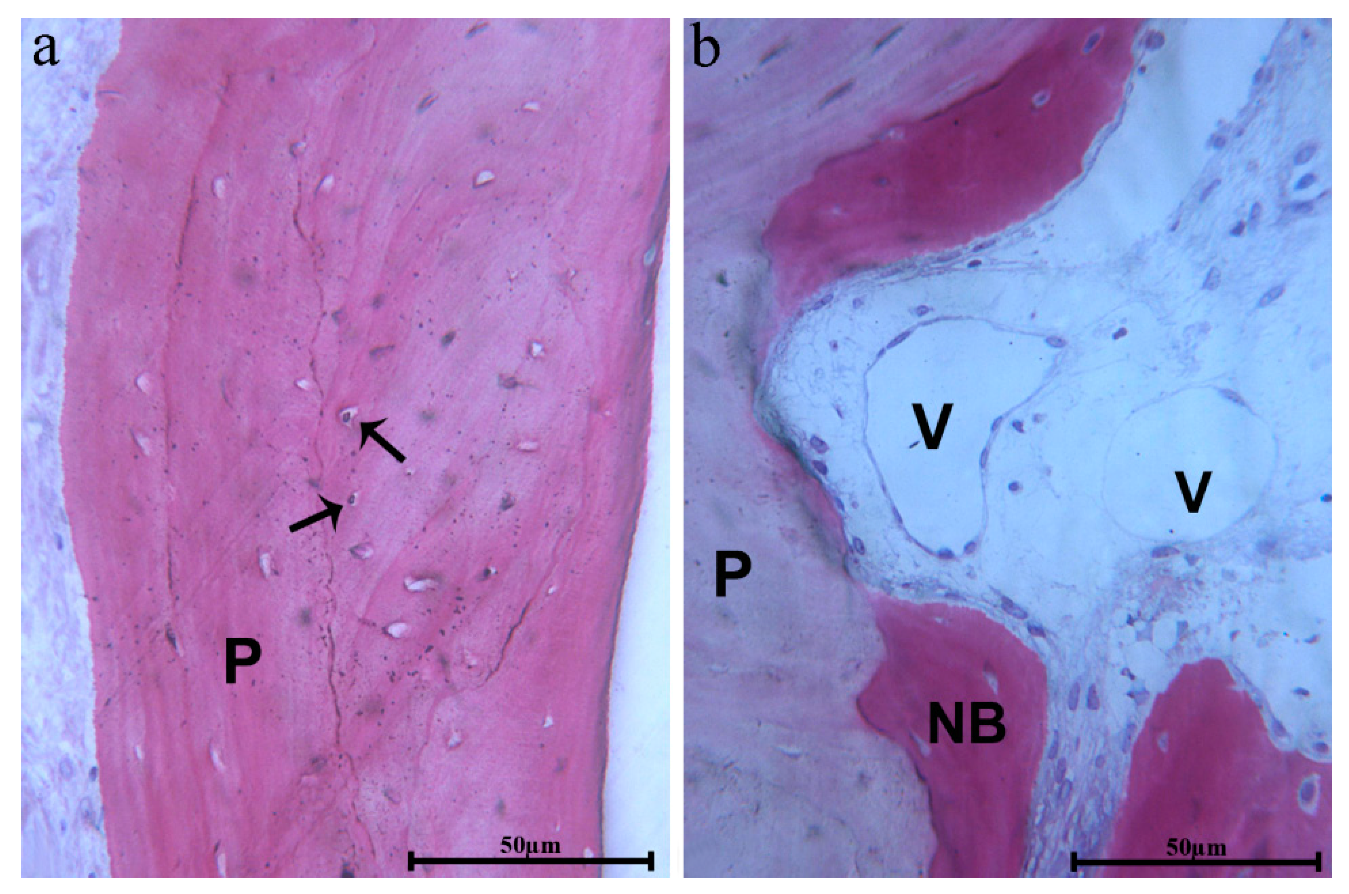
Figure 8a). Close to the biomaterial, osteoblasts depositing osteoid matrix, newly formed bone, and blood vessels were present (
Figure 8b). In some portions, the connective tissue, close to the biomaterial, appeared dense with many fibroblasts and without an inflammatory infiltrate. Resorption or remodeling of the scaffold seemed not related to the presence of osteoclasts or multinucleated cells.
At 2-year control CBCT, no signs of inflammation and bone resorption were observed at the grafted sites and around implants (
Figure 9).
4. Discussion
Given the constant updating of surgical bone grafting-techniques, the approach described in this short communication for the reconstruction of horizontal atrophic edentulous maxillae aimed to offer a potential alternative.
In the recent years, the use of bone substitutes in the augmentation procedures has gained attention to overcome the drawbacks of the autogenous bone, including the additional surgery for harvesting, the risk of donor site morbidity, the limited availability, the possible need of general anesthesia and hospitalization, and the unpredictable graft resorption [
20].
An organic bovine bone substitute is one of the best-documented biomaterials, being commercially available in a wide range of products, and its osteoconductive properties and high biocompatibility have been tested in many clinical trials [
21,
22].
The bovine-derived xenohybrid composite bone has proven to have effective integration and bone regeneration when it is used in oral and orthopedic fields [
23]. Its composite structure is based on a deproteinized bovine-derived bone matrix reinforced with biodegradable aliphatic polymers and bioactive agents. The bovine-derived matrix, made of calcium hydroxyapatite (HA, Ca5(PO4)3(OH)), maintains an adequate 3D structure for strength. The biopolymers (poly(L-lactic acid) and poly(e-caprolactone) enhance mechanical properties. The bioactive agents (RGD-containing collagen fragments, obtained by purified gelatin) promote cell adhesion, proliferation, and high hydrophilicity. Furthermore, morphological analysis has shown a well-diffused open-porosity and microstructure comparable in terms of size and cell seeding spaces to human cortical bone.
Furthermore, the possibility to custom-make scaffolds using CAD/CAM technologies speeds up the surgical procedure and limits any potential biological and technical complications. Indeed, the manual cutting and shaping of the blocks and the adaptation at recipient sites during surgery increase the risk of intraoral and extraoral contamination and result in a procedure that is time-consuming and highly dependent on the clinician’s skill and experience. Additionally, the size and shape of the conventional bone grafts often are imprecise, and the difficulty of achieving a stable position on the native bone might jeopardize the integration process. Manufacturing of customized block grafts offers some advantages also compared to the grafts manually shaped on the 3D stereolithographic model of patients’ jaws. The manual adjustment of the prefabricated block leads to less precise fitting, needs a greater quantity of material, and exposes to a risk of graft infection due to the prolonged contact to possible sources of contamination, such as the gloves of the surgeon, oral fluids of the patient, burs, and other environmental factors [
24].
The therapeutic approach using bovine-derived bone blocks combined with 3D-CT and CAD/CAM technologies seems to be promising to overcome the above-mentioned limits of conventional bock grafting procedures.
The use of deproteinized bovine bone, not requiring additional surgery for bone harvesting, avoids the risk of donor sites, reduces postoperative morbidity, and provides an unlimited biomaterial availability.
3D-CT and the surgical planning software allow virtually to analyze the patient’s anatomical and prosthetic parameters, to plan the exact implant position and direction, to verify bone defects at implant sites, and to design the graft blocks.
CAD/CAM technologies manufacture on the exact 3D virtual planning models saved in STL files the prefabricated blocks of the xenogenic material, decreasing the amount of graft material required for conventional bone regeneration. Cutting bone substitute blocks into the most appropriate 3D shape facilitates graft adaptation, improves scaffold fitting and stability, and minimizes dead spaces, promoting regeneration [
25]. Furthermore, the customization of the graft blocks is less dependent on the clinician’s skill and experience and reduces the surgical invasiveness and operating times because the manual shaping of the blocks and its adaptation at recipient sites are not necessary.
The decrease in postoperative morbidity, complications and discomfort at the donor site, the lack of need for general anesthesia and hospitalization, and the shortened treatment period increase patient’s acceptance and satisfaction.
Nevertheless, the procedure presents some limitations, such as computer and digital skills, added costs of the biomaterial, and the CAD/CAM manufacturing, and, finally, the preoperative virtual modeling session is time-consuming.
Besides, caution must be used to interpret these results due to the lack of controlled trials and the long-term follow-up to investigate the efficacy of the procedure.