3.2. Effect of Cultivar on Sensory Properties of Currants
A comparison of individual cultivars within each of the three variants revealed significant differences (
p ≤ 0.05) in all evaluated properties, with the exception of astringency. Astringency was consistently low across all red and white cultivars, while being higher in all black cultivars. A detailed comparison of the sensory characteristics of individual cultivars within each variant is provided in
Table 1, with the best (most acceptable) cultivars within each variant marked with an asterisk.
Sweetness in white cultivars (
Table 1a) ranged from 32.5% to 52.4%, acidity ranged from 38.2% to 72.3%, and astringency was very low (19.1–34.7%). Among the white cultivars, ‘Victoria’ was evaluated as the most acceptable in both years (68.7% and 72.1%) due to its excellent flavor, very good odor, sweetness with minimal astringency, and firm, crispy texture. ‘Orion’ also had very good flavor, attributed to high sweetness and very low astringency, but its less crispy texture lowered its overall rating. ‘Jantar’ was considered the least acceptable (64.2% and 50.9%) due to its less appealing flavor, lower sweetness, and noticeable astringency. Its texture was also softer and less crispy. A distinct earthy off-flavor was detected in ‘Primus’, which was negatively perceived by the evaluators.
In red currants (
Table 1b), all cultivars were mildly sweet (24.8–51.5%) and more acidic (39.3–69.6%), with low astringency (23.8–29.4%). This low astringency slightly contradicts Schwarz and Hofmann [
2], who stated that both acidic and astringent tastes are key determinants of red currant flavor. Among the red cultivars, ‘Rubigo’ was evaluated as the most acceptable in both years (70.2% and 62.2%) due to its superior flavor, odor, and texture. ‘Tatran’ was the least acceptable (56.3% and 52.2%) due to its strong acid and astringent taste and a distinct earthy off-flavor.
The overall acceptability of black cultivars (
Table 1c) varied considerably (44.3–68.3%). ‘Demon’ (68.3% and 68.1%) and ‘Moravia’ (64.9% and 66.6%) were considered the best in both years, with a pleasantly sweet flavor, very good odor, and a balanced, characteristic “blackcurrant” note, although their texture was less crispy. ‘Ben Gairn’ had the lowest acceptability (46.4% and 44.3%), likely due to its less appealing flavor with an atypical rancid off-flavor that masked the characteristic “blackcurrant” note. This may indicate increased sensitivity of this cultivar and its specific storage requirements.
These results are difficult to compare with existing literature due to the scarcity of studies on the sensory quality of fresh currants. Most studies focus on juices, a commercially significant product. However, the sensory characteristics (flavor and aroma) of fresh berries and processed juice can differ significantly due to processing interventions such as heat [
13] or enzyme treatment [
7] and sucrose addition [
20], which can substantially alter the flavor.
Descriptor selection in this study was inspired by the work of Boccorh et al. [
15,
19] and Brennan et al. [
20], who first identified basic aroma descriptors for black currant juices, including sweet, acidic, and astringent tastes and fruity, grassy, citrus, caramel, and “blackcurrant” aromas. These attributes were also used in other studies on black currant juices [
3,
4,
5,
21], sometimes with additional descriptors such as “catty”, “minty”, and “earthy”. The only study focusing on fresh black currant berries, by Jung et al. [
6], provided a brief evaluation of their aroma profile based on GC-olfactometry. No detailed sensory evaluation of red and white currants has been performed to date. The work of Schwarz and Hofmann [
2], who evaluated an extract from fresh mashed red currant berries and described phenolics as key astringent compounds using sensory analysis, is a relevant exception.
The main objective of this study was to compare currant cultivars, as cultivar is a key factor affecting fruit flavor, along with post-harvest processing and storage. Studies comparing currant cultivars [
6] or juices produced from different cultivars [
3,
4,
20] are very scarce. Nevertheless, in accordance with our results, they all confirm differences in sensory characteristics, especially flavor. No scientific studies specifically addressing the cultivars analyzed in this study were found, although Jung [
26] conducted a brief evaluation involving ‘Rovada’, but its aroma profile was not described in detail. Some brief information can be found in growers’ manuals, which generally describe currants as having a firm texture and juicy flavor, with white and black currants being sweet and red currants being sweet tart, although each variety should have its unique flavor profile and texture.
3.3. Volatile Compounds Identified in Currant Samples Using HS-SPME-GC-FID/MS Method
Fruit flavor is closely related to the content and composition of volatile (aroma) compounds, which can be measured instrumentally. Therefore, the sensory evaluation was supplemented by volatile compound analysis. This approach has been particularly understudied in red and white currants.
A total of 54 volatile compounds were identified in the 15 analyzed currant cultivars using HS-SPME-GC-FID/MS. They were classified into six groups based on their chemical characteristics: alcohols (14), aldehydes (11), esters (12), terpenes and terpenoids (14), ketones (2), and an acid (1). An overview, indicating their presence in each variant (red/white/black), is provided in
Supplementary Material Table S1. All these compounds, except ethyl heptanoate, have been previously found in black currants by numerous authors [
6,
7,
10,
12,
13,
14,
17] and are known to be aroma-active. However, as shown in
Table S1, their aroma descriptions from the open-access PubChem database can vary significantly depending on factors such as their concentration in sample, the sensitivity of the evaluator, etc.
The volatile profile of black currants has been investigated for several decades, with over 200 aroma compounds identified. Most authors report alcohols, esters, terpenes, and terpenoids as major components [
9,
12,
13,
16,
17]. This study also found aldehydes to be a substantial group.
Alcohols were the most abundant compounds identified in all samples. Several metabolic pathways are involved in their biosynthesis. Six primary alcohols—ethanol, butan-1-ol, pentan-1-ol, hexan-1-ol, octan-1-ol, and nonan-1-ol—were identified. These are formed by enzymatic reduction of corresponding aldehydes by alcohol dehydrogenases. Free ethanol is a product of fermentation and is found in very small quantities in fruit; however, bound in esters, it is a common component of fruit aroma [
31]. Other short-chain (<C
18) primary alcohols contribute to the characteristic flavors of fruits, but according to Liu et al. [
17], they do not significantly contribute to currant aroma in their free form. They are less important than their aldehyde homologs due to higher odor thresholds [
31]. Among these, only hexan-1-ol, generated from linoleic acid as part of the C
6 group, was recognized as an important contributor to black currant aroma [
6]. The identified secondary alcohols, butan-2-ol and pentan-2-ol, are assumed to be formed by enzymatic reduction of corresponding methyl ketones. The branched-chain alcohols, 2-methylpropan-1-ol and 3-methylbutan-1-ol, are derived from amino acids (Leu, Ile) [
31]. Among these, 3-methylbutan-1-ol, along with the unsaturated C
6 alcohols (E)-2-hexen-1-ol and the isomers (E)- and (Z)-3-hexen-1-ol, was previously recognized as a key component of black currant aroma [
13,
16]. These are formed from the unsaturated linoleic and linolenic acids via the lipoxygenase pathway after plant tissue disruption [
6]. 1-Octen-3-ol, known in cheese for its typical mushroom aroma, has also been isolated from plants and, surprisingly, identified as a key component of black currant aroma, contributing a floral note [
12].
Five straight-chain aldehydes—ethanal, hexanal, octanal, nonanal, and decanal—were identified. They are formed via β-oxidation of unsaturated fatty acids; saccharides (glucose, fructose, and sucrose) or amino acids can also be precursors [
31]. Octanal and nonanal were previously recognized as key aroma components of black currants [
12,
13,
14,
16,
17]. Three unsaturated aldehydes—(E)-2-hexenal, (Z)-3-hexenal, and (E)-2-octenal—were identified. These, along with their corresponding unsaturated alcohols, are generated from the oxidative degradation of linolenic and linoleic acids by the lipoxygenase pathway [
6]. (E)-2-hexenal was previously recognized as a key component of black currants [
12]. These C
6 and C
8 compounds are important contributors to the characteristic flavors of many fruits [
31], but their profile can be rapidly altered by isomerization or reduction; accordingly, Jung et al. [
6] identified the constitutional isomer (E)-3-hexenal. The branched-chain aldehydes 2-methylbutanal and 3-methylbutanal are formed from amino acids, similar to the corresponding alcohols. Benzaldehyde, derived from the shikimate pathway via phenylalanine [
31], has been identified as a key component of black currants [
14,
17].
Esters are one of the largest groups of volatile constituents in nearly all fruits, contributing to their fruity flavor; they are also important in black currant berries [
6,
12,
17]. They can be formed by different reactions: esterification, alcoholysis, acidolysis, or transesterification. These reactions can occur spontaneously or be catalyzed by esterases and lipases [
14]. Fatty acids, particularly those with 2, 4, and 6 carbon straight chains, play a major role in ester synthesis, while the source of alcohols and aldehydes for ester synthesis in fruit is not fully understood [
31]. Acetic acid and ethanol are most often bound in esters, and Jung [
26] also mentions butanoic acid esters as quantitatively important. Four acetates (methyl, ethyl, butyl, and octyl acetate) and five ethyl esters (ethyl butanoate, propanoate, hexanoate, heptanoate, and decanoate) were identified in the samples. Ethyl butanoate and hexanoate were previously recognized as key components of black currants [
13,
14,
16]. Based on GC-olfactometry, Varming et al. [
16] consider the following esters as the top five most potent compounds in black currant juice aroma: methyl acetate, ethyl propanoate, methyl butanoate, ethyl butanoate, and ethyl hexanoate.
Terpenes and their oxygenated derivatives are important volatile compounds in nearly all fruit types, including black currants [
8], and their contribution to fruit aroma is well established. Many are stored in plants as glycosides, released by glycosidase activity during maturation. Consequently, they are reported to be indicators of fruit freshness, maturity, botanical and geographical origin, quality, and authenticity [
7]. They are derived from saccharides through the metabolic intermediate mevalonic acid, which provides the basic structural isoprene unit; many others are formed through transformation of the initial products by oxidation, dehydrogenation, acylation, and other reactions [
31]. There has been particular interest in terpenes in recent years, mainly because of the biological activity shown by some [
11]. Consistent with the literature [
7,
9], terpenes were numerous in the samples. Eight monoterpenes (β-pinene, sabinene, limonene, δ-3-carene, α-terpinene, γ-terpinene, terpinolene, and β-phellandrene) and five monoterpenoids (linalool, α-terpineol, terpinen-4-ol, β-damascenone, and rose oxide) were identified. Terpenoids are known for their strong flavor and represent the characteristic black currant flavor, while sesquiterpenes are not considered contributors to black currant aroma. The only sesquiterpene found in this study was β-caryophyllene. Limonene, β-phellandrene, linalool, α-terpineol, β-damascenone, and rose oxide are known as key aroma components of black currants [
12,
13].
Although most ketones have relatively high flavor thresholds, some, such as the alkan-2-ones, are still important to flavor. They are formed by β-oxidation and decarboxylation of fatty acids, and short-chain (C
5–C
11) alkan-2-ones are highly potent flavor molecules found in numerous plants. They are also assumed to be precursors of aroma-active secondary alcohols [
31]. Only heptan-2-one was identified in the samples, consistent with Jung et al. [
6]. 1-Octen-3-one, along with the corresponding alcohol 1-octen-3-ol, is derived from linoleic acid. These are important components of cheese aroma (with a typical mushroom note) but also occur naturally in some plants and have, surprisingly, been identified as key components of black currant aroma [
6,
12,
13].
Carboxylic acids are important components of plant foods. Acids up to C
10 play a significant role in aroma, not only directly but also as precursors to other aromatic substances (esters, lactones, etc.). Only acetic acid was identified in the samples; it is formed by sugar degradation (glucose, fructose, and sucrose) and, along with malic and citric acids, is a major contributor to fruit sourness [
31].
Sulfur compounds should also be mentioned. Although present at very low concentrations, they are known to contribute to the characteristic flavor of black currants [
15,
19]. They were not detected in the samples, likely because their concentrations were below the detection limit of the method used. Their thermal lability might be another reason for their absence.
In contrast to black currants, there are very few studies on red/white currants. Yu et al. [
18] investigated the chemical composition of various berry fruits (e.g., sea buckthorn, blueberry), including red currants. They identified 18 volatile compounds, of which only the esters methyl, ethyl, and octyl acetate were consistent with our findings. The only more comprehensive study is that of Jung [
26], who studied volatile constituents of fresh red and white currants in greater detail. Using liquid–liquid extraction, they identified a total of 139 compounds in red currants and 92 in white currants. The major classes were C
6 compounds (i.e., C
6 alcohols and aldehydes) and acids, with (Z)-3-hexen-1-ol, (Z)-3-hexenal, (E)-2-hexenal, 1-octen-3-one, acetic acid, linalool, and β-damascenone identified as key components of red currant aroma. With the exception of (Z)-3-hexenal, all of these were also identified in our samples of red/white currants, although not in all varieties (see
Table S1).
In total, 40, 39, and 54 volatile compounds were identified in red, white, and black currants, respectively (see
Table S1). As expected, and consistent with Jung [
26], the red and white currants were quite similar in terms of identified compounds, as they belong to the same species,
R. rubrum, while black currants had the most substances, mainly from the group of terpenoids. Of all identified terpenes, sabinene, δ-3-carene, and rose oxide have not yet been identified in red/white currants and could, therefore, be tentatively considered specific to black currants. Alcohols, aldehydes, and esters were the most numerous in all currant variants (red/white/black). In contrast to black currants, terpenes and terpenoids constituted only a minor compound class in the red variants, which is also consistent with Jung [
26].
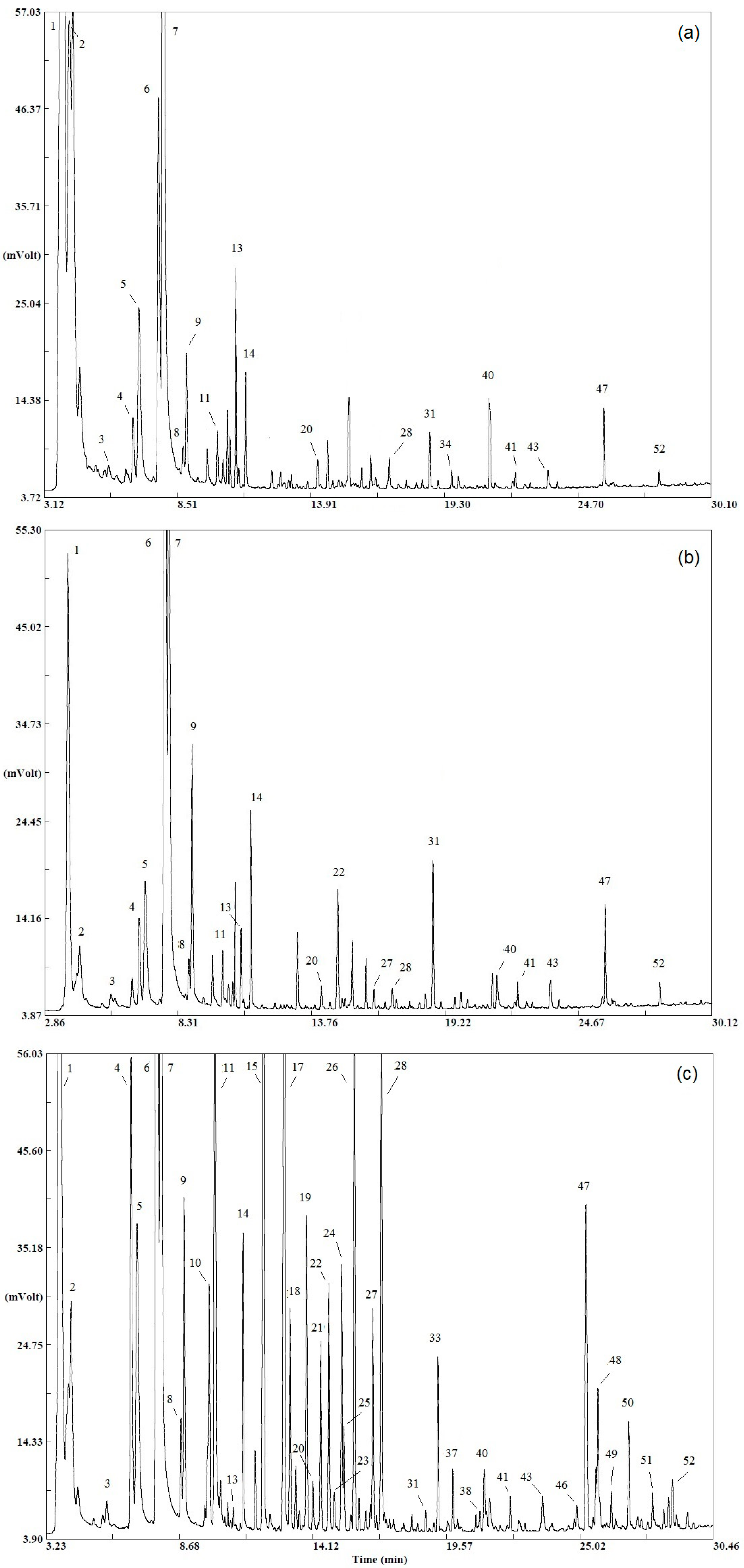
Regarding the individual cultivars within the red/white/black variants, they were similar in terms of identified compounds (see below). Typical HS-SPME-GC-FID chromatograms of compounds identified in selected representatives (the sensorially most acceptable) of red/white/black cultivars are depicted in
Figure 1, showing clear differences in the volatile composition between black and red/white currants.
3.4. Effect of Cultivar on Volatile Composition of Currants
As confirmed by many authors, fruit volatile profiles can be influenced by various pre-harvest (e.g., cultural practices) and post-harvest (e.g., processing, storage) factors [
31]. Considering that the tested currants were cultivated under the same conditions, harvested at full ripeness, and analyzed fresh, the cultivar is the main factor influencing their variability. However, very few publications compare currant cultivars. We can mention studies by Jung et al. [
6], Marsol-Vall et al. [
7], Kampuss et al. [
10], and Liu et al. [
17], who compared several black currant cultivars (none of which overlapped with ours) and confirmed the significant effect of cultivar on aroma compound composition. Only Ruiz del Castillo and Dobson [
11] analyzed differences in the terpene composition of ‘Ben Hope’, a well-established cultivar grown in the UK, describing the terpene fraction as largely associated with the distinctive black currant aroma. In the case of red currants, Jung [
26] analyzed ‘Jonkheer van Tets’, ‘Rovada’, and ‘Tatran’—cultivars also included in our study. Many studies do not mention cultivars at all, meaning that most of the cultivars we analyzed have not yet been the subject of published research.
The variance in the content of compounds identified in red/white/black currants is provided in
Supplementary Material Table S2. Consistent with previous studies, alcohols, aldehydes, and esters were the major classes of volatile compounds and quantitatively predominated in nearly all varieties. In contrast, Jung et al. [
6] also found terpenes to be predominant in several black currant cultivars.
Despite the similar number of compounds identified, significant differences in the proportions of most volatiles were observed between cultivars within both the red/white and black currant groups (see
Table S2). This is consistent with the findings of other authors [
7,
9], who also found differences between cultivars in the quantitative rather than the qualitative composition of compounds.
Comparing individual compound classes, alcohols ranged from 11.5% to 48.5% in red/white currants and were less abundant in black currants (9.2–37.4%). The following alcohols were present in all cultivars (concentration range in parentheses): ethanol (87.6–1090.2 µg/kg), butan-1-ol (0–53.2 µg/kg), pentan-1-ol (0–14.4 µg/kg), hexan-1-ol (4.7–45.8 µg/kg), butan-2-ol (3.4–98.2 µg/kg), and 1-octen-3-ol (0–38.5 µg/kg), with ethanol quantitatively predominating. The differences between cultivars were significant for all detected alcohols except (E)-3-hexen-1-ol (
p = 0.33). This compound was found in minimal concentrations (0–6.2 µg/kg) and only in eight red and black cultivars (see
Table S3). Consistent with Jung [
26], it was not present in white currants. (E)- and (Z)-3-hexen-1-ol, in contrast to Jung [
26], where these compounds were found to be predominant in black currants, were present only in small quantities (<6.6 µg/kg) in our samples.
Aldehydes ranged from 15.1% to 30.2% in red/white cultivars and from 21.1% to 37.4% in black cultivars, with ethanal predominating in nearly all cultivars. Ethanal (19.6–455.7 µg/kg), hexanal (18.9–85.6 µg/kg), 2-methylbutanal (13.7–231.1 µg/kg), 3-methylbutanal (37.3–266.6 µg/kg), octanal (3.3–136.6 µg/kg), and nonanal (0–17.9 µg/kg) were present in all cultivars. Liu et al. [
17] found that hexanal, nonanal, and (E)-2-octenal to significantly contribute to the overall aroma of black currants. These compounds were also predominantly present in our black cultivars. Significant differences were found between cultivars for all detected aldehydes except decanal (
p = 0.09). In contrast to Liu et al. [
17], who considered this compound a significant contributor to black currant aroma, it was found in minimal concentrations (0–6.4 µg/kg) in 10 red and black cultivars.
Esters ranged from 26.3% to 57.0% in red/white cultivars and from 23.5% to 43.4% in black cultivars, with ethyl propanoate significantly predominating in all cultivars. In contrast to Jung [
26], a quantitative preponderance of ethyl esters compared to methyl esters was observed. Nearly all identified esters were present in all cultivars: methyl acetate (0–104.2 µg/kg), ethyl acetate (4.9–38.3 µg/kg), butyl acetate (4.9–112.6 µg/kg), ethyl propanoate (80.9–859.0 µg/kg), methyl butanoate (4.7–57.9 µg/kg), ethyl butanoate (8.7–149.2 µg/kg), and octyl acetate (0–13.2 µg/kg). Consistent with Jung [
26], they varied considerably depending on the cultivar, except for ethyl decanoate (
p = 0.09), which was present in a range of 4.0–34.6 µg/kg, while published studies have found it only in traces in black currants (see
Table S2). Consistent with our results, Liu et al. [
17] and Jung [
26] state that black currant berry profiles are dominated by short-chain esters, mentioning methyl and ethyl butanoate as the main ones, which also significantly prevailed in our black cultivars over the red ones. Harb et al. [
14] also found high amounts of hexyl esters, which were not present in our samples.
Terpenes and terpenoids, the largest group of plant secondary metabolites, are not present in abundant levels in fruits [
17]. They were more typical and abundant in black cultivars (6.0–20.4%) compared to red/white cultivars (1.8–10.6%). Consistent with our results, Jung [
26] and Ruiz del Castillo and Dobson [
9] observed large variations in the proportions of most terpenes and terpenoids. β-Pinene (0–46.6 µg/kg), linalool (3.5–74.5 µg/kg), and β-damascenone (3.0–11.5 µg/kg) were present in all cultivars. Marsol-Vall et al. [
7], consistent with our results, stated that monoterpenoids are the most abundant among terpenoids, with limonene, δ-3-carene, and γ-terpinene prevailing. Liu et al. [
17] also mentioned β-damascenone, but it was only present in small quantities in our samples.
Ketones, consistent with the study by Liu et al. [
17], showed low levels (<4%) in all cultivars, as did acids.
Comparing our results with the literature is difficult because relatively few studies analyze fresh berries. More often, different products are analyzed [
8,
13], and the aromatic profile is then influenced by technological operations such as homogenization, juice extraction, or heat treatment. Moreover, only some studies report exact quantities (µg/kg) [
6,
17,
26]; others express results as relative percentages [
9,
12]. For clarity,
Table S2 shows the concentration range of each compound reported in the literature. As can be seen, the variability of individual compounds between studies is quite large, as in our case, but all coincide on the presence of significant differences between cultivars, mainly in compound content.
Regarding red/white cultivars, the quantitative representation of chemical groups was as follows: esters > alcohols > aldehydes > terpenes > ketones > acids. As mentioned previously, these have been scarcely investigated, and a direct comparison of red/white versus black currants has not yet been published. Jung [
26] is one of the few who analyzed red currants and compared them to black currants. In contrast to our results, they found a high concentration of acetic acid as a distinctive feature of red currants; however, they did not find any esters, while Yu et al. [
18] found esters to make up more than 40% of the aroma profile of
R. rubrum. Similarly, in our study, esters were quantitatively the most important group, constituting 30.8–57.0% in white cultivars and 26.3–49.7% in red cultivars. As in the case of black currants, C
6 compounds formed a significant group, with (E)-2-hexenal predominating, and relatively low concentrations of terpenes were found (<10%). Comparing red versus white currants, the aroma composition was very similar, as also concluded by Jung [
26].
3.5. Integrated Evaluation of Instrumental and Sensory Data Using PCA Analysis
To better visualize the differences between samples (black vs. red vs. white cultivars and the 2020 vs. 2021 seasons) and the relationships between instrumental (volatile composition) and sensory characteristics, all acquired results were subjected to PCA. PCA was performed using the averaged ratings (
n = 20) of all sensory characteristics and the averaged peak area (
n = 2) of identified chemical groups of volatile compounds. The results for the first two principal components (PCs) are shown in
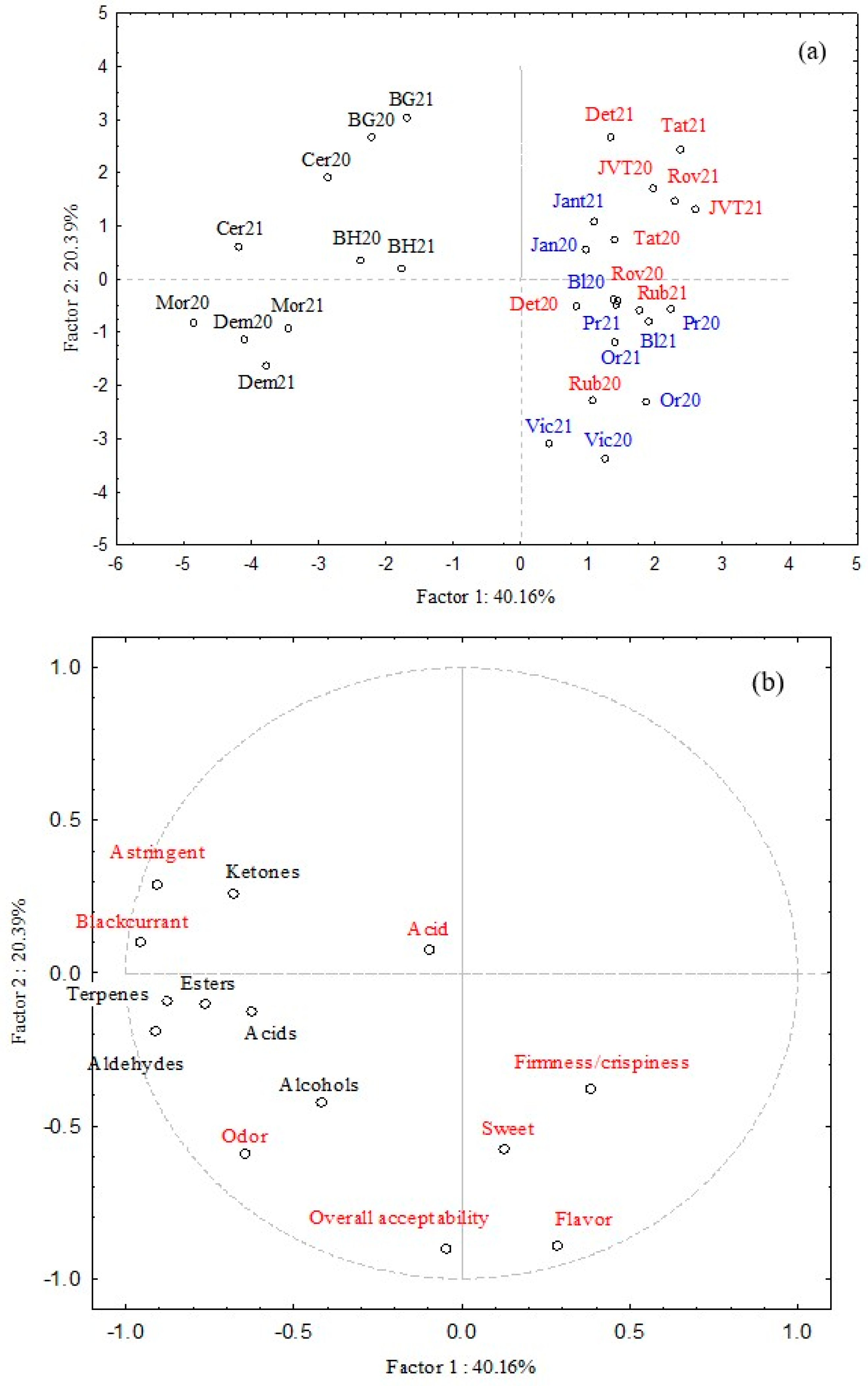
Figure 2. The first three PCs cumulatively explained more than 70% of the total dataset variability.
Figure 2a demonstrates a distinct separation between samples, although seasonal variability (2020 vs. 2021) is less prominent. While individual cultivars cluster closely, they remain clearly differentiated. Principal component 2 (PC2) effectively distinguishes between the two years for all studied cultivars. The influence of season (2020 vs. 2021) on the aroma profile of currants is further illustrated in
Supplementary Material Figure S1 (based on peak area). This figure also highlights the significantly higher total content of compounds, particularly terpenoids, in black currants.
Limited research exists on the effect of season on aroma compounds in currants, and the impact of season on sensory properties, has not been extensively investigated. Marsol-Vall et al. [
7] and Boccorh et al. [
15,
19] reported significant environmental effects, with temperature and radiation identified as key factors. Conversely, Jung et al. [
6,
26] observed consistent levels of selected compounds in black currants across two years. In this study, Kruskal–Wallis and ANOVA analyses revealed significant differences (
p ≤ 0.05), primarily in flavor and odor (see
Table 1). Notably, the previously identified best cultivars (‘Victoria’, ‘Rubigo’, and ‘Demon’) did not exhibit significant differences in sensory properties, indicating their stability and resilience to varying climatic conditions. However, almost all cultivars showed significant differences in aroma composition, especially in alcohol content (see
Table S3), confirming the substantial influence of environmental factors. Pinpointing specific effects remains challenging due to compound-specific variations. Climatic conditions for the harvest years (2020/2021) are summarized in
Supplementary Material Figure S2 for reference.
Although 2020 was generally warmer than 2021 in the Czech Republic, temperatures during the currant ripening period (typically June to August) were comparable, with a maximum difference of approximately 3 °C.
The effect of currant type (red/white/black) is evident (see
Figure 2a), with two distinct clusters. All black currant cultivars are located in the left part of the plot, correlating negatively with PC1. The second cluster, correlating positively with PC1, includes red and white cultivars, indicating their similarity in composition and sensory properties. The red and white cultivar clusters partially overlap, but separation can be observed along PC2. Most red cultivars are located in the upper part, correlating positively with PC2, except for ‘Rubigo’, which was sensorially evaluated as the most acceptable and is, thus, clearly separated from other red cultivars.
White cultivars are at the bottom, correlating with flavor, firmness, and overall acceptability. ‘Jantar’ is the only exception, being considered the least acceptable of the white cultivars. This suggests that white currants were sensorially preferred over red currants, likely due to their sweeter taste, better flavor, and texture, particularly their firmness/crispiness.
PCA also confirmed the distinctly different composition of black currant cultivars, which (unlike red/white cultivars) show a strong correlation with aldehydes, ketones, acids, esters, and terpenes. They are also characterized by their astringent and “blackcurrant” flavor notes [
13,
15,
19], which were strongly correlated with each other. However, these properties correlated negatively with overall flavor, indicating that they are generally perceived negatively by consumers. The flavor of black currants is generally less preferred than that of red/white cultivars. As expected, flavor contributed most significantly to the overall acceptability of the samples. Sweetness correlated negatively with acidity and astringency (see
Figure 2b), which, consequently, contributed negatively to sample acceptability. It can be inferred that assessors/consumers prefer sweeter, less acidic currants.
To estimate which compounds contribute to sample flavor, odor activity values (OAVs)—the ratio of a compound’s concentration to its odor threshold—were calculated. Because currant berries primarily consist of water, odor thresholds in water, obtained from the literature, were used.
In black currants, the following compounds had OAVs ≥ 1 (exact values in parentheses) and were, therefore, considered to contribute to their aroma (aroma descriptions are provided in
Table S1): β-damascenone (1520) > 1-octen-3-one (298) > δ-3-carene (55) > 3-methylbutan-1-ol (30) > (Z)-3-hexenal (17) > ethyl butanoate (12) ≈ ethyl hexanoate (12) ≈ rose oxide (12) > hexanal (9) > ethyl propanoate (8) > 1-octen-3-ol (6) > octanal (5) > nonanal (4) > ethyl heptanoate (2) ≈ (E)-2-octenal (2). The remaining compounds with OAVs < 1 likely make minor or no contributions. This is comparable with Jung [
26], who, based on the OAV concept, detected the C
6 compounds hexanal, (Z)-3-hexenal, (E)-2-hexenal, and (Z)-3-hexenol; the short-chain esters ethyl butanoate and methyl butanoate; and the terpenes 1,8-cineole, α-pinene, rose oxide, 1-octen-3-one, and 1-octen-3-ol as odor-active compounds. Of these, β-damascenone, 3-methylbutan-1-ol, octanal, nonanal, ethyl propanoate, and ethyl hexanoate were also recognized as key components in other studies on black currant berries [
12,
13,
16,
17].
In red/white currants, the following compounds showed OAVs ≥ 1: β-damascenone (>1000) > 1-octen-3-one (>100) > ethyl propanoate (11) > ethyl hexanoate (5–6) > octanal (5) ≈ hexanal (5) > ethyl butanoate (4–5) > 1-octen-3-ol (4) ≈ nonanal (4). Consistent with our results, the only study dealing with OAV values in red currants [
26] identified β-damascenone and 1-octen-3-one as odor-active. However, (E)-2-hexenal and acetic acid, which they found to be important, were detected in concentrations that were too low in our samples, and (Z)-3-hexenal was not detected in our cultivars at all. As shown, β-damascenone (0.002 μg/L) and 1-octen-3-one (0.005 μg/L), compounds with low odor thresholds, had the highest OAVs in all currant variants. According to Liu et al. [
17], fruity, floral, and sweet notes are the major aroma features in currants, to which, according to our results, mainly terpenes and esters with high OAVs likely contribute.