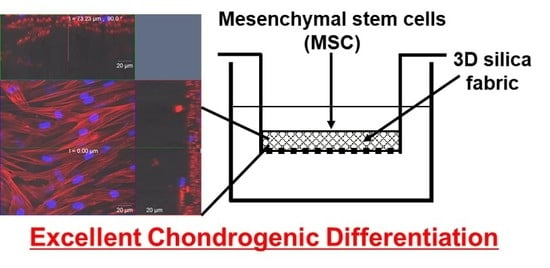
Cartilage Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells in Three-Dimensional Silica Nonwoven Fabrics
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Preparation of Silica Nonwoven Fabrics
2.4. Induction of Chondrogenic Differentiation of MSCs
2.5. Confocal-Laser Scanning Microscopic (CLSM) Observation
2.6. Cell Number Analysis
2.7. Quantification of Glycosaminoglycan and Collagen Content
2.8. Real-time Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
3. Results
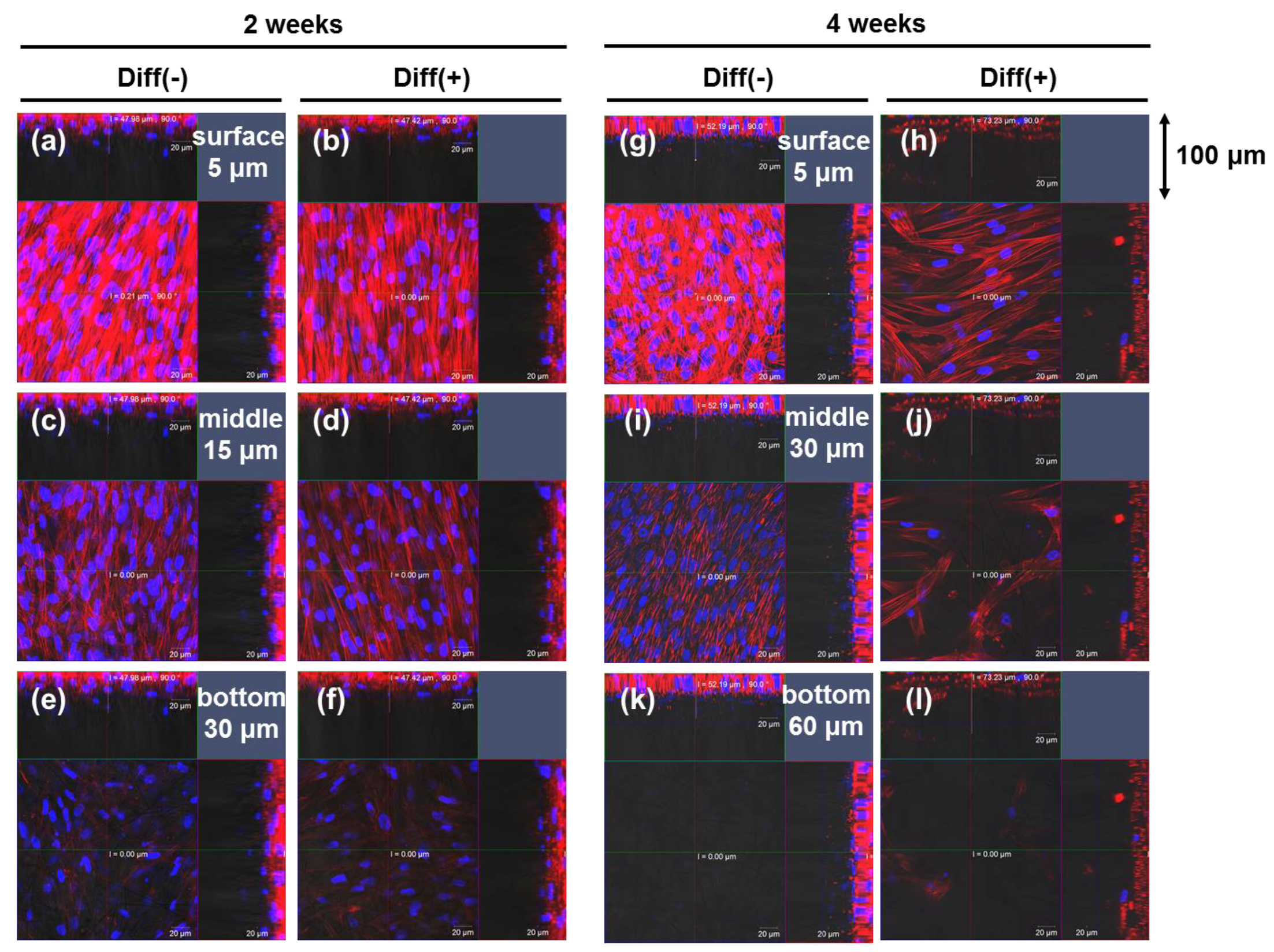
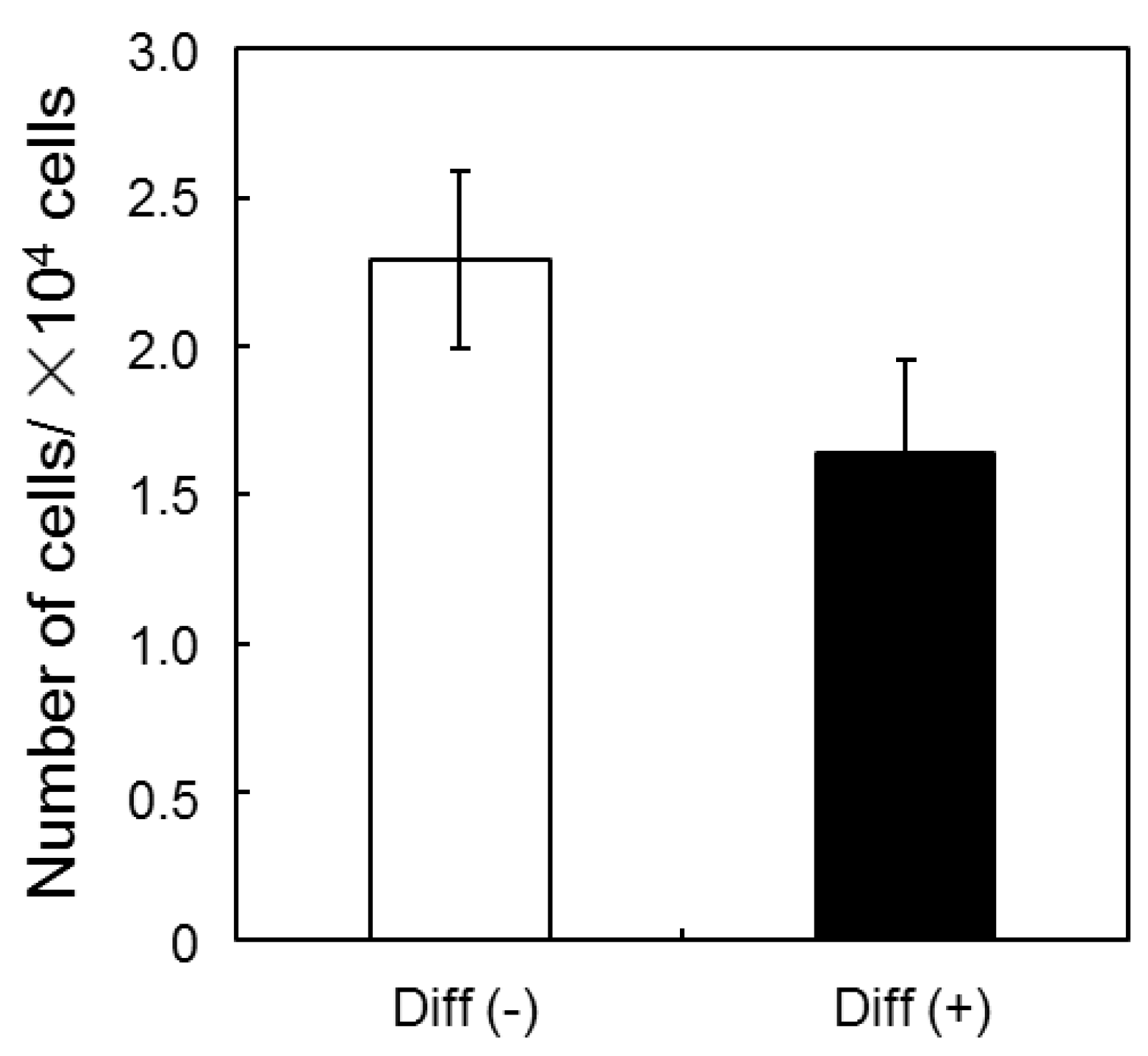
3.1. Proliferation and Morphology of Chondrogenic MSCs Cultured in 3D Silica Fabrics
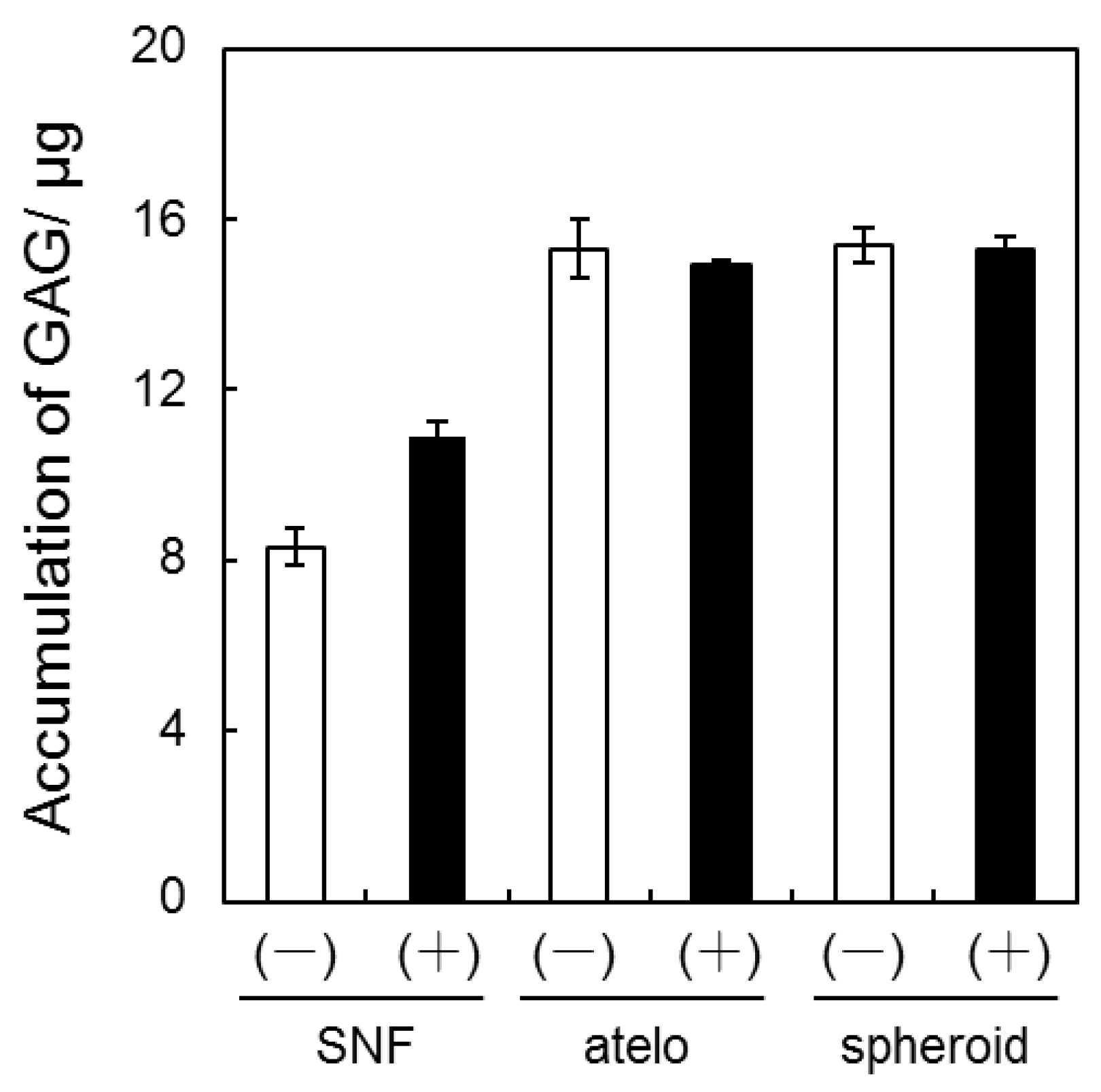
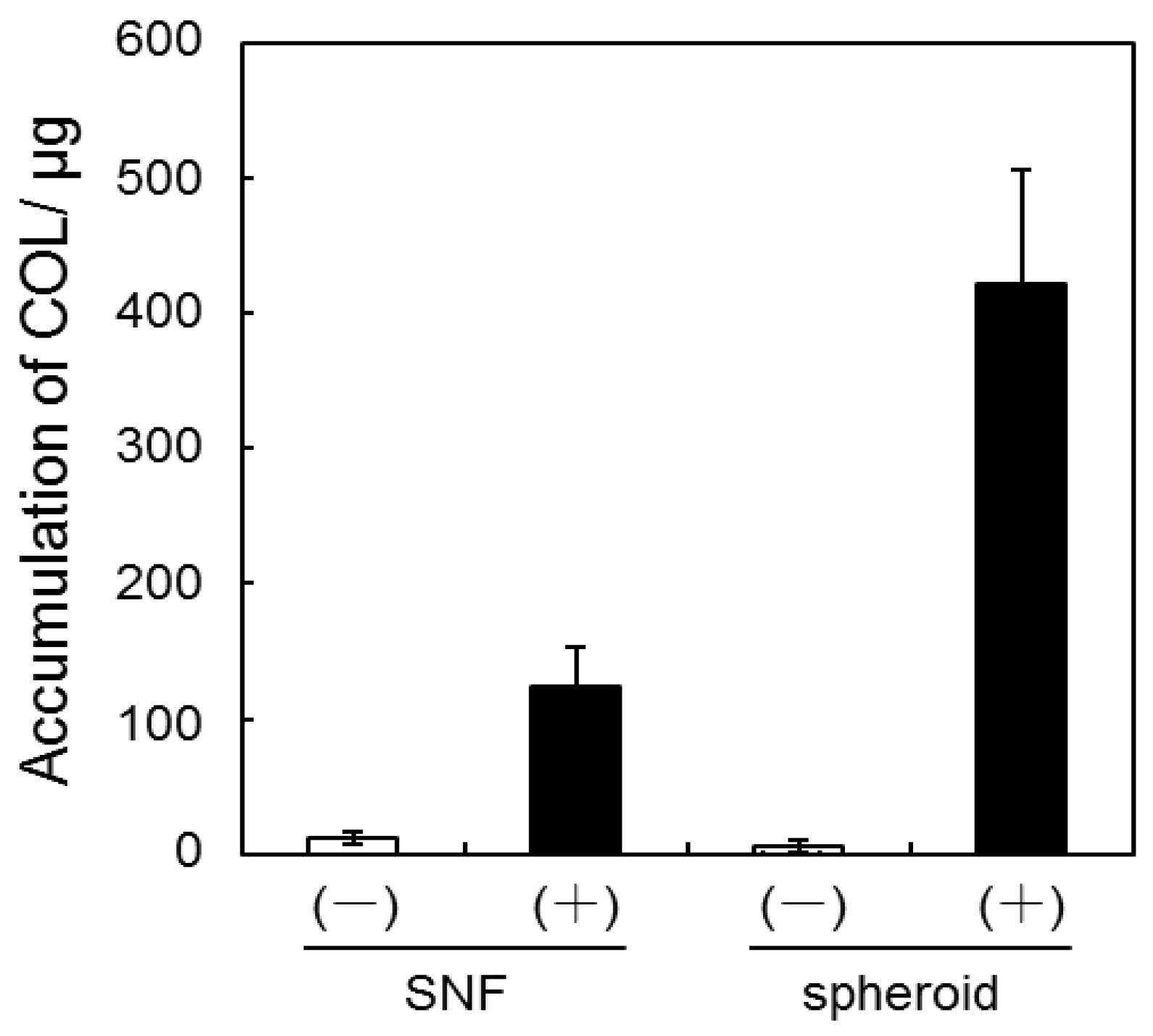
3.2. Quantification of GAG and Collagen Content
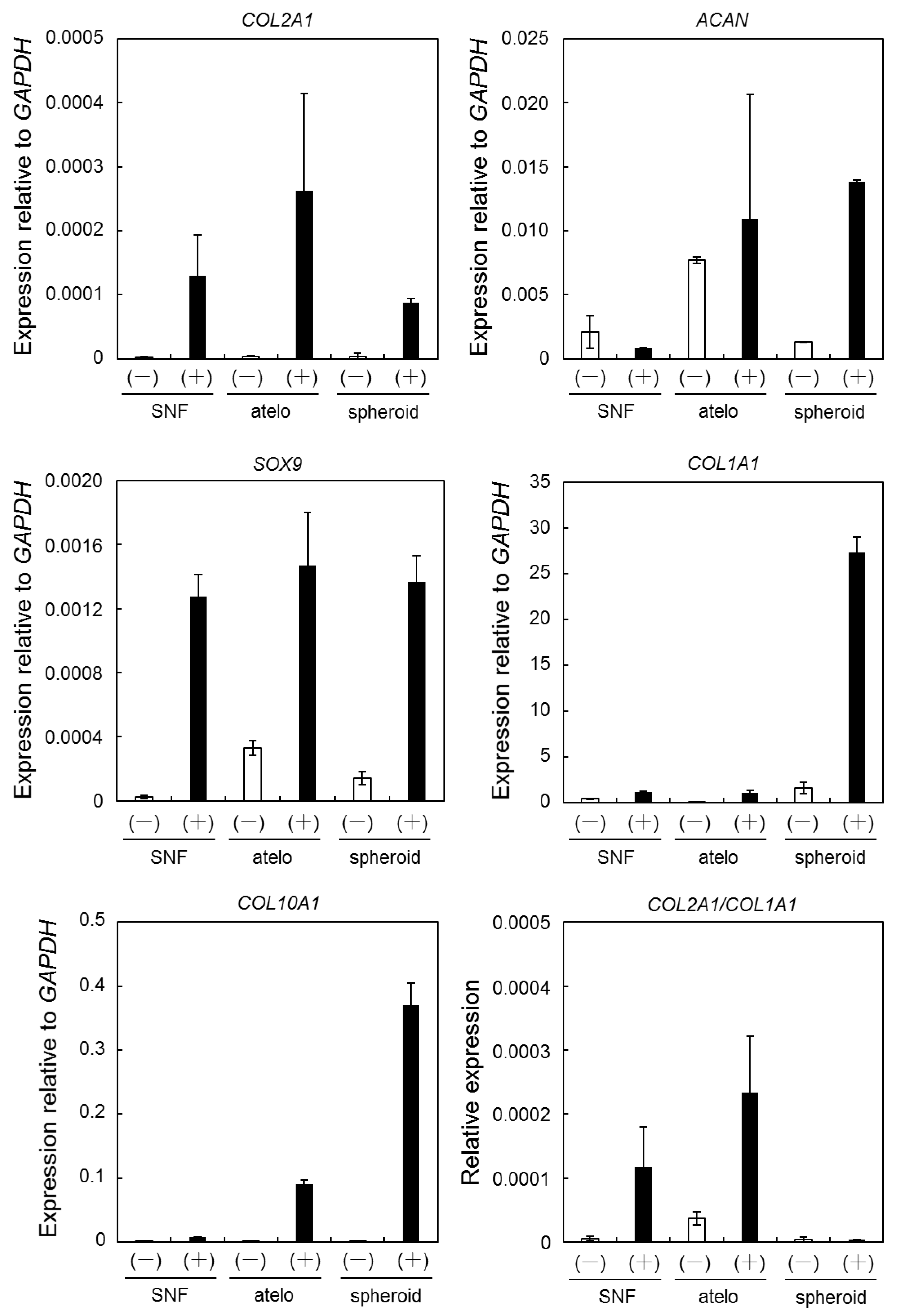
3.3. Expression of Chondrogenic Differentiation Marker Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Clar, C.; Cummins, E.; Mclntyre, L.; Thomas, S.; Bain, L.; Jobanputra, P.; Waugh, N. Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation. Health Technol. Assess. 2005, 9, 1–82. [Google Scholar] [CrossRef]
- Knutsen, G.; Engebretsen, L.; Ludvigsen, T.C.; Drogset, J.O.; Grøntvedt, T.; Solheim, E.; Strand, T.; Roberts, S.; Isaksen, V.; Johansen, O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J. Bone Joint Surg. Am. 2004, 86, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Robert, B.; Dzioba, M.D. The classification and treatment of acute articular cartilage lesions. Arthroscopy 1988, 4, 72–80. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Sherwood, J.K.; Riley, S.L.; Palazzolo, R.; Brown, S.C.; Monkhouse, D.C.; Coates, M.; Griffith, L.G.; Landeen, L.K.; Ratcliffe, A. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials 2002, 23, 4739–4751. [Google Scholar] [CrossRef]
- Jiang, C.C.; Chiang, H.; Liao, C.J.; Lin, Y.J.; Kuo, T.F.; Shieh, C.S.; Huang, Y.Y.; Tuan, R.S. Repair of porcine articular cartilage defect with a biphasic osteochondral composite. J. Orthop. Res. 2007, 25, 1277–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hangody, L.; Füles, P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints. J. Bone Joint Surg. Am. 2003, 85, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.P.; Murphy, J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 2002, 10, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 2, 143–147. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M.; Du, J.; Aldrich, S.; Lisberg, A.; Low, W.C.; Largaespada, D.A.; Verfaillie, C.M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002, 418, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- C Murphy, C.A.; Costa, J.B.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Collins, M.N. Biopolymers and polymers in the search of alternative treatments for meniscal regeneration: State of the art and future trends. Applied Materials Today 2018, 12, 51–71. [Google Scholar] [CrossRef]
- Hung, A.H.; Farrell, M.J.; Mauck, R.L. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J. Biomech. 2010, 43, 128–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaoka, H.; Tanaka, Y.; Nishizawa, S.; Asawa, Y.; Hoshi, K. The application of atelocollagen gel in combination with porous scaffolds for cartilage tissue engineering and its suitable conditions. J. Bio. Mat. Res. A 2009, 93, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Charriere, G.; Bejot, M.; Schnitzler, L.; Ville, G.; Hartmann, D.J. Reactions to a bovine collagen implant. Clinical and immunologic study in 705 patients. J. Am. Acad. Dermatol 1989, 21, 1203–1208. [Google Scholar] [CrossRef]
- Shepherd, D.E.; Seedhom, B.B. The ‘instantaneous’ compressive modulus of human articular cartilage in joints of the lower limb. Rheumatology 1998, 38, 124–132. [Google Scholar] [CrossRef]
- Hennig, T.; Lorenz, H.; Thiel, A.; Goetzke, K.; Dickhut, A.; Geiger, F.; Richter, W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFβ receptor and BMP profile and is overcome by BMP-6. J. Cell. Physiol. 2007, 211, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Hsieh, P.S.; Tseng, C.S.; Hsu, S.H. The substrate-dependent regeneration capacity of mesenchymal stem cell spheroids derived on various biomaterial surfaces. Biomater. Sci. 2014, 2, 1652–1660. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Takagi, S.; Okumura, M.; Fujinaga, T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol. Bioeng. 2006, 93, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Man, Z.; Dai, L.; Huang, H.; Zhang, X.; Hu, X.; Shao, Z.; Zhu, J.; Zhang, J.; Fu, X.; Duan, X.; Ao, Y. A composite scaffold of MSC affinity peptide-modified demineralized bone matrix particles and chitosan hydrogel for cartilage regeneration. Sci. Rep. 2015, 5, 17802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Kim, U.J.; Blasioli, D.J.; Kim, H.J.; Kaplan, D.L. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials 2005, 26, 7082–7094. [Google Scholar] [CrossRef] [PubMed]
- Karkhaneh, A.; Naghizadeh, Z.; Shokrgozar, M.S.; Bonakdar, S. Evaluation of the chondrogenic differentiation of mesenchymal stem cells on hybrid biomimetic scaffolds. J. Appl. Plym. Sci. 2014, 131, 40635. [Google Scholar] [CrossRef]
- Li, W.J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Souness, A.; Zamboni, F.; Walker, G.M.; Collins, M.N. Influence of scaffold design on 3D printed cell constructs. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Tuli, R.; Huang, X.; Laquerriere, P.; Tuan, R.S. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials 2005, 26, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, D.; Shang, C.; Yang, S.T.; Wang, J.; Wang, X. Three-dimensional culture of human mesenchymal stem cells in a polyethylene terephthalate matrix. Biomed. Mater. 2010, 5, 065013. [Google Scholar] [CrossRef] [PubMed]
- Ardeshirylajimi, A.; Mossahebi-Mohammadi, M.; Vakilian, S.; Langroudi, L.; Seyedjafari, E.; Atashi, A.; Soleimani, M. Comparison of osteogenic differentiation potential of human adult stem cells loaded on bioceramic-coated electrospun poly (L-lactide) nanofibres. Cell Prolif. 2015, 48, 47. [Google Scholar] [CrossRef] [PubMed]
- Pournaqi, F.; Ghiaee, A.; Vakilian, S.; Ardeshirylajimi, A. Improved proliferation and osteogenic differentiation of mesenchymal stem cells on polyaniline composited by polyethersulfone nanofibers. Biologicals 2017, 45, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Soleimani, M.; Chamheidari, G.A.; Seyedjafari, E.; Dodel, M.; Atashi, A.; Gheisari, Y. Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. J. Biomed. Mater. Res. A 2011, 99, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Lee, C.H.; Cho, I.H.; Kim, Y.J.; Lee, Y.J.; Kim, I.A.; Park, K.D.; Yui, N.; Shin, J.W. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J. Biomater. Sci. Polym. Ed. 2006, 17, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Sakai, S.; Watanabe, R.; Tarao, T.; Kawakami, K. Heat treatment of electrospun silicate fiber substrates enhances cellular adhesion and proliferation. J. Biosci. Bioengin. 2010, 109, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Deng, D.; Sato, Y.; Hou, Y.T.; Watanabe, R.; Sasaki, K.; Kawabe, M.; Hirano, E.; Morinaga, T. Silicate fiber-based 3D cell culture system for anticancer drug screening. Anticancer Res. 2013, 33, 5301–5309. [Google Scholar] [PubMed]
- Otsuka, H.; Sasaki, K.; Okimura, S.; Nagamura, M.; Watanabe, R.; Kawabe, M. Contribution of fibroblasts cultured on 3D silica nonwoven fabrics to cocultured hepatocytes function. Chem. Lett. 2014, 43, 343–345. [Google Scholar] [CrossRef]
- Kascholke, C.; Hendrikx, S.; Flath, T.; Kuzmenka, D.; Dörfler, H.M.; Schumann, D.; Gressenbuch, M.; Schulze, F.P.; Schulz-Siegmund, M.; Hacker, M.C. Biodegradable and adjustable sol-gel glass based hybrid scaffolds from multi-armed oligomeric building blocks. Acta Biomater. 2017, 63, 336. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kiyono, T.; Imabayashi, H.; Takeda, Y.; Tsuchiya, K.; Miyoshi, S.; Makino, H.; Matsumoto, K.; Saito, H.; Ogawa, S.; Sakamoto, M.; Hata, J.; Umezawa, A. Combination of hTERT and bmi-1, E6, or E7 induces prolongation of the life span of bone marrow stromal cells from an elderly donor without affecting their neurogenic potential. Mol. Cell. Biol. 2005, 25, 5183–5195. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.M.; Shortkro, S.; Schneider, T.O.; Breinan, H.A.; Yannas, L.V.; Spector, T.O. Meniscus cells seeded in type I and type II collagen–GAG matrices in vitro. Biomaterials 1999, 20, 701–709. [Google Scholar] [CrossRef]
- Bahney, C.S.; Hsu, S.W.; Yoo, J.U.; West, J.L.; Johnstone, B. A bioresponsive hydrogel tuned to chondrogenesis of human mesenchymal stem cells. FASEB 2011, 25, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Woessner, J.F. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef]
- Yamashita, A.; Morioka, M.; Yahara, Y.; Okada, M.; Kobayashi, T.; Kuriyama, S.; Matsuda, S.; Tsumaki, N. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Reports 2015, 4, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Burdick, J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng. Part A 2009, 15, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Ludeman, M.; Cheng, K.; Hayami, T.; Lotz, J.C.; Kapila, S. Chondrogenic Differentiation of Human Mesenchymal Stem Cells in Three-Dimensional Alginate Gels. Tissue Eng. Part A 2008, 14, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Yang, C.Y.; Yang, S.R.; Ku, K.L.; Tsao, C.K.; Chuang, D.C.C.; Chu, I.M.; Cheng, M.H. Cartilage formation through alterations of amphiphilicity of poly(ethylene glycol)–poly(caprolactone) copolymer hydrogels. RSC Adv. 2013, 3, 25769–25779. [Google Scholar] [CrossRef]
- Lin, H.; Cheng, A.W.M.; Alexander, P.G.; Beck, A.M.; Tuan, R.S. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng. Part A 2014, 20, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Miyazono, K.; ten Dijke, P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Huang, J.S. TGF-β control of cell proliferation. J. Cell Biochem. 2005, 96, 447–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, A.S.; Sable, R.B.; Kothari, R.M. An update on transforming growth factor-β (TGF-β): sources, types, functions and clinical applicability for cartilage/bone healing. J. Cell. Physiol. 2011, 226, 3094–3103. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, A.; Dudek, K.A.; Murphy, C.L. Regulation of human chondrocyte function through direct inhibition of cartilage master regulator SOX9 by microRNA-145 (miRNA-145). J. Biol. Chem. 2012, 287, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Dy, P.; Wang, W.; Bhattaram, P.; Wang, Q.; Wang, L.; Ballock, R.T.; Lefebvre, V. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev. Cell 2012, 22, 597–609. [Google Scholar] [CrossRef]
- Tsirimonaki, E.; Fedonidis, C.; Pneumaticos, S.G.; Tragas, A.A.; Michalopoulos, I.; Mangoura, D. PKCε signalling activates ERK1/2, and regulates aggrecan, ADAMTS5, and miR377 gene expression in human nucleus pulposus cells. PLoS One 2013, 8, e82045. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.T.; Chen, C.T.; Chen, J.P. Osteogenic differentiation and ectopic bone formation of canine bone marrow-derived mesenchymal stem cells in injectable thermo-responsive polymer hydrogel. Tissue Eng. Part C Methods 2011, 17, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Melchels, F.P.; Jeon, J.E.; van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrigues, N.W.; Little, D.; Sanchez-Adams, J.; Ruch, D.S.; Guilak, F. Electrospun cartilage-derived matrix scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. A 2014, 102, 3998–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shariati, S.R.P.; Moeinzadeh, S.; Jabbari, E. Nanofiber Based Matrices for Chondrogenic Differentiation of Stem Cells. J. Nanosci. Nanotechnol. 2016, 16, 8966–8977. [Google Scholar] [CrossRef]
- Schlichting, K.; Schell, H.; Kleemann, R.U.; Schill, A.; Weiler, A.; Duda, G.N.; Epari, D.R. Influence of scaffold stiffness on subchondral bone and subsequent cartilage regeneration in an ovine model of osteochondral defect healing. Am. J. Sports Med. 2008, 36, 2379–2391. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, D. Cartilage tissue engineering using PHBV and PHBV/Bioglass scaffolds. Mol. Med. Rep. 2014, 10, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicate®—A multipurpose, highly bioactive glass-ceramic. In vitro, in vivo and clinical trials. J. Non-Cryst. Solids 2016, 432, 90–110. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, S.; Iijima, K.; Sasaki, K.; Hashizume, M.; Kawabe, M.; Otsuka, H. Cartilage Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells in Three-Dimensional Silica Nonwoven Fabrics. Appl. Sci. 2018, 8, 1398. https://doi.org/10.3390/app8081398
Ishikawa S, Iijima K, Sasaki K, Hashizume M, Kawabe M, Otsuka H. Cartilage Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells in Three-Dimensional Silica Nonwoven Fabrics. Applied Sciences. 2018; 8(8):1398. https://doi.org/10.3390/app8081398
Chicago/Turabian StyleIshikawa, Shohei, Kazutoshi Iijima, Kohei Sasaki, Mineo Hashizume, Masaaki Kawabe, and Hidenori Otsuka. 2018. "Cartilage Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells in Three-Dimensional Silica Nonwoven Fabrics" Applied Sciences 8, no. 8: 1398. https://doi.org/10.3390/app8081398
APA StyleIshikawa, S., Iijima, K., Sasaki, K., Hashizume, M., Kawabe, M., & Otsuka, H. (2018). Cartilage Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells in Three-Dimensional Silica Nonwoven Fabrics. Applied Sciences, 8(8), 1398. https://doi.org/10.3390/app8081398