Influence of the Scanning Temperature on the Classification of Whisky Samples Analysed by UV-VIS Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
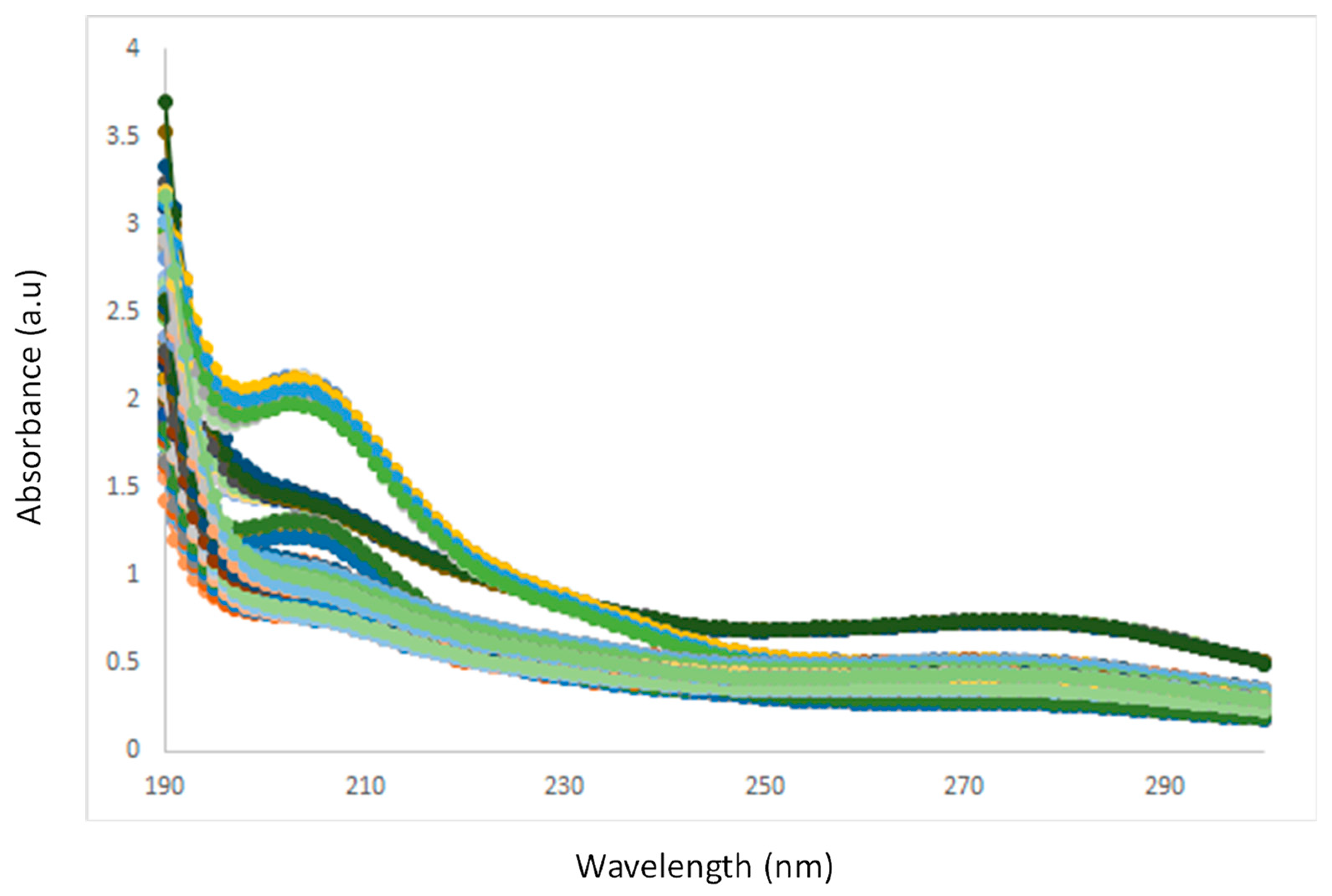
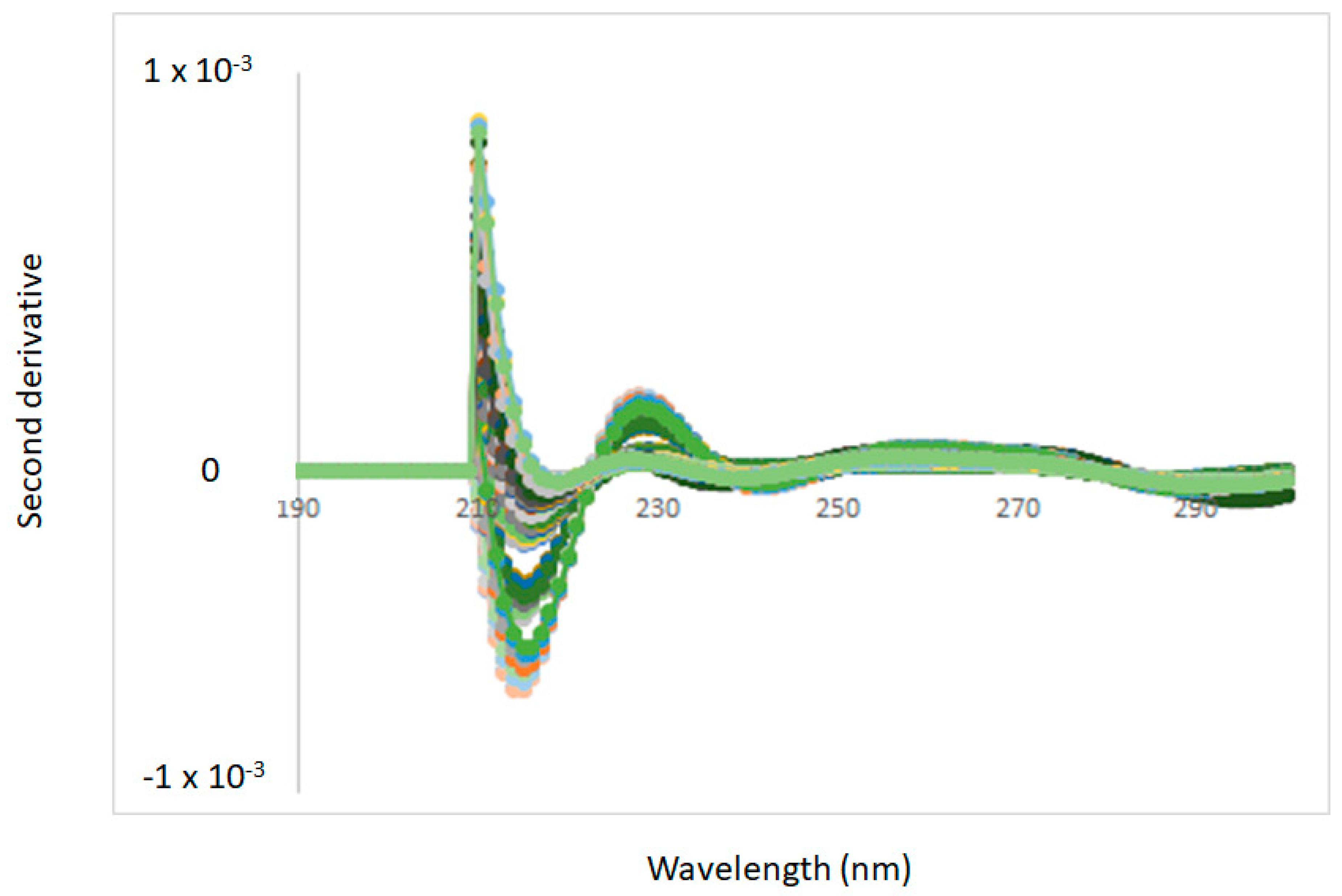
3.1. Spectra Interpretation
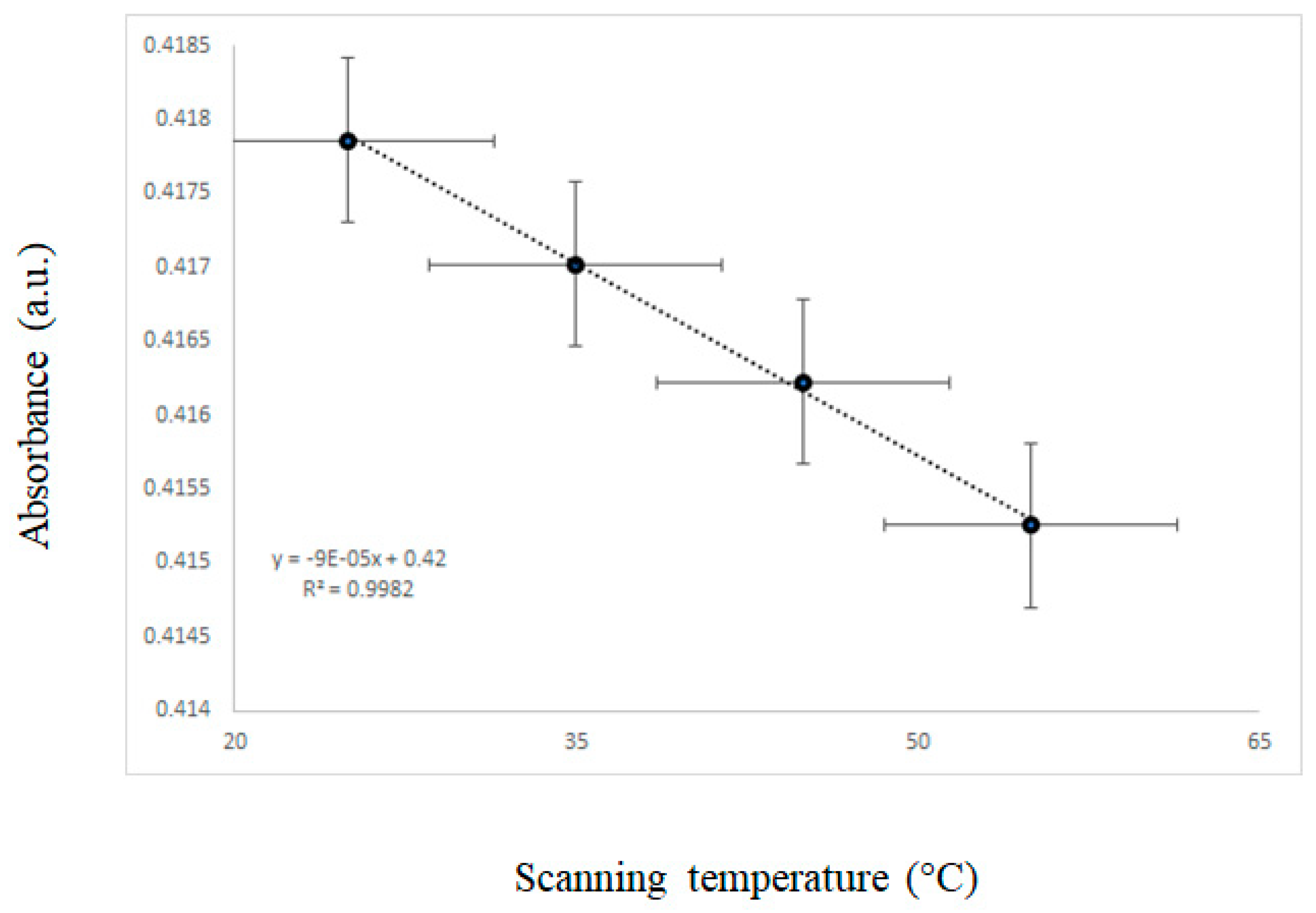
3.2. Effect of Temperature on the UV-VIS Spectra
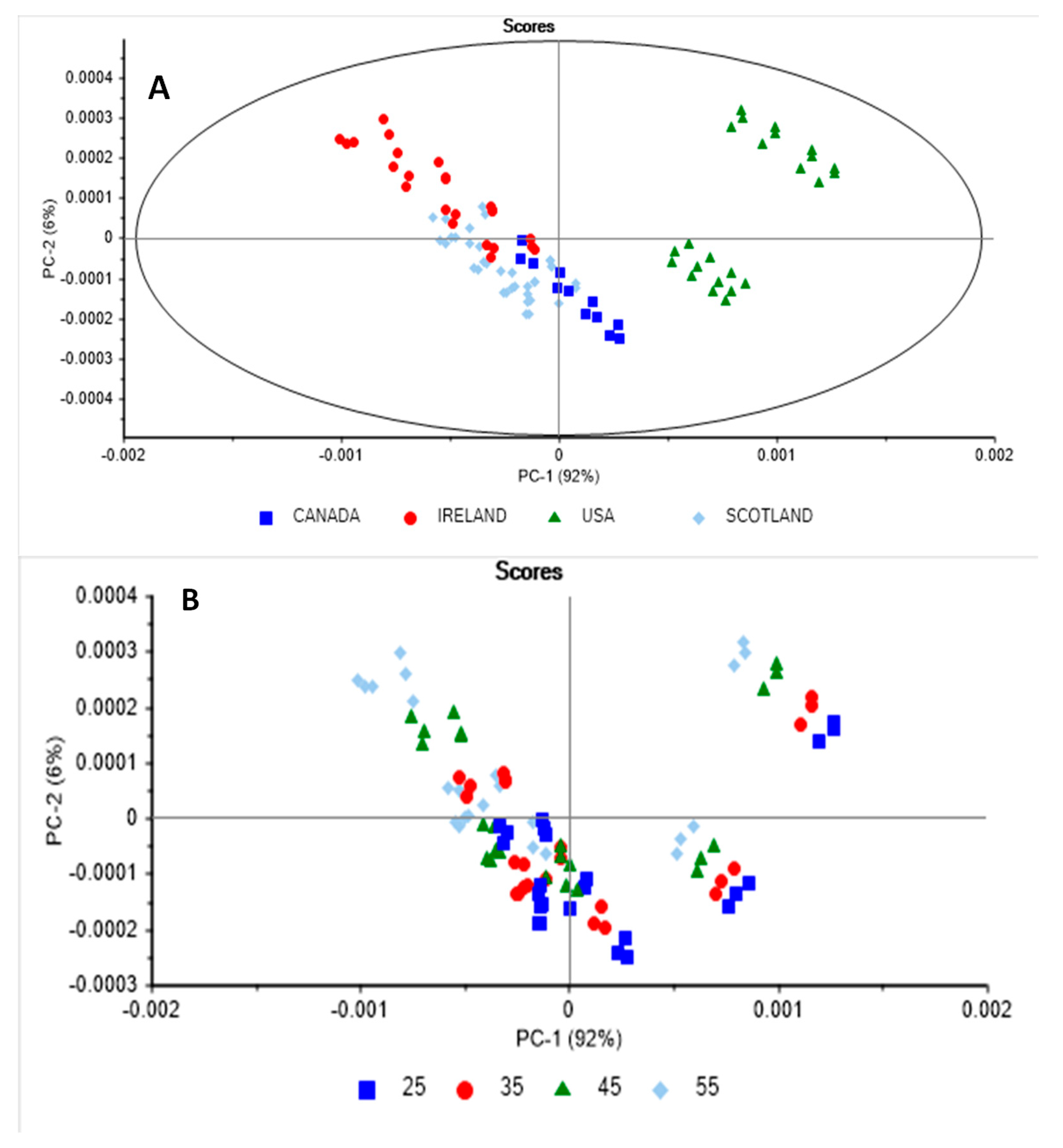
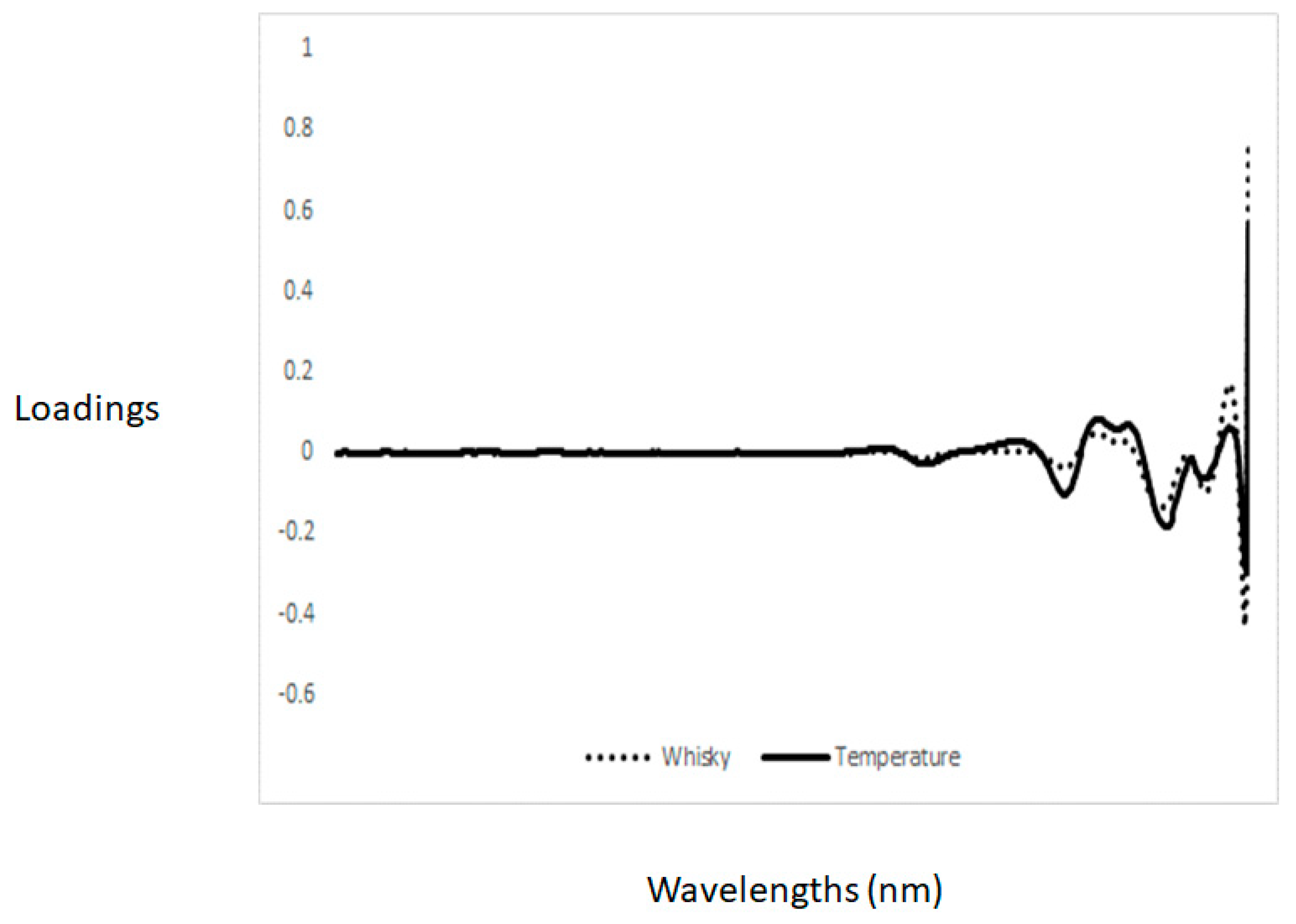
3.3. Principal Component Analysis and Discriminant Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Chemical composition analysis and authentication of whisky. J. Sci. Food Agric. 2015, 95, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Mignani, A.G.; Ciaccheri, L.; Gordillo, B.; Azelio Mencaglia, A.; González-Miret, M.L.; Heredia, F.J.; Culshaw, B. Identifying the production region of single-malt Scotch whiskies using optical spectroscopy and pattern recognition techniques. Sens. Actuators B Chem. 2012, 171, 458–462. [Google Scholar] [CrossRef]
- MacKenzie, W.M.; Aylott, R.I. Analytical strategies to confirm Scotch whisky authenticity. Part II: Mobile brand authentication. Analyst 2004, 129, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.A. Whiskey: A Fascinating Journey Through the Most Famous Whiskey and Distilleries Worldwide; Parragon Inc.: Bath, UK, 2008. [Google Scholar]
- Aoshima, H.; Tsunoue, H.; Koda, H.; Kiso, Y. Aging of whiskey increases 1, 1-diphenyl-2-picrylhydrazyl radical scavenging activity. J. Agric. Food Chem. 2004, 52, 5240–5244. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, M.; Tanaka, T.; Suwa, Y.; Koshimizu, S.; Kouno, I. Isolation and structure of whiskey polyphenols produced by oxidation of oak wood ellagitannins. J. Agric. Food Chem. 2008, 56, 7305–7310. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Authentication of whisky due to its botanical origin and way of production by instrumental analysis and multivariate classification methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.J.; Jimenez, J.; Maldonado, S.; Domínguez, E.; Narváez, A. Classification of Wines Produced in Specific Regions by UV−Visible Spectroscopy Combined with Support Vector Machines. J. Agric. Food Chem. 2007, 55, 6842–6849. [Google Scholar] [CrossRef]
- Urbano, M.; De Castro, M.D.L.; Pérez, P.M.; García-Olmo, J.; Gómez-Nieto, M.A. Ultraviolet–visible spectroscopy and pattern recognition methods for differentiation and classification of wines. Food Chem. 2006, 97, 166–175. [Google Scholar] [CrossRef]
- Azcarate, S.M.; Cantarelli, M.A.; Pellerano, R.G.; Marchevsky, E.J.; Camiña, J.M. Classification of Argentinean Sauvignon blanc wines by UV spectroscopy and chemometric methods. J. Food Sci. 2013, 78, C432–C436. [Google Scholar] [CrossRef]
- Uríčková, V.; Sádecká, J. Determination of geographical origin of alcoholic beverages using ultraviolet, visible and infrared spectroscopy: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 148, 131–137. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Giusti, M.M.; Shotts, M. Advances in Infrared Spectroscopy for Food Authenticity Testing. In Advances in Food Authenticity Testing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 71–116. [Google Scholar]
- Cozzolino, D. Near Infrared Spectroscopy and Food Authenticity. In Advances in Food Traceability Techniques and Technologies; Elsevier: Amsterdam, The Netherlands, 2016; pp. 119–136. [Google Scholar]
- Tosato, F.; Correia, R.M.; Oliveira, B.G.; Fontes, A.M.; França, H.S.; Coltro, W.K.; Romão, W. Paper spray ionization mass spectrometry allied to chemometric tools for quantification of whisky adulteration with additions of sugarcane spirit. Anal. Methods 2018, 10, 1952–1960. [Google Scholar] [CrossRef]
- Kerslake, F.; Longo, R.; Dambergs, R. Discrimination of Juice Press Fractions for Sparkling Base Wines by a UV-Vis Spectral Phenolic Fingerprint and Chemometrics. Beverages 2018, 4, 45. [Google Scholar] [CrossRef]
- Kew, W.; Goodall, I.; Clarke, D.; Uhrín, D. Chemical diversity and complexity of scotch whisky as revealed by high-resolution mass spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Sharma, S. Derivative UV-vis absorption spectra as an invigorated spectrophotometric method for spectral resolution and quantitative analysis: Theoretical aspects and analytical applications: A review. TrAC Trends Anal. Chem. 2016, 77, 44–53. [Google Scholar] [CrossRef]
- Wold, S. Chemometrics; what do we mean with it, and what do we want from it? Chemom. Intell. Lab. Syst. 1995, 30, 109–115. [Google Scholar] [CrossRef]
- Wold, S.; Albano, C.W.J.D.; Dunn, W.J.; Edlund, U.; Esbensen, K.; Geladi, P.; Hellberg, S.; Johansson, E.; Lindberg, W.; Sjöström, M. Multivariate Data Analysis in Chemistry; Springer: Berlin/Heidelberg, Germany, 1984; pp. 17–95. [Google Scholar]
- Fuwa, K.; Valle, B. The Physical Basis of Analytical Atomic Absorption Spectrometry. The Pertinence of the Beer-Lambert Law. Anal. Chem. 1963, 35, 942–946. [Google Scholar] [CrossRef]
- Perkampus, H.-H. Recent developments in UV-VIS spectroscopy. In UV-VIS Spectroscopy and Its Applications; Springer: Berlin/Heidelberg, Germany, 1992; pp. 81–130. [Google Scholar]
- Cozzolino, D.; Liu, L.; Cynkar, W.U.; Dambergs, R.G.; Janik, L.; Colby, C.B.; Gishen, M. Effect of temperature variation on the visible and near infrared spectra of wine and the consequences on the partial least square calibrations developed to measure chemical composition. Anal. Chim. Acta 2007, 588, 224–230. [Google Scholar] [CrossRef]
- Espiñeira, M.; Santaclara, F.J. Advances in Food Traceability Techniques and Technologies: Improving Quality Throughout the Food Chain; Woodhead Publishing is an Imprint of Elsevier: Duxford, UK, 2016. [Google Scholar]
- Martin, C.; Bruneel, J.-L.; Castet, F.; Fritsch, A.; Teissedre, P.-L.; Jourdes, M.; Guillaume, F. Spectroscopic and theoretical investigations of phenolic acids in white wines. Food Chem. 2017, 221, 568–575. [Google Scholar] [CrossRef]
- Martens, H.; Næs, T. Multivariate calibration. I. Concepts and distinctions. TrAC Trends Anal. Chem. 1984, 3, 204–210. [Google Scholar] [CrossRef]
- Næs, T.; Martens, H. Multivariate calibration. II. Chemometric methods. TrAC Trends Anal. Chem. 1984, 3, 266–271. [Google Scholar] [CrossRef]
- Martins, A.R.; Talhavini, M.; Vieira, M.L.; Zacca, J.J.; Braga, J.W.B. Discrimination of whisky brands and counterfeit identification by UV–Vis spectroscopy and multivariate data analysis. Food Chem. 2017, 229, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Boscolo, M.; Andrade-Sobrinho, L.G.; Lima-Neto, B.S.; Franco, D.W.; Ferreira, M.M.C. Spectrophotometric determination of caramel content in spirits aged in oak casks. J. AOAC Int. 2002, 85, 744–750. [Google Scholar] [PubMed]
- Somers, T.C.; Ziemelis, G. Spectral evaluation of total phenolic components in Vitis vinifera: Grapes and wines. J. Sci. Food Agric. 1985, 36, 1275–1284. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, I.; Truong, V.K.; Elbourne, A.; Chapman, J.; Cozzolino, D. Influence of the Scanning Temperature on the Classification of Whisky Samples Analysed by UV-VIS Spectroscopy. Appl. Sci. 2019, 9, 3254. https://doi.org/10.3390/app9163254
Joshi I, Truong VK, Elbourne A, Chapman J, Cozzolino D. Influence of the Scanning Temperature on the Classification of Whisky Samples Analysed by UV-VIS Spectroscopy. Applied Sciences. 2019; 9(16):3254. https://doi.org/10.3390/app9163254
Chicago/Turabian StyleJoshi, Ishita, Vi Khanh Truong, Aaron Elbourne, James Chapman, and Daniel Cozzolino. 2019. "Influence of the Scanning Temperature on the Classification of Whisky Samples Analysed by UV-VIS Spectroscopy" Applied Sciences 9, no. 16: 3254. https://doi.org/10.3390/app9163254
APA StyleJoshi, I., Truong, V. K., Elbourne, A., Chapman, J., & Cozzolino, D. (2019). Influence of the Scanning Temperature on the Classification of Whisky Samples Analysed by UV-VIS Spectroscopy. Applied Sciences, 9(16), 3254. https://doi.org/10.3390/app9163254








