An Experimental and Numerical Study of CO2–Brine-Synthetic Sandstone Interactions under High-Pressure (P)–Temperature (T) Reservoir Conditions
Abstract
1. Introduction
2. Samples and Methods
2.1. Sample Descriptions
2.2. Physical Experimental Conditions
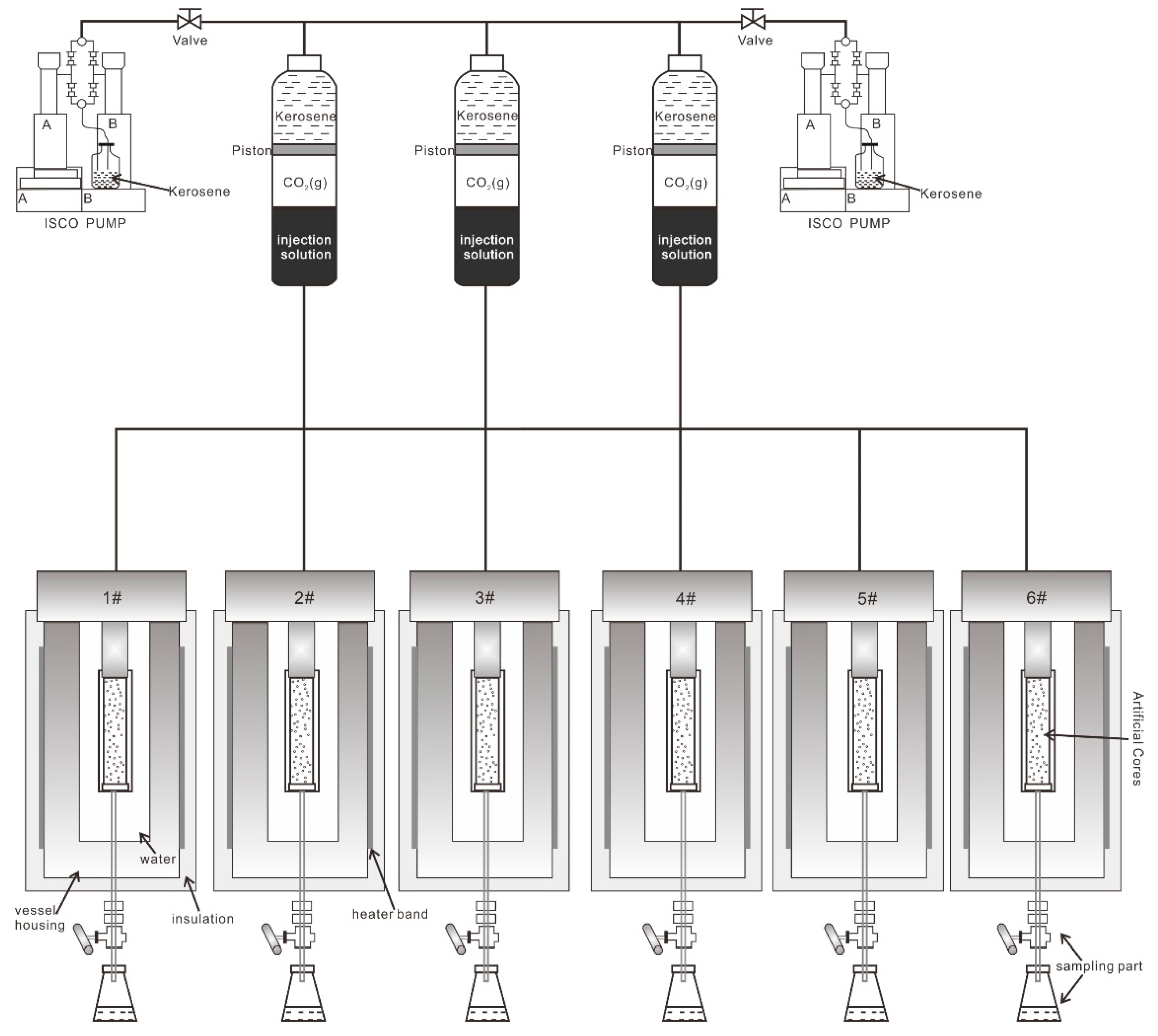
2.3. Experimental Apparatus
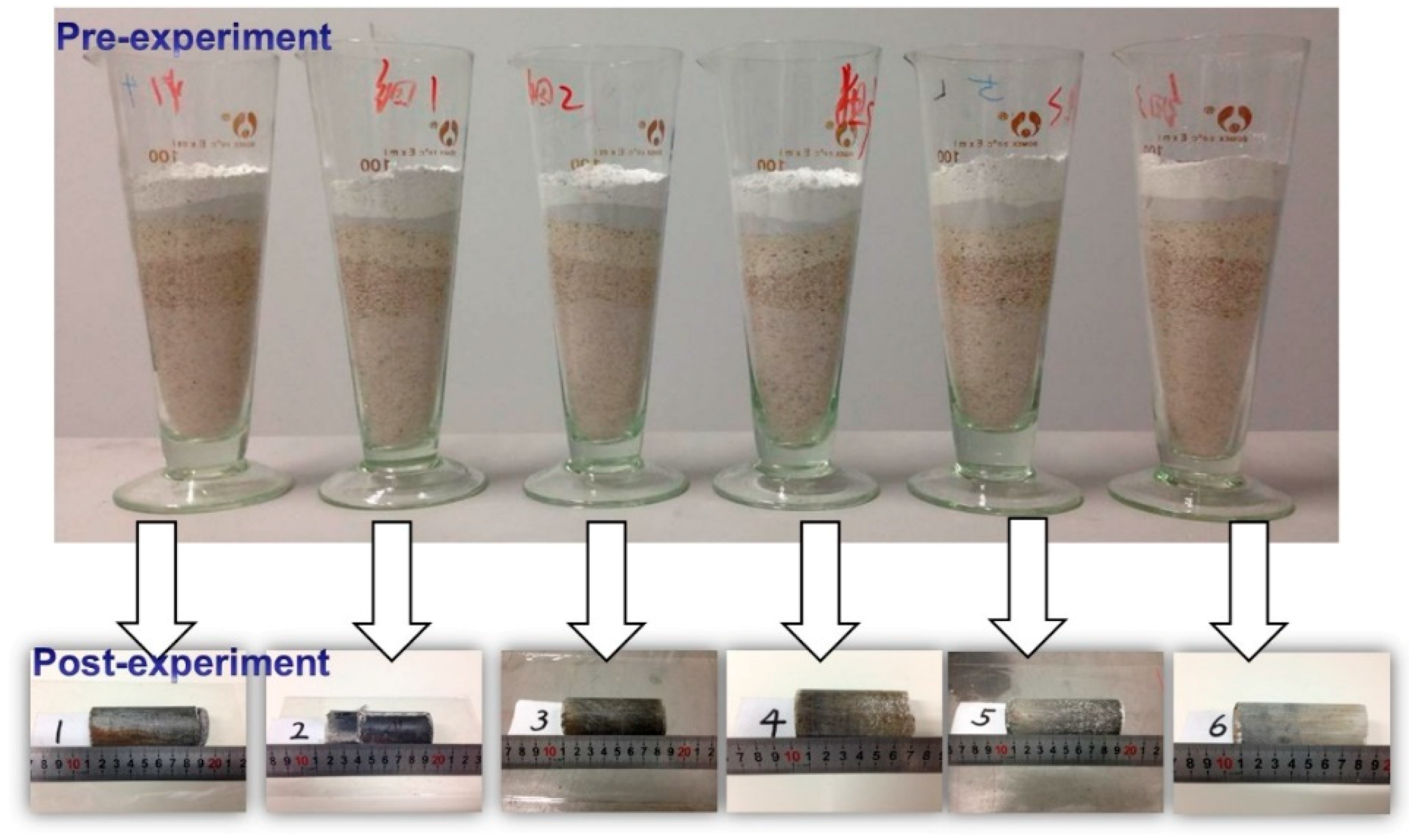
2.4. Physical Simulation Workflow and Analysis
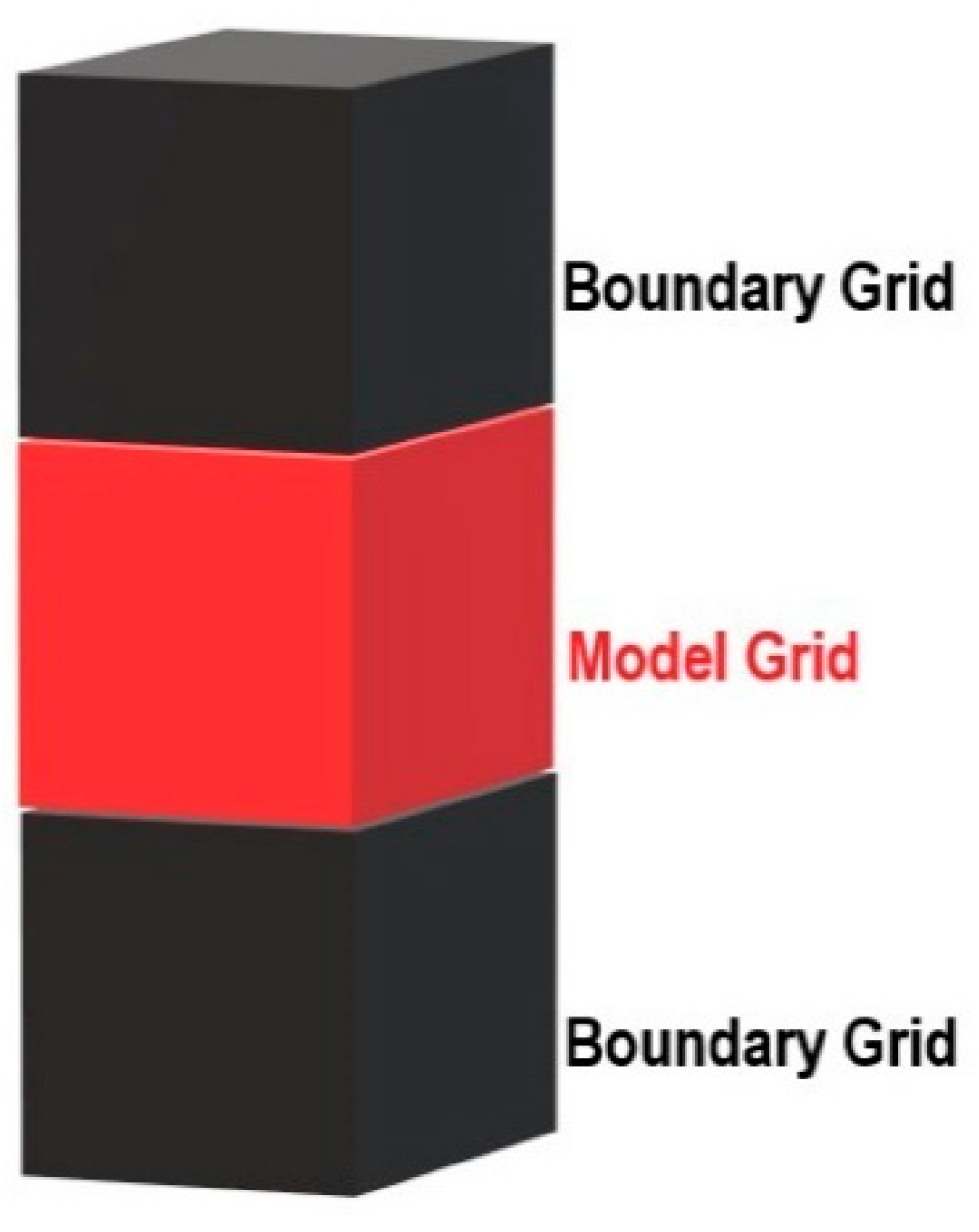
2.5. Numerical Simulation
3. Results
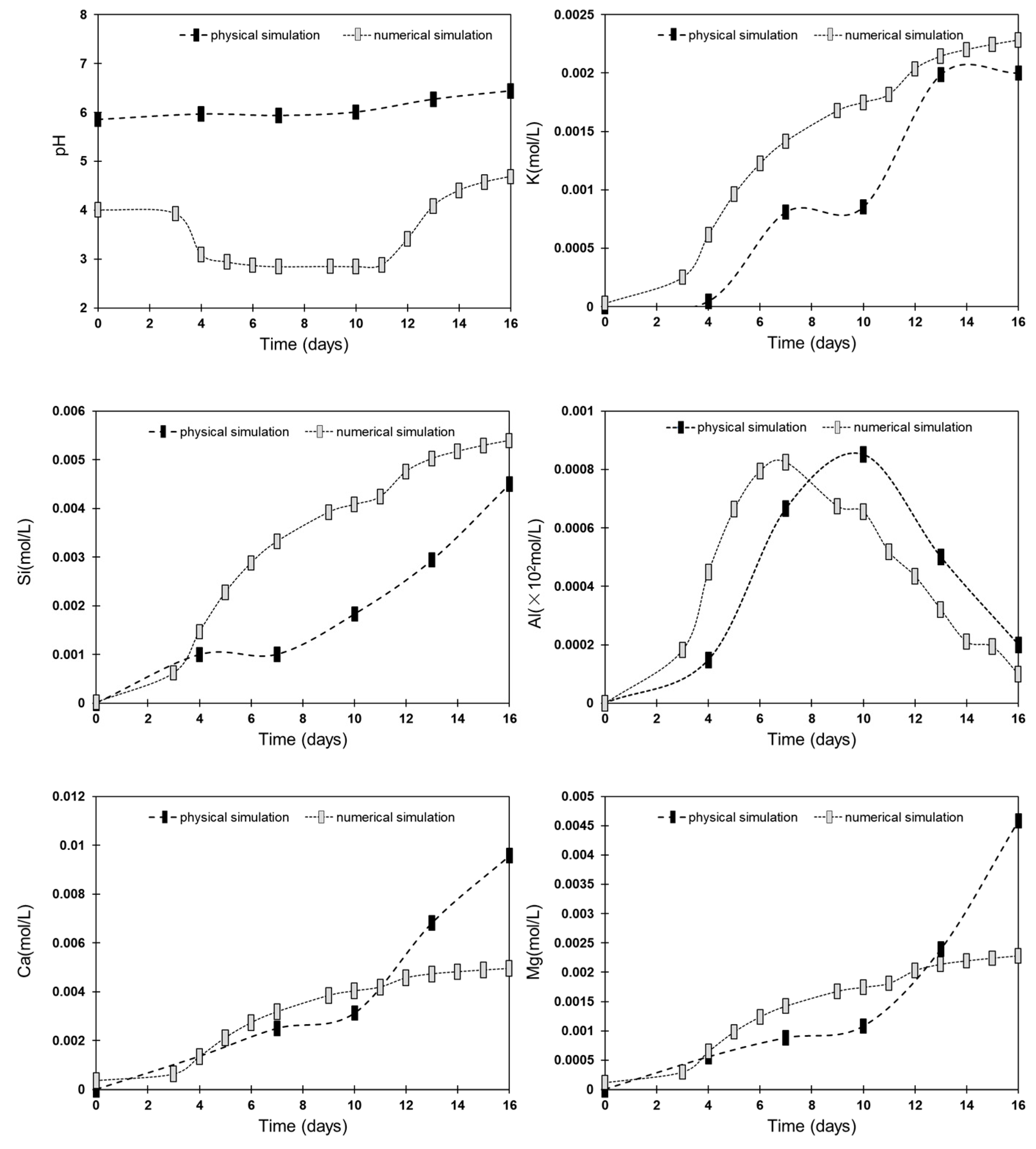
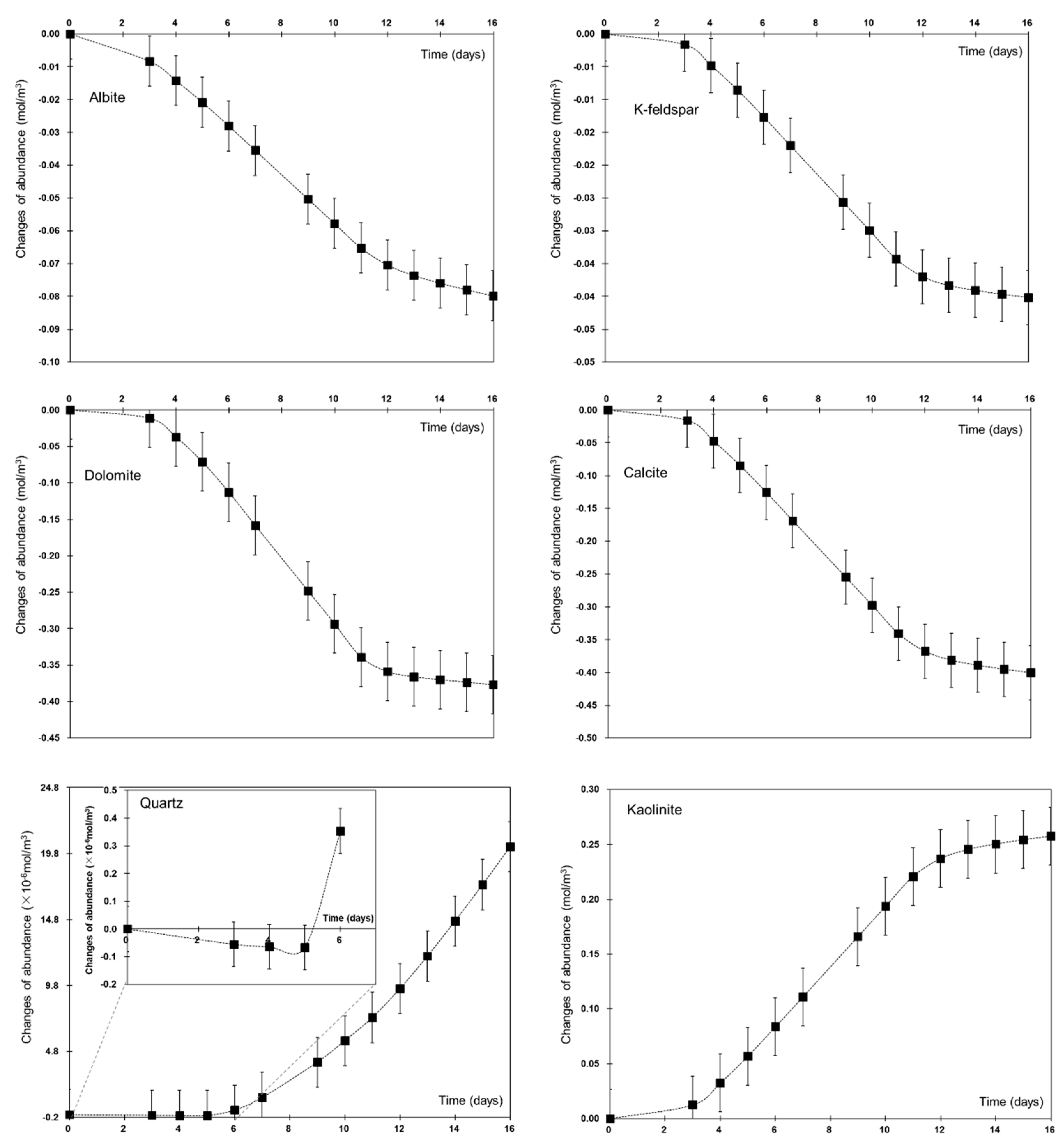
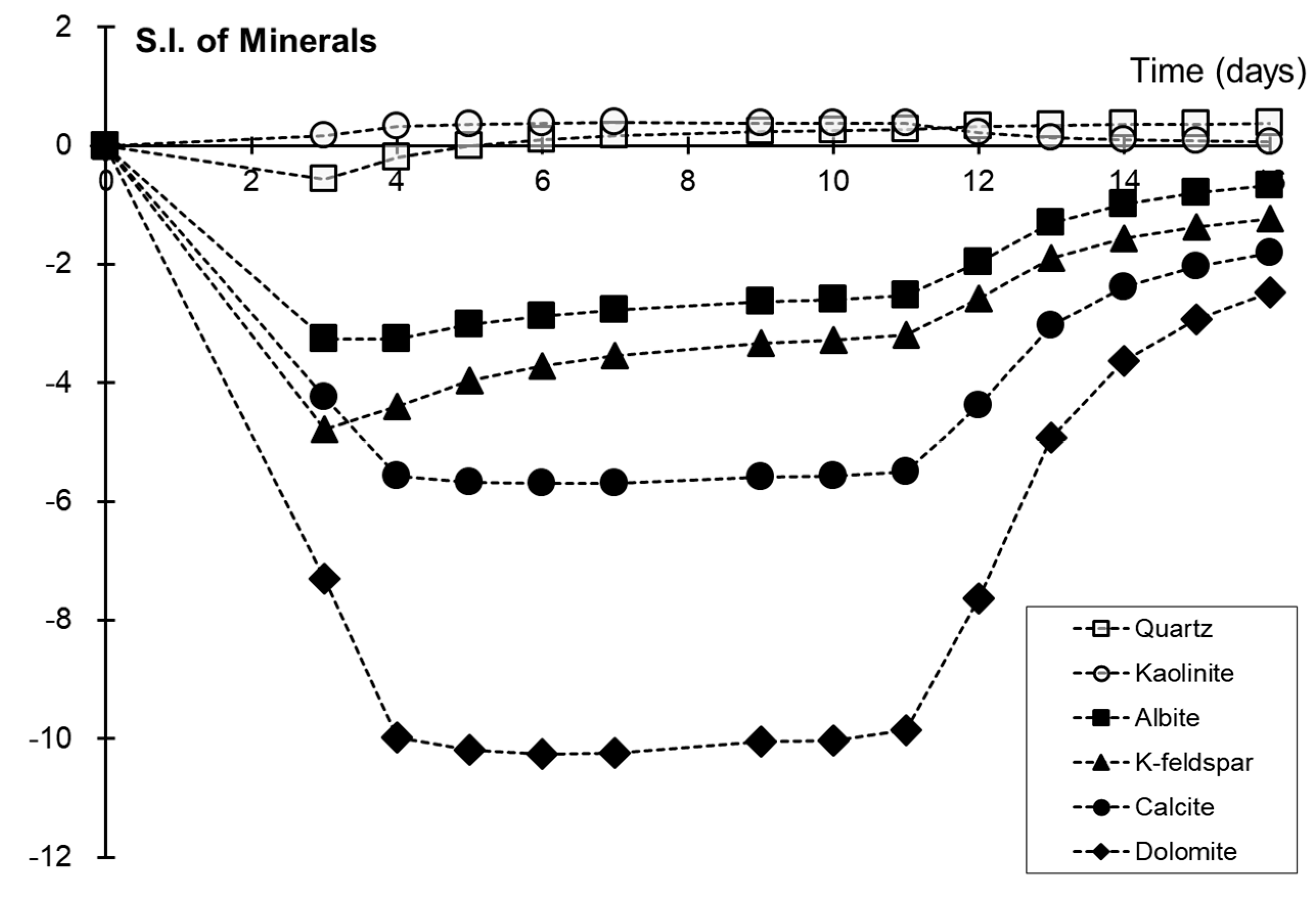
3.1. Changes in Fluid Chemistry
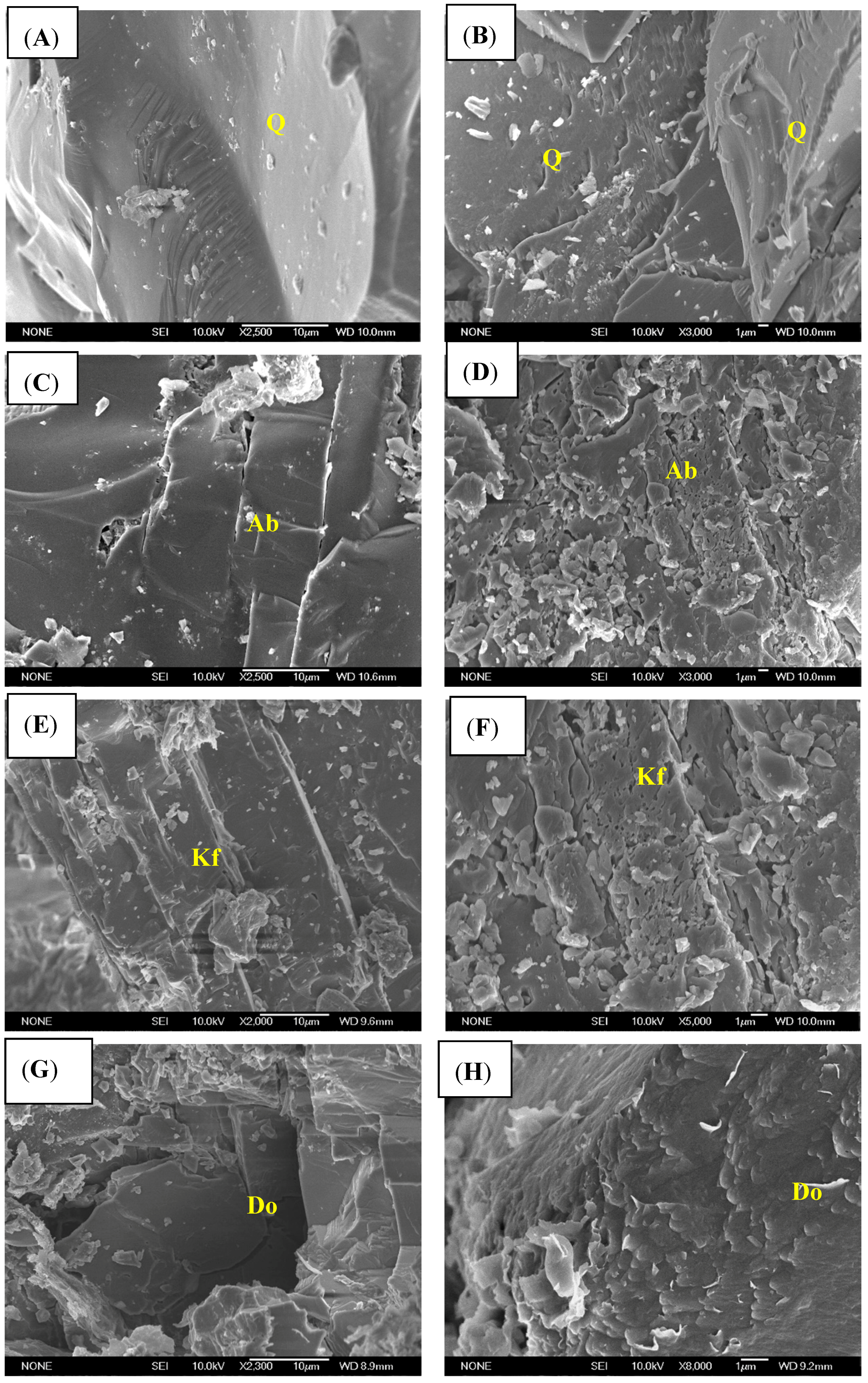
3.2. Changes in Mineral Morphology during the Physical Simulation
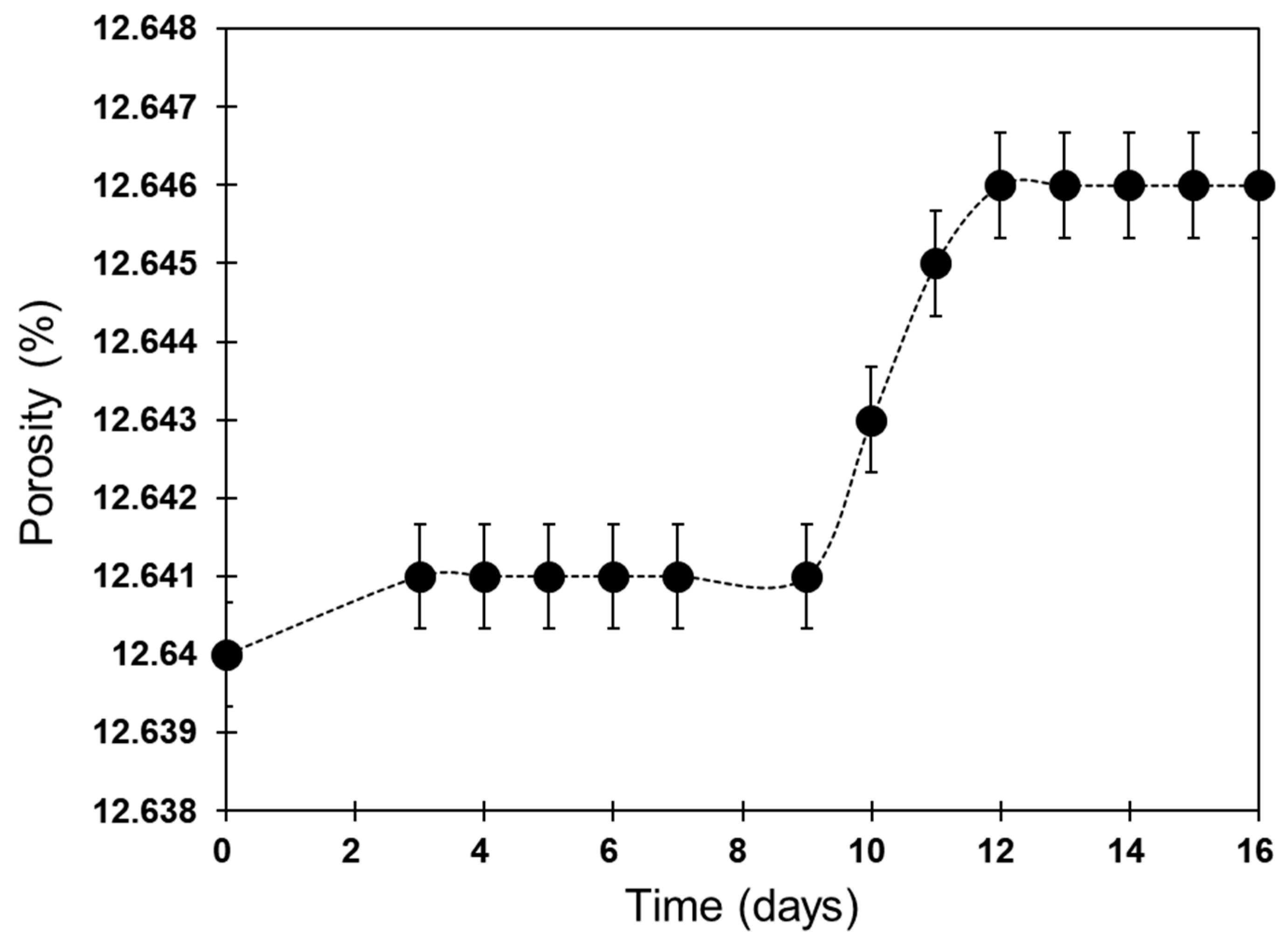
3.3. Changes in Porosity
4. Discussion
4.1. Mineral Dissolution and Precipitation
4.2. Porosity Changes
5. Conclusions
- (1)
- No significant short-term CO2-rock-formation-water geochemical reactions are induced by CO2 injection.
- (2)
- Neither physical nor numerical simulation found significant core porosity variations after CO2 injection.
- (3)
- Minor amounts of kaolinite and quartz were precipitated during the numerical modelling but were not observed in the physical simulation.
- (4)
- Physical and numerical simulations conducted in tandem can be used to verify each other and improve their reliability.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Kinetic Rate Law for Mineral Dissolution and Precipitation
References
- Intergov Panel on Climate Change. Climate change 2014: Mitigation of Climate Change Working Group III Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 10013–12473. [Google Scholar]
- Fischer, S.; Liebscher, A.; Wandrey, M.; the CO2 SINK Group. CO2–brine–rock interaction—First results of long-term exposure experiments at in situ P–T conditions of the Ketzin CO2 reservoir. Chem. Erde 2010, 70, 155–164. [Google Scholar] [CrossRef]
- Hutcheon, I.; Shevalier, M.; Durocher, K.; Bloch, J.; Johnson, G.; Nightingale, M.; Mayer, B. Interactions of CO2 with formation waters, oil and minerals and CO2 storage at the Weyburn IEA EOR site, Saskatchewan, Canada. Int. J. Greenh. Gas Control 2016, 53, 354–370. [Google Scholar] [CrossRef]
- IEA. CO2 Emissions from Fuel Combustion, 2012th ed.; OECD/IEA: Paris, France, 2012; p. 136. Available online: http://www.iea.org/ publications/freepublications/publication/CO2emissionfromfuelcombustionHIGHLIGHTS.pdf (accessed on 25 November 2012).
- Bachu, S.; Gunter, W.D.; Perkins, E.H. Aquifer disposal of CO2: Hydrodynamic and mineral trapping. Energy Convers. Manag. 1994, 35, 269–279. [Google Scholar] [CrossRef]
- Hitchon, B.; Gunter, W.D.; Gentzis, T.; Baileyc, R.T. Sedimentary basins and greenhouse gases: A serendipitous association. Energy Convers. Manag. 1999, 40, 825–843. [Google Scholar] [CrossRef]
- Sayegh, S.G.; Krause, F.F.; Girard, M.; DeBree, C. Rock/fluid interactions of carbonated brines in a sandstone reservoir: Pembina Cardium, Alberta, Canada. SPE Form. Eval. 1990, 5, 399–405. [Google Scholar] [CrossRef]
- Tobergte, D.R.; Curtis, S. Experimental perspectives of mineral dissolution and precipitation due to carbon dioxide-water-rock interactions. J. Chem. Inf. Model. 2013, 53, 1689–1699. [Google Scholar]
- Lombard, J.M.; Azaroual, M.; Pironon, J.; Broseta, D.; Egermann, P.; Munier, G.; Mouronval, G. CO2 injectivity in geological storages: An overview of program and results of the GeoCarbone-Injectivity project. Oil Gas Sci. Technol. 2010, 65, 533–539. [Google Scholar] [CrossRef]
- Miri, R.; van Noort, R.; Aagaard, P.; Hellevang, H. New insights on the physics of salt precipitation during injection of CO2 into saline aquifers. Int. J. Greenh. Gas Control 2015, 43, 10–21. [Google Scholar] [CrossRef]
- Kampman, N.; Bickle, M.; Wigley, M.; Dubacq, B. Fluid flow and CO2–fluid–mineral interactions during CO2-storage in sedimentary basins. Chem. Geol. 2014, 369, 22–50. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Golubev, S.V.; Schott, J.; Castillo, A. Calcite, dolomite and magnesite dissolution kinetics in aqueous solutions at acid to circumneutral pH, 25 to 150 C and 1 to 55 atm pCO2: New constraints on CO2 sequestration in sedimentary basins. Chem. Geol. 2009, 265, 20–32. [Google Scholar] [CrossRef]
- Alemu, B.L.; Aker, E.; Soldal, M.; Johnsen, Ø.; Aagaard, P. Effect of sub-core scale heterogeneities on acoustic and electrical properties of a reservoir rock: A CO2 flooding experiment of brine saturated sandstone in a computed tomography scanner. Geophys. Prospect 2013, 61, 235–250. [Google Scholar] [CrossRef]
- Luquot, L.; Andreani, M.; Gouze, P.; Camps, P. CO2 percolation experiment through chlorite/zeolite-rich sandstone (Pretty Hill Formation–Otway Basin–Australia). Chem. Geol. 2012, 294–295, 75–88. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, L.; Yang, S.; Li, S.; Yang, Y. An experimental study of CO2–brine–rock interaction at in situ pressure–temperature reservoir conditions. Chem. Geol. 2012, 326–327, 88–101. [Google Scholar] [CrossRef]
- Nitao, J.J. User’s Manual for the USNT Module of NUFT Code; Version 2.0. LLNL.; Lawrence Livermore National Laboratory Report UCRL-MA-130653; Lawrence Livermore National Laboratory: Livermore, CA, USA, 1998.
- Lichtner, P.C. FLOWTRAN User Manual; Tech. Report, LA-Ur-01-2349; Las Alamos National Laboratory: Las Alamos, NM, USA, 2001.
- Nghiem, L.; Sammon, P.; Grabenstetter, J.; Ohkuma, H. Modeling CO2 storage in aquifers with fully-coupled geochemical EOS compositional simulator. Soc. Pet. Eng. J. 2004, 89474. [Google Scholar] [CrossRef]
- White, M.D.; Oostrom, M. STOMP: Subsurface Transport over Multiple Phases; Version 4.0; User’s Guide; Pacific Northwest National Laboratory: Richland, WA, USA, 2006; p. 120.
- Xu, T.; Sonnenthal, E.L.; Spycher, N.; Pruess, K. TOUGHREACT: A simulation program for non-isothermal multiphase reactive geochemical transport in variably saturated geologic media. Comput. Geosci. 2006, 32, 145–165. [Google Scholar] [CrossRef]
- Xu, T.; Spycher, N.; Sonnenthal, E.L.; Zhang, G.; Zheng, L.; Pruess, K. TOUGHREACT Version 2.0: A simulator for subsurface reactive transport under non-isothermal multiphase flow conditions. Comput. Geosci. 2011, 37, 763–774. [Google Scholar] [CrossRef]
- Audigane, P.; Gaus, I.; Czernichowski-Lauriol, I.; Pruess, K.; Xu, T. Two dimensional reactive transport modeling of CO2 injection in a saline aquifer at the Sleipner Site. Am. J. Sci. 2007, 307, 974–1008. [Google Scholar] [CrossRef]
- Gherardi, F.; Xu, T.; Pruess, K. Numerical modeling of self-limiting and self-enhancing cap rock alteration induced by CO2 storage in a depleted gas reservoir. Chem. Geol. 2007, 244, 103–129. [Google Scholar] [CrossRef]
- Okuyama, Y.; Sasaki, M.; Nakanishi, S.; Norifumi, T.; Ajima, S. Geochemical CO2 trapping in open aquifer storage—The Tokyo Bay model. Energy Procedia 2009, 1, 3253–3258. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Apps, J.A.; Zhang, Y.Q.; Xu, T.; Birkholzer, J.T. Reactive Transport Simulations to Study Groundwater Quality Changes in Response to CO2 Leakage from Deep Geological Storage. Phys. Procedia 2009, 1, 1887–1894. [Google Scholar] [CrossRef]
- Xu, T.; Kharaka, Y.; Doughty, C.; Freifeld, B.M.; Daley, T.M. Reactive transport modeling to study changes in water chemistry induced by CO2 injection at the Frio-I brine pilot. Chem. Geol. 2010, 271, 153–164. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, K.; Zhao, M.; Liu, S.; Zhuo, Q.; Lu, X. Characterization of Diagenesis and the Petroleum Charge in Kela 2 Gas Field, Kuqa Depression, Tarim Basin. Earth Sci. 2015, 41, 533–545, (In Chinese with English Abstract). [Google Scholar]
- Duan, Z.H.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Palandri, J.; Kharaka, Y.K. A Compilation of Rate Parameters of Water–Mineralinteraction Kinetics for Application to Geochemical Modeling; US Geological Survey Open File Report (of 2004–1068); USGS: Reston, VA, USA, 2004; p. 64.
- Bertier, P.; Swennen, R.; Laenen, B.; Lagrou, D.; Dreesen, R. Experimental identification of CO2–water–rock interactions caused by sequestration of CO2 in Westphalia and Buntsandstein sandstones of the Campine Basin (NE-Belgium). J. Geochem. Explor. 2006, 89, 10–14. [Google Scholar] [CrossRef]
- Wandrey, M.; Fischera, S.; Zemkea, K.; Liebschera, A.; Scherfb, A.K.; Hillebrandb, A.V.; Zettlitzerc, M.; Würdemann, H. Monitoring petrophysical, mineralogical, geochemical and microbiological effects of CO2 exposure–Results of long-term experiments under in situ conditions. Energy Procedia 2011, 4, 3644–3650. [Google Scholar] [CrossRef][Green Version]
- Xu, T.; Apps, J.A.; Pruess, K. Numerical simulation of CO2 disposal by mineral trapping in deep aquifers. Appl. Geochem. 2004, 19, 917–936. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K. Mineral sequestration of carbon dioxide in a sandstone–shale system. Chem. Geol. 2005, 217, 295–318. [Google Scholar] [CrossRef]
- Zerai, B.; Saylor, B.Z.; Matisoff, G. Computer simulation of CO2 trapped through mineral precipitation in the Rose Run Sandstone. Ohio Appl. Geochem. 2006, 21, 223–240. [Google Scholar] [CrossRef]
- Bowker, K.A.; Shuler, P.J. Carbon dioxide injection and resultant alteration of the Weber sandstone, Rangely Field, Colorado. Am. Assoc. Pet. Geol. Bull. 1991, 75, 1489–1499. [Google Scholar]
- Assayag, N.; Matter, J.; Ader, M.; Goldberg Agrinier, P. Water–rock interactions during a CO2 injection field-test: Implications on host rock dissolution and alteration effects. Chem. Geol. 2009, 265, 227–235. [Google Scholar] [CrossRef]
- Pearce, J.M.; Holloway, S.; Wacker, H.; Nelis, M.K.; Rochelle, C.; Bateman, K. Natural occurrences as analogues for the geological disposal of carbon dioxide. Energy Convers. 1996, 37, 1123–1128. [Google Scholar] [CrossRef]
- Wilkinson, M.; Haszeldine, R.S.; Fallick, A.E.; Odling, N.; Stoker, S.J.; Gatliff, R.W. CO2-mineral reaction in a natural analogue for CO2 storage–implications for modeling. J. Sediment. Res. 2009, 79, 486–494. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2–rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Shiraki, R.; Dunn, T.L. Experimental study on water–rock interactions during CO2 flooding in the Tensleep Formation, Wyoming, USA. Appl. Geochem. 2000, 15, 265–279. [Google Scholar] [CrossRef]
- Weibel, R.; Kjoller, C.; Bateman, K.; Nielsen, L.H.; Frykman, P.; Springer, N.; Laier, T. Mineral changes in CO2 experiments–Examples from Danish onshore saline aquifers. Energy Procedia 2011, 4, 4495–4502. [Google Scholar] [CrossRef]
- Flukiger, F.; Bernard, D. A new numerical model for pore scale dissolution of calcite due to CO2 saturated water flow in 3D realistic geometry: Principles and first results. Chem. Geol. 2009, 265, 171–180. [Google Scholar] [CrossRef]
- Ketzer, J.M.; Iglesias, R.; Einloft, S.; Dullius, J.; Ligabue, R.; Lima, V. Water–rock–CO2 interactions in saline aquifers aimed for carbon dioxide storage: Experimental and numerical modeling studies of the Rio Bonito Formation (Permian), southern Brazil. Appl. Geochem. 2009, 24, 760–767. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Luhmann, A.J.; Kong, X.Z.; Saar, M.O.; Seyfried, W.E. CO2 sequestration in feldspar-rich sandstone: Coupled evolution of fluid chemistry, mineral reaction rates, and hydrogeochemical properties. Geochim. Cosmochim. Acta 2015, 160, 132–154. [Google Scholar] [CrossRef]
- Ross, G.D.; Todd, A.C.; Tweedie, J.A.; Will, A.G.S. The Dissolution Effects of CO2-Brine Systems on the Permeability of U.K. and North Sea Calcareous Sandstones; Proc.3rd Joint SPE/DOE Symposium Enhanced Oil Recovery; (SPE/DOE 10685); Society of Petroleum Engineers Enhanced Oil Recovery Symposium: Tulsa, OK, USA, 1982; pp. 149–162. [Google Scholar]
- Ehrenberg, S.; Walderhaug, O.; Bjørlykke, K. Carbonate porosity creation by mesogenetic dissolution: Reality or illusion? AAPG 2012, 96, 217–233. [Google Scholar] [CrossRef]
- Dunham, R.J. Early vadose silt in Townsend mound (reef), New Mexico. In Depositional Environments in Carbonate Rocks: A Symposium; Friedman, G.M., Ed.; SEPM Special Publication: Los Angeles, CA, USA, 1969; Volume 14, pp. 139–181. [Google Scholar]
- Bathurst, R.G.C. Carbonate Sediments and their Diagenesis; Elsevier: Amsterdam, The Netherlands, 1975; p. 658. [Google Scholar]
- Scholle, P.A.; Halley, R.B. Burial diagenesis: Out of sight, out of mind! In Carbonate Cements; Mann, N.S., Harris, P.M., Eds.; SEPM Special Publication: Malden, MA, USA, 1985; Volume 36, pp. 309–334. [Google Scholar]
- Morse, J.W.; Mackenzie, F.T. Geochemical constraints on CaCO3 transport in subsurface sedimentary environments. Chem. Geol. 1993, 105, 181–196. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Kong, X.Z.; Seyfried, W.E.; Saar, M.O. High performance reactive transport simulations examining the effects of thermal, hydraulic, and chemical (THC) gradients on fluid injectivity at carbonate CCUS reservoir scales. Int. J. Greenh. Gas Control 2015, 39, 285–301. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Xu, T.; Cheng, H.; Zheng, Y.; Xiong, P. Long-term variations of CO2 trapped in different mechanisms in deep saline formations: A case study of the Songliao Basin, China. Int. J. Greenh. Gas Control 2009, 3, 161–180. [Google Scholar] [CrossRef]
- Yang, L.; Xu, T.; Liu, K.; Peng, B.; Yu, Z.; Xu, X. Fluid–rock interactions during continuous diagenesis of sandstone reservoirs and their effects on reservoir porosity. Sedimentology 2017, 64, 1303–1321. [Google Scholar] [CrossRef]
- Lasaga, A.C.; Steefel, C.I. A coupled model for transport of multiple chemical species and kinetic precipitation/dissolution reactions with applications to reactive flow in single phase hydrothermal system. Am. J. Sci. 1994, 294, 529–592. [Google Scholar]
| Mineral Types | Quartz | K-Feldspar | Albite | Calcite | Kaolinite | Dolomite |
|---|---|---|---|---|---|---|
| Content (wt. %) | 37.5 | 23.3 | 20.8 | 9.5 | 1.5 | 7.4 |
| Mineral | A/(cm2/g) | Geochemical Kinetic Rate Constants | ||
|---|---|---|---|---|
| K25/(mol/(m2·s)) | Ea/(kJ/mol) | n H+ | ||
| Quartz | 9.8 | |||
| Kaolinite | 151.6 | 4.9 × 10−12 | 65.9 | 0.8 |
| Illite | 151.6 | 1.0 × 10−11 | 23.6 | 0.3 |
| K-feldspar | 9.8 | 8.7 × 10−11 | 51.7 | 0.5 |
| albite | 9.8 | 6.9 × 10−11 | 65.0 | 0.5 |
| Chlorite | 9.8 | 7.8 × 10−12 | 88.0 | 0.5 |
| Calcite | 9.8 | 5.0 × 10−1 | 14.4 | 1.0 |
| Dolomite | 9.8 | 6.5 × 10−4 | 36.1 | 0.5 |
| Siderite | 9.8 | 6.5 × 10−4 | 36.1 | 0.5 |
| Ankerite | 9.8 | 1.6 × 10−4 | 36.1 | 0.5 |
| Dawsonite | 9.8 | 1.6 × 10−4 | 36.1 | 0.5 |
| Magnesite | 9.8 | 4.2 × 10−7 | 14.4 | 1.0 |
| Pyrite | 12.9 | 3.0 × 10−8 | 56.9 | −0.5 |
| Physical Simulation | Reaction Time (d) | pH | K | Si | Ca | Mg | Al |
| mol/L | mol/L | mol/L | mol/L | mol/L | |||
| 0 | 5.86 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | |
| 4 | 5.97 | 0.000046 | 0.001000 | 0.001360 | 0.000554 | 0.000148 | |
| 7 | 5.94 | 0.000810 | 0.001004 | 0.002500 | 0.000879 | 0.000667 | |
| 10 | 6.01 | 0.000854 | 0.001832 | 0.003125 | 0.001079 | 0.000852 | |
| 13 | 6.27 | 0.001987 | 0.002943 | 0.006825 | 0.002396 | 0.000500 | |
| 16 | 6.44 | 0.002000 | 0.004500 | 0.009575 | 0.004583 | 0.000200 | |
| Numerical simulation | Reaction Time (d) | pH | K | Si | Ca | Mg | Al |
| mol/L | mol/L | mol/L | mol/L | mol/L | |||
| 0 | 4.01 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | |
| 3 | 3.93 | 0.000252 | 0.000625 | 0.000631 | 0.000298 | 0.000182 | |
| 4 | 3.09 | 0.000616 | 0.001471 | 0.001344 | 0.000648 | 0.000450 | |
| 5 | 2.94 | 0.000964 | 0.002277 | 0.002119 | 0.000985 | 0.000664 | |
| 6 | 2.87 | 0.001226 | 0.002881 | 0.002733 | 0.001238 | 0.000795 | |
| 7 | 2.84 | 0.001417 | 0.003323 | 0.003178 | 0.001423 | 0.000825 | |
| 9 | 2.85 | 0.001678 | 0.003922 | 0.003846 | 0.001676 | 0.000675 | |
| 10 | 2.85 | 0.001749 | 0.004083 | 0.004035 | 0.001744 | 0.000656 | |
| 11 | 2.88 | 0.001818 | 0.004243 | 0.004192 | 0.001812 | 0.000518 | |
| 12 | 3.41 | 0.002036 | 0.004758 | 0.004568 | 0.002029 | 0.000435 | |
| 13 | 4.08 | 0.002144 | 0.005024 | 0.004732 | 0.002137 | 0.000321 | |
| 14 | 4.40 | 0.002201 | 0.005174 | 0.004819 | 0.002196 | 0.000211 | |
| 15 | 4.57 | 0.002245 | 0.005295 | 0.004891 | 0.002242 | 0.000194 | |
| 16 | 4.68 | 0.002282 | 0.005395 | 0.004953 | 0.002281 | 0.000100 |
| Correlation Matrix | K | Ca | Mg | Si | Fe | Al |
|---|---|---|---|---|---|---|
| K | 1.000 | |||||
| Ca | 0.943 | 1.000 | ||||
| Mg | 0.989 | 0.979 | 1.000 | |||
| Si | 0.921 | 0.932 | 0.955 | 1.000 | ||
| Fe | 0.877 | 0.978 | 0.938 | 0.931 | 1.000 | |
| Al | −0.035 | −0.143 | −0.126 | −0.301 | −0.330 | 1.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Yang, S.; Liu, K.; Zhuo, Q.; Yang, L. An Experimental and Numerical Study of CO2–Brine-Synthetic Sandstone Interactions under High-Pressure (P)–Temperature (T) Reservoir Conditions. Appl. Sci. 2019, 9, 3354. https://doi.org/10.3390/app9163354
Yu Z, Yang S, Liu K, Zhuo Q, Yang L. An Experimental and Numerical Study of CO2–Brine-Synthetic Sandstone Interactions under High-Pressure (P)–Temperature (T) Reservoir Conditions. Applied Sciences. 2019; 9(16):3354. https://doi.org/10.3390/app9163354
Chicago/Turabian StyleYu, Zhichao, Siyu Yang, Keyu Liu, Qingong Zhuo, and Leilei Yang. 2019. "An Experimental and Numerical Study of CO2–Brine-Synthetic Sandstone Interactions under High-Pressure (P)–Temperature (T) Reservoir Conditions" Applied Sciences 9, no. 16: 3354. https://doi.org/10.3390/app9163354
APA StyleYu, Z., Yang, S., Liu, K., Zhuo, Q., & Yang, L. (2019). An Experimental and Numerical Study of CO2–Brine-Synthetic Sandstone Interactions under High-Pressure (P)–Temperature (T) Reservoir Conditions. Applied Sciences, 9(16), 3354. https://doi.org/10.3390/app9163354




