Advances in Tuberculous Meningitis: Research, Challenges, and Future Perspectives
Abstract
1. Introduction
2. Pathogenic Mechanisms in Tuberculous Meningitis
2.1. Brief Insight into TBM Pathogenesis
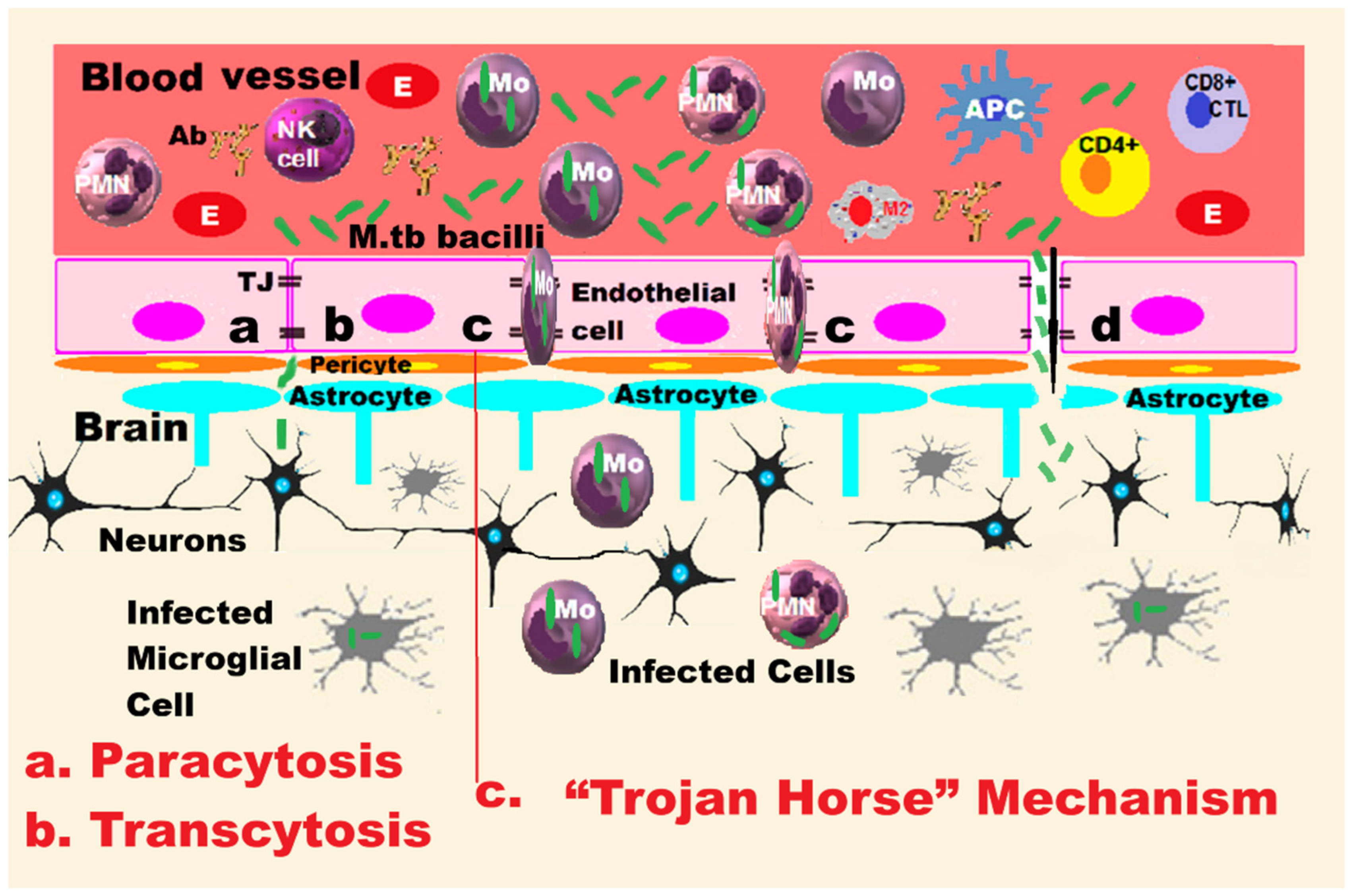
2.2. Hypotheses of Blood–Brain Barrier Penetration
2.2.1. The “Trojan Horse” Mechanism
2.2.2. Hypothesis of Transcellular Mechanisms
2.3. Immune System Response After BBB Penetration
2.4. Implications of Genetic Changes in Mycobacterium Tuberculosis
2.5. Anatomic-Pathological Changes in the Central Nervous System
3. Clinical Manifestations of Tuberculous Meningitis
- The initial phase of the disease is called prodromal and encompasses a period of 1–3 weeks from the BK infection, manifesting itself completely nonspecifically in both children and adults through a state similar to a viral disease or similar to that of other forms of chronic meningitis, with subfebrile moments or moderate fever, loss of appetite, a state of apathy, general discomfort, irritability or psychomotor agitation, insomnia, nausea, vomiting, photophobia, and then behavioral changes.
- The meningitic stage follows, expressed by headache in older children and adults, a persistent symptom that increases and becomes more severe, neck muscle contracture (meningismus), a state of confusion.
- The final, paralytic, stage is expressed by paresthesia, tremors, chorea, myoclonus, localized paralysis of the cranial nerves, convulsions, hemiparesis, stupor, and coma.
| Stages of the Evolving Disease | Children | Adults |
|---|---|---|
| Prodromal | Vomiting (morning) Loss of appetite/difficulty feeding agitation/apathy Headache (common in older children), behavioral/personality disorders, altered general condition, and fatigue weight loss/underweight Moderate fever | Vomiting Loss of appetite Agitation/apathy Persistent and progressive headache Behavioral/personality disorders Night sweats Nausea |
| Meningitic | Persistent, worsening headache Neck muscle contracture (rigidity), cranial nerve VI (abducens) palsy, causing diplopia and limited eye movement Seizures Focal neurological signs | Increasingly intense to severe headache Neck muscle contraction (rigidity), sometimes absent Cranial nerve palsy: III, IV and VI with vision and eye movement disorders Photophobia Symptoms of active infection: low-grade fever or fever, chronic cough and weight loss |
| Paralytic or advanced | Disturbance of consciousness: stupor or coma Motor deficits: hemiparesis or other focal neurological deficits Opisthotonus (decerebration or decortication attitude) Hydrocephalus with increased intracranial pressure causing: visual disturbances or blindness; hearing loss; movement disorders Complications: vasculitis, stroke, hearing loss, psychobehavioral dysfunction | Disturbance of consciousness: stupor or coma Motor deficits: hemiparesis or other focal neurological deficits Seizures: generalized or focal Increased intracranial pressure and hydrocephalus causing severe deterioration of neurological functions Complications: vasculitis, stroke, deafness, blindness, memory and concentration dysfunction |
| Symptoms specific to infants | Infants may have an atypical presentation with the absence of neck stiffness (sometimes), bulging of the anterior fontanelle, shrill crying, agitation, irritability, capricious appetite, opisthotonus contracture, regression in neuropsychic development (they may lose acquisitions they have already acquired). | |
| Atypical symptoms in older adults | Common manifestations: mental status disturbance and severe headache. Uncommon manifestations: vomiting and neck stiffness. Older adults may have more severe signs, rapid clinical deterioration and a high death rate. | |
4. Diagnosis of Tuberculous Meningitis
4.1. Clinical Data and Medical History
4.2. Cerebrospinal Fluid Study
4.3. Brain Imaging Data
4.4. Evidence of Tuberculosis Elsewhere
4.5. Molecular Diagnostic Tests for Tuberculous Meningitis
4.6. Nanotechnologies in Diagnosis of TBM
- Nanopore sequencing technologies, such as Oxford Nanopore Technology (ONT), a type of third-generation sequencing technology that uses a barrel-shaped protein called α-hemolysin (which occurs naturally as a “pore” in the cell membrane and allows the passage of a single strand of DNA), which is embedded in an artificial membrane inside the cell being sequenced and measures changes in electrical current, can rapidly detect M.tb resistance genes in nucleic acids, offering high sensitivity and specificity in a short time. ONT has some advantages over other technologies because it can generate ultralong reads (up to several million base pairs), thus facilitating easy sequencing of an entire genome. At the same time, the instrument is very small—from the size of a phone to a microwave oven—which gives it the advantage of being used anywhere desired by attaching it to a laptop [112,113,114].
- Nanoparticle-based assays are used in diagnostic tests, such as calorimetric assays, to rapidly detect M.tb in cultures, which could lead to faster diagnoses. NPs are valuable in the early stages of tuberculosis infection, when the number of mycobacteria may be low, and in excluding other infectious agents with similar symptoms. The calorimetric assay using mesoporous silica NPs incorporating gold on in vitro cultures of M.tb can be used for the rapid diagnosis of TB, as well as for its treatment. Although the results are promising, there are limitations and issues regarding the stability of the biosensors, biocompatibility, and long-term performance. These diagnostic techniques need to be validated in clinical use to ensure safety, efficacy, and compliance with current regulations. Further research is needed to improve sensor design, nanomaterial fabrication, and interpretation of the generated parameters [110,115,116].
- Discovery of new biomarkers is possible due to progress in the implementation of nanotechnologies that manage to analyze cellular and molecular changes in the CNS of patients with TBM.
- Detection of genetic mutations has been developed through a new technique using magnetic nanospheres labeled with streptavidin and biotin [117].
5. Treatment of Tuberculosis Meningitis
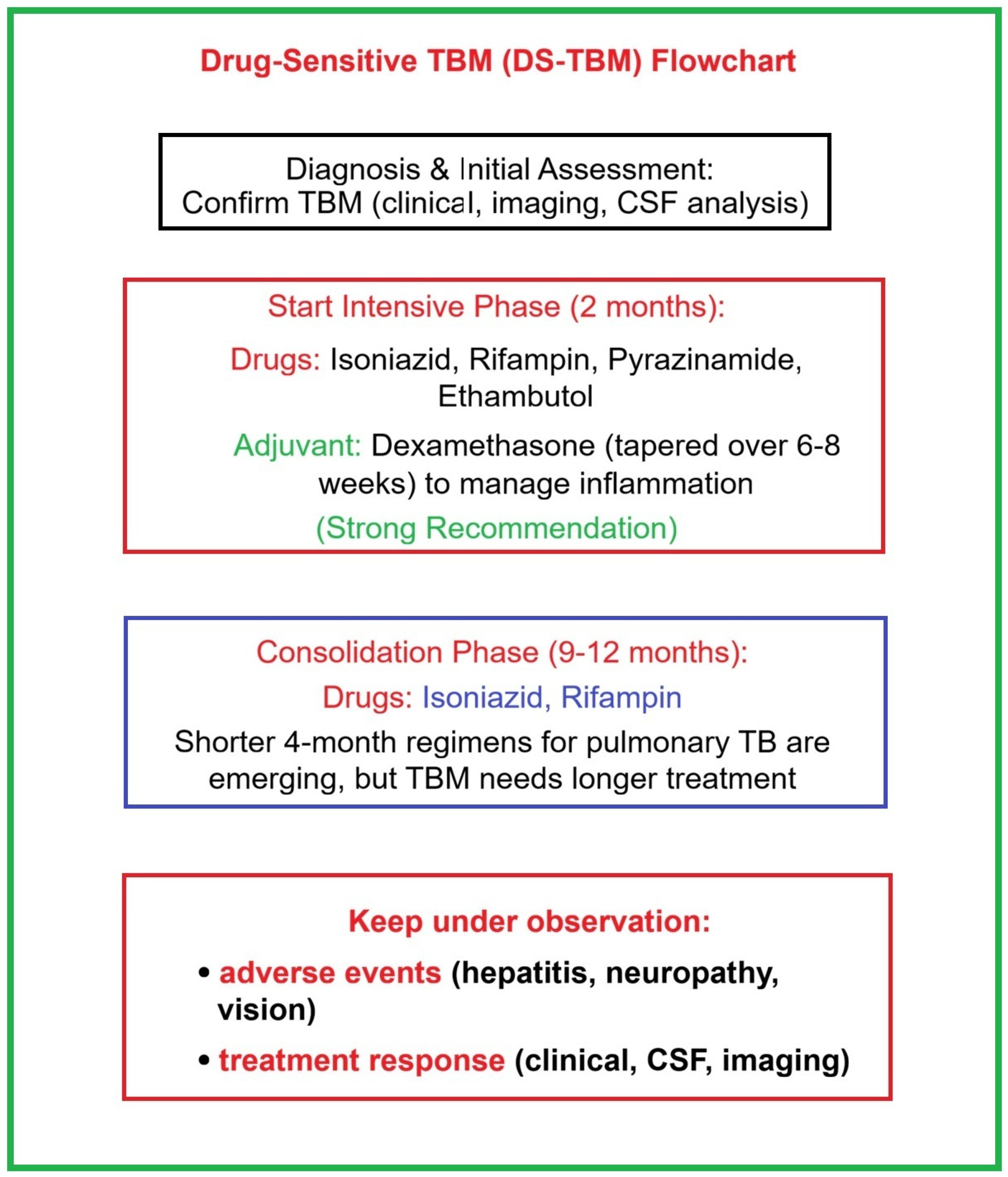
5.1. Standard Therapy Protocol for Drug-Sensitive TBM
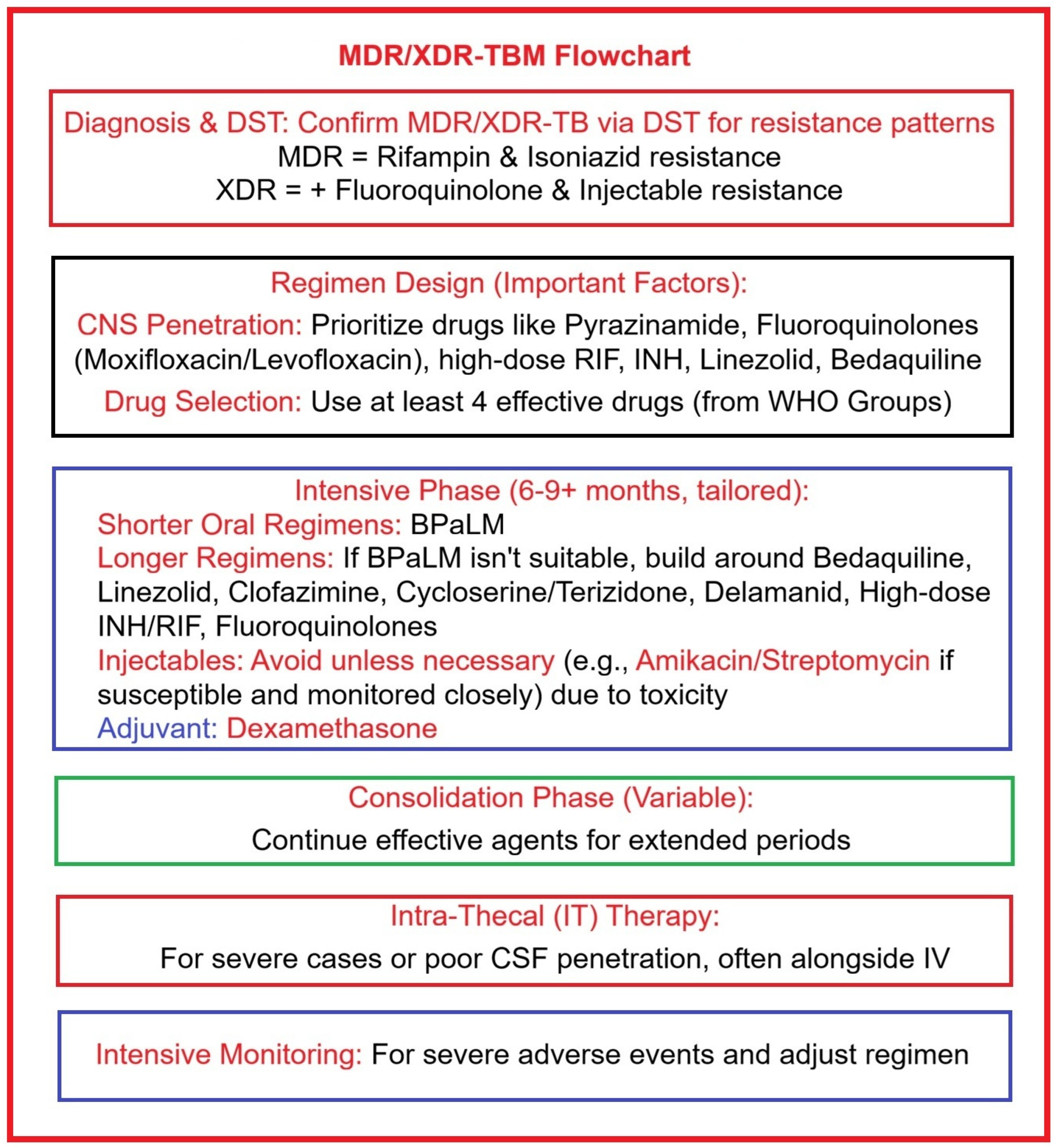
5.2. Treatment Strategies for Drug-Resistant TBM
5.3. Host-Directed Therapy
5.4. Nanotechnologies in Treatment and Prevention of TBM
- Improved drug delivery can be achieved through NPs that are capable of encapsulating antituberculosis drugs to enhance their stability, solubility, and ability to cross the BBB to reach the desired target and work without causing adverse reactions. Nanotechnology-based NPs are very easily modifiable and specific in their characteristics and structure, which guarantees their use for managing drugs in a particular way. Nanocarriers that are currently in a research-and-clinical-trial process for use in TB therapy include silica nanoparticles, chitosan, micelles, dendrimers, liposomes, metallic nanoparticles (AuNPs, AgNPs, magnetic nanoparticles—iron, nickel, cobalt and oxides, quantum dots, carbon-based nanomaterials—fullerene, carbon nanotubes, nanodiamonds, and graphene), and fluorescent nanodiamonds. New drug delivery technologies are focused on optimizing drug delivery to the brain while minimizing damage to non-targeted tissues. These include focused ultrasound-mediated BBB opening and microbubble technology that can temporarily and locally open the BBB, facilitating drug penetration. Magnetic targeting offers excellent potential because it uses magnetic NPs to direct drugs to specific areas. Receptor-mediated delivery, polymeric nanoparticle systems, gene therapy that can repair or replace diseased genes, cell-penetrating peptides, exosomes, stem cells, and smart nanoparticle systems are used for BBB penetration, slow release, and drug stability [118,160,161,162,163,164].
- Reduced toxicity and side effects can be limited by drug-loaded nanoparticles that are guided and delivered to the site of infection.
- With NPs designed to overcome the emergence of drug resistance through high loading concentration and delivery directly to the site of infection, nanoengineered solutions are revolutionizing the fight against extensively drug-resistant tuberculosis (XDR-TB), a major public health challenge. Nanoengineering allows the simultaneous administration of multiple drugs through a single NP, a high-tech solution to the multidrug regimen required for XDR-TB [165].
- Improving patient compliance through the development of intelligent drug delivery systems can allow for less frequent dosing and address the problems associated with poor patient compliance.
- Theranostic agents or “theranostics” are manufactured for simultaneous diagnostic and therapeutic action by combining imaging capabilities (such as MRI or PET scans) with the role of drug delivery. Simultaneous incorporation of diagnostic agents into NPs could implement a theranostic approach for simultaneous real-time monitoring of therapy and TBM progression. Although nanocarrier-based systems have proven high drug-loading capacities, high fixity, good tolerability of pharmaceutical products, decreased multidrug resistance, controlled release, and targeted administration with greater efficiency compared to conventional treatments, numerous studies are still needed, since currently physical and biological factors (pH, phagocytosis, various proteins, enzymes, renal clearance, etc.) are hostile factors for nanocarriers [166].
- Vaccine development can be achieved with the help of NPs used to generate new vaccine formulations to prevent TB infection. To better understand and improve knowledge about the immunology and pathogenesis of TBM, complementary studies on immune cell categories, specificity and reactions in CSF and peripheral blood of patients are needed. These data may provide information about new potential therapeutic targets for vaccines and the host. BCG (bacillus Calmette–Guérin) is a live-attenuated vaccine made from a weakened strain of Mycobacterium bovis, usually administered as a single injection typically under the skin of the upper arm during the neonatal period, that has proven its effectiveness against severe forms of BK infection and dissemination in the CNS in children, being the only vaccine with this capacity. However, BCG has low efficiency in preventing cavernous TB in adults, which justifies the need to expand research to discover a new effective vaccine against pulmonary TB. BCG vaccine has highly variable efficacy (0–80%) in different clinical trials conducted in adults due to the complexity of M.tb pathogenesis in the lungs and the interactions of the M.tb with the environment, which the vaccine is not optimized to combat. For an in-depth explanation, in tropical and subtropical regions, where there is a strong interference of M.tb with the environment, preexisting immunity induced by these common microorganisms may produce a cross-reaction, leading to altered, i.e., harmful, T-cell responses in the form of Treg cells, which will restrict the effective control of M.tb by the immune system later in life [167].
6. State-of-the-Art Clinical and Experimental Studies in TBM
6.1. Clinical Studies Applied in TBM
6.2. Experimental Studies Applied in TBM
7. Final Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADA | adenosine deaminase |
| AFB | acid-fast bacillus/acid-fast bacilli |
| Ag | silver |
| AI | artificial intelligence |
| AMT | absorption-mediated transcytosis |
| ANG-1 | angiopoietin 1 |
| ARDS | acute respiratory distress syndrome |
| ATT | antituberculosis therapy |
| Au | gold |
| BBB | blood–brain barrier |
| BCG | bacillus Calmette–Guérin |
| bFGF | basic fibroblast growth factor |
| BK | Koch’s bacillus |
| BPaLM | bedaquiline (B), pretomanid (Pa), linezolid (L), moxifloxacin (M) |
| CBF | cerebral blood flow |
| CBNAAT | cartridge-based nucleic acid amplification test |
| CCR5 or CD195 | CC chemokine receptor type 5 |
| CFU | colony-forming unit |
| CNS | central nervous system |
| CNS TB | central nervous system tuberculosis |
| CSF | cerebrospinal fluid |
| CT | computed tomography |
| CXCR-4 | C-X-C chemokine receptor type 4 |
| CXRs | chest X-rays |
| DCs | dendritic cells |
| DNA | deoxyribonucleic acid |
| DRTB | drug-resistant TB |
| DRTBM | drug-resistant TBM |
| DST | drug susceptibility test |
| DS-TBM | drug-sensitive TBM |
| E | ethambutol |
| epTB/EPTB | extrapulmonary TB |
| Eto | ethionamide |
| FDA | U.S. Food and Drug Administration |
| GCxGC-TOFMS | two-dimensional gas chromatography–time-of-flight mass spectrometry |
| GDNF | glial cell line-derived neurotrophic factor |
| 1H-NMR | proton magnetic resonance spectroscopy |
| H/INH | isoniazid |
| HDT | host-directed therapy |
| HIV | human immunodeficiency virus |
| HIV-1 | human immunodeficiency virus 1 |
| ICU | intensive care unit |
| IFN-γ | interferon gamma |
| IGRA | interferon gamma release assay |
| IL-1 | interleukin 1 |
| IL-6 | interleukin 6 |
| I-PCR | immuno-PCR |
| IT | information technology |
| IV | intravenous |
| IVF-ET | in vitro fertilization with embryo transfer |
| LAM | lipoarabinomannan |
| LAMP | loop-mediated isothermal amplification |
| LC | liquid chromatography |
| LECs | lymphatic endothelial cells |
| LRP1 | lipoprotein receptor-related protein 1 |
| LRP2 | lipoprotein receptor-related protein 2 |
| LTBI | latent tuberculosis infection |
| MALDI | matrix assisted laser desorption–ionization |
| MDR | multidrug-resistant |
| MDR/RR-TB | multidrug-resistant/rifampicin-resistant tuberculosis |
| MDR-TB | multidrug-resistant TB |
| MDR-TBM | multidrug-resistant TBM |
| MDR/XDR-TBM | multidrug-resistant TBM/extensively drug-resistant TBM |
| ML | machine learning |
| mNGS | metagenomic next-generation sequencing |
| M-PCR | multiplex polymerase chain reaction |
| MPTB | miliary pulmonary tuberculosis |
| MRI | magnetic resonance imaging |
| MS | mass spectrometry |
| MT | miliary tuberculosis |
| M.tb or MTB | Mycobacterium tuberculosis |
| MTBVAC | live-attenuated M.tb vaccine |
| MWFGs | multiple windows and feature granularities |
| NAAT | nucleic acid amplification test |
| NP | nanoparticle |
| NTM | non-tuberculous mycobacteria |
| NTS | nanopore-targeted sequencing |
| NVU | neurovascular unit |
| ONT | Oxford Nanopore Technology |
| PCR | polymerase chain reaction |
| PET–CT | positron-emission tomography–computed tomography |
| PLWH | people living with HIV |
| PPD | purified protein derivative |
| PTB | pulmonary tuberculosis |
| QFT-GIT | QuantiFERON-TB Gold In-Tube |
| QFT-Plus | QuantiFERON-TB Gold Plus |
| RD1 | region of difference 1 |
| R/RIF | rifampicin |
| RMT | receptor–ligand-mediated transcytosis |
| RNA | ribonucleic acid |
| ROC | receiver operator characteristic curve |
| ROS | reactive oxygen species |
| RR | rifampicin-resistant |
| RR-TB | rifampicin-resistant tuberculosis |
| SINAN | Brazilian Notifiable Diseases Information System |
| SSRIs | selective serotonin reuptake inhibitors |
| TB | tuberculosis |
| TBM | tuberculous meningitis |
| TJs | tight junctions |
| TNF-α | tumor necrosis factor alpha |
| tNGS | targeted next-generation sequencing |
| T-SPOT.TB | T-cell spot of tuberculosis assay |
| TST | tuberculin skin test |
| Ultra | Xpert MTB/RIF Ultra |
| VEGF | vascular endothelial growth factor |
| XDR-TB | extensively drug-resistant tuberculosis |
| XDR-TBM | extensively drug-resistant tuberculous meningitis |
| Xpert | Xpert MTB/RIF test for detection of M.tb |
| X-ray | radiography |
| Z | pyrazinamide |
| ZN | Ziehl–Neelsen |
| WHO | World Health Organization |
| Increased | ↑ |
| Decreased | ↓ |
| Present | + |
| Absent/missing | − |
References
- Jeong, Y.J.; Park, J.S.; Kim, H.W.; Min, J.; Ko, Y.; Oh, J.Y.; Lee, E.H.; Yang, B.; Lee, M.K.; Kim, Y.S.; et al. Deaths from tuberculosis: Differences between tuberculosis-related and non-tuberculosis-related deaths. Front. Public Health 2023, 11, 1207284. [Google Scholar] [CrossRef]
- Mousavi-Sagharchi, S.M.A.; Afrazeh, E.; Seyyedian-Nikjeh, S.F.; Meskini, M.; Doroud, D.; Siadat, S.D. New insight in molecular detection of Mycobacterium tuberculosis. AMB Express 2024, 14, 74. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024. Available online: https://www.who.int/publications/i/item/9789240101531 (accessed on 11 August 2025).
- Mello, F.C.Q.; Silva, D.R.; Dalcolmo, M.P. Tuberculosis: Where are we? J. Bras. Pneumol. 2018, 44, 82. [Google Scholar] [CrossRef] [PubMed]
- Ohene, S.A.; Bakker, M.I.; Ojo, J.; Toonstra, A.; Awudi, D.; Klatser, P. Extra-pulmonary tuberculosis: A retrospective study of patients in Accra, Ghana. PLoS ONE 2019, 14, e0209650. [Google Scholar] [CrossRef] [PubMed]
- Patrascu, R.E.; Cucu, A.I.; Costea, C.F.; Cosman, M.; Blaj, L.A.; Hristea, A. Brain Tuberculosis: An Odyssey through Time to Understand This Pathology. Pathogens 2023, 12, 1026. [Google Scholar] [CrossRef] [PubMed]
- Mousavi-Sagharchi, S.M.A.; Ghorbani, A.; Meskini, M.; Siadat, S.D. Historical examination of tuberculosis; from ancient affliction to modern challenges. J. Infect. Public Health 2025, 18, 102649. [Google Scholar] [CrossRef]
- Seddon, J.A.; Tugume, L.; Solomons, R.; Prasad, K.; Bahr, N.C.; Tuberculous Meningitis International Research Consortium. The current global situation for tuberculous meningitis: Epidemiology, diagnostics, treatment and outcomes. Wellcome Open Res. 2019, 4, 167. [Google Scholar] [CrossRef]
- Dodd, P.J.; Osman, M.; Cresswell, F.V.; Stadelman, A.M.; Lan, N.H.; Thuong, N.T.T.; Muzyamba, M.; Glaser, L.; Dlamini, S.S.; Seddon, J.A. The global burden of tuberculous meningitis in adults: A modelling study. PLoS Glob. Public Health 2021, 1, e0000069. [Google Scholar] [CrossRef]
- Ieque, A.L.; Palomo, C.T.; Castro Moreira, D.; Meneguello, J.E.; Murase, L.S.; Silva, L.L.; Baldin, V.P.; Caleffi-Ferracioli, K.R.; Dias Siqueira, V.L.; Cardoso, R.F.; et al. Systematic review of tuberculous meningitis in high-risk populations: Mortality and diagnostic disparities. Future Microbiol. 2025, 20, 559–571. [Google Scholar] [CrossRef]
- Oo, N.; Agrawal, D.K. Epidemiology, Pathogenesis, Clinical Manifestations, and Management Strategies of Tuberculous Meningitis. Arch. Intern. Med. Res. 2025, 8, 48–58. [Google Scholar] [CrossRef]
- Li, R.; Yin, R.; Li, Y.; Wei, Y.; Zhao, B.; Ge, C. Advances in the Treatment and Clinical Management Strategies of Tuberculous Meningitis. Int. J. Gen. Med. 2025, 18, 3267–3276. [Google Scholar] [CrossRef] [PubMed]
- Manyelo, C.M.; Solomons, R.S.; Walzl, G.; Chegou, N.N. Tuberculous Meningitis: Pathogenesis, Immune Responses, Diagnostic Challenges, and the Potential of Biomarker-Based Approaches. J. Clin. Microbiol. 2021, 59, e01771-20. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, N.D.P.; Laya, B.F.; Andronikou, S.; Daltro, P.A.N.; Sanchez, M.O.; Uy, J.A.U.; Lim, T.R.U. Standardized radiographic interpretation of thoracic tuberculosis in children. Pediatr. Radiol. 2017, 47, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, L.; Byard, R.W. An atlas overview of characteristic features of tuberculosis that may be encountered at autopsy. Forensic Sci. Med. Pathol. 2020, 16, 143–151. [Google Scholar] [CrossRef]
- Donald, P.R.; Diacon, A.H.; Thee, S. Anton Ghon and His Colleagues and Their Studies of the Primary Focus and Complex of Tuberculosis Infection and Their Relevance for the Twenty-First Century. Respiration 2021, 100, 557–567. [Google Scholar] [CrossRef]
- Malik, M.A.; Kamran, A.; Ahsan, D.; Amjad, A.; Moatter, S.; Noor, A.; Sohaib, A.; Shaukat, M.; Masood, W.; Hasanain, M.; et al. Advances in management and treatment of tubercular meningitis—A narrative review. Ann. Med. Surg. 2025, 87, 3673–3681. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef]
- Will, C.; Fromm, M.; Müller, D. Claudin tight junction proteins: Novel aspects in paracellular transport. Perit. Dial. Int. 2008, 28, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From Tight Junctions to Biological Systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57–58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Birkelund, S.; Burkhart, A.; Stensballe, A.; Moos, T. Synthesis and deposition of basement membrane proteins by primary brain capillary endothelial cells in a murine model of the blood-brain barrier. J. Neurochem. 2017, 140, 741–754. [Google Scholar] [CrossRef]
- Wright, S.A.; Lennon, R.; Greenhalgh, A.D. Basement membranes’ role in immune cell recruitment to the central nervous system. J. Inflamm. 2024, 21, 53. [Google Scholar] [CrossRef]
- Lacoste, B.; Prat, A.; Freitas-Andrade, M.; Gu, C. The Blood-Brain Barrier: Composition, Properties, and Roles in Brain Health. Cold Spring Harb. Perspect. Biol. 2025, 17, a041422. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2018, 4, 78–82. [Google Scholar] [CrossRef]
- Yan, L.; Moriarty, R.A.; Stroka, K.M. Recent progress and new challenges in modeling of human pluripotent stem cell-derived blood-brain barrier. Theranostics 2021, 11, 10148–10170. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.-M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef]
- Alves de Lima, K.; Rustenhoven, J.; Kipnis, J. Meningeal Immunity and Its Function in Maintenance of the Central Nervous System in Health and Disease. Annu. Rev. Immunol. 2020, 38, 597–620. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E. What Are the Roles of Pericytes in the Neurovascular Unit and Its Disorders? Neurology 2023, 100, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Evans, T. How Pathogens Penetrate the Blood-Brain Barrier. 17 April 2020. Available online: https://asm.org/articles/2020/april/how-pathogens-penetrate-the-blood-brain-barrier (accessed on 11 August 2025).
- Barnacle, J.R.; Davis, A.G.; Wilkinson, R.J. Recent advances in understanding the human host immune response in tuberculous meningitis. Front. Immunol. 2024, 14, 1326651. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Dong, Y.; Wang, H.; Liang, Z.; Yue, R.; Zhou, X. Trojan horse strategy and TfR/LDLR-Mediated transcytosis determine the dissemination of mycobacteria in tuberculous meningoencephalitis. Microbiol. Res. 2025, 297, 128172. [Google Scholar] [CrossRef]
- Strazielle, N.; Ghersi-Egea, J.F. Efflux transporters in blood-brain interfaces of the developing brain. Front. Neurosci. 2015, 9, 21. [Google Scholar] [CrossRef]
- van Leeuwen, L.M.; Boot, M.; Kuijl, C.; Picavet, D.I.; van Stempvoort, G.; van der Pol, S.M.A.; de Vries, H.E.; van der Wel, N.N.; van der Kuip, M.; van Furth, A.M.; et al. Mycobacteria employ two different mechanisms to cross the blood-brain barrier. Cell. Microbiol. 2018, 20, e12858. [Google Scholar] [CrossRef]
- Villaseñor, R.; Lampe, J.; Schwaninger, M.; Collin, L. Intracellular transport and regulation of transcytosis across the blood-brain barrier. Cell. Mol. Life Sci. 2019, 76, 1081–1092. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.Y.; Haqqani, A.S.; Leclerc, S.; Liu, Z.; Fauteux, F.; Baumann, E.; Delaney, C.E.; Ly, D.; Star, A.T.; et al. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS 2020, 17, 47. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G.; Chiran, D.A. Celiac Disease and Targeting the Molecular Mechanisms of Autoimmunity in COVID Pandemic. Int. J. Mol. Sci. 2022, 23, 7719. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Infection, Dysbiosis and Inflammation Interplay in the COVID Era in Children. Int. J. Mol. Sci. 2023, 24, 10874. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shi, C.; Li, X.; Matsui, T. Impact of Peptide Transport and Memory Function in the Brain. Nutrients 2024, 16, 2947. [Google Scholar] [CrossRef] [PubMed]
- Baghirov, H. Mechanisms of receptor-mediated transcytosis at the blood-brain barrier. J. Control. Release 2025, 381, 113595. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Kshirsagar, P.; Agrawal, P.; Murry, D.J. Crossing the Blood–Brain Barrier: Innovations in Receptor- and Transporter-Mediated Transcytosis Strategies. Pharmaceutics 2025, 17, 706. [Google Scholar] [CrossRef]
- Lerner, T.R.; de Souza Carvalho-Wodarz, C.; Repnik, U.; Russell, M.R.; Borel, S.; Diedrich, C.R.; Rohde, M.; Wainwright, H.; Collinson, L.M.; Wilkinson, R.J.; et al. Lymphatic endothelial cells are a replicative niche for Mycobacterium tuberculosis. J. Clin. Investig. 2016, 126, 1093–1108. [Google Scholar] [CrossRef]
- Davis, A.G.; Rohlwink, U.K.; Proust, A.; Figaji, A.A.; Wilkinson, R.J. The pathogenesis of tuberculous meningitis. J. Leukoc. Biol. 2019, 105, 267–280. [Google Scholar] [CrossRef]
- Zaharie, S.D.; Franken, D.J.; van der Kuip, M.; van Elsland, S.; de Bakker, B.S.; Hagoort, J.; Roest, S.L.; van Dam, C.S.; Timmers, C.; Solomons, R.; et al. The immunological architecture of granulomatous inflammation in central nervous system tuberculosis. Tuberculosis 2020, 125, 102016. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, J.; Kong, X.; Zeng, Y.; Chen, Z.; Liu, H.; Liu, L.; Lu, S.; Wang, X. Interactions between CNS and immune cells in tuberculous meningitis. Front. Immunol. 2024, 15, 1326859. [Google Scholar] [CrossRef]
- Majambere, J.C.; Zaidi, S.; Errami, A.; Marih, L.; Marhoum El Filali, K.; Bousfiha, A.A.; Lahsen, A.O. Tuberculous Meningitis Genetic predisposition: Understanding cellular interactions, molecular mechanisms and genetic dimensions. Tunis. Med. 2024, 102, 440–446. [Google Scholar] [CrossRef]
- Gemici Karaaslan, B.; Rosain, J.; Bustamante, J.; Kıykım, A. Interferon Gamma in Sickness Predisposing to Mycobacterial Infectious Diseases. Balk. Med. J. 2024, 41, 326–332. [Google Scholar] [CrossRef]
- Donald, P.R.; Schaaf, H.S.; Schoeman, J.F. Tuberculous meningitis and miliary tuberculosis: The Rich focus revisited. J. Infect. 2005, 50, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.M. Central Nervous System Tuberculosis. Microbiol. Spectr. 2017, 5, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, H.; Chen, D.; Zhang, H.; Liao, Y. Autophagy in mycobacterial infections: Molecular mechanisms, host-pathogen interactions, and therapeutic opportunities. Front. Cell. Infect. Microbiol. 2025, 15, 1640647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Solomon, R.; Gautam, P.; Inbaraj, L.R.; Sivadasan, A.; Michael, J.S.; Karthik, R.; Varghese, G.M.; Christudass, C.S.; Samuel, P.; et al. Leukotriene A4 hydrolase (LTA4H rs17525495) gene polymorphisms and paradoxical reactions in extrapulmonary tuberculosis. Sci. Rep. 2023, 13, 3746. [Google Scholar] [CrossRef]
- Méchaï, F.; Bouchaud, O. Tuberculous meningitis: Challenges in diagnosis and management. Rev. Neurol. 2019, 175, 451–457. [Google Scholar] [CrossRef]
- Daniel, B.D.; Grace, G.A.; Natrajan, M. Tuberculous meningitis in children: Clinical management & outcome. J. Med. Res. 2019, 150, 117–130. [Google Scholar] [CrossRef]
- van Toorn, R.; Solomons, R. Diagnosis and Management of Tuberculous Meningitis in Children- an Update. Semin. Pediatr. Neurol. 2023, 47, 101071. [Google Scholar] [CrossRef]
- Garg, R.K. Updates in the clinical management of tuberculous meningitis. Expert Rev. Anti Infect. Ther. 2025, ahead of print, 1–18. [Google Scholar] [CrossRef]
- Slane, V.H.; Unakal, C.G. Tuberculous Meningitis. In StatPearls [Internet]; [Updated 2 September 2024]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541015/ (accessed on 24 November 2025).
- Hammami, F.; Koubaa, M.; Chakroun, A.; Rekik, K.; Feki, W.; Marrakchi, C.; Smaoui, F.; Jemaa, M.B. Comparative analysis between tuberculous meningitis and other forms of extrapulmonary tuberculosis. Germs 2021, 11, 23–31. [Google Scholar] [CrossRef]
- Ssebambulidde, K.; Gakuru, J.; Ellis, J.; Cresswell, F.V.; Bahr, N.C. Improving Technology to Diagnose Tuberculous Meningitis: Are We There Yet? Front. Neurol. 2022, 13, 892224. [Google Scholar] [CrossRef]
- Boyles, T.; Stadelman, A.; Ellis, J.P.; Cresswell, F.V.; Lutje, V.; Wasserman, S.; Tiffin, N.; Wilkinson, R. The diagnosis of tuberculous meningitis in adults and adolescents: Protocol for a systematic review and individual patient data meta-analysis to inform a multivariable prediction model. Wellcome Open Res. 2021, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Marais, S.; Thwaites, G.; Schoeman, J.F.; Török, M.E.; Misra, U.K.; Prasad, K.; Donald, P.R.; Wilkinson, R.J.; Marais, B.J. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect. Dis. 2010, 10, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Li, M.; Xu, T.; Yu, X.; Wang, L.; Li, K. Clinical features, outcomes and prognostic factors of tuberculous meningitis in adults worldwide: Systematic review and meta-analysis. J. Neurol. 2019, 266, 3009–3021. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Ma, Y.; Ji, X.; Jiang, H.; Liu, F.; Chu, N.; Li, Q. A study of risk factors for tuberculous meningitis among patients with tuberculosis in China: An analysis of data between 2012 and 2019. Front. Public Health 2023, 10, 1040071. [Google Scholar] [CrossRef]
- Abubakar, I.; Lalvani, A.; Southern, J.; Sitch, A.; Jackson, C.; Onyimadu, O.; Lipman, M.; Deeks, J.J.; Griffiths, C.; Bothamley, G.; et al. Two interferon gamma release assays for predicting active tuberculosis: The UK PREDICT TB prognostic test study. Health Technol. Assess. 2018, 22, 1–96. [Google Scholar] [CrossRef]
- Surve, S.; Bhor, V.; Naukariya, K.; Begum, S.; Munne, K.; Tipre, P.; Sutar, N.; Jaiswal, A.; Bhonde, G.; Chauhan, S.; et al. Discordance between TST and QFT-TBGold Plus for Latent Tuberculosis Screening among Under-Five Children: An Interim Analysis. J. Trop. Pediatr. 2021, 67, fmab103. [Google Scholar] [CrossRef]
- Buonsenso, D.; Noguera-Julian, A.; Moroni, R.; Hernández-Bartolomé, A.; Fritschi, N.; Lancella, L.; Cursi, L.; Soler-Garcia, A.; Krüger, R.; Feiterna-Sperling, C.; et al. Performance of QuantiFERON-TB Gold Plus assays in paediatric tuberculosis: A multicentre PTBNET study. Thorax 2023, 78, 288–296. [Google Scholar] [CrossRef]
- Diel, R.; Breuer, C.; Bös, L.; Geerdes-Fenge, H.; Günther, A.; Häcker, B.; Hannemann, J.; Nienhaus, A.; Priwitzer, M.; Witte, P.; et al. Empfehlungen für die Umgebungsuntersuchungen bei Tuberkulose—Update 2023 [Recommendations for contact tracing for tuberculosis—Update 2023]. Pneumologie 2023, 77, 607–631. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Shi, W.; Shi, W.; Hu, M.; Kong, D.; Long, R.; He, J.; Chen, N. Comparing the diagnostic performance of QuantiFERON-TB Gold Plus with QFT-GIT, T-SPOT.TB and TST: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 40. [Google Scholar] [CrossRef]
- Siddiqi, O.K.; Birbeck, G.L.; Ghebremichael, M.; Mubanga, E.; Love, S.; Buback, C.; Kosloff, B.; Ayles, H.; Atadzhanov, M.; Dheda, K.; et al. Prospective Cohort Study on Performance of Cerebrospinal Fluid (CSF) Xpert MTB/RIF, CSF Lipoarabinomannan (LAM) Lateral Flow Assay (LFA), and Urine LAM LFA for Diagnosis of Tuberculous Meningitis in Zambia. J. Clin. Microbiol. 2019, 57, e00652-19. [Google Scholar] [CrossRef]
- Hou, J.; Liu, X.J.; He, Y.; Zhang, Y.A.; Wang, M.S. Cerebrospinal fluid findings of infant tuberculous meningitis: A scoping review. Ann. Med. 2022, 54, 2516–2520. [Google Scholar] [CrossRef]
- Nuwagira, E.; Huppler Hullsiek, K.; Jjunju, S.; Rutakingirwa, M.; Kasibante, J.; Tadeo, K.K.; Kagimu, E.; Tugume, L.; Ssebambulidde, K.; Musubire, A.K.; et al. Diagnostic and Prognostic Value of Cerebrospinal Fluid Lactate and Glucose in HIV-Associated Tuberculosis Meningitis. Microbiol. Spectr. 2022, 10, e0161822. [Google Scholar] [CrossRef] [PubMed]
- Nardel, A.E.; Muzny, A.C. Extrapulmonary Tuberculosis (TB). 2025. Available online: https://www.msdmanuals.com/professional/infectious-diseases/mycobacteria/extrapulmonary-tuberculosis-tb (accessed on 5 November 2025).
- Zaghba, N.; El Hachimi, K.; Benjelloun, H.; Yassine, N. La miliaire tuberculeuse, une série rétrospective marocaine [Miliary tuberculosis]. Rev. Pneumol. Clin. 2018, 74, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yang, Q.; Zhou, X.; Lv, K.; Zhou, Z.; Sun, F.; Ruan, Q.; Zhang, J.; Shao, L.; Geng, D. Cerebral Infarction and Evan’s Ratio on MRI Affect the Severity and Prognosis of Tuberculosis Meningitis Patients. Diagnostics 2022, 12, 1264. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, F.; Liang, C.; He, G.; Chen, H.; Wu, Y.; Chen, Y.; Shuai, J.; Yang, Y.; Dai, C.; et al. MRI advances in the imaging diagnosis of tuberculous meningitis: Opportunities and innovations. Front. Microbiol. 2023, 14, 1308149. [Google Scholar] [CrossRef]
- Milburn, J.; Williams, C.G.; Lechiile, K.; Siamisang, K.; Owen, L.; Gwakuba, E.; Milton, T.; Machiya, T.; Leeme, T.; Barton, H.E.; et al. Computed Tomography of the Head Before Lumbar Puncture in Adults With Suspected Meningitis in High-HIV Prevalence Settings. Open Forum Infect. Dis. 2024, 11, ofae565. [Google Scholar] [CrossRef]
- Vohra, S.; Dhaliwal, H.S. Miliary Tuberculosis. In StatPearls [Internet]; [Updated 30 January 2024]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562300/ (accessed on 11 November 2025).
- van den Bos, F.; Terken, M.; Ypma, L.; Kimpen, J.L.; Nel, E.D.; Schaaf, H.S.; Schoeman, J.F.; Donald, P.R. Tuberculous meningitis and miliary tuberculosis in young children. Trop. Med. Int. Health 2004, 9, 309–313. [Google Scholar] [CrossRef]
- Kumar, V.; Dalton, T.L.; Armstrong, L.R.; Whitesell, A.; Li, R.; Starks, A.M. Use of Nucleic Acid Amplification Testing for Rapid Detection of Mycobacterium tuberculosis Complex Among US Tuberculosis Patients, 2011–2017. Open Forum Infect. Dis. 2021, 8, ofab528. [Google Scholar] [CrossRef]
- Gaudemer, A.; Covier, N.; Henry-Feugeas, M.C.; Timsit, J.F.; Lavallée, P.C.; de Montmollin, E.; Lecler, A.; Khalil, A.; Sonneville, R.; Couffignal, C. Neuroimaging for prognosis of central nervous system infections: A systematic review and meta-analysis. Ann. Intensive Care 2025, 15, 101. [Google Scholar] [CrossRef]
- Mehta, P.K.; Dahiya, B.; Sharma, S.; Singh, N.; Dharra, R.; Thakur, Z.; Mehta, N.; Gupta, K.B.; Gupta, M.C.; Chaudhary, D. Immuno-PCR, a new technique for the serodiagnosis of tuberculosis. J. Microbiol. Methods 2017, 139, 218–229. [Google Scholar] [CrossRef]
- Carranza, C.; Pedraza-Sanchez, S.; de Oyarzabal-Mendez, E.; Torres, M. Diagnosis for Latent Tuberculosis Infection: New Alternatives. Front. Immunol. 2020, 11, 2006. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Singh, R.; Sharma, S.; Singh, V.; Sheoran, A.; Soni, A.; Dhull, V.; Gill, P.S.; Yadav, A.; Chaudhary, D.; et al. Diagnosis of osteoarticular tuberculosis by immuno-PCR assay based on mycobacterial antigen 85 complex detection. Lett. Appl. Microbiol. 2022, 74, 17–26. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, S.M.; Kussen, G.M.B.; Cogo, L.; Carvalho, J.H.; Nogueira, K. Diagnostic characteristics of Xpert MTB/RIF assay for the diagnosis of tuberculous meningitis and rifampicin resistance in Southern Brazil. Arq. Neuropsiquiatr. 2020, 78, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Sharma, M.; Shree, R.; Modi, M.; Goyal, M.; Narang, D.; Sharma, A.; Lal, V.; Sharma, N.; Ray, P. Xpert MTB/RIF ultra for the diagnosis of tuberculous meningitis: A diagnostic accuracy study from India. Tuberculosis 2020, 125, 101990. [Google Scholar] [CrossRef]
- Hernandez, A.V.; de Laurentis, L.; Souza, I.; Pessanha, M.; Thota, P.; Roman, Y.M.; Barboza-Meca, J.; Boulware, D.R.; Vidal, J.E. Diagnostic accuracy of Xpert MTB/RIF for tuberculous meningitis: Systematic review and meta-analysis. Trop. Med. Int. Health 2021, 26, 122–132. [Google Scholar] [CrossRef]
- Huang, M.; Wang, G.; Sun, Q.; Jiang, G.; Li, W.; Ding, Z.; Jia, H.; Gao, M.; Huang, H.; Li, Q. Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in a clinical practice setting of China. Diagn. Microbiol. Infect. Dis. 2021, 100, 115306. [Google Scholar] [CrossRef]
- Dahiya, B.; Mehta, N.; Soni, A.; Mehta, P.K. Diagnosis of extrapulmonary tuberculosis by GeneXpert MTB/RIF Ultra assay. Expert Rev. Mol. Diagn. 2023, 23, 561–582. [Google Scholar] [CrossRef]
- Khan, A.; Khan, N.; Singh, R. Tuberculosis diagnosis versus GeneXpert®MTB/RIF formats. Bioanalysis 2024, 16, 843–848. [Google Scholar] [CrossRef]
- Wang, C.; Forsman, L.D.; Wang, S.; Wang, S.; Shao, G.; Xiong, H.; Bao, Z.; Hu, Y. The diagnostic performance of GeneXpert MTB/RIF in tuberculosis meningitis: A multicentre accuracy study. Diagn. Microbiol. Infect. Dis. 2024, 109, 116277. [Google Scholar] [CrossRef]
- Mehta, P.K.; Sebastian, J. Diagnosis of extrapulmonary tuberculosis by Truenat® MTB/MTB Plus assay. Tuberculosis 2025, 155, 102688. [Google Scholar] [CrossRef]
- Lin, F. Tuberculous meningitis diagnosis and treatment: Classic approaches and high-throughput pathways. Front. Immunol. 2025, 15, 1543009. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, W.; Guo, Y.; Zeng, Y. Nanopore-based targeted next-generation sequencing (tNGS): A versatile technology specialized in detecting low bacterial load clinical specimens. PLoS ONE 2025, 20, e0324003. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, E.L.; Jaszczyszyn, Y.; Naquin, D.; Thermes, C. The Third Revolution in Sequencing Technology. Trends Genet. 2018, 34, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Blessing, F.; Fimpler, L.; Wenzel, F. Nanopore Sequencing in a Clinical Routine Laboratory: Challenges and Opportunities. Clin. Lab. 2020, 66, 1097. [Google Scholar] [CrossRef]
- Wei, N.; Du, F.; Nie, W.; Nong, Y.; Lin, Y.; Huang, A.; Xi, S.; Lan, Y.; Luo, X.; Yang, S.; et al. An exploratory study on the application of nanopore sequencing for detecting Mycobacterium tuberculosis drug resistance in respiratory specimens. BMC Pulm. Med. 2025, 25, 279. [Google Scholar] [CrossRef]
- Kurien, R.; Sudarsanam, T.D.; Samantha, S.; Thomas, K. Tuberculous meningitis: A comparison of scoring systems for diagnosis. Oman Med. J. 2013, 28, 163–166. [Google Scholar] [CrossRef]
- Shah, M.I.; Mishra, S.; Yadav, V.K.; Chauhan, A.; Sarkar, M.; Sharma, S.K.; Rout, C. Ziehl-Neelsen sputum smear microscopy image database: A resource to facilitate automated bacilli detection for tuberculosis diagnosis. J. Med. Imaging 2017, 4, 027503. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Xie, B.D. Progress on Diagnosis of Tuberculous Meningitis. Methods Mol. Biol. 2018, 1754, 375–386. [Google Scholar] [CrossRef]
- Pathrikar, G.T.; Bansal, P.V.; Mulay, V.M.; Ghogare, S.H. Comparison of Ziehl-Neelsen Smear Microscopy and AFB Culture in a Resource Limited Setting from Various Clinical Samples. Int. J. Health Sci. Res. 2020, 10, 46–51. Available online: https://www.ijhsr.org/IJHSR_Vol.10_Issue.4_April2020/7.pdf (accessed on 11 November 2025).
- Sulaiman, T.; Medi, S.; Erdem, H.; Senbayrak, S.; Ozturk-Engin, D.; Inan, A.; Civljak, R.; Nechifor, M.; Akbulut, A.; Crisan, A.; et al. The diagnostic utility of the “Thwaites’ system” and “lancet consensus scoring system” in tuberculous vs. non-tuberculous subacute and chronic meningitis: Multicenter analysis of 395 adult patients. BMC Infect. Dis. 2020, 20, 788. [Google Scholar] [CrossRef]
- Karabela, Ş.N.; Ünlü, G.; Şenoğlu, S.; Canbolat Ünlü, E.; Korkusuz, R.; Kart Yaşar, K. The Roles of Thwaites’ and Marais’ Diagnostic Scoring Indexes and a Clinical Prediction Model in the Diagnosis of Tuberculous Meningitis. Med. J. Bakirkoy. 2021, 17, 221–226. [Google Scholar] [CrossRef]
- Luo, Y.; Xue, Y.; Lin, Q.; Mao, L.; Tang, G.; Song, H.; Liu, W.; Wu, S.; Liu, W.; Zhou, Y.; et al. Diagnostic Model for Discrimination Between Tuberculous Meningitis and Bacterial Meningitis. Front. Immunol. 2021, 12, 731876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cao, M.; Shao, N.; Qin, Y.; Liu, L.; Zhang, Q.; Yang, X. Development and validation of a new model for the early diagnosis of tuberculous meningitis in adults based on simple clinical and laboratory parameters. BMC Infect. Dis. 2023, 23, 901. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, N.S.; Bijlsma, M.W.; van Zeggeren, I.E.; Staal, S.L.; Tanck, M.W.T.; van de Beek, D.; Brouwer, M.C. Diagnostic prediction models for bacterial meningitis in children with a suspected central nervous system infection: A systematic review and prospective validation study. BMJ Open 2024, 14, e081172. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, H.; Chen, L. Recent advances in nanomaterials for the detection of Mycobacterium tuberculosis (Review). Int. J. Mol. Med. 2025, 55, 36. [Google Scholar] [CrossRef]
- Metcalf, T.; Soria, J.; Montano, S.M.; Ticona, E.; Evans, C.A.; Huaroto, L.; Kasper, M.; Ramos, E.S.; Mori, N.; Jittamala, P.; et al. Evaluation of the GeneXpert MTB/RIF in patients with presumptive tuberculous meningitis. PLoS ONE 2018, 13, e0198695. [Google Scholar] [CrossRef]
- Deserranno, K.; Tilleman, L.; Rubben, K.; Deforce, D.; Van Nieuwerburgh, F. Targeted haplotyping in pharmacogenomics using Oxford Nanopore Technologies’ adaptive sampling. Front. Pharmacol. 2023, 14, 1286764. [Google Scholar] [CrossRef]
- Deserranno, K.; Tilleman, L.; Deforce, D.; Van Nieuwerburgh, F. Comparative evaluation of Oxford Nanopore Technologies’ adaptive sampling and the Twist long-read PGx panel for pharmacogenomic profiling. Front. Pharmacol. 2025, 16, 1653999. [Google Scholar] [CrossRef]
- YG Your Genome. What is Oxford Nanopore Technology (ONT) Sequencing? Explore Genomics. Methods and Technology. Available online: https://www.yourgenome.org/theme/what-is-oxford-nanopore-technology-ont-sequencing/ (accessed on 11 November 2025).
- Kumar, S.; Wang, Z.; Zhang, W.; Liu, X.; Li, M.; Li, G.; Zhang, B.; Singh, R. Optically Active Nanomaterials and Its Biosensing Applications—A Review. Biosensors 2023, 13, 85. [Google Scholar] [CrossRef]
- Parveen, A.; Chatterjee, A.; Karak, P. Biomedical Applications of Carbon-Based Nanomaterials: Exploring Recent Advances in Therapeutics, Diagnostics, and Tissue Engineering. Adv. Pharm. Bull. 2025, 15, 232–247. [Google Scholar] [CrossRef]
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnol. 2020, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Liu, X.; Li, W.; Fu, H.; Liu, X.; Zhang, X.; Zhou, X.; Yang, B.; Yao, J.; et al. Comparative diagnostic utility of metagenomic next-generation sequencing, GeneXpert, modified Ziehl-Neelsen staining, and culture using cerebrospinal fluid for tuberculous meningitis: A multi-center, retrospective study in China. J. Clin. Lab. Anal. 2022, 36, e24307. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Xiong, Y.; Wang, Y.; Yu, Q.; Ma, Y.; Xie, Y. Comparative performance of microbiological methods for the detection of tuberculous meningitis pathogens in cerebrospinal fluid. Diagn. Microbiol. Infect. Dis. 2023, 107, 116025. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Hong, J.C.; Jiang, Z.J.; Zhang, W.Q.; Fan, Q.C.; Yao, X.P. Performance of metagenomic next-generation sequencing in cerebrospinal fluid for diagnosis of tuberculous meningitis. J. Med. Microbiol. 2024, 73, 001818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dai, X.; Hu, P.; Tian, L.; Li, C.; Ding, B.; Yang, X.; He, X. Comparison of targeted next-generation sequencing and the Xpert MTB/RIF assay for detection of Mycobacterium tuberculosis in clinical isolates and sputum specimens. Microbiol. Spectr. 2024, 12, e0409823. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A. Metagenomic Next-Generation Sequencing in Infectious Diseases: Clinical Applications, Translational Challenges, and Future Directions. Diagnostics 2025, 15, 1991. [Google Scholar] [CrossRef]
- Lu, H.J.; Guo, D.; Wei, Q.Q. Potential of Neuroinflammation-Modulating Strategies in Tuberculous Meningitis: Targeting Microglia. Aging Dis. 2024, 15, 1255–1276. [Google Scholar] [CrossRef]
- Inbaraj, L.R.; Manesh, A.; Ponnuraja, C.; Bhaskar, A.; Srinivasalu, V.A.; Daniel, B.D. Comparative evaluation of intensified short course regimen and standard regimen for adults TB meningitis: A protocol for an open label, multi-center, parallel arms, randomized controlled superiority trial (INSHORT trial). Trials 2024, 25, 294. [Google Scholar] [CrossRef]
- WHO. WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment and Care. Guideline. 15 April 2025. Available online: https://www.who.int/publications/i/item/9789240107243 (accessed on 4 November 2025).
- WHO. WHO Operational Handbook on Tuberculosis. Module 5: Management of Tuberculosis in Children and Adolescents; World Health Organization: Geneva, Switzerland, 2022; Available online: https://tbksp.who.int/en/node/1981 (accessed on 4 November 2025).
- Global Programme on Tuberculosis & Lung Health. 2025 WHO. 5.2.6.1. Treatment of TB Meningitis and Osteoarticular TB. Available online: https://tbksp.who.int/en/node/2141 (accessed on 4 November 2025).
- Updated Guideline on the Treatment of Drug-Susceptible and Drug-Resistant TB (ATA/CDC/ERS/IDSA, 2025). American Thoracic Society, US Centers for Disease Control and Prevention, European Respiratory Society, and Infectious Diseases Society of America. 10 March 2025. Available online: https://reference.medscape.com/viewarticle/updated-guideline-treatment-drug-susceptible-and-drug-2025a10004yx?&icd=login_success_email_match_fpf (accessed on 4 November 2025).
- Huynh, J.; Chabala, C.; Sharma, S.; Choo, L.; Singh, V.; Sankhyan, N.; Mujuru, H.; Nguyen, N.; Trinh, T.H.; Phan, P.H.; et al. Effectiveness and safety of shortened intensive treatment for children with tuberculous meningitis (SURE): A protocol for a phase 3 randomised controlled trial evaluating 6 months of antituberculosis therapy and 8 weeks of aspirin in Asian and African children with tuberculous meningitis. BMJ Open 2025, 15, e088543. [Google Scholar] [CrossRef]
- Wang, G.; Wang, S.; Jiang, G.; Yang, X.; Huang, M.; Huo, F.; Ma, Y.; Dai, G.; Li, W.; Chen, X.; et al. Xpert MTB/RIF Ultra improved the diagnosis of paucibacillary tuberculosis: A prospective cohort study. J. Infect. 2019, 78, 311–316. [Google Scholar] [CrossRef]
- WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment. Guideline. 20 March 2019. Available online: https://www.who.int/publications/i/item/9789241550529 (accessed on 9 November 2025).
- Kohli, M.; Schiller, I.; Dendukuri, N.; Yao, M.; Dheda, K.; Denkinger, C.M.; Schumacher, S.G.; Steingart, K.R. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 2021, 1, CD012768. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.E.; Avaliani, T.; Gujabidze, M.; Bakuradze, T.; Kipiani, M.; Sabanadze, S.; Smith, A.G.C.; Avaliani, Z.; Collins, J.M.; Kempker, R.R. Long term outcomes of patients with tuberculous meningitis: The impact of drug resistance. PLoS ONE 2022, 17, e0270201. [Google Scholar] [CrossRef] [PubMed]
- WHO. Module 4: Treatment-Drug-Resistant Tuberculosis Treatment. In WHO Consolidated Guidelines on Tuberculosis; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.aidsdatahub.org/sites/default/files/resource/who-consolidated-guidelines-tuberculosis-module-4-treatment-2020.pdf (accessed on 9 November 2025).
- Paradkar, M.S.; Devaleenal, D.B.; Mvalo, T.; Arenivas, A.; Thakur, K.T.; Wolf, L.; Nimkar, S.; Inamdar, S.; Giridharan, P.; Selladurai, E.; et al. Randomized Clinical Trial of High-Dose Rifampicin With or Without Levofloxacin Versus Standard of Care for Pediatric Tuberculous Meningitis: The TBM-KIDS Trial. Clin. Infect. Dis. 2022, 75, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Zhang, X.; Li, Q.; Fan, F.; Qin, M.; Lin, F. Cerebrospinal Fluid Parameters Predicting Contralateral Isolated Lateral Ventricle in Adult Tuberculous Meningitis with Hydrocephalus Post-Ventriculoperitoneal Shunt. World Neurosurg. 2024, 189, e204–e210. [Google Scholar] [CrossRef]
- Wasserman, S.; Davis, A.; Wilkinson, R.J.; Meintjes, G. Key considerations in the pharmacotherapy of tuberculous meningitis. Expert Opin. Pharmacother. 2019, 20, 1791–1795. [Google Scholar] [CrossRef]
- Liu, X.; Tang, D.; Qi, M.; He, J. Efficacy of linezolid in the treatment of tuberculous meningitis: A meta-analysis. Arch. Med. Sci. 2024, 20, 1038–1042. [Google Scholar] [CrossRef]
- Sahib, A.; Bhatia, R.; Srivastava, M.V.P.; Singh, M.B.; Komakula, S.; Vishnu, V.Y.; Rajan, R.; Gupta, A.; Srivastava, A.K.; Wig, N.; et al. Escalate: Linezolid as an add on treatment in the intensive phase of tubercular meningitis. A randomized controlled pilot trial. Tuberculosis 2023, 142, 102351. [Google Scholar] [CrossRef]
- Fei, Z.T.; Huang, W.; Zhou, D.P.; Yang, Y.; Liu, P.; Gan, N.; He, P.P.; Ye, D.; Liu, H.R.; Liu, X.H.; et al. Clinical efficacy of linezolid in the treatment of tuberculous meningitis: A retrospective analysis and literature review. BMC Infect. Dis. 2025, 25, 467. [Google Scholar] [CrossRef]
- Kempker, R.R.; Smith, A.G.; Avaliani, T.; Gujabidze, M.; Bakuradze, T.; Sabanadze, S.; Avaliani, Z.; Collins, J.M.; Blumberg, H.M.; Alshaer, M.H.; et al. Cycloserine and linezolid for tuberculosis meningitis: Pharmacokinetic evidence of potential usefulness. Clin. Infect. Dis. 2022, 75, 682–689. [Google Scholar] [CrossRef]
- Drugs.com. Know More. Be Sure. Ethionamide Side Effects. Available online: https://www.drugs.com/sfx/ethionamide-side-effects.html (accessed on 9 November 2025).
- Cresswell, F.V.; Meya, D.B.; Kagimu, E.; Grint, D.; Te Brake, L.; Kasibante, J.; Martyn, E.; Rutakingirwa, M.; Quinn, C.M.; Okirwoth, M.; et al. High-Dose Oral and Intravenous Rifampicin for the Treatment of Tuberculous Meningitis in Predominantly Human Immunodeficiency Virus (HIV)-Positive Ugandan Adults: A Phase II Open-Label Randomized Controlled Trial. Clin. Infect. Dis. 2021, 73, 876–884. [Google Scholar] [CrossRef]
- Charlie, L.; Abay, S.M.; Tesfaye, A.; Mlera, R.N.; Mwango, S.; Goretti, M. Safety and efficacy of high-dose rifampicin in the management of tuberculosis meningitis: Systematic review and meta-analysis. Int. J. Mycobacteriol. 2021, 10, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, T.; He, K.; Xue, J.; Wang, X.; Liang, J. High-dose rifampicin for the treatment of tuberculous meningitis: A meta-analysis of randomized controlled trials. J. Clin. Pharm. Ther. 2022, 47, 445–454. [Google Scholar] [CrossRef]
- Abdelgawad, N.; Wasserman, S.; Gausi, K.; Davis, A.; Stek, C.; Wiesner, L.; Meintjes, G.; Wilkinson, R.J.; Denti, P. Population Pharmacokinetics of Rifampicin in Plasma and Cerebrospinal Fluid in Adults With Tuberculosis Meningitis. J. Infect. Dis. 2025, 232, e234–e241. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Jiang, L.; Liu, Q.B.; Bai, Y.; Yang, Y.; Sun, F.J.; Jia, Y.T.; Zhou, Y.J.; Zhang, Z.Z. Efficacy and safety of first-line anti-tuberculosis drugs combined with Linezolid for the treatment of pediatric tuberculous meningitis in real-word practice. Chin. J. Pediatr. 2024, 62, 715–720. [Google Scholar] [CrossRef]
- Madadi, A.K.; Sohn, M.J. Comprehensive Therapeutic Approaches to Tuberculous Meningitis: Pharmacokinetics, Combined Dosing, and Advanced Intrathecal Therapies. Pharmaceutics 2024, 16, 540. [Google Scholar] [CrossRef]
- Tornheim, J.A.; Dooley, K.E. Tuberculosis Associated with HIV Infection. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Paliwal, V.K.; Das, A.; Anand, S.; Mishra, P. Intravenous steroid days and predictors of early oral steroid administration in tuberculous meningitis: A retrospective study. Am. J. Trop. Med. Hyg. 2019, 101, 1083–1086. [Google Scholar] [CrossRef]
- Rohilla, R.; Shafiq, N.; Malhotra, S. Efficacy and safety of aspirin as an adjunctive therapy in tubercular meningitis: A systematic review and meta-analysis. eClinicalMedicine 2021, 34, 100819. [Google Scholar] [CrossRef]
- Saluja, A.; Vibha, D.; Pandit, A.K.; Shukla, G.; Srivastava, A.K.; Tripathi, M.; Srivastava, M.V.P.; Prasad, K.; Dwivedi, S.N. Comparison of dexamethasone regimens in tubercular meningitis (TBM): A randomized open label clinical trial. J. Infect. Dev. Ctries. 2023, 17, 1769–1774. [Google Scholar] [CrossRef]
- Gao, Y.; Su, J.; Ma, Y.; Sun, Y.; Cui, J.; Jin, X.; Li, Y.; Chen, Z. Efficacy and safety of intrathecal dexamethasone combined with isoniazid in the treatment of tuberculous meningitis: A meta-analysis. BMC Neurol. 2024, 24, 194. [Google Scholar] [CrossRef]
- Tian, N.; Chu, H.; Li, Q.; Sun, H.; Zhang, J.; Chu, N.; Sun, Z. Host-directed therapy for tuberculosis. Eur. J. Med. Res. 2025, 30, 267. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lin, J.; Taggart, C.C.; Bengoechea, J.A.; Scott, C.J. Nanodelivery strategies for the treatment of multidrug-resistant bacterial infections. J. Interdiscip. Nanomed. 2018, 3, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, P.N.; Consalvi, S.; Forte, J.; Cabiddu, G.; De Logu, A.; Poce, G.; Rinaldi, F.; Biava, M.; Carafa, M.; Marianecci, C. Nano-Based Drug Delivery Systems of Potent MmpL3 Inhibitors for Tuberculosis Treatment. Pharmaceutics 2022, 14, 610. [Google Scholar] [CrossRef] [PubMed]
- Borah Slater, K.; Kim, D.; Chand, P.; Xu, Y.; Shaikh, H.; Undale, V. A Current Perspective on the Potential of Nanomedicine for Anti-Tuberculosis Therapy. Trop. Med. Infect. Dis. 2023, 8, 100. [Google Scholar] [CrossRef]
- Nair, A.; Greeny, A.; Nandan, A.; Sah, R.K.; Jose, A.; Dyawanapelly, S.; Junnuthula, V.; Athira, K.V.; Sadanandan, P. Advanced drug delivery and therapeutic strategies for tuberculosis treatment. J. Nanobiotechnol. 2023, 21, 414. [Google Scholar] [CrossRef]
- Kia, P.; Ruman, U.; Pratiwi, A.R.; Hussein, M.Z. Innovative Therapeutic Approaches Based on Nanotechnology for the Treatment and Management of Tuberculosis. Int. J. Nanomed. 2023, 18, 1159–1191. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, P.; Gupta, M.K.; Vyas, S. Colloidal carriers: A rising tool for therapy of tuberculosis. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 299–353. [Google Scholar] [CrossRef]
- Futane, A.; Narayanamurthy, V.; Jadhav, P.; Srinivasan, A. Aptamer-based rapid diagnosis for point-of-care application. Microfluid. Nanofluidics 2023, 27, 15. [Google Scholar] [CrossRef]
- Al-Sayadi, G.M.H.; Verma, A.; Choudhary, Y.; Sandal, P.; Patel, P.; Singh, D.; Gupta, G.D.; Kurmi, B.D. Solid Lipid Nanoparticles (SLNs): Advancements in Modification Strategies Toward Drug Delivery Vehicle. Pharm. Nanotechnol. 2023, 11, 138–154. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, L.; Wang, R.; Song, G.; Fu, J.; Wang, J.; Gao, N.; Wang, H. Drug Delivery Across the Blood-Brain Barrier: A New Strategy for the Treatment of Neurological Diseases. Pharmaceutics 2024, 16, 1611. [Google Scholar] [CrossRef]
- Singh, D.; Krishna, V.; Kumari, N.; Banerjee, A.; Kapoor, P. Nano-engineered solutions for extensively drug-resistant tuberculosis (XDR-TB): A novel nanomedicine. Nano Struct. Nano Objects 2024, 40, 101390. [Google Scholar] [CrossRef]
- Chopra, H.; Mhanta, Y.K.; Rauta, P.R.; Ahmed, R.; Mahanta, S.; Mishra, P.; Panda, P.; Rabaan, A.A.; Alshehri, A.A.; Othman, B.; et al. An Insight into Advances in Developing Nanotechnology Based Therapeutics, Drug Delivery, Diagnostics and Vaccines: Multidimensional Applications in Tuberculosis Disease Management. Pharmaceuticals 2023, 16, 581. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.; Doherty, T. The success and failure of BCG—Implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005, 3, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Zhong, Q.; Zhang, Z.; Kong, L.; Zhou, M.; Wang, R.; Pi, X.; Qiao, S. Construction and expression of multi-stage antigen fusion protein RPC4 vaccine for Mycobacterium tuberculosis and its immunogenicity analysis in combination with adjuvant DIMQ. Tuberculosis 2025, 152, 102635. [Google Scholar] [CrossRef]
- Sadigurschi, G.; Kuschnir, M.C.C.; Dos Santos, E.A.P.; da Silva, B.R.A.; Marques, C.M.C.; de Andrade, R.C.; Vianna, C.M.; de Barros, D.G.; Mazzi, M.T.; Lago, E.A.; et al. Challenges in developing new tuberculosis vaccines. Mem. Inst. Oswaldo Cruz 2025, 120, e240236. [Google Scholar] [CrossRef]
- Geddes, L. Gavi, the Vaccine Alliance. VaccinesWork. How New Vaccines Could Revolutionise Our Relationship with Tuberculosis. Available online: https://www.gavi.org/vaccineswork/how-new-vaccines-could-revolutionise-our-relationship-tuberculosis (accessed on 24 November 2025).
- Seddon, J.A.; Wilkinson, R.; van Crevel, R.; Figaji, A.; Thwaites, G.E. Tuberculous Meningitis International Research Consortium. Knowledge gaps and research priorities in tuberculous meningitis. Wellcome Open Res. 2019, 4, 188. [Google Scholar] [CrossRef]
- Subbaraman, R.; Jhaveri, T.; Nathavitharana, R.R. Closing gaps in the tuberculosis care cascade: An action-oriented research agenda. J. Clin. Tuberc. Other Mycobact. Dis. 2020, 19, 100144. [Google Scholar] [CrossRef]
- Spatola, M.; Nziza, N.; Irvine, E.B.; Cizmeci, D.; Jung, W.; Van, L.H.; Nhat, L.T.H.; Ha, V.T.N.; Phu, N.H.; Ho, D.T.N.; et al. Distinctive antibody responses to Mycobacterium tuberculosis in pulmonary and brain infection. Brain A J. Neurol. 2024, 147, 3247–3260. [Google Scholar] [CrossRef]
- Chen, X.; Arun, B.; Nino-Meza, O.J.; Sarhan, M.O.; Singh, M.; Jeon, B.; Mane, K.; Shah, M.; Tucker, E.W.; Carroll, L.S.; et al. Dynamic PET reveals compartmentalized brain and lung tissue antibiotic exposures of tuberculosis drugs. Nat. Commun. 2024, 15, 6657. [Google Scholar] [CrossRef]
- Hai, H.T.; Thanh Hoang Nhat, L.; Tram, T.T.B.; Vinh, D.D.; Nath, A.P.; Donovan, J.; Thu, N.T.A.; Van Thanh, D.; Bang, N.D.; Ha, D.T.M.; et al. Whole blood transcriptional profiles and the pathogenesis of tuberculous meningitis. eLife 2024, 13, RP92344. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Gao, Y.; Lu, J.; Zhang, G.; Chen, X.; Zhang, R.; Kong, W.; Shang, L. Clinical profiles and related factors in tuberculosis patients with positive sputum smear Mycobacterium tuberculosis tests. Sci. Rep. 2024, 14, 20376. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liang, Y.; Chen, Y.; Li, L.; Bian, H. Early screening of miliary tuberculosis with tuberculous meningitis based on few-shot learning with multiple windows and feature granularities. Sci. Rep. 2024, 14, 23620. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.; Solomons, R.; Mason, S. Targeted metabolomics investigation of metabolic markers of Mycobacterium tuberculosis in the cerebrospinal fluid of paediatric patients with tuberculous meningitis. PLoS ONE 2024, 19, e0314854. [Google Scholar] [CrossRef]
- Yang, C.; Wang, T.; Guo, Y.; Zeng, Y.; Gao, W. Nanopore-targeted sequencing (NTS) for intracranial tuberculosis: A promising and reliable approach. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 89. [Google Scholar] [CrossRef]
- Nanda, S.; Bansal, M.K.; Singh, P.; Shrivastav, A.K.; Malav, M.K.; Prakash, C. Evaluation of the Cerebrospinal Fluid (CSF)-Truenat Assay: A Novel Chip-Based Test in the Diagnosis and Management of Tubercular Meningitis at a Tertiary Care Hospital. Cureus 2024, 16, e74522. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, X.; Zhang, S.; Li, D.; Wang, D.; Wang, Y.; Tang, Y.; Tong, F.; Xu, W.; Li, G.; et al. Comparative study of pathogen detection methods for central nervous system infections: Laboratory testing of tuberculous meningitis. BMC Infect. Dis. 2024, 24, 1172. [Google Scholar] [CrossRef]
- Milburn, J.; Ntwayagae, O.; Ngoni, K.; Suresh, R.; Lemme, N.; Northcott, C.; Penney, J.; Kinsella, M.; Mechie, I.; Ensor, S.; et al. The Impact of GeneXpert Cerebrospinal Fluid Testing on Tuberculous Meningitis Diagnosis in Routine Care in Botswana. Open Forum Infect. Dis. 2024, 11, ofae489. [Google Scholar] [CrossRef]
- Canas, L.S.; Dong, T.H.K.; Beasley, D.; Donovan, J.; Cleary, J.O.; Brown, R.; Thuong, N.T.T.; Nguyen, P.H.; Nguyen, H.T.; Razavi, R.; et al. Computer-aided prognosis of tuberculous meningitis combining imaging and non-imaging data. Sci. Rep. 2024, 14, 17581. [Google Scholar] [CrossRef]
- VidyaRaj, C.K.; Vadakunnel, M.J.; Mani, B.R.; Anbazhagi, M.; Pradhabane, G.; Venkateswari, R.; Palavesam, S.; Venkatesh, K.; Usharani, B.; Sriramkumar, S.R.; et al. Prevalence of extrapulmonary tuberculosis and factors influencing successful treatment outcomes among notified cases in South India. Sci. Rep. 2025, 15, 8290. [Google Scholar] [CrossRef]
- Urmenyi, L.G.; Vinhaes, C.L.; Villalva-Serra, K.; Araujo-Pereira, M.; Andrade, B.B. Influence of HIV co-infection on clinical presentation and disease outcome in hospitalized adults with tuberculous meningitis in Brazil: A nationwide observational study. Front. Public Health 2025, 13, 1600104. [Google Scholar] [CrossRef]
- Winichakoon, P.; Watcharasaksilp, K.; Butphet, S.; Wongworapat, K.; Pantip, C.; Khamnoi, P.; Supparatpinyo, K.; Salee, P. Sequential testing with Xpert MTB/RIF assay for diagnosis of tuberculous meningitis in Maharaj Nakorn Chiang Mai University Hospital. Sci. Rep. 2025, 15, 3675. [Google Scholar] [CrossRef]
- Jian, Y.; Bao, Y.; Yang, F.; Zhu, M. The role of isoniazid dosage and NAT2 gene polymorphism in the treatment of tuberculous meningitis. Front. Immunol. 2025, 15, 1535447. [Google Scholar] [CrossRef] [PubMed]
- Hueda-Zavaleta, M.; de la Torre, J.C.G.; Barletta-Carrillo, C.; Tapia-Sequeiros, G.; Flores, C.; Piscoche, C.; Miranda, C.; Mendoza, A.; Sánchez-Tito, M.; Benites-Zapata, V.A. Cytochemical analysis of cerebrospinal fluid in tuberculous meningitis versus other etiologies. PLoS ONE 2025, 20, e0318398. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.M.; Nasrin, R.; Kabir, S.; Kabir, F.; Rahman, A.M.R.; Uddin, M.K.M.; Islam, A.S.M.I.; Khatun, R.; Ahmed, S.; Mondal, M.B.A.; et al. Performance of Xpert MTB/RIF ultra for the diagnosis of tuberculous meningitis in children using cerebrospinal fluid. Sci. Rep. 2025, 15, 13060. [Google Scholar] [CrossRef] [PubMed]
- Urteneche, M.I.; González, N.E.; Ginestet, E.; Macha Marín, E.; Pereda, R.; Pablo, M.; Cassanelli, P.M. Diagnosis of central nervous system tuberculosis in pediatric patients. Diagnóstico de tuberculosis del sistema nervioso central en pacientes pediátricos. Arch. Argent. Pediatr. 2025, 123, e202410592. [Google Scholar] [CrossRef]
- Tameris, M.; Rozot, V.; Imbratta, C.; Geldenhuys, H.; Mendelsohn, S.C.; Kany Luabeya, A.K.; Shenje, J.; Tredoux, N.; Fisher, M.; Mulenga, H.; et al. Safety, reactogenicity, and immunogenicity of MTBVAC in infants: A phase 2a randomised, double-blind, dose-defining trial in a TB endemic setting. eBioMedicine 2025, 114, 105628. [Google Scholar] [CrossRef]
- Guo, L.; Wu, X.; Cao, L. Clinical Characteristics of Miliary Pulmonary Tuberculosis in Pregnancy After In Vitro Fertilization-Embryo Transfer: A Retrospective Clinical Study. Health Sci. Rep. 2025, 8, e70705. [Google Scholar] [CrossRef]
- Kim, J.; Spears, I.; Erice, C.; Kim, H.H.; Porter, N.A.; Tressler, C.; Tucker, E.W. Spatially heterogeneous lipid dysregulation in tuberculous meningitis. Neurobiol. Dis. 2024, 202, 106721. [Google Scholar] [CrossRef]
- Wasserman, S.; Antilus-Sainte, R.; Abdelgawad, N.; Odjourian, N.M.; Cristaldo, M.; Dougher, M.; Kaya, F.; Zimmerman, M.; Denti, P.; Gengenbacher, M. Rifabutin central nervous system concentrations in a rabbit model of tuberculous meningitis. Antimicrob. Agents Chemother. 2024, 68, e0078324. [Google Scholar] [CrossRef]
- Proust, A.; Wilkinson, K.A.; Wilkinson, R.J. Effects of M. tuberculosis and HIV-1 infection on in vitro blood-brain barrier function. J. Neuroinflammation 2025, 22, 141. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Photobiomodulation in Alzheimer’s Disease—A Complementary Method to State-of-the-Art Pharmaceutical Formulations and Nanomedicine? Pharmaceutics 2023, 15, 916. [Google Scholar] [CrossRef] [PubMed]

| TBM Features | Pulmonary TB Features | |
|---|---|---|
| Primary Site | Brain/meninges | Respiratory system |
| Symptom Onset | Slowly over weeks/months with vague signs | Sometimes subacute, with evident respiratory manifestations |
| Neurological versus Respiratory | Brain/spinal cord symptoms | Cough/breathing difficulties |
| Symptoms | Specific neurological symptoms Severe headache Stiff neck Confusion Focal deficits (e.g., vision loss, paralysis) Another important clinical sign Fever Nonspecific Fatigue Malaise Possible worse neurological outcomes | Respiratory symptoms Significant cough Sputum discharge Chest pain Hemoptysis Other clinical signs Fever Night sweats Fatigue Weight loss Often respiratory complications |
| Distinguishing Signs | Stiff neck/confusion/focal deficits | Cough |
| Similar Symptoms | Both share systemic features like fever and fatigue. | |
| Key Features | Slow onset Neurologically focused High risk of severe long-term complications (hydrocephalus, stroke, permanent deficits) | Lung-centered symptoms Important chest X-ray abnormalities |
| Prognosis | Higher mortality and neurological sequelae | Better prognosis than TBM if there are no complications |
| Criteria | Score | |
|---|---|---|
| Clinical and medical history | Long-term contact with a tuberculosis patient in the last 12 months | 2 |
| Clinical manifestations lasting more than 5 days | 4 | |
| Prolonged clinical signs beyond two weeks (night sweats, cough, weight loss or failure to thrive in infants and young children, etc.) | 2 | |
| Focal neurological deficits (for example: left side of the face, right arm, tongue, speech, vision or hearing problems, etc.), ruling out cranial nerve palsies | 1 | |
| Cranial nerve palsies | 1 | |
| Impaired consciousness | 1 | |
| Maximum score | 6 | |
| Cerebrospinal fluid study | Clear color—crystalline, like rock water | 1 |
| Increased cellularity to 10–500/μL | 1 | |
| Mononuclear pleocytosis with lymphocytes > 50% | 1 | |
| Proteins in cerebrospinal fluid > 1g‰ | 1 | |
| CSF/plasma glucose ratio < 50% or low absolute glucose level in CSF < 2.2 mmol/L | 1 | |
| Maximum score | 4 | |
| Brain imaging data | Hydrocephalus | 1 |
| Basal meningeal enhancement | 2 | |
| Tuberculoma | 2 | |
| Cerebral infarction | 1 | |
| Pre-contrast basal hyperdensity | 2 | |
| Maximum score | 6 | |
| Evidence of tuberculosis elsewhere | Chest X-ray suggesting signs of active TB | 2 |
| X-ray appearance of miliary TB | 4 | |
| CT scan/MRI/ultrasound positive for TB in other body segments | 2 | |
| Identification of acid-fast bacilli or Ziehl–Neilson stain and positive culture from blood, sputum, gastric lavage, urine, lymph node biopsy | 4 | |
| Nucleic acid amplification test (NAAT) positive for M. tuberculosis from extraneuronal specimens | 4 | |
| Maximum score | 4 | |
| Diagnostic Method | Advantages | Disadvantages | |
|---|---|---|---|
| Classic CSF analysis Positive results for TBM Low glucose High protein Lymphocyte predominance | Indispensable classic analysis, low cost, specific TBM profile | Low sensitivity for M.tb and late culture results | |
| Imaging techniques | MRI Specific for brainstem/meninges enhancement Accurately detects complications (hydrocephalus, infarctions) CT better for acute hydrocephalus | Cannot indicate a positive diagnosis Must be corroborated with clinical and laboratory data | |
| Microbiological tests | Traditional microscopy (Ziehl–Neelsen stain for AFB) | Easy, fast, cheap, widely available | Low sensitivity (0–87%), requires high bacterial load Detects only ~10–20% of M.tb |
| Slow cultures (liquid/solid media) | Gold standard for positive diagnosis, results after weeks or months, higher sensitivity (40–83%), but slow. | Still low sensitivity for TBM | |
| Molecular tests NAATs (Xpert MTB/RIF, TB-LAMP) Xpert MTB/RIF Ultra | Rapid (h), high sensitivity/specificity (especially Xpert Ultra detects M.tb DNA) and rifampicin resistance in CSF Significantly speeding up diagnosis and guiding early, appropriate treatment for severe forms Xpert Ultra an advancement with improved sensitivity Highly valuable tools recommended by the WHO | High cost, requires special equipment, negative results do not rule out TBM | |
| Immunological tests (IGRA, ADA) | Crucial biomarkers | IGRA Very reliable for ruling out TBM (negative results are certain) High sensitivity/specificity in CSF ADA Suggests TB (especially >40 U/L) but requires context (there are other causes when elevated). Useful as a low-cost screening test for TBM | IGRA Potential false-positive results Helps identify infection, not always disease ADA Limited specificity. A high value is not definitive. Neither test should be used alone |
| Emerging mNGS and other omics technologies | Sequencing all DNA/RNA to identify all microbes present, plus host genetic material. | Non-targeted, broad-spectrum screening, excellent for rare, novel, or difficult-to-culture pathogens where traditional methods fail. | Very expensive, requires validation and integration |
| Xpert Ultra | Nanopore-Targeted Sequencing (NTS) NTS | mNGS | |
|---|---|---|---|
| Main application | Identification of TB/RIF through quick screening | Complete outline of drug resistance | Recognition of scarce or unrevealed germs, and hard-to-detect infectious diseases |
| Specificity | High | Very high for bacteria | High |
| Sensitivity | ≤90% for PTB | 92–95% | 60–90%, varies depending on the pathogen, sample type (CSF, blood, etc.) and patient immunosuppression |
| Turnaround time | Fast (120 min) | Rapid (hours) | Quick (4–24 h) on ultrarapid devices, and 24–72 h for standard protocols |
| Cost | High | Medium ≤ USD100 | High (USD100–400 per sample) |
| Sample requirements | Need: sputum ~1 mL unprocessed, CSF ~2 mL | High-quality, high-molecular-weight DNA, good purity, adequate concentration (>20 ng/µL) | Sufficient volumes in sterile boxes, quickly refrigerated or fresh |
| Infrastructure needs | Same platform as Xpert, but recalibration for 10-color technology Specific cartridges | Core lab equipment, sequencing hardware, flow cells, computer technologies, and specialized reagents | Complex hardware and software, specialized labs, skilled staff |
| Drug | CNS Penetration | |
|---|---|---|
| Good/Excellent Penetration | Isoniazid | Rapid penetration, reaching high concentrations, key drug for CNS tuberculosis |
| Pyrazinamide | Readily crosses BBB, good for inflammation. | |
| Ethionamide | Excellent penetration | |
| Cycloserine or terizidone | High, excellent for MDR-TBM | |
| Moxifloxacin | Good penetration (60–80%) | |
| Levofloxacin | Good penetration (60–80%) | |
| Linezolid | Moderate/significant penetration (30–70%) | |
| Moderate/Lower Penetration | Rifampicin | Moderate penetration, better with inflammation, important for overall regimen |
| Ethambutol | Poor/moderate (10–50%) | |
| Clofazimine | Low penetration/very low in CSF (e.g., ~0.13% of plasma levels) |
| Reference | Study Characteristics | No. Patients/ Diagnosis | Clinical Protocol | Results | Conclusions |
|---|---|---|---|---|---|
| Spatola, M. et al., 2024 [173] | 10 different M.tb antigens | HIV-negative adults with pulmonary TB (n = 10) versus TBM (n = 60) | IgG, IgM, IgA and subclasses IgG1–4; M.tb-specific antibodies binding to Fc receptors or C1q. T cells controlling Mbt infection | Highly specific antibodies in CSF, with exclusive and compartmentalized humoral reactions against M.tb in TBM. Phagocytosis and complement-mediated antibodies may contribute to a milder type of disease in the brain. | Distinct antibody responses, functionally divergent humoral reactions depending on the site of infection (lungs or brain). |
| Chen, X. et al., 2024 [174] | Dynamic PET with antibiotics active against MDR M.tb strains in human and animal studies. | Dynamic PET for 50–60 min immediately after IV injection of 18F-pretomanid in 8 human subjects (six healthy volunteers and 2 newly diagnosed TB patients); median age 29 | Compartmentalized antibiotic exposures in mouse studies. All animals also received adjunctive dexamethasone (standard for TBM). Monte Carlo simulations (n = 1000 subjects for four antibiotics). | A rich set of concentration-time data in multiple compartments in 3D space simultaneously illustrated antibiotic-specific compartmentalization. | Pretomanid had higher brain exposures than lung tissue, while opposite was observed for bedaquiline. |
| Hai, H.T. et al., 2024 [175] | Whole-blood RNA sequencing. Hub genes and pathways linked to TBM severity and mortality. | 4-RNAseq cohorts: 281 adults with TBM (n = 207, HIV-negative; n = 74, HIV-positive); n = 295 patients (PTB); n = 30 healthy controls | Hub genes and pathways in TBM are stratified by HIV status. Validation of hub genes in the qPCR confirmation cohort (i.e., HIV-negative TBM). Comparison of hub genes and pathways associated with TBM mortality in all 4 RNAseq cohorts. | Prediction models for TBM prognostics in HIV-negative TBM, and in HIV-positive TBM cohorts. TNF signaling, Toll-like receptors, NF-kappa B and neutrophil extracellular trap formation were correlated with TBM mortality. A four-gene host response signature in blood was identified as a new biomarker for the highest risk of death, regardless of HIV status. | Mortality from TBM has been linked to increased acute inflammatory responses, their regulation, and neutrophil activation. Dysregulation of both innate and adaptive immune responses is strongly associated with death from TBM. |
| Yu, S. et al., 2024 [176] | Smear M.tb tests (I); Sputum Gene Xpert tests (II). | 1612 patients recently diagnosed with PTB, divided into 2 groups: -Positive for test I, i.e., “Sputum smear positive” group (n = 432). -Negative for I, but positive for test II, i.e., “smear negative, but GeneXpert positive” (n = 1180) | Data collection and statistical analysis of: - Clinical symptoms (wheeze and coughing up of blood, spitting up phlegm, night perspirations, pyrexia, shortness of breath etc. - Extrapulmonary tuberculosis status (pleurisy, meningitis, TB peritonitis, intestinal TB, joint TB, urinary TB). - Other concomitant diseases (diabetes, hypertension, etc.). | Percentage of TBM (3.7% vs. 1.3%, p < 0.05), TB pleurisy (8.1% vs. 2.7%, p < 0.001), and TB peritonitis (4.4% vs. 0.9%, p < 0.001) were higher in group I. Age stratification (p < 0.001) and BMI stratification (p < 0.01). | Patients with PTB aged 75–89 yrs. have bacterial secretions and high risk of transmitting TB. Underweight patients (BMI < 18.5 kg/m2) have highly infectious TB and require rapid isolation. Immunocompromised patients will develop extrapulmonary tuberculosis, including TBM, contributing to high levels of infectiousness. |
| Tian, Y. et al., 2024 [177] | Computer modeling: 40-case learning with multiple windows and feature granularities (MWFG). | 40 MT patients | Complete highlights from a set of 40 thoracic CT images of MT patients. | Successful early screening model of MT-TBM. | Prediction of MT-TBM by computer-aided diagnosis. Linking MT and MT-TBM by chest CT images to help doctors in the prevention and early treatment of MT-TBM in patients with MT. |
| Samuel, V. et al., 2024 [178] | Targeted M.tb-related metabolites in CSF | 46 children divided into two groups: G1 (n = 21, TBM group) and G2 (n = 25, control group). | M.tb-related target metabolites were investigated in the CSF of TBM patients by 1H-NMR and GCxGC-TOFMS analysis. | Four targeted metabolites were elevated in the TBM group, statistically significant: D-mannose, D-arabinose, propanoic acid (FDR p-value < 0.0001) and nonanoic acid. | Nonanoic acid is a downstream degradation product of the cell wall of M.tb and a prospective M.tb-specific marker of TBM. |
| Yang, C. et al., 2024 [179] | 5 testing methods for a variety of intracranial TB, including NTS. | 122 patients suspected of intracranial TB | Diagnostic efficiency of NTS, Xpert, MTB culture, PCR, AFB smear | Positive NTS tests could be an important mark for the danger of intracranial TB. | Multicenter and larger sample size research are necessary to validate the use of NTS in highlighting drug resistance in intracranial TB. |
| Nanda, S. et al., 2024 [180] | CSF samples tested with CBNAAT, comparatively with and Truenat. | 150 patients with significant clinical signs and symptoms for TBM, as follows: 46.7% (15–35 years); 39.3% (36–55 years) and 14% > 55 years; 104 men (69.3%) and 46 women (30.7% of total participants). | Clinical history and neurological examinations. Laboratory data were collected. Radiological investigations and brain MRI with contrast were performed (if necessary). CSF samples (4 mL) were collected; last 2 mL were processed for CBNAAT and Truenat tests. Rifampicin resistance was tested by both methods. | Diagnostic accuracy of Truenat in comparison to CBNAAT: sensitivity = 83.75%, specificity = 88.57%, overall accuracy = 86%. | Truenat is a portable device, easy to use in low-resource settings and in a short time, cost-effective, gives similar results compared to CBNAAT, but still in its early stages. More studies in larger populations are needed. |
| Liu, Z. et al., 2024 [181] | Comparative study on the clinical performance and efficacy of mNGS with other standard microbiological tests, to identify the best diagnostic master plan for TBM in clinical practice. | 514 patients, diagnosed by clinicians as having a CNS infection. | CSF analysis, mNGS, GeneXpert, and microbial cultures. Comparison of the consistency of results of TBM detection methods. | GeneXpert and microbial culture methods still have detection limitations. mNGS has the potential to overcome these constraints by simultaneously detecting known and unknown genes of all pathogenic microorganisms in the database. As databases improve and new machine learning approaches are developed, mNGS will be more widely used in the future. | Diagnosis and treatment of TBM are challenging due to the similarity of symptoms to other CNS infections. A unique diagnostic technique frequently is unsuccessful to provide error-free results. Combining manifold diagnostic approaches for TBM patients is likely to provide more accurate outcomes. |
| Milburn, J. et al., 2024 [182] | CSF analysis whose results were stored in an integrated patient management system was extracted for samples collected between 2016 and 2022. | 6934 CSF samples were analyzed. | 1114 were investigated using TB-specific tests: 787 Xpert MTB/RIF, 340 smear microscopies, and 177 M.tb cultures. | Patients with a specific test for TB had a higher median age (39.1 vs. 35.2 years), a higher prevalence of HIV (61.3% vs. 51.0%), higher rates of pleocytosis in CSF, increased CSF protein >1 mg/mL and a positive extraneural sample for M.tb. | No. of CSF samples that underwent specific TB-testing increased from 4.5% in 2016 to 29.0% in 2022, due to analysis with Xpert MTB/RIF (from 0.9% to 23.2%), and confirmed TBM cases increased from 0.4% to 1.2%. |
| Canas, L.S. et al., 2024 [183] | A prospective longitudinal study using a machine learning model for clinical and brain MRI data in TBM. | 216 adults with TBM; 73 (34%) were HIV-positive. | A novel prognostic model consisting of an end-to-end approach designed to model TBM using clinical and imaging longitudinal data, integrating the symptoms of the disease and their severity. | The results showed that clinical parameters and imaging aspects can contribute to the prognosis of TBM, especially signaling the most unfavorable outcomes. Limitations of the modeling were lower accuracy for intermediate stages of TBM severity. | The model anticipates possible adverse events that could delay or affect the patient’s recovery within the clinic framework. |
| VidyaRaj, C.K. et al., 2025 [184] | Prevalence of epTB, including TBM, and factors controlling therapeutic outcomes | 4526 patients with epTB | GeneXpert MTB/RIF assay Clinical diagnosis of EpTB and EP rifampicin-resistant TB | Highest positivity rates of epTB in: abscesses (47.06%), lymph nodes (38.37%), pus (31.95%), tissues (18.06%), gastric lavage (15.82%), pleural fluid (12.45%), other fluids (10.39%), and TBM (8.88%). | Poor results were mainly due to patients lost to follow-up, including TBM. Raising clinical awareness, targeted screening in high-risk groups, and better monitoring and counseling is the key to success. |
| Urmenyi, L.G. et al., 2025 [185] | TBM and in-hospital mortality in cases of HIV co-infection | 1819 hospitalized TBM patients (2007–2021) from the SINAN-meningitis database in Brazil | Frequency of clinical signs and symptoms of TBM by HIV status. Characteristics and differentiations between HIV+ and HIV- adults with TBM; and between deceased and surviving patients. | 57% of TBM cases (as PLWH) had fewer episodes of vomiting, nuchal rigidity, meningeal inflammation, or coma; and lower CSF leukocyte counts compared to HIV- patients. Mortality was indicated by seizures, nuchal rigidity, age > 64 years. | HIV was not an independent predictor of mortality in this population. |
| Winichakoon, P. et al., 2025 [186] | Sequential testing of TBM score and Xpert MTB/RIF assay for speedy and accurate diagnosis of TBM | 65 patients aged 18 and over hospitalized for subacute lymphocytic meningitis | 65 eligible CSF samples, with a concentration of 8–10 mL, were collected and sent to the TB lab for analysis. Sensitivity, specificity, and congruence between Xpert MTB/RIF and MGIT culture. | Xpert MTB/RIF sensitivity = 83.33%; specificity = 96.23%; concordance between Xpert MTB/RIF and MGIT culture was 93.85%. | A protocol that includes the TBM score increases accuracy, speed of diagnosis, and outcomes in resource-limited hospitals. |
| Jian, Y. et al., 2025 [187] | Management of TBM according to NAT2 gene polymorphism and INH dose. | 119 patients with TBM, divided into two groups: group 1 with standard INH dose; group 2 with double INH dose (600 mg/day). | Distribution of NAT2 genotypes among patients. Neurological manifestations and disease severity. CSF and imaging findings. Mortality and disability outcomes between the two groups. | Impairments and percentage of deaths varied depending on the NAT2 gene polymorphism and the dose of INH. Genotype-based INH dosing could reduce the side effects of therapy. | TBM management must be personalized. Higher doses of INH in genotype IA may reduce disability and mortality. Other factors, as well as CSF and MRI data, are essential in predicting TBM outcomes. |
| Hueda-Zavaleta, M. et al., 2025 [188] | Comparative analysis of CSF characteristics in TBM with other bacterial, viral or cryptococcal meningitis. | 450 CSF samples of suspected meningitis. | Cytochemical and biochemical analysis, as well as by smear microscopy, Xpert® MTB/RIF or Xpert MTB/RIF Ultra, and by culture. | M.tb (8.9%), Cryptococcus neoformans (6.0%), Streptococcus pneumoniae (2.4%), and Listeria monocytogenes (1.5%); viral meningitis (1.8%). In TBM patients: 57.5 cells/μL (red blood cells); 91.5 cells/μL (leukocytes); 70% (mononuclear cells); 22.5 mg/dL (median glucose) and 218.3 mg/dL (protein). | There are essential differences in CSF characteristics according to etiology. |
| Rahman, S.M.M. et al., 2025 [189] | Xpert MTB/RIF Ultra (Ultra); Xpert MTB/RIF (Xpert); smear microscopy, culture and drug susceptibility testing. | 187 children suspected of TBM. | Patients were clinically evaluated. CSF samples were taken for complete laboratory investigations by Ultra, Xpert, culture and microscopy. | Ultra detected TBM in 23.4% of cases, comparatively with only 9.1% by Xpert. Ultra had a sensitivity of 88%, noticeably better than Xpert (34%) and L-J culture (30%). Ultra’s sensitivity was 100% against the CMRS. AFB microscopy had a very low sensitivity (2.3%). | Ultra detected 26 cases of TBM missed by other tests and proved the highest sensitivity for diagnosing TBM by CSF analysis. |
| Urteneche, M.I. et al., 2025 [190] | Observational, retrospective study including CNS TB patients. | 26 cases of CNS TB out of 1013 cases of TB. | Clinical elements: fever and neurological symptoms (vomiting, headache, seizures, sensory disturbances and focal signs). Results of CT/MRI of the brain, Marais criteria. Pulmonary X ray. Cytochemical study of CSF. Direct ZN examination, M.tb culture, immunochromatography, Xpert MTB/RIF™, phenotypic sensitivity for R and INH (SIRE, MGIT-BD™). | Negative smear microscopy in all cases. Xpert MTB/RIF™ and culture revealed 61% and 75% sensitivity, respectively. Bacteriological proof was 81%. | CNS TB can be recognized more easily by simultaneous analysis of epidemiological data, pulmonary involvement, neurological signs and the contribution of molecular biology, facilitating timely treatment in children. |
| Tameris, M. et al., 2025 [191] | Phase 2a randomized, double-blind, dose-defining trial in a TB-endemic setting from South Africa. | 99 healthy BCG-naïve infants, HIV-unexposed, were randomized into 4 groups (G1–G4). | G1 (n = 24) received a single intradermal dose of BCG: 2.5 × 105 CFU. G2, G3 and G4 received MTBVAC, as follows: G1 (n = 25) =2.5 × 104 CFU G2 (n = 25) =2.5 × 105 CFU G3 (n = 25) = 2.5 × 106 CFU | 63 (of 99) infants experienced mild adverse reactions. Induration, swelling, and erythema were more common with increasing doses of MTBVAC. One patient treated with BCG was diagnosed with unconfirmed TBM, 9 days after vaccination. One patient died 182 days after MTBVAC vaccination due to bronchopneumonia. 9 infants were treated for TB. | Research identified the optimal dose of the MTBVAC vaccine for a phase 3 trial (NCT04975178) that will enroll 7000 infants from 6 African areas. |
| Guo, L. et al., 2025 [192] | Retrospective and statistical study after IVF-ET (January 2018–December 2021). | 21 patients with MPTB after IVF-ET. | Fever and vaginal bleeding were monitored as clinical symptoms. Laboratory parameters: sputum M.tb smears and cultures; Xpert; PCR-TB; TB antibody and other routine TB data had low positive rates. Chest imaging being restricted in pregnancy, delayed diagnosis. | Approximately 33% of patients had chills, night sweats, and dyspnea. 3 were admitted to ICU with critical illness, such as septic shock and ARDS; 7 had TBM or tuberculous encephalitis. 14 patients suffered from mild liver impairment secondary to ATT. CXRs and CT revealed bilateral signs of MPTB. | Prior to IVF-ET, patients should undergo exhaustive screening for TB, as they may develop severe forms of TB, including MPTB and CNS TB in some cases, which can endanger the life of the patient and the fetus. |
| Reference | Experimental Model | Study Protocol | Experiments and Parameters | Results | Conclusions |
|---|---|---|---|---|---|
| Kim, J. et al., 2024 [193] | Rabbit model of TB meningitis | Infection with M.tb was incubated for three weeks preliminary to tissue harvest and processing (rabbits were euthanized). | MALDI-MSI was performed on prepared samples, and imaging; lipid identification and verification using LIPID MAPS publication database. Sterol and oxysterol LC-MS/MS. | Spatially heterogeneous lipid dysregulation in TBM. Extended lipid peroxidation and lower lipid concentration may trigger poor neurodevelopmental consequences in children after TBM. | Direct infection is a key source of microglial activation and neuroinflammation in TBM, but alternations in the lipid homeostasis may be another machinery of microglial activation. |
| Wasserman, S. et al., 2024 [194] | Rabbit model of TBM | 7 rabbits were infected with 104 CFUs M.tb HN878 via the cisterna magna and treated with rifabutin once daily at 15 mg/kg for 3 days by oral gavage after reaching a predefined neurological score. | Blood was collected from the middle ear artery before dosing, and at various time intervals after drug administration on day 1, and up to the time of necropsy on day 3. Concentrations in the CNS, plasma, and lung compartments were analyzed. | Highest concentrations of rifabutin were found in the meninges and spinal cord, compared to plasma, CSF, and other CNS tissues. | Rabbit TBM model has been optimized to reproduce human TBM disease for future drug selection in human clinical trials. |
| Proust, A. et al., 2025 [195] | In vitro BBB model with human CNS cells. | BBB model and CNS cells were infected with M.tb and/or HIV-1. | Flow cytometry for M.tb growth inside CNS. BBB permeability for M.tb. Viral and bacterial cytopathogenicity (xCELLigence). Metabolic activity & ROS (colorimetric assays). Fluorometric assay for extracellular glutamate. Inflammatory response by Luminex. Quantitative PCR for endoplasmic reticulum stress. | M.tb increases the permeability of BBB, with its translocation. Pathological effects at the cellular level are: increase in markers of cellular stress and ROS; cell-type specific inflammatory mediators and effectors; astrocyte neurotoxicity and excitotoxic secretion of glutamate. | M.tb infects and multiplies in all CNS cell types, with HIV-1 potentiating its entry into astrocytes and pericytes, the latter growing more rapidly together with HIV-1-positive endothelial cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Advances in Tuberculous Meningitis: Research, Challenges, and Future Perspectives. Appl. Sci. 2026, 16, 232. https://doi.org/10.3390/app16010232
Ailioaie LM, Ailioaie C, Litscher G. Advances in Tuberculous Meningitis: Research, Challenges, and Future Perspectives. Applied Sciences. 2026; 16(1):232. https://doi.org/10.3390/app16010232
Chicago/Turabian StyleAilioaie, Laura Marinela, Constantin Ailioaie, and Gerhard Litscher. 2026. "Advances in Tuberculous Meningitis: Research, Challenges, and Future Perspectives" Applied Sciences 16, no. 1: 232. https://doi.org/10.3390/app16010232
APA StyleAilioaie, L. M., Ailioaie, C., & Litscher, G. (2026). Advances in Tuberculous Meningitis: Research, Challenges, and Future Perspectives. Applied Sciences, 16(1), 232. https://doi.org/10.3390/app16010232









