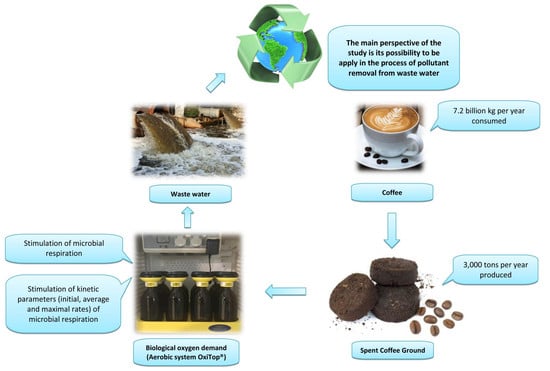
The Possibility of Using Spent Coffee Grounds to Improve Wastewater Treatment Due to Respiration Activity of Microorganisms
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campos-Vega, R.; Loarca-Pina, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Using cow dung and spent coffee grounds to enhance the two-stage co-composting of green waste. Bioresour. Technol. 2017, 245, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Hardgrove, S.J.; Livesley, S.J. Applying spent coffee grounds directly to urban agriculture soils greatly reduces plant growth. Urban For. Urban Green. 2016, 18, 8. [Google Scholar] [CrossRef]
- Lessa, E.F.; Nunes, M.L.; Fajardo, A.R. Chitosan/waste coffee-grounds composite: An efficient and eco-friendly adsorbent for removal of pharmaceutical contaminants from water. Carbohydr. Polym. 2018, 189, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Yang, B.; Jahng, D. Spent coffee ground as a new bulking agent for accelerated biodrying of dewatered sludge. Water Res. 2018, 138, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Caetano, N.S.; Silva, V.F.; Mata, T.M. Valorization of coffee grounds for biodiesel production. Ital. Assoc. Chem. Eng. 2012, 26, 267–272. [Google Scholar]
- Gómez-de la Cruz, F.J.; Cruz-Peragón, F.; Casanova-Pelaez, P.J.; Palomar-Carnicero, J.M. A vital stage in the large-scale production of biofuels from spent coffee grounds: The drying kinetics. Fuel Process. Technol. 2015, 130, 188–196. [Google Scholar] [CrossRef]
- Calixto, F.; Fernandes, J.; Couto, R.; Hernandez, E.J.; Najdanovic-Visak, V.; Simoes, P.C. Synthesis of fatty acid methyl esters via direct transesterification with methanol/carbon dioxide mixtures from spent coffee grounds feedstock. Green Chem. 2011, 13, 1196–1202. [Google Scholar] [CrossRef]
- Deligiannis, A.; Papazafeiropoulou, A.; Anastopoulos, G.; Zannikos, F. Waste coffee grounds as an energy feedstock. In Proceedings of the 3rd International CEMEPE & SECOTOX Conference, Skiathos, Greece, June 19–24 2011; pp. 617–622. [Google Scholar]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Pasin, L.A.A.P.; Abreu, M.S.D.; Souza, I.P. Influence of the fungi population on the physicochemical and chemical composition of coffee (Coffea arabica L.). Food Sci. Technol. 2011, 31, 681–687. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Naidu, M.M. Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Fardiaz, S. Antimicrobial activity of coffee (Coffea robusta) extract. ASEAN Food J. 1995, 10, 103–106. [Google Scholar]
- Vítěz, T.; Koutný, T.; Šotnar, M.; Chovanec, J. On the spent coffee grounds biogas production. Acta Univ. Agric. Silvic. Mendel. Brun. 2016, 64, 1279–1282. [Google Scholar] [CrossRef]
- Kushkevych, I.; Vítězová, M.; Vítěz, T.; Bartoš, M. Production of biogas: Relationship between methanogenic and sulfate-reducing microorganisms. Open Life Sci. 2017, 12, 82–91. [Google Scholar] [CrossRef]
- Kushkevych, I.; Vítězová, M.; Vítěz, T.; Kováč, J.; Kaucká, P.; Jesionek, W.; Bartoš, M.; Barton, L. A new combination of substrates: Biogas production and diversity of the methanogenic microorganisms. Open Life Sci. 2018, 13, 119–128. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kováč, J.; Vítězová, M.; Vítěz, T.; Bartoš, M. The diversity of sulfate-reducing bacteria in the seven bioreactors. Arch. Microbiol. 2018, 200, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; Bize, A.; Bureau, C.; Bouchez, T.; Chapleur, O. Community shifts within anaerobic digestion microbiota facing phenol inhibition: Towards early warning microbial indicators? Water Res. 2016, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Lavecchia, R. Spent coffee grounds as a valuable source of phenolic compounds and bioenergy. J. Clean. Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Bertrand, B.; Guyot, B.; Anthony, F.; Lashermes, P. Impact of the Coffea canephora gene introgression on beverage quality of C. arabica. Theor. Appl. Genet. 2003, 107, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Soares, C.; Casal, S.; Fernandes, J.O.; Beatriz, M.; Oliveira, P.P. Acrylamide in espresso coffee: Influence of species, roast degree and brew length. Food Chem. 2010, 119, 929–934. [Google Scholar] [CrossRef]
- Kushkevych, I.; Vítězová, M.; Fedrová, P.; Vochyanová, Z.; Paráková, L.; Hošek, J. Kinetic properties of growth of intestinal sulphate-reducing bacteria isolated from healthy mice and mice with ulcerative colitis. Acta Vet. Brno 2017, 86, 405–411. [Google Scholar] [CrossRef]
- Katalinić, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols, Food Chem. 2006, 94, 550–557. Food Chem 2006, 94, 550–557. [Google Scholar]
- Dobrinas, S.; Soceanu, A.; Popescu, V.; Stanciu, G.; Smalberger, S. Optimization of a UV-VIS spectrometric method for caffeine analysis in tea, coffee and other beverages. St. Cerc. St. CICBIA 2013, 14, 071–078. [Google Scholar]
- Naegele, E. Determination of chlorogenic acid in coffee products according to DIN 10767. In Food Test. Agric.-Food Authenticity; Agilent Technologies, Inc.: Waldbronn, Germany, 2016; pp. 1–8. [Google Scholar]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166–172. [Google Scholar] [CrossRef]
- Cecconet, D.; Bolognesi, S.; Daneshgar, S.; Callegari, A.; Capodaglio, A.G. Improved process understanding and optimization by multivariate statistical analysis of Microbial Fuel Cells operation. Int. J. Hydrog. Energy 2018, 43, 16719–16727. [Google Scholar] [CrossRef]
- Ibrahim, S.; Shukor, M.Y.; Syed, M.A.; Rahman, N.A.A.; Khalil, A.K.; Khalid, A.; Ahmad, S.A. Bacterial degradation of caffeine: A review. Asian J. Plant Biol. 2014, 2, 18–27. [Google Scholar]
- Mazzafera, P. Degradation of caffeine by microorganisms and potential use of decaffeinated coffee husk and pulp in animal feeding. Sci. Agric. 2002, 59, 815–821. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Extraction of polysaccharides by autohydrolysis of spent coffee grounds and evaluation of their antioxidant activity. Carbohydr. Polym. 2017, 157, 258–266. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Optimization of autohydrolysis conditions to extract antioxidant phenolic compounds from spent coffee grounds. J. Food Eng. 2017, 199, 8. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Duda, A.; Wajda, L.W.; Tarko, T.; Sroka, P.; Satora, P. A review of the interactions between acrylamide, microorganisms and food components. Food Funct. 2016, 7, 1282–1295. [Google Scholar] [CrossRef]







| Sample | Kinetic Parameters | |||
|---|---|---|---|---|
| Initial Rate V0 (mg O2/L/h) | Average Rate Vaverage (mg O2/L/h) | Maximal Rate Vmax (mg O2/L/h) | Time t (hours) | |
| Control (without coffee ground) | 15.93 ± 1.45 | 14.73 ± 1.34 | 25.54 ± 2.32 | 2 |
| Arabica (50%)/Robusta (50%) | 15.94 ± 1.44 | 35.95 ± 3.27 | 49.99 ± 4.54 | 38 |
| Arabica (80%)/Robusta (20%) | 26.54 ± 2.41 | 36.47 ± 3.32 | 47.51 ± 4.32 | 21 |
| Arabica (100%) | 26.55 ± 2.43 | 35.46 ± 3.22 | 45.56 ± 4.14 | 32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vítězová, M.; Jančiková, S.; Dordević, D.; Vítěz, T.; Elbl, J.; Hanišáková, N.; Jampílek, J.; Kushkevych, I. The Possibility of Using Spent Coffee Grounds to Improve Wastewater Treatment Due to Respiration Activity of Microorganisms. Appl. Sci. 2019, 9, 3155. https://doi.org/10.3390/app9153155
Vítězová M, Jančiková S, Dordević D, Vítěz T, Elbl J, Hanišáková N, Jampílek J, Kushkevych I. The Possibility of Using Spent Coffee Grounds to Improve Wastewater Treatment Due to Respiration Activity of Microorganisms. Applied Sciences. 2019; 9(15):3155. https://doi.org/10.3390/app9153155
Chicago/Turabian StyleVítězová, Monika, Simona Jančiková, Dani Dordević, Tomáš Vítěz, Jakub Elbl, Nikola Hanišáková, Josef Jampílek, and Ivan Kushkevych. 2019. "The Possibility of Using Spent Coffee Grounds to Improve Wastewater Treatment Due to Respiration Activity of Microorganisms" Applied Sciences 9, no. 15: 3155. https://doi.org/10.3390/app9153155
APA StyleVítězová, M., Jančiková, S., Dordević, D., Vítěz, T., Elbl, J., Hanišáková, N., Jampílek, J., & Kushkevych, I. (2019). The Possibility of Using Spent Coffee Grounds to Improve Wastewater Treatment Due to Respiration Activity of Microorganisms. Applied Sciences, 9(15), 3155. https://doi.org/10.3390/app9153155