1. Introduction
By the year 2050, an estimated 3.6 million persons will be living with amputations within the United States [
1]. In the fiscal year 2016, 22% (
n = 20,158) of US veterans who received amputation care at Veterans Affairs (VA) medical facilities had experienced an upper limb amputation [
2]. Finger amputations are the most common amputations of upper limbs. Finger amputations influence hand function, general functioning, and quality of life [
3]. Despite advances in upper limb prostheses, there is a high rate of user abandonment [
4]. Up to 52% of upper limb amputees reject or abandon their prosthesis [
2]. Fitting a patient with a prosthesis within 4 weeks after amputation will increase the likelihood of acceptance of the device [
5]. This time is known as the ‘golden period’ of upper extremity prosthetic rehabilitation and may be the most vital factor in a patient’s acceptance of the prosthesis [
6]. During this period, contractures, muscle atrophy, and infections are common risk factors that can affect prosthesis use and overall function. The use of transitional prostheses decreases the burden on the contralateral limb, increases function by offering an additional grasp with bimanual tasks, assists in body symmetry, and improves self-image [
7]. It has been reported that the use of an immediate post-operative functional prosthesis (i.e., a transitional prosthesis) can also improve the range of motion and strength of the affected hand [
8]. However, these functional transitional devices are often made by hand, requiring long construction times and highly skilled technicians to manufacture them [
8,
9]. Furthermore, patients using socket-based prostheses face multiple skin disorders and are susceptible to bacterial and fungal infections [
10]. These skin disorders have a significant detrimental impact in the function of activities of daily living and quality of life of patients who have experienced limb loss [
4].
Previous investigations [
11,
12] have shown that copper compounds have a high potential for use in the development of low-cost medical devices with powerful antibacterial properties. These positive characteristics and the high environmental safety of copper makes it capable of replacing silver and other antimicrobial compounds in the development of a wide range of medical devices [
11]. Furthermore, a previous investigation reported side effects after using compounds with silver, including local skin irritation, discoloration, or staining, which are harmless and usually reversible [
13]. Copper ions function by altering proteins and inhibiting their biological activity, membrane lipid peroxidation, and plasma membrane permeabilization [
14]. Copper has also been found to improve the healing process of wounds, as it plays a key role in the enhancement of angiogenesis via the induction of vascular endothelial growth factor, which upregulates the activity of copper-dependent enzymes, cell proliferation and reepithelization [
15]. Specifically, the addition of copper nanoparticles to polymers has been shown to provide strong antimicrobial properties, producing novel biocide materials and allowing the development of a broad range of polymer nanocomposites with a high release of metal ions facilitating the antimicrobial properties [
12]. It has been suggested [
12] that the addition of copper nanoparticles to polymers and the resulting antimicrobial properties have promising applications in the development of medical devices associated with bacterial development, such as socket-based prostheses, among many others. Recent technological advances in additive manufacturing (i.e., 3D printing) [
16] and a new antimicrobial 3D printing filament offer the unique possibility of manufacturing low-cost, customized, and antibacterial upper-limb 3D printed prostheses [
16,
17,
18]. The development and effectiveness of manufacturing upper limb prostheses using antimicrobial filaments, however, has not been tested [
12]. Therefore, the purpose of the present investigation was two-fold: (i) to describe the development of 3D printed prostheses using antibacterial filaments and (ii) to verify the antibacterial properties of the 3D printed prostheses. This information is crucial for the implementation of 3D printed prostheses as post-operative or transitional prostheses. Based on previous investigations [
11,
12,
13,
14,
16,
17,
18,
19], we hypothesized that (i) antibacterial 3D printed filaments can be used for the development of functional upper-limb prostheses and (ii) the antibacterial properties of the 3D printing filament after extrusion and development of the prosthesis do not affect the antibacterial properties of the filament.
2. Materials and Methods
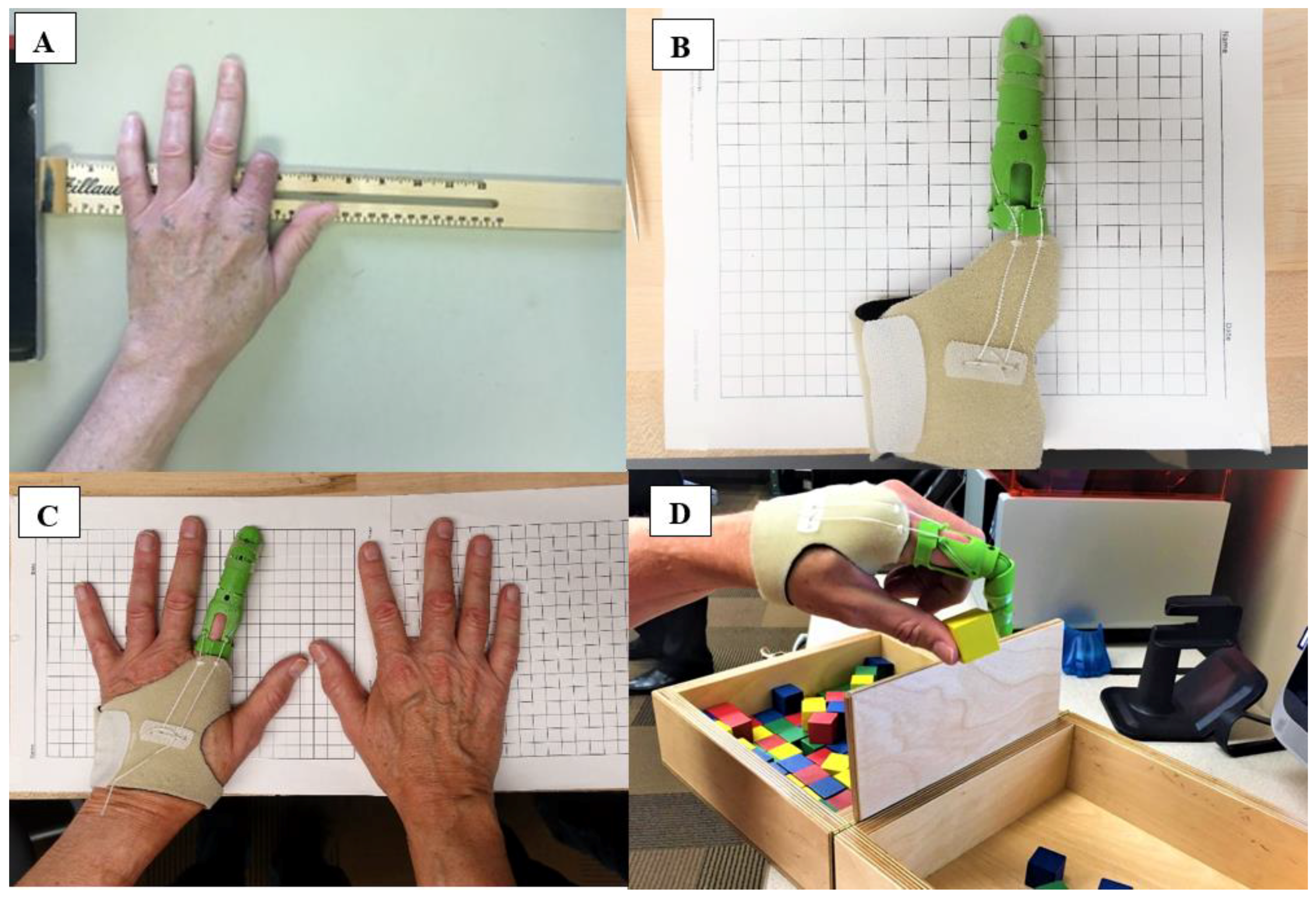
This investigation describes the development of 3D printed antibacterial finger prostheses and the verification of their antibacterial properties. Furthermore, the current investigation also describes patient satisfaction outcomes and function with the prosthesis. Two patients were recruited for the present investigation. Patient 1 was a 65-year-old male (height 177.8 cm and weight 81.6 kg) with a traumatic index finger amputation at the proximal phalanx of the left (non-dominant) hand (
Figure 1A). The residual finger at the proximal phalange was 4.5 cm in length and 7 cm in circumference. The non-affected index finger was 9.5 cm in length and 7 cm in circumference. Patient 2 was a 40-year-old male (height 180 cm and weight 104 kg) with a traumatic index finger amputation at the proximal phalanx of the left (non-dominant) hand. The residual finger at the proximal phalange was 2 cm in length and 7.2 cm in circumference. The non-affected index finger was 9 cm in length and 7.2 cm in circumference. Prior the laboratory visit, the research participants provided pictures of both hands (affected and non-affected) for remote prosthetic fitting [
16]. The research participants visited the laboratory on two occasions. During the first visit, the participants completed an orientation session, were introduced to the test procedures, and completed an informed consent form. During the second visit, the participants were fitted with a 3D printed antibacterial finger prosthesis (
Figure 1B,C) and performed the Box and Block Test of manual gross dexterity (
Figure 1D). The Box and Block Test has been suggested as a measure of unilateral gross dexterity [
20,
21] and has been previously used to assess upper limb prosthetic performance and motor learning [
22]. The Box and Block Test consists of a wooden box with dimensions of 53.7 cm by 25.4 cm by 8.5 cm. A partition is placed at the middle of the box creating two containers of 25.4 cm each [
20]. In the current study, after instructions had been provided, the research subjects were allowed a 15-s familiarization period prior to testing. Immediately before testing began, the subjects were asked to place their hands on the sides of the box. When testing started, each subject was asked to grasp one block at a time, transport the block over the partition, and release it into the opposite compartment. This task was performed for a duration of one minute.
The participants reported using the 3D printed antibacterial finger prosthesis for 12 and 15 h a week, respectively. After two weeks of prosthesis use, the participants completed a satisfaction survey. Prosthesis use and satisfaction were assessed using the Quebec User Evaluation of Satisfaction with assistive Technology (QUEST 2.0) [
23]. The research participants were informed about the study, and a consent form was explained and signed. The study was approved by the University of Nebraska Medical Center Institutional Review Board.
2.1. Antibacterial Testing
The antibacterial properties of the filament use to 3D print the antibacterial fingers were tested by two independent laboratories following standard procedures for ISO 22196. Six flat test samples (5 cm × 5 cm × 1 cm) were manufactured and tested. The ISO 22196 was designed to measure the antimicrobial properties of a solid plastic surface incubated with methicillin-resistant Staphylococcus aureus, standard Staphylococcus aureus, and Escherichia coli. These bacteria were chosen because they are known to be the main causes of a variety of home- and hospital-acquired infections. The basis of this test was the incubation of the bacterial inoculum in contact with the 3D printed antibacterial finger material for a 24-h period. Following this exposure, the inoculated bacteria were recovered, and the concentration of each organism was determined. The antimicrobial performance was determined by comparison of the recovered organism incubated in a control material with the 3D printed antibacterial finger material after a 24-h incubation period. Laboratory 1 tested the antibacterial efficacy against methicillin-resistant Staphylococcus aureus and Escherichia coli, which are common bacteria associated with skin infections and gastrointestinal distress, respectively. Laboratory 2 tested the antibacterial efficacy against Staphylococcus aureus and Escherichia coli. In particular, methicillin-resistant Staphylococcus aureus is one of the most common hospital-acquired infections and represents a serious public health concern.
2.2. 3D Printed Antibacterial Finger Prosthesis Description
The 3D printed antibacterial finger prosthesis was a voluntary-closing prosthesis powered by metacarpophalangeal (MCP) flexion. A detailed technical drawing and rendering of the finger design can be found in
Figure 2 (
Figure 2A–C). The device was secured using a customized neoprene strap in the palm of the hand. The prosthesis was designed to be proportional to the length and circumference of the participant’s non-affected finger. The 3D printed antibacterial finger prosthesis allowed pinch grasping actions actuated by flexion of the MCP. A MCP flexion of 40° produced 1 inch of cable travel for full operation. A silicone finger grip was added onto the fingertip to increase friction and to prevent slippage of gripped objects. The scaling of the prosthesis was performed remotely and began with instructing the patient to photograph both the affected and unaffected limbs including a known measurable scale, such as metric grid paper (
Figure 1A). This photogrammetric method allowed the extraction of several anthropometric measurements from the photographs. This photo was then uploaded to Autodesk Fusion 360 and used as a backdrop. The software measuring system was calibrated with the ruler included in the photograph. Once the prosthesis had been scaled to the patient’s arm and measurements had been confirmed by a certified prosthetist, the files were uploaded to a desktop 3D Printer (Ultimaker 2 extended, Ultimaker B.V., Geldermalsen, The Netherlands). The prosthesis was manufactured using PLACTIVE
TM (PLACTIVE
TM 1% Antibacterial Nanoparticles additive, Copper3D, Santiago, Chile), which is a high quality polylactic acid polymer, using an internationally patented additive containing copper nanoparticles. Copper nanoparticles have been showed to be effective in eliminating fungi, viruses, and bacteria, but are harmless to humans. PLACTIVE
TM was chosen as it uses a sound and proven antibacterial mechanism, is a low-cost material that is biodegradable, and possesses thermoforming characteristics that facilitate post-processing and final adjustments of 3D printed prostheses. PLACTIVE
TM has similar physical (relative viscosity = 4.0 g/dL, clarity = transparent, peak melt temperature = 145–160 °C, glass transition temperature = 55–60 °C) and mechanical (tensile yield strength = 8700 psi, tensile strength at break = 7700 psi, tensile modulus = 524,000 psi, tensile elongation = 6%, flexural strength = 12,000 psi, and heat distortion temperature at 66 psi = 55 °C) properties to standard polylactic acid filaments.
The average printing time for the 3D printed finger prostheses was 60 ± 5.6 min. The cost of a 750 g spool of antibacterial filament is
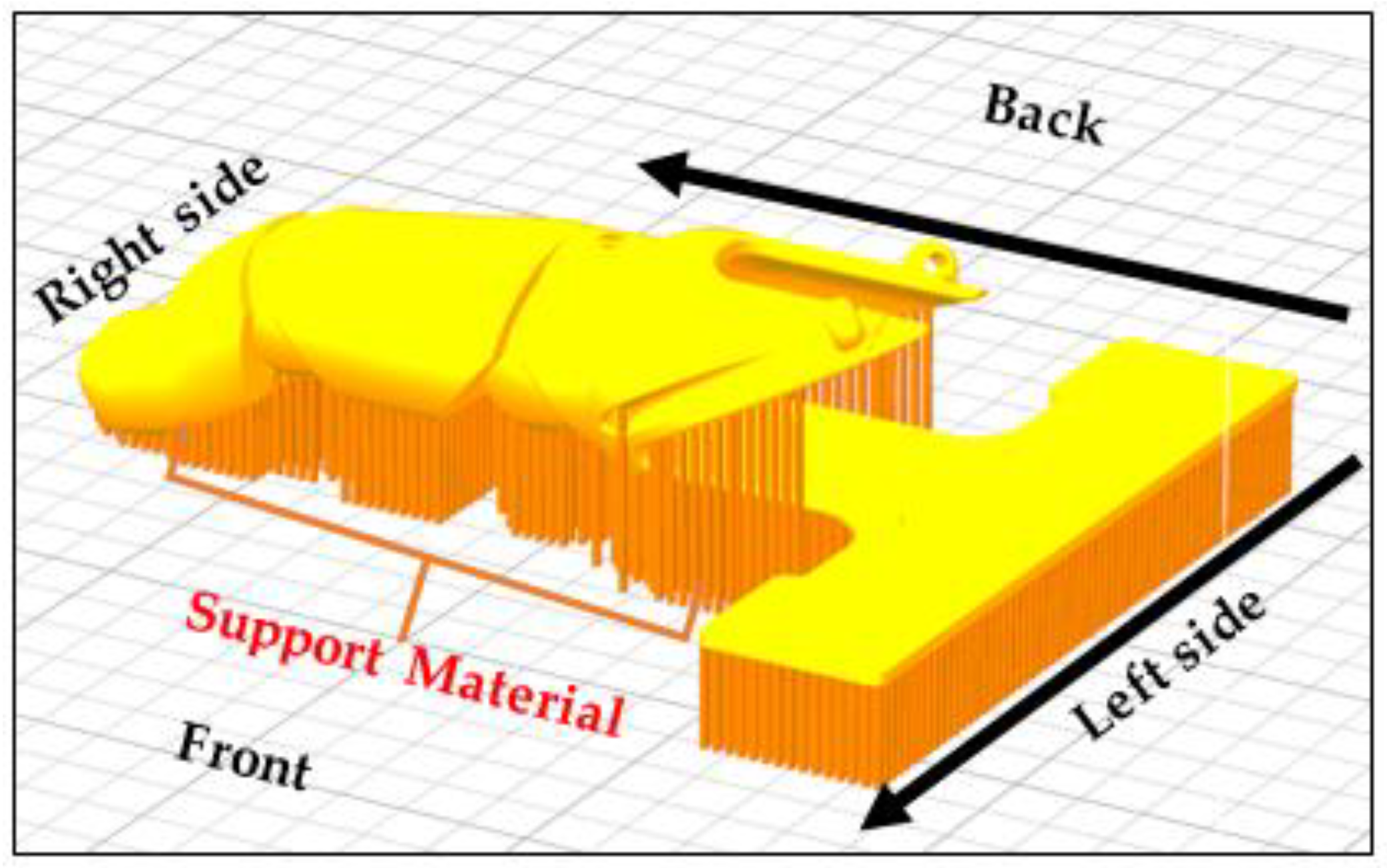
$92 USD. All parts were printed at 40% infill (hexagon pattern) with a 50 mm/s print speed and a 150–200 mm/s travel speed on a 50 °C heated bed at a printing temperature of 200 °C with a 0.15 mm layer height and 1 mm shell thickness. Post-processing consisted of support removal and filing of rough areas in the joints and prosthetic socket area in contact with the skin. The location and generation of the support as well as the build orientation on the building platform are illustrated in
Figure 3. The post-processing of the 3D printed antibacterial prosthesis took 10 min, and assembly took 30 min.
4. Discussion
The main findings of the current investigation were that the antibacterial 3D printed filament, PLACTIVE
TM, can be effectively used for the development of functional 3D printed finger prostheses. Furthermore, the antibacterial properties of the 3D printing filament after extrusion were not affected (
Table 1). The thermoforming properties of polylactic acid were not affected by the addition of copper nanoparticles and allowed for necessary post-processing modifications for the final fitting of the 3D printed antibacterial finger prosthesis.
Previous investigations have described the use of silver compounds to develop antibacterial lower limbs [
24] and dental prostheses [
25]. A recent review [
24] identified silver as being the main compound used to reduce sweat and odor build-up at the socket interface. Similarly, Yamada et al. [
25] described the use of silver compounds for dental prostheses with the objective of reducing bacterial adhesion to dental materials and thus, reducing the incidence of caries and periodontitis [
25]. However, there is some evidence that the use of silver compounds may result in local skin irritation and discoloration [
13]. Furthermore, copper compounds have been described as a low-cost alternative to silver, with high potential for the development of medical devices with powerful antibacterial properties [
11,
12]. The addition of nanoparticles of copper to polymers and the resulting antimicrobial properties have promising applications in the development of transitional prostheses [
12]. The term “post-operative or preparatory prosthesis” has been widely used in the field of prosthetics [
10,
26]. More recently, these types of devices have been referred as “temporary prostheses,” “initial prostheses,” or “transitional prostheses” [
9]. Previous investigations have used transitional prosthetic devices with the objective of restoring and preserving strength and range of motion in children with upper-limb reduction deficiencies [
8,
27]. A transitional prosthesis can be used while the patient’s residual limb is still healing to decrease edema and allow the patient to improve wear tolerance. A recently amputated limb, however, is particularly susceptible to infections and skin disorders [
10]. The current findings suggest that the 3D printing antibacterial filament may also be used to manufacture more effective and sanitary transitional prostheses. Furthermore, the fabrication of antibacterial sockets of definitive upper limb prostheses may have the potential to alleviate the majority of skin disorders associated with bacterial and fungal infections [
10,
12].
The development of an antibacterial 3D printing filament with thermoforming capabilities has the potential to revolutionize patient care in the orthotic and prosthetic industry. The addition of copper nanoparticles to polymers, and the resulting antimicrobial properties have promising applications in the development of medical devices associated with bacterial development [
12]. These applications are not limited to post-operative prostheses [
12,
18], but can also be used as other types of medical devices, such as wound dressings [
13] and surgical instruments [
28]. Wound dressings are external barriers that isolate the injury site from the external environment and provide an optimal environment for the wound to heal. When a wound occurs due to trauma or disease, the barrier becomes compromised. This can increase the susceptibility of the wound site to microbial infections originating from endogenous and exogenous sources. A previous investigation [
13] used zinc, copper, and silver particles incorporated into polycaprolactone to develop patient-specific 3D printed antimicrobial wound dressings. The authors found that wound dressings manufactured using 3D printing filament containing particles of silver and copper had the most potent bactericidal properties. Specifically, these wound dressings showed the most bactericidal properties against methicillin-resistant
Staphylococcus aureus which is a common cause of bacterial skin infections. Similarly, the current investigation found that polylactic acid with 1% of copper nanoparticles additives was up to 99.99% effective against
Staphylococcus aureus and
Escherichia coli after a 24-h incubation period. Nanoparticles of copper are preferable over silver due to the lower cost of copper and the reported side effects of using silver nanoparticles including local skin irritation, discoloration, or staining [
13].
3D printed antibacterial filaments also provide the possibility of developing antibacterial surgical instruments. A previous investigation [
28] used polylactic acid filaments to develop a low cost Army/Navy retractor strong enough to be used in the operating room (75% infill was capable of supporting 13.6 kg before fracture). Polylactic acid has been shown to be a safe and suitable material for use in surgical instruments [
28]. Polylactic acid is extruded at temperatures well above the 121 °C recommended for steam sterilization or even the 170 °C recommended for dry heat sterilization. However, other sterilization methods, such as autoclaving, compromise the structural integrity of polylactic acid, limiting the use of these devices in surgery [
28]. Although lower temperature methods of sterilization, such as ethylene oxide “gas” sterilization, do not impact the strength of polylactic acid, the high levels of ethylene oxide residue produced in this process are a serious concern [
28]. The hypoallergenic and safe nature of polylactic acid has been previously verified by the U.S. Food and Drug Administration and approved as a semi-permanent dermal filler and suture material [
28,
29]. Therefore, the development of a polylactic acid 3D printing filament with antibacterial properties has several impactful medical applications and could revolutionize the manufacture of medical devices associated with bacterial development. The present investigation used PLACTIVE
TM 3D printing filaments that combine the versatility of polylactic acid and the high antibacterial properties of copper nanoparticles to develop antibacterial, thermoforming, and functional 3D printed finger prostheses.
The increase in manual gross dexterity and high patient satisfaction scores after using the 3D printed partial finger prosthesis suggest that the finger prosthesis was functional, easy to use, comfortable, and effective (
Table 2). The large difference observed in the Box and Block performance with and without the prosthesis for subject 2 (
Table 2) may be due to the short residual finger of subject 2 compared to subject 1. Subject 2 had only 2 cm in length and subject 1 had 4.5 cm in length. While a longer residual index can increase gross dexterity, a shorter residual segment may increase the difficulty in performing this task. Although, the functional nature of a prosthesis is inherent to its design, the thermoforming and antibacterial properties of PLACTIVE
TM discussed in the current manuscript could be used to develop a variety of prostheses [
16,
17,
18], orthoses, assistive devices, wound dressing [
13], and surgical instruments [
28].
The potential limitations of the present investigation are related to the low number of subjects using the 3D printed antibacterial finger prosthesis, the limited number of materials tested, the use of a single antibacterial testing protocol (i.e., ISO 22196), and the limited diversity of bacteria used (methicillin-resistant Staphylococcus aureus, Staphylococcus aureus and Escherichia coli). Furthermore, whilst our results indicated that the antibacterial properties of the 3D printing filament after extrusion were not affected, the longevity of the antibacterial properties was not tested. The main findings of the current investigation were that the antibacterial 3D printed filament, PLACTIVETM, can be effectively used for the development of functional 3D printed finger prostheses. Furthermore, the antibacterial properties of the 3D printing filament after extrusion were not affected. Future investigations should test a large sample size using different types of antibacterial sockets and prostheses. Furthermore, the use of a more comprehensive testing protocol, such as the ISO 10993 test series (20 tests) that can assess biocompatibility on more diverse bacterial strains as well as the longevity of the antibacterial properties could significantly strengthen these finding.
Overall, the findings from current investigation suggest that the antibacterial 3D printed filament, PLACTIVETM, can be effectively used for the development of functional 3D printed finger prostheses. Furthermore, the present investigation also confirmed that antibacterial and thermoforming properties of the 3D printing filament after extrusion were not affected allowing for post-processing modifications necessary for the final fitting. The use of antibacterial 3D printed filament has promising potential applications for the development of medical devices associated with bacterial development, such as postoperative prostheses, wound dressings, and surgical equipment.