Acellular Small-Diameter Tissue-Engineered Vascular Grafts
Abstract
1. Introduction
2. Background
- Mechanical properties: the TEVG must be able to maintain its shape under a certain pressure;
- Degradability: the scaffold of the TEVG should be degradable, and the rate of degradability should match the rate of tissue regeneration;
- Biocompatibility: the scaffold should not cause an immune reaction or a toxic reaction;
- Surface structure and biological activity: the inner surface of the vascular graft should be favorable to cell adhesion, growth, and proliferation.
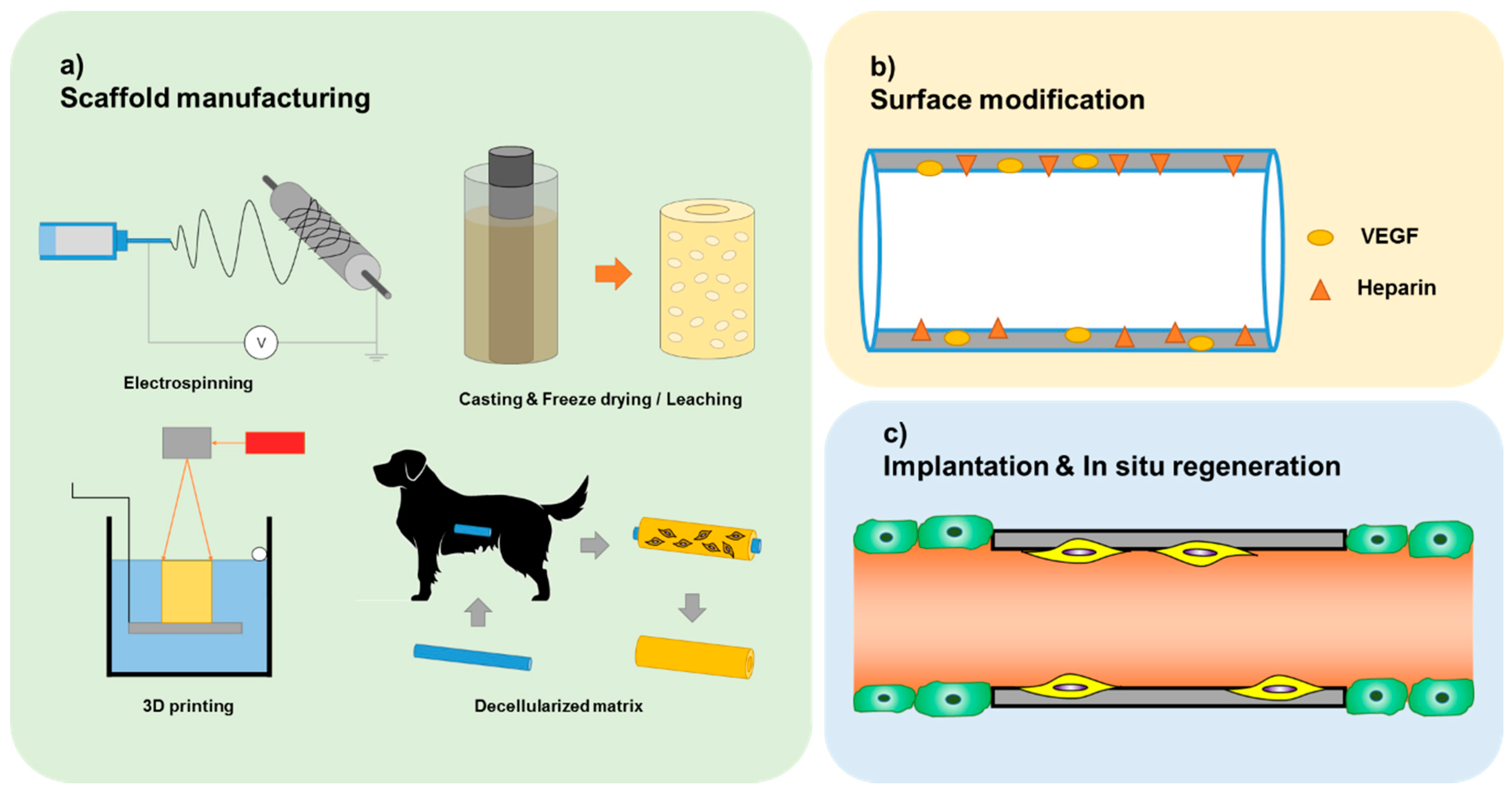
3. Current State of Acellular TEVG
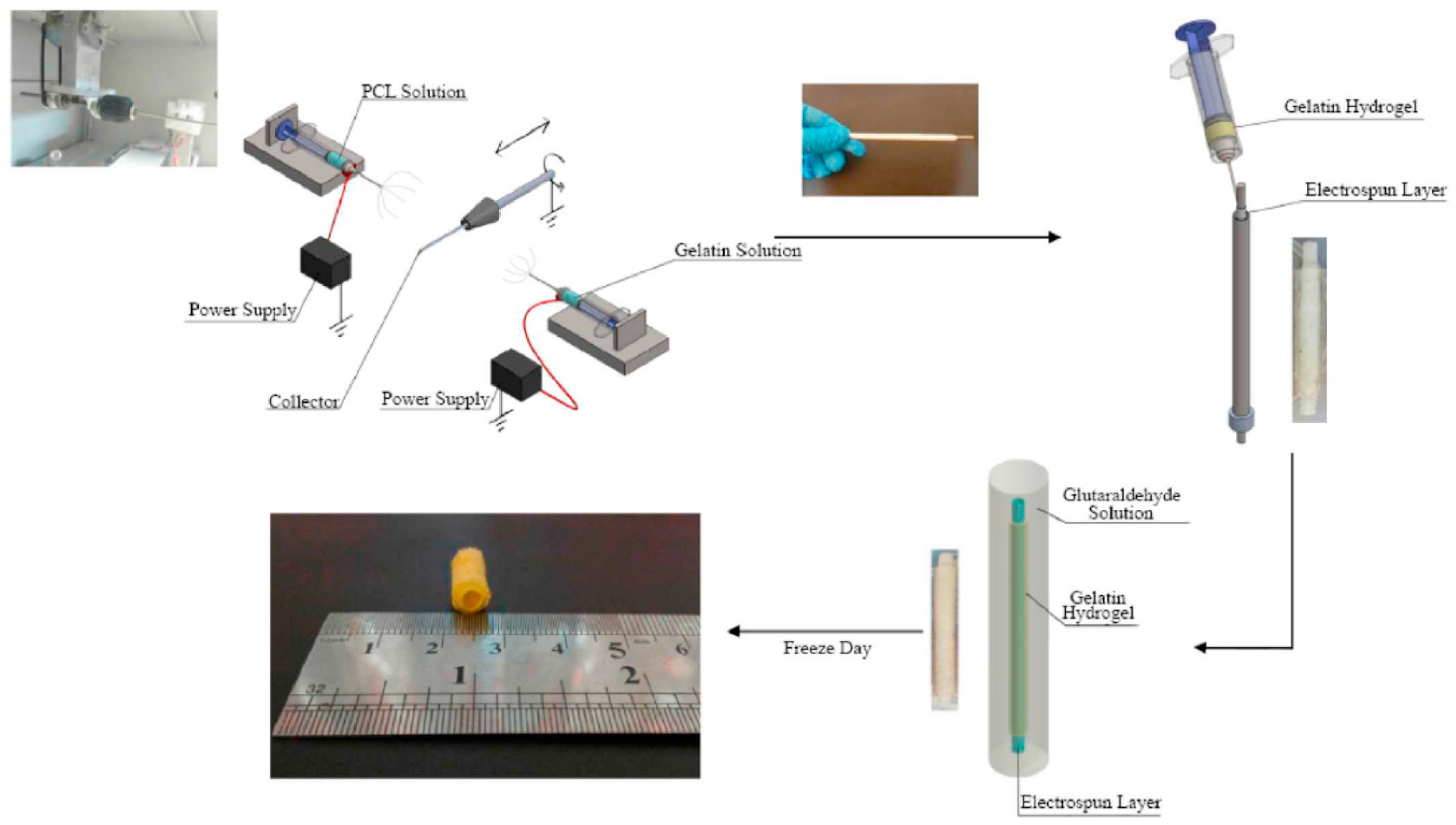
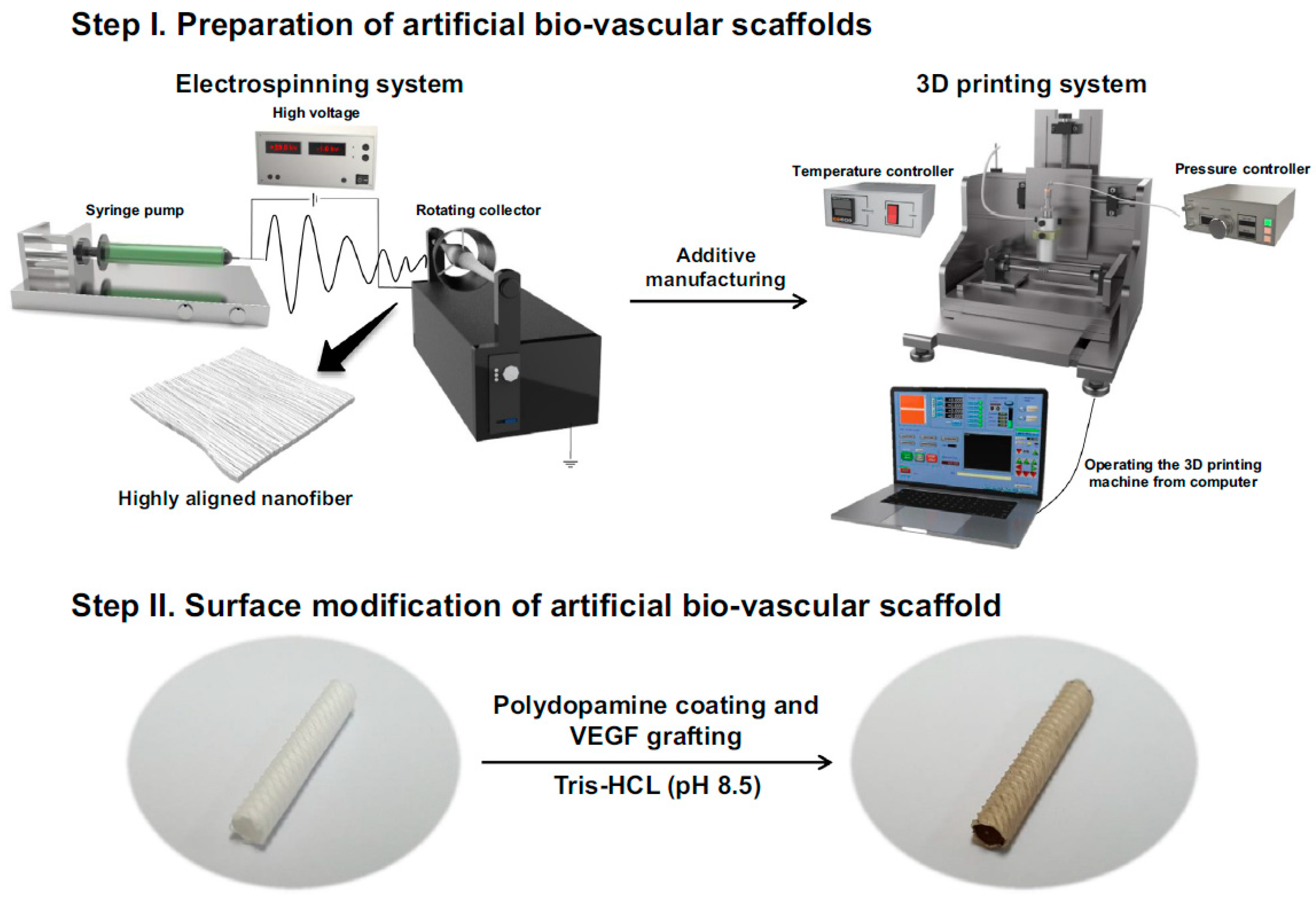
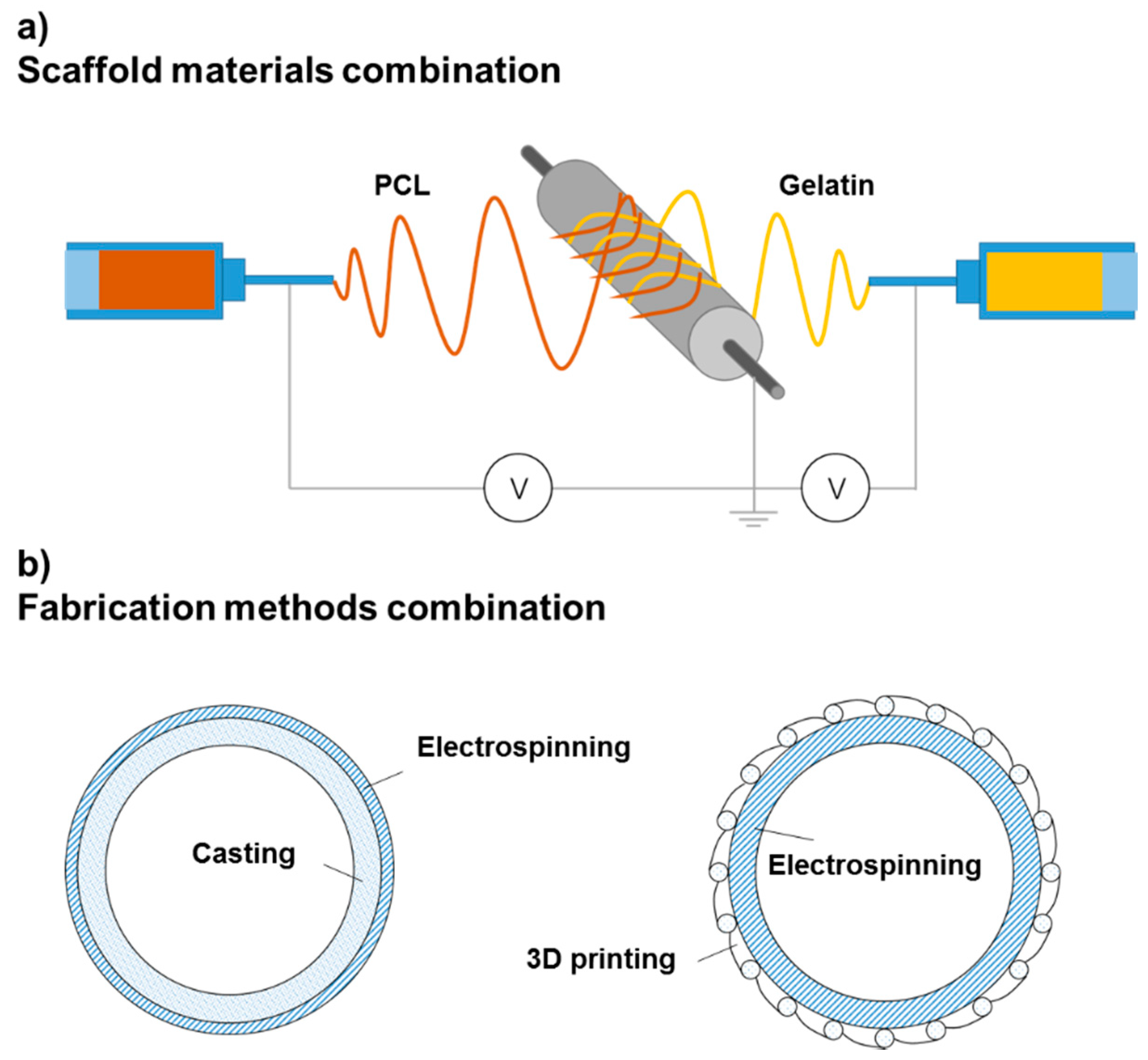
3.1. Electrospinning
3.2. Casting
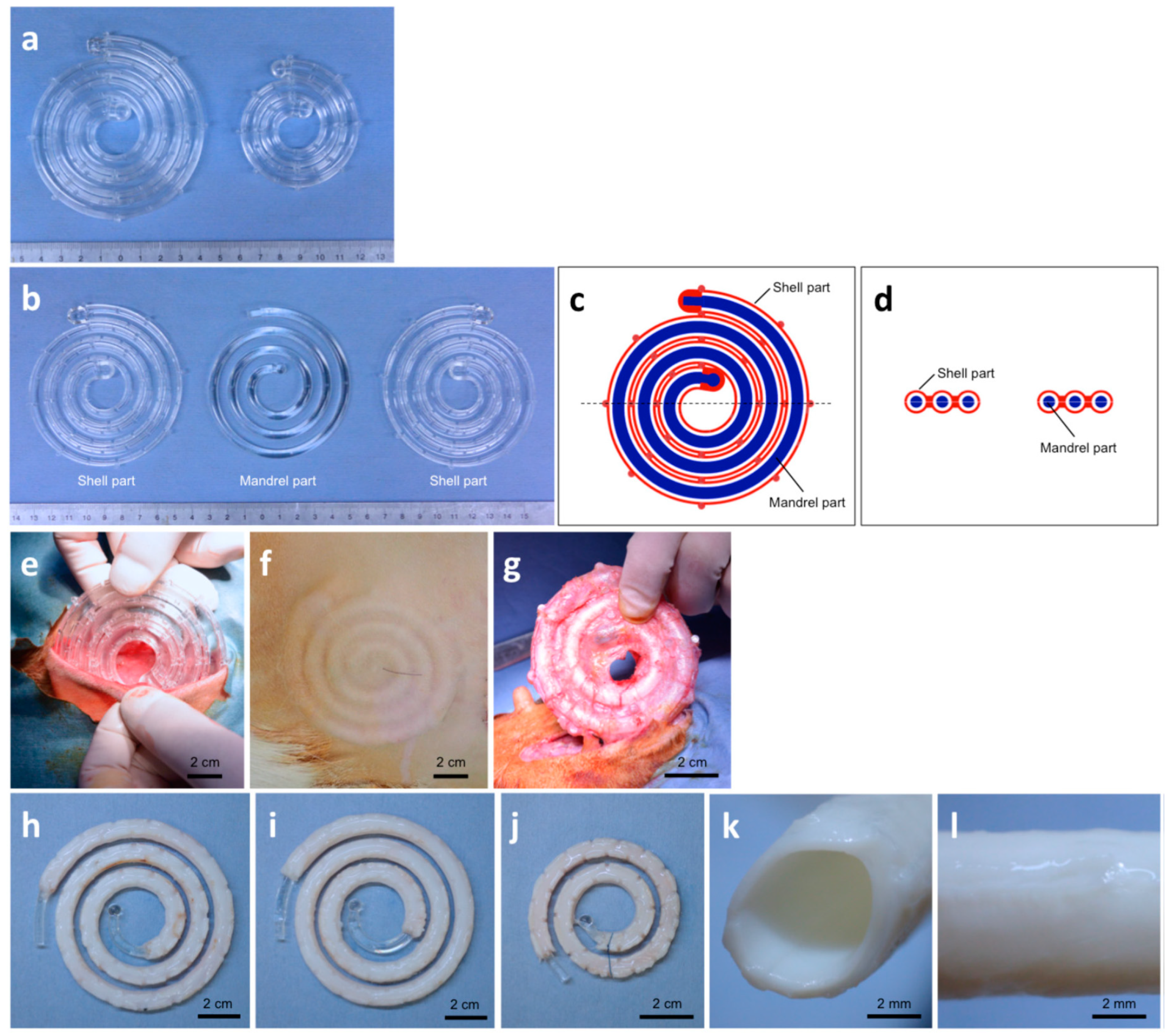
3.3. Three-Dimensional Printing
3.4. Decellularized Matrix
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development. Adv. Healthc. Mater. 2018, 7, e1701461. [Google Scholar] [CrossRef] [PubMed]
- Nemeno-Guanzon, J.G.; Lee, S.; Berg, J.R.; Jo, Y.H.; Yeo, J.E.; Nam, B.M.; Koh, Y.; Lee, J.I. Trends in Tissue Engineering for Blood Vessels. J. Biomed. Biotechnol. 2012, 2012, 956345. [Google Scholar] [CrossRef] [PubMed]
- Drews, J.D.; Miyachi, H.; Shinoka, T. Tissue-engineered vascular grafts for congenital cardiac disease: Clinical experience and current status. Trends Cardiovas. Med. 2017, 27, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Li, S. Advances in vascular tissue engineering. J. Med Biomech. 2016, 31, 333–339. [Google Scholar]
- Melchiorri, A.J.; Hibino, N.; Fisher, J.P. Strategies and Techniques to Enhance the In Situ Endothelialization of Small-Diameter Biodegradable Polymeric Vascular Grafts. Tissue Eng. Part B Rev. 2013, 19, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Seifu, D.G.; Purnama, A.; Mequanint, K.; Mantovani, D. Small-diameter vascular tissue engineering. Nat. Rev. Cardiol. 2013, 10, 410–421. [Google Scholar] [CrossRef]
- Carrabba, M.; Madeddu, P. Current Strategies for the Manufacture of Small Size Tissue Engineering Vascular Grafts. Front. Bioeng. Biotechnol. 2018, 6, 41. [Google Scholar] [CrossRef]
- Maschhoff, P.; Heene, S.; Lavrentieva, A.; Hentrop, T.; Leibold, C.; Wahalla, M.; Stanislawski, N.; Blume, H.; Scheper, T.; Blume, C. An intelligent bioreactor system for the cultivation of a bioartificial vascular graft. Eng. Life Sci. 2017, 17, 567–578. [Google Scholar] [CrossRef]
- Diamantouros, S.E.; Hurtado-Aguilar, L.G.; Schmitz-Rode, T.; Mela, P.; Jockenhoevel, S. Pulsatile Perfusion Bioreactor System for Durability Testing and Compliance Estimation of Tissue Engineered Vascular Grafts. Ann. Biomed. Eng. 2013, 41, 1979–1989. [Google Scholar] [CrossRef]
- Hielscher, D.; Kaebisch, C.; Braun, B.J.V.; Gray, K.; Tobiasch, E. Stem Cell Sources and Graft Material for Vascular Tissue Engineering. Stem. Cell. Rev. Rep. 2018, 14, 642–667. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, S.; Wang, Y.; Wang, S. Progress and prospect of small-diameter vascular grafts research. Chin. J. Biomed. Eng. 2016, 35, 357–364. [Google Scholar]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft—Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Zhou, X.; Huang, C.Y.; Fukunishi, T.; Zhang, H.; Hibino, N. Tissue engineered vascular grafts: Current state of the field. Expert Rev. Med. Devices 2017, 14, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, S.; Vanerio, N.; Catto, V.; Bonandrini, B.; Tironi, M.; Figliuzzi, M.; Remuzzi, A.; Kock, L.; Redaelli, A.C.L.; Greco, F.G.; et al. A novel hybrid silk-fibroin/polyurethane three-layered vascular graft: Towards in situ tissue-engineered vascular accesses for haemodialysis. Biomed. Mater. 2019, 14, 025007. [Google Scholar] [CrossRef] [PubMed]
- Teebken, O.E.; Haverich, A. Tissue engineering of small diameter vascular grafts. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, J.; Li, Q.; Guo, J.; Ren, X.; Xia, S.; Zhang, W.; Feng, Y. Biofunctionalized Electrospun PCL-PIBMD/SF Vascular Grafts with PEG and Cell-Adhesive Peptides for Endothelialization. Macromol. Biosci. 2019, 19, e1800386. [Google Scholar] [CrossRef]
- Norouzi, S.K.; Shamloo, A. Bilayered heparinized vascular graft fabricated by combining electrospinning and freeze drying methods. Mater. Sci. Eng. C-Mater. 2019, 94, 1067–1076. [Google Scholar] [CrossRef]
- Shi, J.; Chen, S.; Wang, L.; Zhang, X.; Gao, J.; Jiang, L.; Tang, D.; Zhang, L.; Midgley, A.; Kong, D.; et al. Rapid endothelialization and controlled smooth muscle regeneration by electrospun heparin-loaded polycaprolactone/gelatin hybrid vascular grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2040–2049. [Google Scholar] [CrossRef]
- Shafiq, M.; Wang, L.; Zhi, D.; Zhang, Q.; Wang, K.; Wang, L.; Kim, D.; Kong, D.; Kim, S.H. In situ blood vessel regeneration using neuropeptide substance P-conjugated small-diameter vascular grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1669–1683. [Google Scholar] [CrossRef]
- Henry, J.J.D.; Yu, J.; Wang, A.; Lee, R.; Fang, J.; Li, S. Engineering the mechanical and biological properties of nanofibrous vascular grafts for in situ vascular tissue engineering. Biofabrication 2017, 9, 035007. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Allen, R.A.; Wang, Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 2012, 18, 1148. [Google Scholar] [CrossRef]
- Melchiorri, A.J.; Hibino, N.; Brandes, Z.R.; Jonas, R.A.; Fisher, J.P. Development and assessment of a biodegradable solvent cast polyester fabric small-diameter vascular graft. J. Biomed. Mater. Res. A 2014, 102, 1972–1981. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Tara, S.; Nakayama, H.; Yi, T.; Lee, Y.; Shoji, T.; Breuer, C.K.; Shinoka, T. Fast-degrading bioresorbable arterial vascular graft with high cellular infiltration inhibits calcification of the graft. J. Vasc. Surg. 2017, 66, 243–250. [Google Scholar] [CrossRef]
- Maina, R.M.; Barahona, M.J.; Finotti, M.; Lysyy, T.; Geibel, P.; D’Amico, F.; Mulligan, D.; Geibel, J.P. Generating vascular conduits: From tissue engineering to three-dimensional bioprinting. Innov. Surg. Sci. 2018, 3, 203. [Google Scholar] [CrossRef]
- Elomaa, L.; Yang, Y.P. Additive Manufacturing of Vascular Grafts and Vascularized Tissue Constructs. Tissue Eng. Part B Rev. 2017, 23, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, A.J.; Hibino, N.; Best, C.A.; Yi, T.; Lee, Y.U.; Kraynak, C.A.; Kimerer, L.K.; Krieger, A.; Kim, P.; Breuer, C.K.; et al. 3D-Printed Biodegradable Polymeric Vascular Grafts. Adv. Healthc. Mater. 2016, 5, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Gao, X.; Wang, J.; Chen, H.; Tong, C.; Tan, Y.; Tan, Z. Triple-Layer Vascular Grafts Fabricated by Combined E-Jet 3D Printing and Electrospinning. Ann. Biomed. Eng. 2018, 46, 1254–1266. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.E.; Nah, H.; Seok, J.M.; Jeong, M.H.; Park, K.; Kwon, I.K.; Lee, J.S.; Park, S.A. Vascular endothelial growth factor immobilized on mussel-inspired three-dimensional bilayered scaffold for artificial vascular graft application: In vitro and in vivo evaluations. J. Colloid Interface Sci. 2019, 537, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Michalopoulos, E.; Dinou, A.; Vlachou, M.S.; Panagouli, E.; Papapanagiotou, A.; Kassi, E.; Giokas, C.S. Development of HLA-matched vascular grafts utilizing decellularized human umbilical artery. Hum. Immunol. 2018, 79, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Muto, A.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Development of Decellularized Human Umbilical Arteries as Small-Diameter Vascular Grafts. Tissue Eng. Part A 2009, 15, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Kajbafzadeh, A.; Khorramirouz, R.; Kameli, S.M.; Fendereski, K.; Daryabari, S.S.; Tavangar, S.M.; Azizi Garajegayeh, B. Three-year efficacy and patency follow-up of decellularized human internal mammary artery as a novel vascular graft in animal models. J. Thorac. Cardiovasc. Surg. 2019, 157, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Ye, Z.; Fu, M.; Wang, Q.; Wu, H.; Lin, S.; Yin, T.; Hu, T.; Wang, G. Design, Preparation, and Performance of a Novel Bilayer Tissue-Engineered Small-Diameter Vascular Graft. Macromol. Biosci. 2019, 19, e1800189. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Ishibashi-Ueda, H.; Takamizawa, K. In vivo tissue-engineered small-caliber arterial graft prosthesis consisting of autologous tissue (Biotube). Cell Transplant. 2004, 13, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Furukoshi, M.; Terazawa, T.; Iwai, R. Development of long in vivo tissue-engineered “Biotube” vascular grafts. Biomaterials 2018, 185, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.J.; Yi, T.; Nasiri, B.; Breuer, C.K.; Andreadis, S.T. Implantation of VEGF-functionalized cell-free vascular grafts: Regenerative and immunological response. FASEB J. 2019, 33, 5089–5100. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hu, C.; Tang, Z.; Yu, Q.; Liu, X.; Chen, H. Tissue-engineered Vascular Grafts: Balance of the Four Major Requirements. Colloid Interface Sci. Commun. 2018, 23, 34–44. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H. Mechanical and degradation properties of small-diameter vascular grafts in an in vitro biomimetic environment. J. Biomater. Appl. 2019, 33, 1017–1034. [Google Scholar] [CrossRef]
- Liu, R.H.; Ong, C.S.; Fukunishi, T.; Ong, K.; Hibino, N. Review of Vascular Graft Studies in Large Animal Models. Tissue Eng. Part B Rev. 2018, 24, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Gade, P.S.; Dong, L.; Zhang, Z.; Aral, A.M.; Gao, J.; Ding, X.; Stowell, C.E.T.; Nisar, M.U.; Kim, K.; et al. A biodegradable synthetic graft for small arteries matches the performance of autologous vein in rat carotid arteries. Biomaterials 2018, 181, 67–80. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, Y.; Li, W.; Dong, X.; Chang, H.; Wang, K.; Wu, P.; Zhang, J.; Fan, G.; Wang, L.; et al. Biodegradable and elastomeric vascular grafts enable vascular remodeling. Biomaterials 2018, 183, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yuan, X.; Wang, L.; Liu, J.; Midgley, A.C.; Wang, Z.; Wang, K.; Liu, J.; Zhu, M.; Kong, D. Construction of a bilayered vascular graft with smooth internal surface for improved hemocompatibility and endothelial cell monolayer formation. Biomaterials 2018, 181, 1–14. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, X.; Wei, Y.; Zhang, Q.; Wang, T.; Zhu, M.; Li, W.; Huang, R.; Liu, R.; Chen, J.; et al. Small-diameter hybrid vascular grafts composed of polycaprolactone and polydioxanone fibers. Sci. Rep.-UK 2017, 7, 3615. [Google Scholar] [CrossRef]
- Guo, H.; Dai, W.; Qian, D.; Qin, Z.; Lei, Y.; Hou, X.; Wen, C. A simply prepared small-diameter artificial blood vessel that promotes in situ endothelialization. Acta Biomater. 2017, 54, 107–116. [Google Scholar] [CrossRef]
- Li, Q.; Mu, L.; Zhang, F.; Mo, Z.; Jin, C.; Qi, W. Manufacture and property research of heparin grafted electrospinning PCU artificial vascular scaffolds. Mater. Sci. Eng. C-Mater. 2017, 78, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Rothuizen, T.C.; Damanik, F.F.R.; Lavrijsen, T.; Visser, M.J.T.; Hamming, J.F.; Lalai, R.A.; Duijs, J.M.G.J.; van Zonneveld, A.J.; Hoefer, I.E.; van Blitterswijk, C.A.; et al. Development and evaluation of in vivo tissue engineered blood vessels in a porcine model. Biomaterials 2016, 75, 82–90. [Google Scholar] [CrossRef]
- Kumar, V.A.; Caves, J.M.; Haller, C.A.; Dai, E.; Liu, L.; Grainger, S.; Chaikof, E.L. Acellular vascular grafts generated from collagen and elastin analogs. Acta Biomater. 2013, 9, 8067–8074. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, Z.; Hu, L.; He, W.; Liu, R.; Qin, Y.; Wang, S. The in vivo performance of small-caliber nanofibrous polyurethane vascular grafts. BMC Cardiovasc. Disord. 2012, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Pektok, E.; Nottelet, B.; Tille, J.; Gurny, R.; Kalangos, A.; Moeller, M.; Walpoth, B.H. Degradation and Healing Characteristics of Small-Diameter Poly(Epsilon-Caprolactone) Vascular Grafts in the Rat Systemic Arterial Circulation. Circulation 2008, 118, 2563–2570. [Google Scholar] [CrossRef]
| Year | Manufacturing Method and Structure of Scaffold | Animal Model | Duration (Week) | Patency | Reference |
|---|---|---|---|---|---|
| 2019 | small intestinal submucosa (SIS) functionalized with heparin/vascular endothelial growth factor (VEGF) | mice | 4 | 100% | [36] |
| 2018 | decellularized film tissue obtained by implanting a mold in vivo in dog or goat | dogs and goats | 12 | - | [35] |
| 2018 | electrospinning of poly-caprolactone (PCL) and gelatin surface modification with heparin | rats | 4 | 100% | [19] |
| 2018 | electrospinning of PCL for inner layer 3D printing of polydopamine (PDA) for outer layer immobilization of VEGF | rats | 4 | 100% | [29] |
| 2018 | electrospinning of a poly(ε-caprolactone)-b-poly(isobutyl-morpholine-2,5-dione)/silk fibroin (PCL-PIBMD/SF) mixed solution surface modification with polyethylene glycol (PEG) and cell-adhesive peptides | rabbits | 10 | 100% | [17] |
| 2018 | casting of poly(glycerol sebacate) (PGS) for inner layer electrospinning of PCL for outer layer | rats | 12 | 90.5% (19/21) | [40] |
| 2018 | wet spinning of circumferentially orientated poly(L-lactide-co-ε-caprolactone) (PLCL) for inner layer electrospinning of PLCL for outer layer | rats | 48 | - | [41] |
| 2018 | 3D printing of aligned structure PCL fiber for inner layer electrospinning of PCL for middle layer co-electrospinning of PCL and PEG for outer layer | rats | 12 | - | [42] |
| 2018 | electrospinning of PCL surface modification with heparin and neuropeptide substance P | rats | 4 | 100% | [28] |
| 2017 | electrospinning of polylactide (PLA) and polyglycolic acid (PGA) for inner layer casting of PLCL for outer layer | mice | 8 | 100% | [24] |
| 2017 | electrospinning of poly-L-lactic acid (PLLA) and PCL surface modification with heparin and VEGF | rats | 4 | 88% (10/11) | [21] |
| 2017 | electrospinning of PCL and polydioxanone (PDS) | rats | 12 | - | [43] |
| 2017 | electrospinning of polyurethane (PU) surface modification of stromal cell-derived factor-1α and VEGF | dogs | 24 | 62.5% (5/8) | [44] |
| 2017 | electospinning of polycarbonate polyurethane (PCU) surface modification of NH3 plasma and heparin | rabbits | 4 | - | [45] |
| 2016 | 3D printing of poly(propylene fumarate) (PPF) | mice | 24 | 100% | [27] |
| 2016 | decellularized film tissue obtained by implanting a mold in vivo in pigs for inner layer electrospinning of PCL for outer layer | pigs | 4 | 87.5% (7/8) | [46] |
| 2013 | comprising collagen fiber networks and elastin-like protein polymers | rats | 2 | 100% | [47] |
| 2012 | electrospinning of PU | dogs | 24 | 83% (5/6) | [48] |
| 2008 | electrospinning of PCL | rats | 24 | 100% | [49] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, X.; Xu, T.; Zhang, L. Acellular Small-Diameter Tissue-Engineered Vascular Grafts. Appl. Sci. 2019, 9, 2864. https://doi.org/10.3390/app9142864
Li Z, Li X, Xu T, Zhang L. Acellular Small-Diameter Tissue-Engineered Vascular Grafts. Applied Sciences. 2019; 9(14):2864. https://doi.org/10.3390/app9142864
Chicago/Turabian StyleLi, Zhen, Xinda Li, Tao Xu, and Lei Zhang. 2019. "Acellular Small-Diameter Tissue-Engineered Vascular Grafts" Applied Sciences 9, no. 14: 2864. https://doi.org/10.3390/app9142864
APA StyleLi, Z., Li, X., Xu, T., & Zhang, L. (2019). Acellular Small-Diameter Tissue-Engineered Vascular Grafts. Applied Sciences, 9(14), 2864. https://doi.org/10.3390/app9142864




