Evaluation of Whole-Body Vibration Exercise on Neuromuscular Activation Through Electromyographic Pattern of Vastus Lateralis Muscle and on Range of Motion of Knees in Metabolic Syndrome: A Quasi-Randomized Cross-Over Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size
2.2. Inclusion and Exclusion Criteria
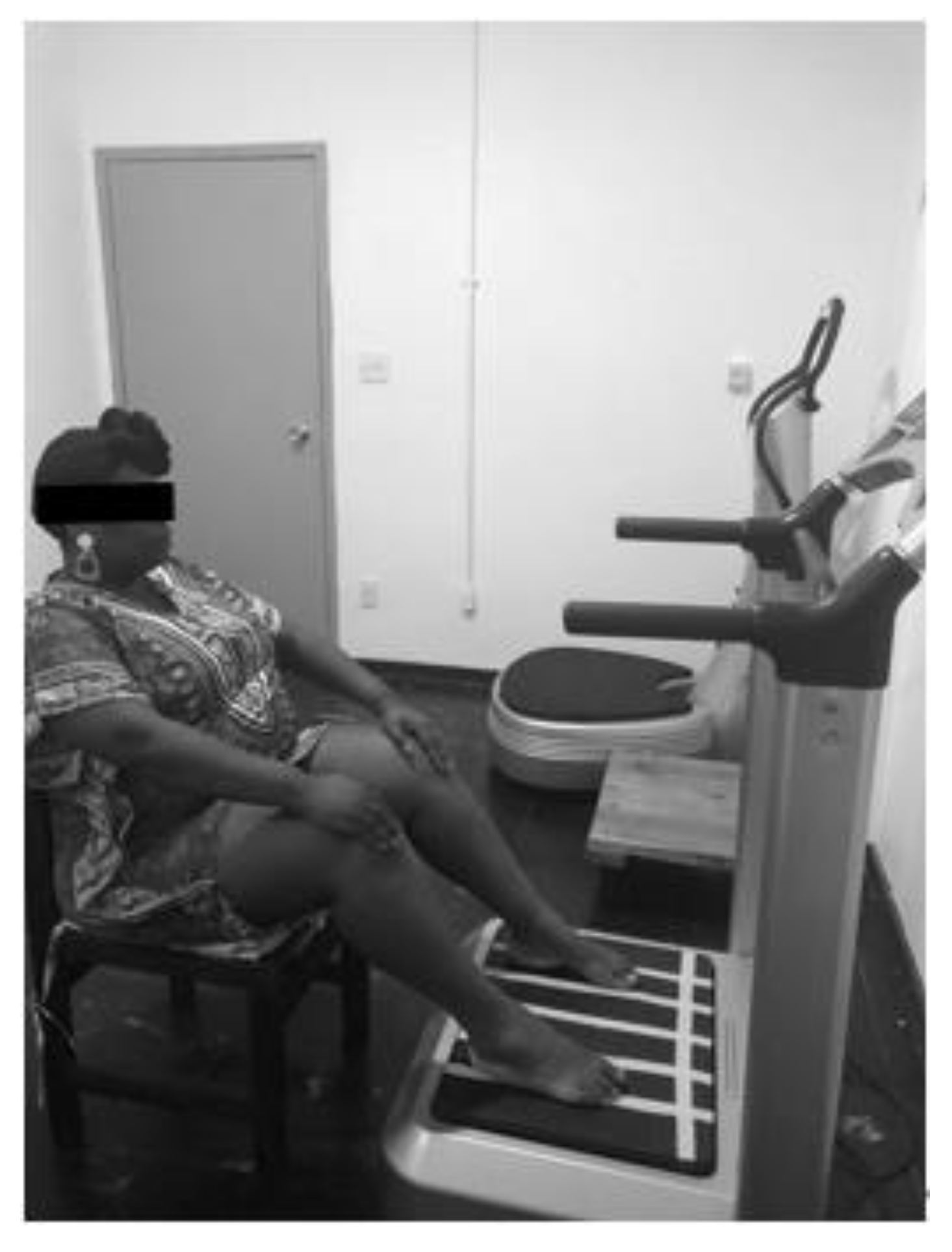
2.3. Interventions
2.4. Outcome Measures
2.4.1. Anthropometric Evaluation
2.4.2. Evaluation of Knee Range of the Motion
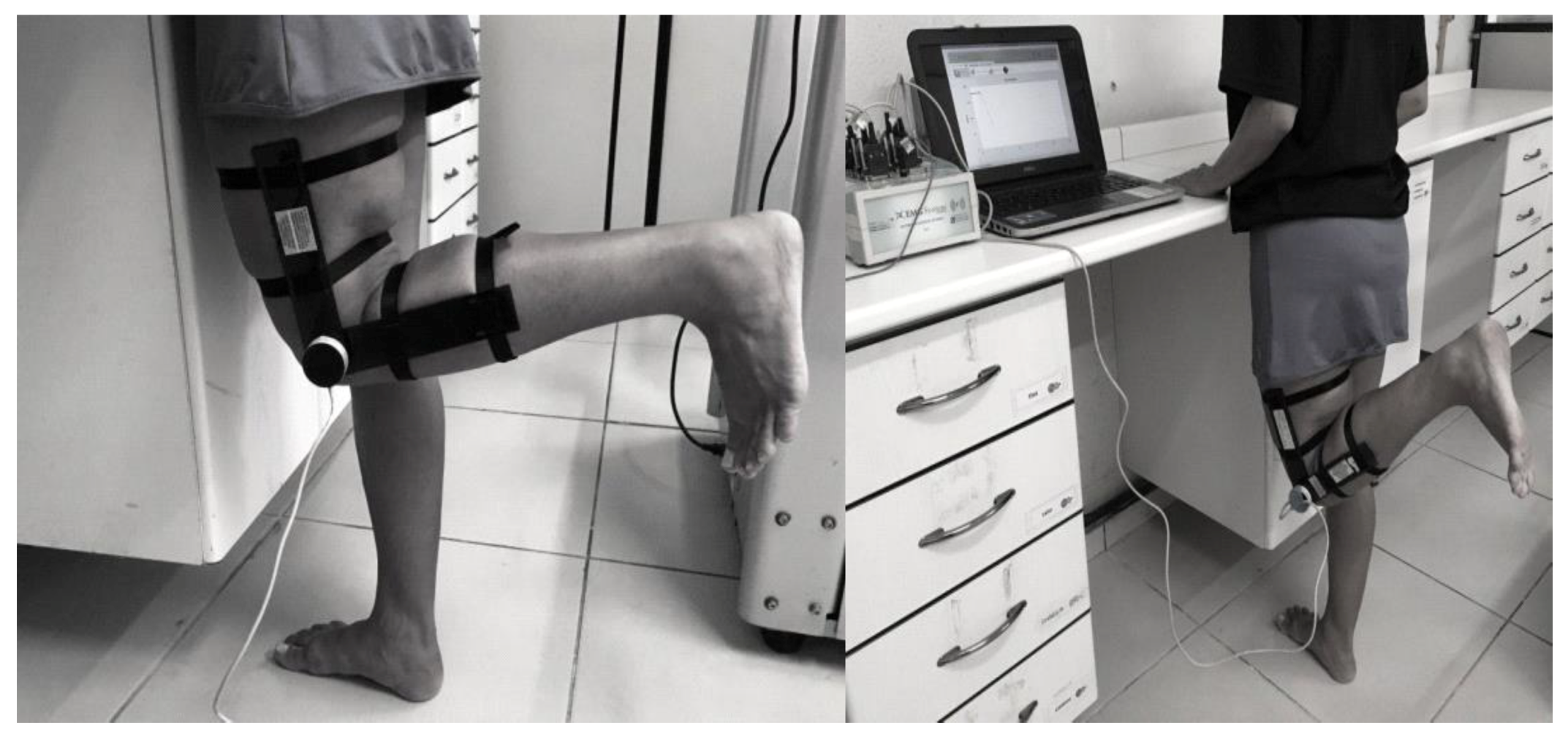
2.5. Surface Electromyography (sEMG) Instrumentation and Measurement
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Effect of Whole Body Vibration Exercise (WBVE) on Range of Motion (ROM)
4.2. Effect of WBVE on sEMG
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CG | control group |
| HUPE | Hospital Universitário Pedro Ernesto |
| IDF | International Diabetes Federation |
| IMAT | intramuscular adipose tissue |
| KOA | knee osteoarthritis |
| LAVIMPI | Laboratório de Vibrações Mecânicas e Práticas Integrativas |
| LEE | Laboratório de Epidemiologia e Estatística |
| MetS | Metabolic syndrome |
| ReBEC | Registro Brasileiro de Ensaios clínicos |
| RMS | root mean square |
| ROM | range of motion |
| sEMG | surface electromyography |
| SENIAM | Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles |
| TG | treatment group |
| UERJ | Universidade do Estado do Rio de Janeiro |
| VL | vastus lateralis |
| VP | vibrating platform |
| WBVE | Whole body vibration exercise |
| WC | waist circumference |
| μV | microvolts |
References
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Worachartcheewan, A.; Schaduangrat, N.; Prachayasittikul, V.; Nantasenamat, C. Data mining for the identification of metabolic syndrome status. EXCLI J. 2018, 17, 72–88. [Google Scholar] [PubMed]
- Yang, C.S.; Wang, H.; Sheridan, Z.P. Studies on prevention of obesity, metabolic syndrome, diabetes, cardiovascular diseases and cancer by tea. J. Food Drug Anal. 2018, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Addison, O.; Marcus, R.L.; Lastayo, P.C.; Ryan, A.S. Intermuscular fat: A review of the consequences and causes. Int. J. Endocrinol. 2014, 2014, 309570. [Google Scholar] [CrossRef]
- Tuttle, L.J.; Sinacore, D.R.; Mueller, M.J. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J. Aging Res. 2012, 2012, 172957. [Google Scholar] [CrossRef]
- Bollinger, L.M. Potential contributions of skeletal muscle contractile dysfunction to altered biomechanics in obesity. Gait Posture 2017, 56, 100–107. [Google Scholar] [CrossRef]
- Capodaglio, P.; Castelnuovo, G.; Brunani, A.; Vismara, L.; Villa, V.; Maria Capodaglio, E. Functional limitations and occupational issues in obesity: A review. Int. J. Occup. Saf. Ergon. 2010, 16, 507–523. [Google Scholar] [CrossRef]
- Ghroubi, S.; Kossemtini, W.; Mahersi, S.; Elleuch, W.; Chaabene, M.; Elleuch, M.H. Contribution of isokinetic muscle strengthening in the rehabilitation of obese subjects. Ann. Phys. Rehabil. Med. 2016, 59, 87–93. [Google Scholar] [CrossRef]
- Fabris, S.M.; Faintuch, J.; Brienze, S.L.A.; Brito, G.B.; Sitta, I.S.; Mendes, E.L.P.; Fonseca, I.C.B.; Cecconello, I. Are knee and foot orthopedic problems more disabling in the superobese? Obes. Surg. 2013, 23, 201–204. [Google Scholar] [CrossRef]
- Gadducci, A.; de Cleva, R.; Santarém, G.; Silva, P.; Greve, J.; Santo, M. Muscle strength and body composition in severe obesity. Clinics 2017, 72, 272–275. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; López, V.; Lazzaro, V.; Pino, J.; Gómez-Reino, J.J.; Gualillo, O. Adipokines, metabolic syndrome and rheumatic diseases. J. Immunol. Res. 2014, 25, 343746. [Google Scholar] [CrossRef] [PubMed]
- Nye, N.S.; Kafer, D.S.; Olsen, C.; Carnahan, D.H.; Crawford, P.F. Abdominal Circumference Versus Body Mass Index as Predictors of Lower Extremity Overuse Injury Risk. J. Phys. Act. Health 2018, 15, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wearing, S.C.; Hennig, E.M.; Byrne, N.M.; Steele, J.R.; Hills, A.P. Musculoskeletal disorders associated with obesity: A biomechanical perspective. Obes. Rev. 2006, 7, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.R.; Karvonen-Gutierrez, C.A. The evolving role of obesity in knee osteoarthritis. Curr. Opin. Rheumatol. 2010, 22, 533–537. [Google Scholar] [CrossRef]
- Holla, J.F.M.; Steultjens, M.P.M.; van der Leeden, M.; Roorda, L.D.; Bierma-Zeinstra, S.M.A.; den Broeder, A.A.; Dekker, J. Determinants of range of joint motion in patients with early symptomatic osteoarthritis of the hip and/or knee: An exploratory study in the CHECK cohort. Osteoarthr. Cart. 2011, 19, 411–419. [Google Scholar] [CrossRef]
- Rowe, P.J.; Myles, C.M.; Walker, C.; Nutton, R. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: How much knee motion is sufficient for normal daily life? Gait Posture 2000, 12, 143–155. [Google Scholar] [CrossRef]
- Clarkson, H. Musculoskeletal Assessment: Joint Range of Motion and Manual Muscle Strength, 2th ed.; Lippincott Wiliams and Wilkin: Philadelphia, PA, USA, 2002. [Google Scholar]
- Pajoutan, M.; Ghesmaty Sangachin, M.; Cavuoto, L.A. Central and peripheral fatigue development in the shoulder muscle with obesity during an isometric endurance task. BMC Musculoskelet Disord 2017, 18, 314. [Google Scholar] [CrossRef]
- Zdziarski, L.A.; Wasser, J.G.; Vincent, H.K. Chronic pain management in the obese patient: A focused review of key challenges and potential exercise solutions. J. Pain Res. 2015, 8, 63–77. [Google Scholar]
- Alvarez-Alvarado, S.; Jaime, S.J.; Ormsbee, M.J.; Campbell, J.C.; Post, J.; Pacilio, J.; Figueroa, A. Benefits of whole-body vibration training on arterial function and muscle strength in young overweight/obese women. Hypertens. Res. 2017, 40, 487–492. [Google Scholar] [CrossRef]
- Patel, V.S.; Ete Chan, M.; Rubin, J.; Rubin, C.T. Marrow Adiposity and Hematopoiesis in Aging and Obesity: Exercise as an Intervention. Curr. Osteoporos. Rep. 2018, 16, 105–115. [Google Scholar] [CrossRef]
- Rauch, F.; Sievanen, H.; Boonen, S.; Cardinale, M.; Degens, H.; Felsenberg, D.; Roth, J.; Schoenau, E.; Verschueren, S.; Rittweger, J. Reporting whole-body vibration intervention studies: Recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J. Musculoskelet. Neuronal Interact. 2010, 10, 193–198. [Google Scholar] [PubMed]
- Pinto, N.S.; Monteiro, M.B.; Arthur, A.P.; Paiva, D.N.; Meyer, P.F.; Santos-Filho, S.D.; Marín, P.J.; Bernardo-Filho, M. Effectiveness of a protocol involving acute whole-body vibration exercises in an adult and health individual with delayed-onset muscle soreness observed after running: A case repor. J. Med. Med. Sci. 2011, 2, 612–617. [Google Scholar]
- Menéndez, H.; Ferrero, C.; Martín-Hernández, J.; Figueroa, A.; Marín, P.J.; Herrero, A.J. Acute effects of simultaneous electromyostimulation and vibration on leg blood flow in spinal cord injury. Spinal Cord 2016, 54, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Lima, R.P.; Sá-Caputo, D.C.; Moreira-Marconi, E.; Dionello, C.; Paineiras-Domingos, L.L.; Sousa-Gonçalves, C.R.; Morel, D.S.; Frederico, E.H.; Neves, M.F.; Oliveira, R.; et al. Quality of life of patients with metabolic syndrome is improved after Whole Body Vibration Exercises. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 59–65. [Google Scholar] [CrossRef]
- Sá-Caputo, D.d.C.; Ronikeili-Costa, P.; Carvalho-Lima, R.P.; Bernardo, L.C.; Bravo-Monteiro, M.O.; Costa, R.; Moraes-Silva, J.d.; Paiva, D.N.; Machado, C.B.; Mantilla-Giehl, P.; et al. Whole Body Vibration Exercises and the Improvement of the Flexibility in Patient with Metabolic Syndrome. Rehabil. Res. Prac. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Dionello, C.F.; De Souza, P.L.; Sá-Caputo, D.; Morel, D.S.; Moreira-Marconi, E.; Paineiras-Domingos, L.L.; Frederico, E.H.F.F.; Guedes-Aguiar, E.; Paiva, P.D.C.; Taiar, R.; et al. Do whole body vibration exercises affect lower limbs neuromuscular activity in populations with a medical condition? Restor. Neurol. Neurosc. 2017, 35, 667–681. [Google Scholar] [CrossRef]
- Herrero, A.J.; Martín, J.; Martín, T.; García-López, D.; Garatachea, N.; Jiménez, B.; Marín, P.J. Whole-body vibration alters blood flow velocity and neuromuscular activity in Friedreich’s ataxia. Clin. Physiol. Funct. Imaging 2011, 31, 139–144. [Google Scholar] [CrossRef]
- Krause, A.; Schoenau, E.; Gollhofer, A.; Duran, I.; Ferrari-Malik, A.; Freyler, K.; Ritzmann, R. Alleviation of Motor Impairments in Patients with Cerebral Palsy: Acute Effects of Whole-body Vibration on Stretch Reflex Response, Voluntary Muscle Activation and Mobility. Front. Neurol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Liao, L.-R.; Ng, G.Y.F.; Jones, A.Y.M.; Huang, M.-Z.; Pang, M.Y.C. Whole-Body Vibration Intensities in Chronic Stroke: A Randomized Controlled Trial. Med. Sci. Sports Exerc. 2016, 48, 1227–1238. [Google Scholar] [CrossRef]
- Van Ruymbeke, B.; Boone, J.; Coorevits, P.; Vanderstraeten, G.; Bourgois, J. Whole-body vibration in breast cancer survivors: A pilot study exploring its effects on muscle activity and subjectively perceived exertion. Int. J. Rehabil. Res. 2014, 37, 371–374. [Google Scholar] [CrossRef]
- Perchthaler, D.; Horstmann, T.; Grau, S. Variations in neuromuscular activity of thigh muscles during whole-body vibration in consideration of different biomechanical variables. J. Sci. Med. Sport 2013, 12, 439–446. [Google Scholar]
- Fattorini, L.; Tirabasso, A.; Lunghi, A.; Di Giovanni, R.; Sacco, F.; Marchetti, E. Muscular forearm activation in hand-grip tasks with superimposition of mechanical vibrations. J. Electromyogr. Kinesiol. 2016, 26, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.T.; Macedo, L.B.; Lins, C.A.A.; Sousa, C.O.; Brasileiro, J.S. Effects of Whole Body Vibration on the Neuromuscular Amplitude of Vastus Lateralis Muscle. J. Sci. Med. Sport 2017, 16, 414–420. [Google Scholar]
- Mosier, E.M.; Herda, T.J.; Trevino, M.A.; Miller, J.D. The influence of prolonged vibration on motor unit behavior. Muscle Nerve 2017, 55, 500–507. [Google Scholar] [CrossRef]
- Simsek, D. Different fatigue-resistant leg muscles and EMG response during whole-body vibration. J. Electromyogr. Kinesiol. 2017, 37, 147–154. [Google Scholar] [CrossRef]
- Cheng, H.-Y.K.; Ju, Y.-Y.; Chen, C.-L.; Chuang, L.-L.; Cheng, C.-H. Effects of whole body vibration on spasticity and lower extremity function in children with cerebral palsy. Hum. Mov. Sci. 2015, 39, 65–72. [Google Scholar] [CrossRef]
- Yang, F.; Finlayson, M.; Bethoux, F.; Su, X.; Dillon, L.; Maldonado, H.M. Effects of controlled whole-body vibration training in improving fall risk factors among individuals with multiple sclerosis: A pilot study. Disabil. Rehabil. 2018, 40, 553–560. [Google Scholar] [CrossRef]
- Wang, P.; Yang, L.; Li, H.; Lei, Z.; Yang, X.; Liu, C.; Jiang, H.; Zhang, L.; Zhou, Z.; Reinhardt, J.D.; et al. Effects of whole-body vibration training with quadriceps strengthening exercise on functioning and gait parameters in patients with medial compartment knee osteoarthritis: A randomised controlled preliminary study. Physiotherapy (UK) 2016, 102, 86–92. [Google Scholar] [CrossRef]
- LEE—Laboratório de Epidemiologia e Estatística. Available online: http://www.lee.dante.br/pesquisa.html (accessed on 10 October 2018).
- O’Keefe, K.; Orr, R.; Huang, P.; Selvadurai, H.; Cooper, P.; Munns, C.F.; Singh, M.A.F. The effect of whole body vibration exposure on muscle function in children with cystic fibrosis: A pilot efficacy trial. J. Clin. Med. Res. 2013, 5, 205–216. [Google Scholar] [CrossRef][Green Version]
- Cardinale, M.; Wakeling, J. Whole body vibration exercise: Are vibrations good for you? J. Sports Med. 2005, 39, 585–589. [Google Scholar] [CrossRef]
- WHO. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Tech. Rep. Ser. 1995, 854, 1–452. [Google Scholar]
- Dos Santos, R.A.; Derhon, V.; Brandalize, M.; Brandalize, D.; Rossi, L.P. Evaluation of knee range of motion: Correlation between measurements using a universal goniometer and a smartphone goniometric application. J. Bodyw. Mov. Ther. 2017, 21, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Milanese, S.; Gordon, S.; Buettner, P.; Flavell, C.; Ruston, S.; Coe, D.; O’Sullivan, W.; McCormack, S. Reliability and concurrent validity of knee angle measurement: Smart phone app versus universal goniometer used by experienced and novice clinicians. Man. Ther. 2014, 19, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Marks, R. Knee Osteoarthritis and Exercise Adherence: A Review. Curr. Aging Sci. 2012, 5, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Neto, S.B.S.; Marconi, E.M.; Kutter, C.R.; Frederico, E.H.F.F.F.; De Paiva, P.d.C.; Meyer, P.F.; Chang, S.; Sá-Caputo, D.; Bernardo-Filho, M. Beneficial effects of whole body mechanical vibration alone or combined with auriculotherapy in the pain and in flexion of knee of individuals with knee osteoarthritis. Acupunct. Electro Ther. Res. 2017, 42, 185–201. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Lee, T.-H.; Choi, J.-D.; Lee, N.-G. Activation timing patterns of the abdominal and leg muscles during the sit-to-stand movement in individuals with chronic hemiparetic stroke. J. Phys. Ther. Sci. 2015, 27, 3593–3595. [Google Scholar] [CrossRef]
- Tomlinson, D.J.; Erskine, R.M.; Winwood, K.; Morse, C.I.; Onambélé, G.L. The impact of obesity on skeletal muscle architecture in untrained young vs. old women. J. Anat. 2014, 225, 675–684. [Google Scholar] [CrossRef]
- Hilton, T.N.; Tuttle, L.J.; Bohnert, K.L.; Mueller, M.J.; Sinacore, D.R. Excessive Adipose Tissue Infiltration in Skeletal Muscle in Individuals With Obesity, Diabetes Mellitus, and Peripheral Neuropathy: Association With Performance and Function. Phys. Ther. 2008, 88, 1336–1344. [Google Scholar] [CrossRef]
- Lafortuna, C.L.; Prinelli, F.; Adorni, F.; Agosti, F.; De Col, A.; Sartorio, A. Effect of mechanical and metabolic factors on motor function and fatigue in obese men and women: A cross-sectional study. J. Endocrinol. Investig. 2013, 36, 1062–1068. [Google Scholar]
- Wang, P.; Yang, L.; Liu, C.; Wei, X.; Yang, X.; Zhou, Y.; Jiang, H.; Lei, Z.; Reinhardt, J.D.; He, C. Effects of Whole Body Vibration Exercise associated with Quadriceps Resistance Exercise on functioning and quality of life in patients with knee osteoarthritis: A randomized controlled trial. Clin. Rehabil. 2016, 30, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Lauwers-Cances, V.; Cristini, C.; van Kan, G.A.; Janssen, I.; Morley, J.E.; Vellas, B. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: The EPIDOS ( EPIDemiologie de l′OSteoporose ) Study 1–3. Am. J. Clin. Nutr. 2009, 89, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Porto, H.C.D.; Pechak, C.M.; Smith, D.R.; Reed-Jones, R.J. Biomechanical Effects of Obesity on Balance. Int. J. Exerc. Sci. 2012, 5, 301–320. [Google Scholar]
- Tihanyi, T.K.; Horváth, M.; Fazekas, G.; Hortobágyi, T.; Tihanyi, J. One session of whole body vibration increases voluntary muscle strength transiently in patients with stroke. Clin. Rehabil. 2007, 21, 782–793. [Google Scholar] [CrossRef]
- Herrero, A.J.; Menéndez, H.; Gil, L.; Martín, J.; Martín, T.; García-López, D.; Gil-Agudo, Á.; Marín, P.J. Effects of whole-body vibration on blood flow and neuromuscular activity in spinal cord injury. Spinal Cord 2011, 49, 554–559. [Google Scholar] [CrossRef]
- Avelar, N.C.P.; Ribeiro, V.G.C.; Mezêncio, B.; Fonseca, S.F.; Tossige-Gomes, R.; da Costa, S.J.; Szmuchrowski, L.; Gripp, F.; Coimbra, C.C.; Lacerda, A.C.R. Influence of the knee flexion on muscle activation and transmissibility during whole body vibration. J. Electromyogr. Kinesiol. 2013, 23, 844–850. [Google Scholar] [CrossRef]
- Annino, G.; Iellamo, F.; Palazzo, F.; Fusco, A.; Lombardo, M.; Campoli, F.; Padua, E. Acute changes in neuromuscular activity in vertical jump and flexibility after exposure to whole body vibration. Medicine (US) 2017, 96, e7629. [Google Scholar] [CrossRef]
- Borges, D.T.; Macedo, L.B.; Lins, C.A.A.; Brasileiro, J.S. Immediate effects of whole-body vibration on neuromuscular performance of quadriceps and oscillation of the center of pressure: A randomized controlled trial. Man. Ther. 2016, 25, 62–68. [Google Scholar] [CrossRef]
- Rubio-Arias, J.Á.; Ramos-Campo, D.J.; Esteban, P.; Martínez, F.; Jiménez, J.F. Effect of 6-weeks WBVT on the behaviour of the lower limb muscle fibres during vertical jumping. J. Sports Sci. 2018, 36, 398–406. [Google Scholar] [CrossRef]

| Anthropometrics | CG (n = 17) Mean ± SE | TG (n = 22) Mean ± SE | P |
|---|---|---|---|
| Height (cm) | 1.62 ± 0.01 | 1.63 ± 0.01 | 0.58 |
| Body mass (kg) | 88.8 ± 4.08 | 83.1 ± 3.65 | 0.46 |
| BMI (kg/m2) | 33.5 ± 1.56 | 31.3 ± 1.12 | 0.25 |
| Age (years) | 58.1 ± 2.07 | 60.7 ± 1.91 | 0.40 |
| WC (cm) | 108 ± 3.58 | 103 ± 2.57 | 0.11 |
| Knee | CG | TG | CG × TG | ||||
|---|---|---|---|---|---|---|---|
| Before Mean ± SE (Degrees) | After Mean ± SE (Degrees) | P | Before Mean ± SE (Degrees) | After Mean ± SE (Degrees) | P | P | |
| Right knee | 94.13 ± 6.89 | 80.63 ± 9.50 | 0.29 | 99.47 ± 7.12 | 97.58 ± 6.86 | 0.62 | 0.59 |
| Left knee | 94.11 ± 6.91 | 100.90 ± 2.74 | 0.25 | 99.37 ± 7.16 | 97.49 ± 6.88 | 0.62 | 0.90 |
| Knee | CG | TG | CG × TG | ||||
|---|---|---|---|---|---|---|---|
| Before Mean ± SE (Degree) | After Mean ± SE (Degree) | P | Before Mean ± SE (Degree) | After Mean ± SE (Degree) | P | P | |
| Right knee | 101.20 ± 3.30 | 103.40 ± 3.49 | 0.49 | 105.50 ± 3.40 | 104.10 ± 3.55 | 0.15 | 0.10 |
| Left knee | 100.90 ± 2.53 | 104.90 ± 3.76 | 0.12 | 102.90 ± 3.22 | 105.00 ± 2.72 | 0.10 | 0.43 |
| Knee | CG | TG | CG × TG | ||||
|---|---|---|---|---|---|---|---|
| Before Mean ± SE (Degree) | After Mean ± SE (Degree) | P | Before Mean ± SE (Degree) | After Mean ± SE (Degree) | P | P | |
| Right knee | 102.70 ± 3.01 | 101.20 ± 3.30 | 0.62 | 94.49 ± 4.43 | 108.00 ± 3.39 | 0.09 | 0.07 |
| Left knee | 95.37 ± 4.28 | 100.90 ± 2.53 | 0.64 | 100.80 ± 2.64 | 108.30 ± 2.59 | 0.07 | 0.27 |
| Muscle | CG | TG | P |
|---|---|---|---|
| Mean ± SE %RMS | Mean ± SE %RMS | ||
| VL right | 93.59 ± 5.50 | 102.10 ± 3.58 | 0.28 |
| VL left | 98.65 ± 3.21 | 94.91 ± 3.66 | 0.99 |
| Muscle | CG | TG | P |
|---|---|---|---|
| Mean ± SE % RMS | Mean ± SE % RMS | ||
| VL right | 95.22 ± 3.18 | 108.00 ± 5.07 | 0.008 * |
| VL left | 96.57 ± 3.69 | 106.20 ± 3.53 | 0.02 * |
| Muscle | CG | TG | P |
|---|---|---|---|
| Mean ± SE % RMS | Mean ± SE % RMS | ||
| VL right | 99.38 ± 5.76 | 105.70 ± 5.40 | 0.63 |
| VL left | 97.10 ± 4.03 | 110.60 ± 7.87 | 0.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa-Gonçalves, C.R.d.; Paineiras-Domingos, L.L.; Teixeira-Silva, Y.; Amadeu, T.; Lírio, A.P.d.S.; Francisca-Santos, A.; De Souza, L.F.F.; Pereira, M.J.d.S.; Melo-Oliveira, M.E.S.; Meirelles, A.G.d.; et al. Evaluation of Whole-Body Vibration Exercise on Neuromuscular Activation Through Electromyographic Pattern of Vastus Lateralis Muscle and on Range of Motion of Knees in Metabolic Syndrome: A Quasi-Randomized Cross-Over Controlled Trial. Appl. Sci. 2019, 9, 4997. https://doi.org/10.3390/app9234997
Sousa-Gonçalves CRd, Paineiras-Domingos LL, Teixeira-Silva Y, Amadeu T, Lírio APdS, Francisca-Santos A, De Souza LFF, Pereira MJdS, Melo-Oliveira MES, Meirelles AGd, et al. Evaluation of Whole-Body Vibration Exercise on Neuromuscular Activation Through Electromyographic Pattern of Vastus Lateralis Muscle and on Range of Motion of Knees in Metabolic Syndrome: A Quasi-Randomized Cross-Over Controlled Trial. Applied Sciences. 2019; 9(23):4997. https://doi.org/10.3390/app9234997
Chicago/Turabian StyleSousa-Gonçalves, Cintia Renata de, Laisa Liane Paineiras-Domingos, Ygor Teixeira-Silva, Thais Amadeu, Adriana Pereira da Silva Lírio, Arlete Francisca-Santos, Luiz Felipe Ferreira De Souza, Mario José dos Santos Pereira, Maria Eduarda Souza Melo-Oliveira, Alexandre Gonçalves de Meirelles, and et al. 2019. "Evaluation of Whole-Body Vibration Exercise on Neuromuscular Activation Through Electromyographic Pattern of Vastus Lateralis Muscle and on Range of Motion of Knees in Metabolic Syndrome: A Quasi-Randomized Cross-Over Controlled Trial" Applied Sciences 9, no. 23: 4997. https://doi.org/10.3390/app9234997
APA StyleSousa-Gonçalves, C. R. d., Paineiras-Domingos, L. L., Teixeira-Silva, Y., Amadeu, T., Lírio, A. P. d. S., Francisca-Santos, A., De Souza, L. F. F., Pereira, M. J. d. S., Melo-Oliveira, M. E. S., Meirelles, A. G. d., Guimarães-Lourenço, G. M., Reis-Silva, A., Moreira-Marconi, E., Moura-Fernandes, M. C., Xavier, V. L., Mulder, A. d. R. P., Lacerda, A. C. R., Mendonça, V. A., Bachur, J. A., ... Bernardo-Filho, M. (2019). Evaluation of Whole-Body Vibration Exercise on Neuromuscular Activation Through Electromyographic Pattern of Vastus Lateralis Muscle and on Range of Motion of Knees in Metabolic Syndrome: A Quasi-Randomized Cross-Over Controlled Trial. Applied Sciences, 9(23), 4997. https://doi.org/10.3390/app9234997












