Influencing Factors, Kinetics, and Pathways of Pesticide Degradation by Chlorine Dioxide and Ozone: A Comparative Review
Abstract
1. Introduction
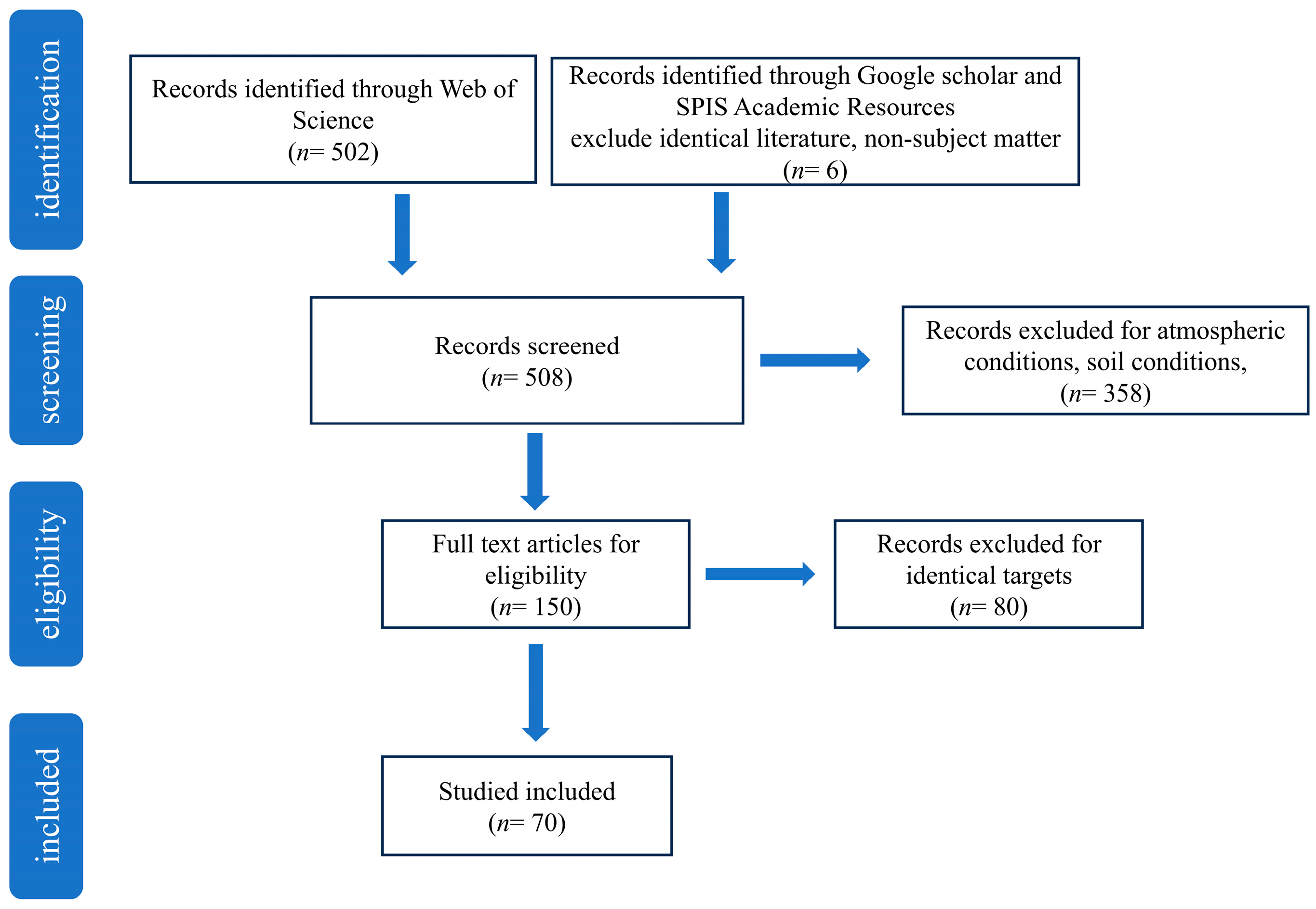
2. Methods
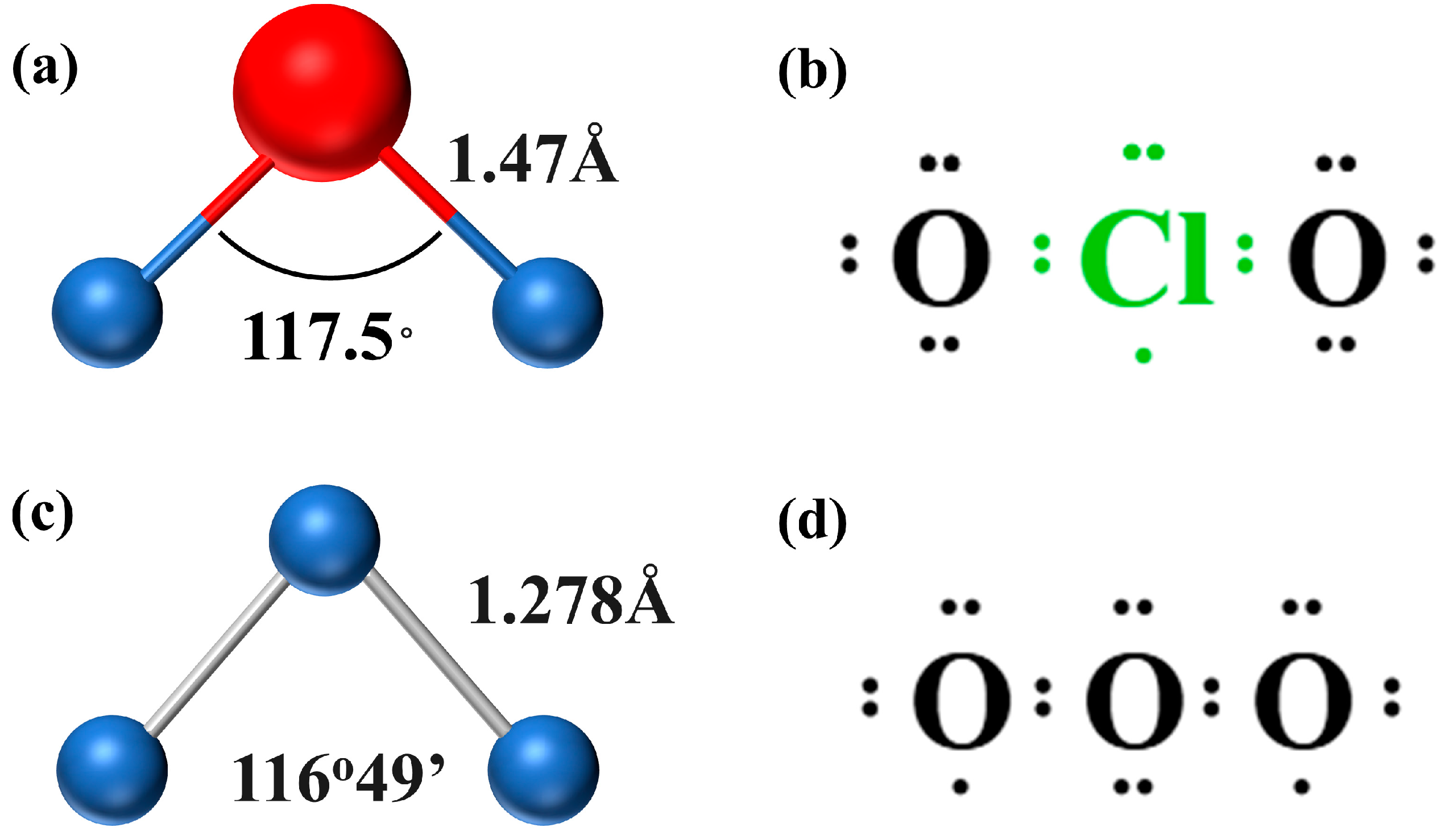
3. Properties and Structure of Chlorine Dioxide and Ozone
3.1. Similarities
3.2. Differences
4. Factors Affecting Pesticide Degradation
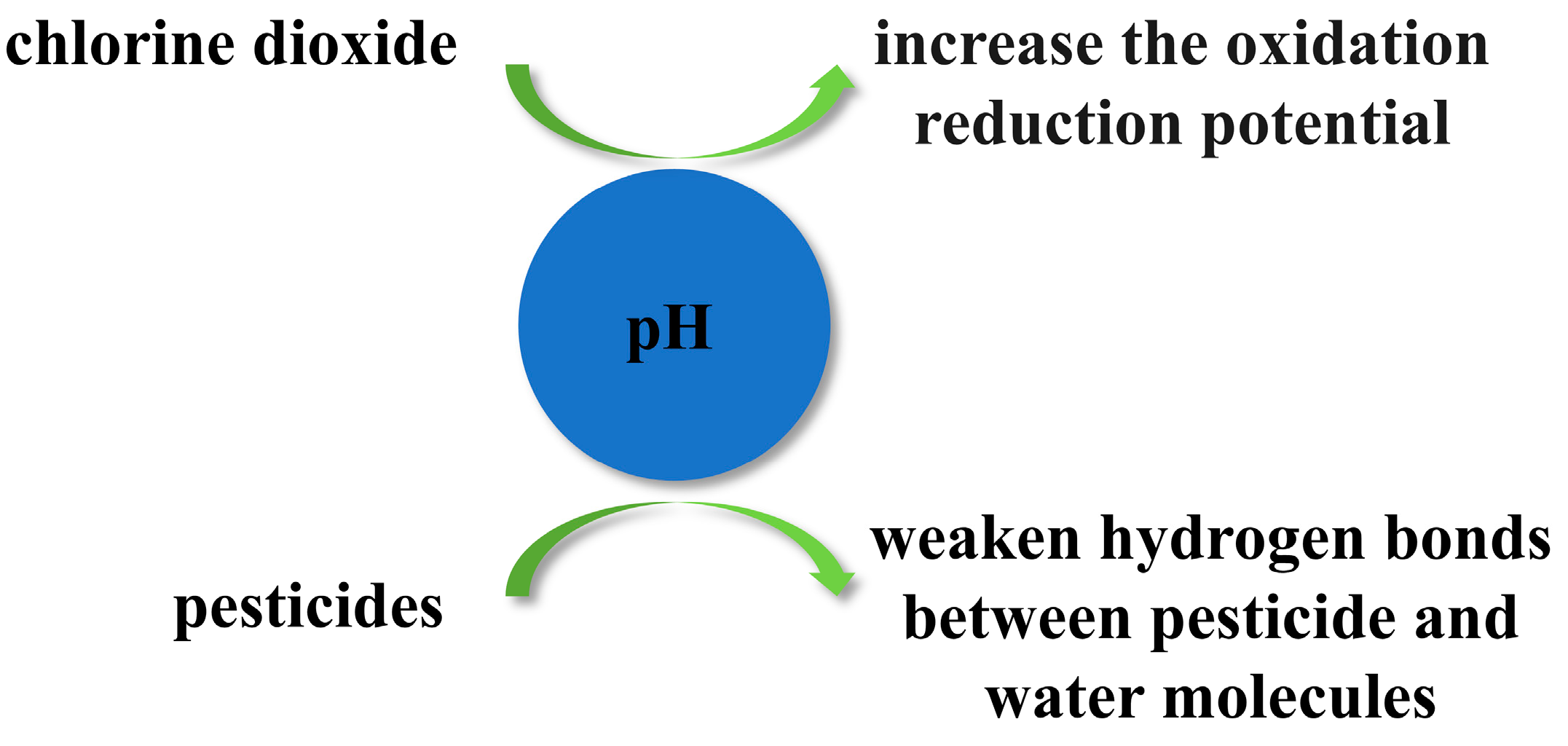
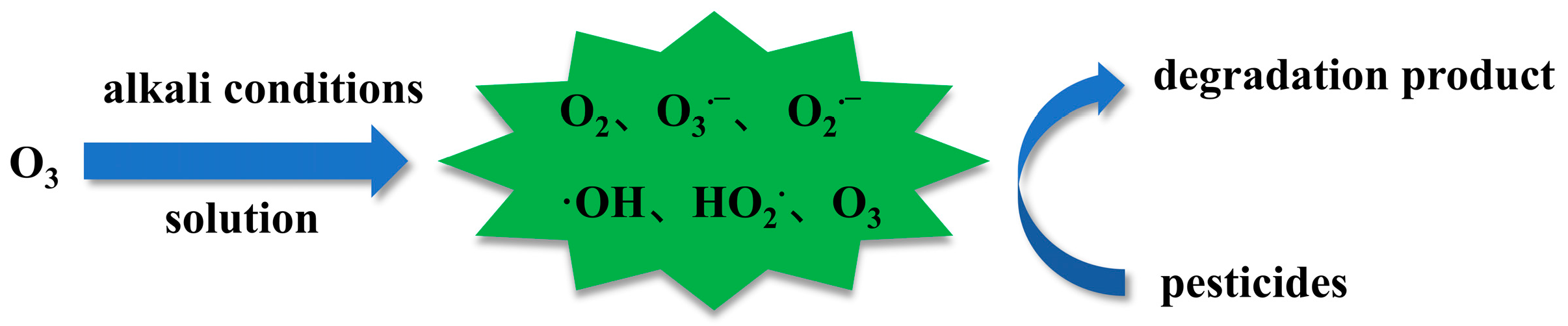
4.1. pH Value
4.2. Concentrations of Chlorine Dioxide and Ozone
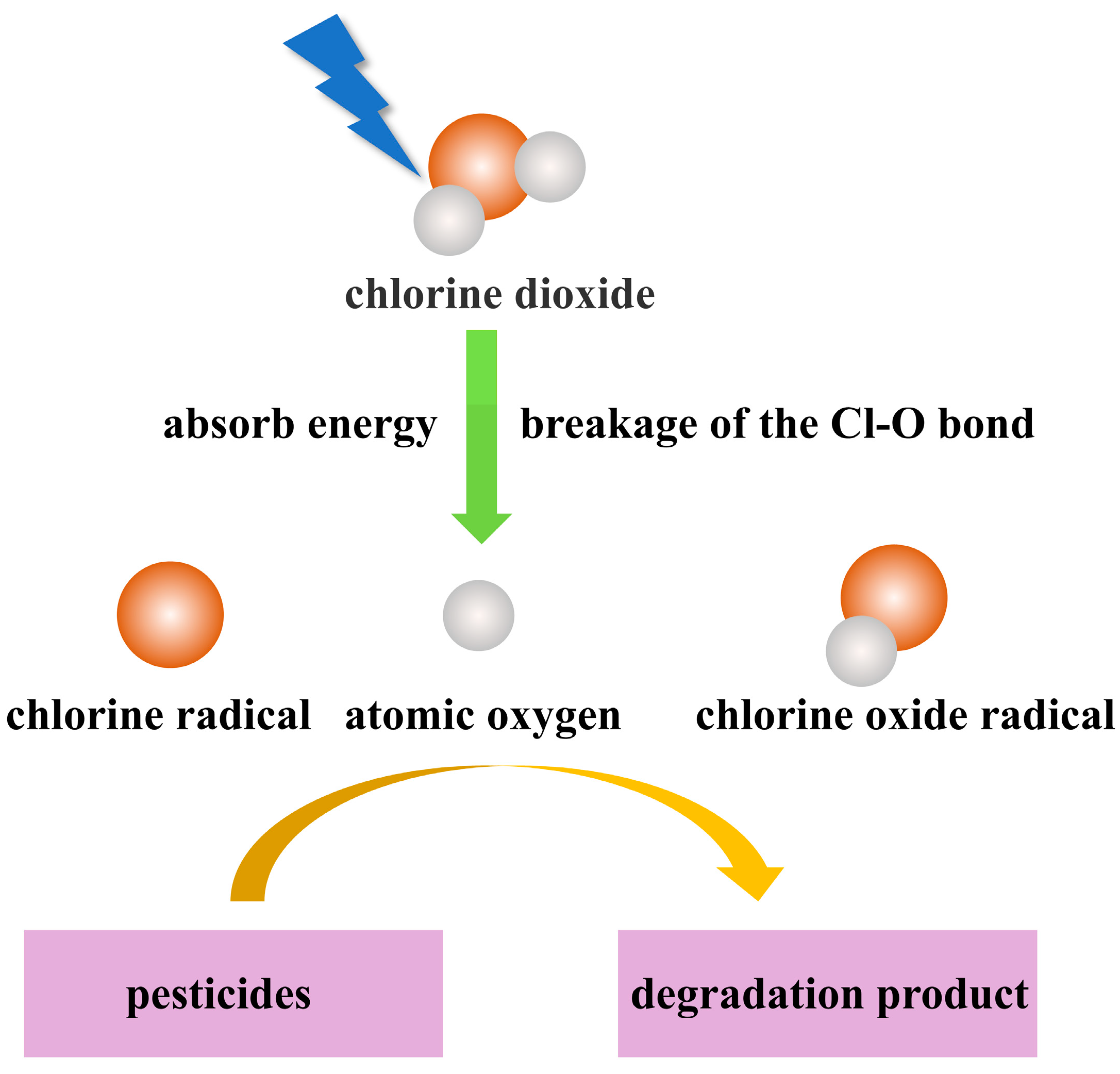
4.3. Light
4.4. Temperature
5. Kinetics of Pesticide Degradation
5.1. Degradation of Pesticides by Chlorine Dioxide
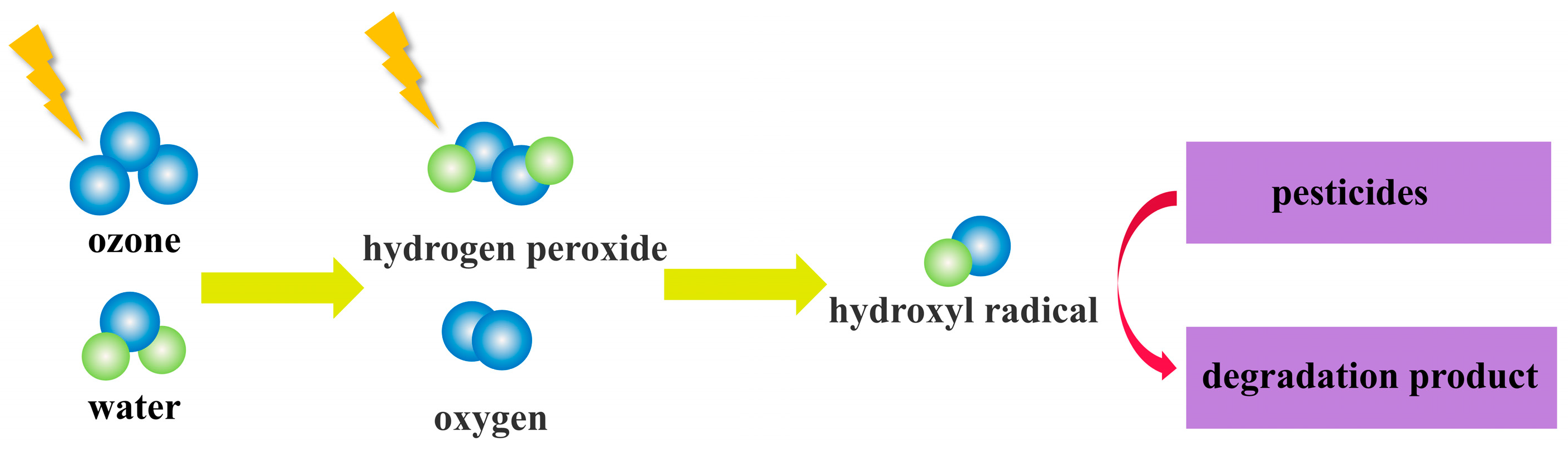
5.2. Degradation of Pesticides by Ozone
| Name | Pesticide Type | Reaction Conditions | Reaction Rate Constant | Reference |
|---|---|---|---|---|
| Thiamethoxam | Nicotine insecticides | tertiary butyl alcohol, pH = 7 | = 3.9 × 109 | [100] |
| Isoprothiolane | Organic sulfur fungicides | tertiary butyl alcohol, pH = 7.5 | = 255.8 | [101] |
| Clothianidin | Nicotine insecticides | tertiary butyl alcohol, pH = 7 | = 3.7 × 109 | [102] |
| Fluopyram | Pyridinylethylbenzamide fungicides | pH = 6.5 | K= 1.002 × 108 | [103] |
| 2-Chloro-N-(2,6-dimethylphenyl) acetamide | Chloroacetamide herbicides | 60 °C | k = 2.40 × 104 | [104] |
| Dichlorvos | Organophosphorus insecticide | tertiary butyl alcohol, pH = 7, 20 °C | = 590 | [39] |
| Alachlor | Amide herbicides | pH = 9.75 | = 3.2 × 1010 | [105] |
| Tembotrione | Triterpenoid herbicides | tertiary butyl alcohol, pH = 7 | = 8.9 × 105 | [106] |
| Sulcotrione | Triterpenoid herbicides | tertiary butyl alcohol, pH = 7 | [106] |
6. Pesticide Degradation Pathways
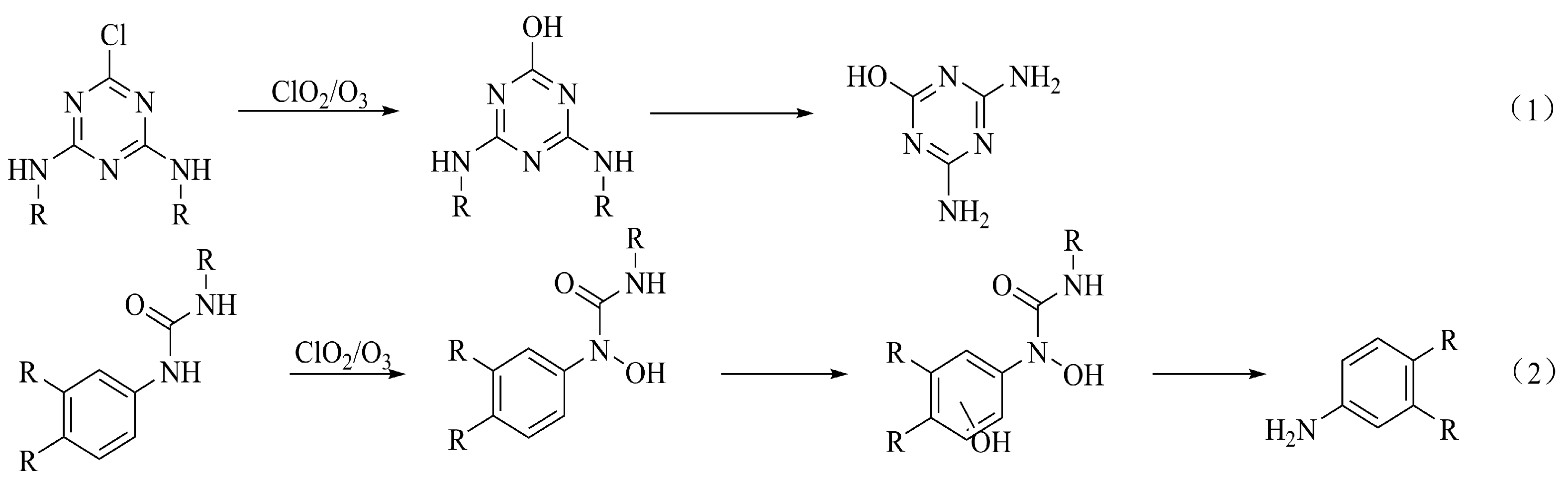
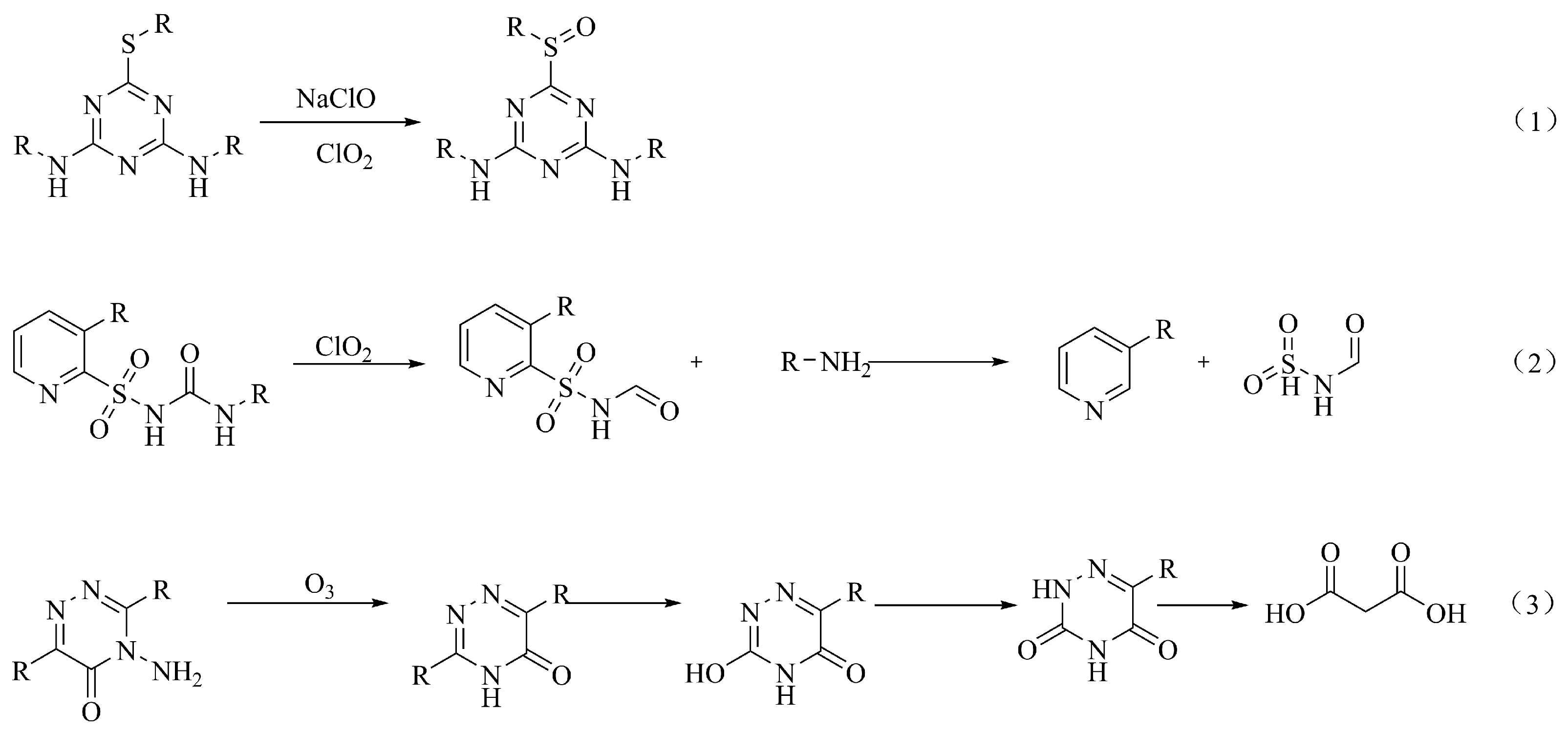
6.1. Herbicides
6.2. Insecticides
6.3. Fungicides
7. Conclusions and Outlook
- (1)
- Currently, there are many studies on the degradation of herbicides and insecticides by chlorine dioxide and ozone, but there is relatively little research on the degradation of fungicides. In the future, emphasis can be placed on the degradation of different types of fungicides.
- (2)
- Current research has focused on the mechanism of pesticide residues in water and on the surface of fruits and vegetables, and not enough attention has been paid to the toxicity of intermediate degradation products, which may be hazardous to human health. Future studies could focus on the toxicity of intermediate degradation products.
- (3)
- Chlorine dioxide and ozone, as oxidants, currently have less research on the degradation of pesticides in gaseous form compared to aqueous solutions. Gaseous chlorine dioxide and ozone are commonly used in the food industry. In the future, we can focus on the degradation of pesticides by gaseous chlorine dioxide and ozone.
- (4)
- Currently, the mechanism of pesticide degradation by chlorine dioxide and ozone requires further investigation. In the process of pesticide degradation, quantum chemical calculation is a means to study the degradation mechanism. In future research, the mechanism of pesticide degradation can be elaborated in more depth by using quantum chemical calculations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations, International Code of Conduct on the Distribution and Use of Pesticides. Available online: http://www.fao.org/3/a-i3604e.pdf (accessed on 10 January 2025).
- Evenson, R.E.; Gollin, D. Assessing the impact of the Green Revolution, 1960 to 2000. J. Sci. 2003, 300, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations, Pesticide Use Data-FAOSTAT. Available online: https://www.fao.org/statistics/highlights-archive/highlights-detail/pesticides-use-and-trade-1990-2022/en (accessed on 1 March 2025).
- El-Nahhal, I.; El-Nahhal, Y. Pesticide residues in drinking water, their potential risk to human health and removal options. J. Environ. Manag. 2021, 299, 113611. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Carrasco Cabrera, L.; Di Piazza, G. The 2021 European Union report on pesticide residues in food. EFSA J. 2023, 21, e07939. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, H.; Li, C.; Qian, H. The present situation of pesticide residues in China and their removal and transformation during food processing. J. Food Chem. 2021, 354, 129552. [Google Scholar] [CrossRef]
- Staley, Z.R.; Harwood, V.J.; Rohr, J.R. A synthesis of the effects of pesticides on microbial persistence in aquatic ecosystems. Crit. Rev. Toxicol. 2015, 45, 813–836. [Google Scholar] [CrossRef]
- Hayes, T.B.; Stuart, A.A.; Mendoza, M.; Collins, A.; Noriega, N.; Vonk, A.; Kpodzo, D. Characterization of atrazine-induced gonadal malformations in African clawed frogs (Xenopus laevis) and comparisons with effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (17β-estradiol): Support for the demasculinization/feminization hypothesis. Environ. Health Perspect. 2006, 114, 134–141. [Google Scholar] [CrossRef]
- Li, X.N.; Zuo, Y.Z.; Qin, L.; Liu, W.; Li, Y.H.; Li, J.L. Atrazine-xenobiotic nuclear receptor interactions induce cardiac inflammation and endoplasmic reticulum stress in quail (Coturnix coturnix coturnix). Chemosphere 2018, 206, 549–559. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, L.; Dou, D.C.; Li, X.N.; Ge, J.; Li, J.L. Atrazine induced oxidative stress and mitochondrial dysfunction in quail (Coturnix C. coturnix) kidney via modulating Nrf2 signaling pathway. Chemosphere 2018, 212, 974–982. [Google Scholar] [CrossRef]
- Green, R.S.; Palatnick, W. Effectiveness of octreotide in a case of refractory sulfonylurea-induced hypoglycemia. J. Emerg. Med. 2003, 25, 283–287. [Google Scholar] [CrossRef]
- Simpson, S.H.; Majumdar, S.R.; Tsuyuki, R.T.; Eurich, D.T.; Johnson, J.A. Dose–response relation between sulfonylurea drugs and mortality in type 2 diabetes mellitus: A population-based cohort study. CMAJ 2006, 174, 169–174. [Google Scholar] [CrossRef]
- Sarikaya, R.; Selvı, M.; Erkoç, F. Investigation of acute toxicity of fenitrothion on peppered corydoras (Corydoras paleatus) (Jenyns, 1842). Chemosphere 2004, 56, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Jokanović, M.; Kosanović, M. Neurotoxic effects in patients poisoned with organophosphorus pesticides. Environ. Toxicol. Pharmacol. 2010, 29, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Jokanović, M. Neurotoxic effects of organophosphorus pesticides and possible association with neurodegenerative diseases in man: A review. Toxicology 2018, 410, 125–131. [Google Scholar] [CrossRef]
- Wacławek, S.; Silvestri, D.; Hrabák, P.; Padil, V.V.; Torres-Mendieta, R.; Wacławek, M.; Dionysiou, D.D. Chemical oxidation and reduction of hexachlorocyclohexanes: A review. Water Res. 2019, 162, 302–319. [Google Scholar] [CrossRef]
- Wu, S.C.; Chang, B.S.; Li, Y.Y. Effect of the coexistence of endosulfan on the lindane biodegradation by Novosphingobium barchaimii and microbial enrichment cultures. Chemosphere 2022, 297, 134063. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Beach, J.; Martin, J.W.; Senthilselvan, A. Pesticide exposures and respiratory health in general populations. J. Environ. Sci. 2017, 51, 361–370. [Google Scholar] [CrossRef]
- Qi, M.; Huang, L.; Xu, X.; Yi, T.; Xu, H.; Zhao, H.; Liu, Y. Synthesis of chlorine dioxide stable solution by combined reduction and its decomposition kinetics. Nord. Pulp Pap. Res. J. 2020, 35, 342–352. [Google Scholar] [CrossRef]
- Peng, X.; Bao, Z.; Zhang, S.; Li, Y.; Ding, L.; Shi, H.; Wang, J. Modulation of Lewis and Brønsted acid centers with oxygen vacancies for Nb2O5 electrocatalysts: Towards highly efficient simultaneously electrochemical ozone and hydrogen peroxide production. Chem. Eng. Sci. 2023, 271, 118573. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wang, T.; Li, C.; Wu, Z. Effects of ozone treatment on pesticide residues in food: A review. Int. J. Food Sci. Technol. 2019, 54, 301–312. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Monteiro, M.M.S.; de Melo Henrique, A.M.; Llanos, J.; Saez, C.; Dos Santos, E.V.; Rodrigo, M.A. A review on the electrochemical production of chlorine dioxide from chlorates and hydrogen peroxide. Curr. Opin. Electrochem. 2021, 27, 100685. [Google Scholar] [CrossRef]
- Chen, Z. Application of chlorine dioxide-based hurdle technology to improve microbial food safety—A review. Int. J. Food Microbiol. 2022, 379, 109848. [Google Scholar] [CrossRef]
- Gavahian, M.; Khaneghah, A.M. Cold plasma as a tool for the elimination of food contaminants: Recent advances and future trends. Crit. Rev. Food Sci. Nutr. 2020, 60, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.M.R.; Ma, H.; Xu, B. Efficacy of ultrasound treatment in the removal of pesticide residues from fresh vegetables: A review. Trends Food Sci. Technol. 2020, 97, 417–432. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Mir, M.M. Current strategies for the reduction of pesticide residues in food products. J. Food Compos. Anal. 2022, 106, 104274. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Kaavya, R.; Khanashyam, A.C. Research trends and emerging physical processing technologies in mitigation of pesticide residues on various food products. Environ. Sci. Pollut. Res. 2022, 29, 45131–45149. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Nunes, M.I. Recent trends and developments in Fenton processes for industrial wastewater treatment—A critical review. Environ. Res. 2021, 197, 110957. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Kim, M.K.; Danish, M.; Jo, W.K. State-of-the-art review on photocatalysis for efficient wastewater treatment: Attractive approach in photocatalyst design and parameters affecting the photocatalytic degradation. Catal. Commun. 2023, 183, 106764. [Google Scholar] [CrossRef]
- Trellu, C.; Vargas, H.O.; Mousset, E.; Oturan, N.; Oturan, M.A. Electrochemical technologies for the treatment of pesticides. Curr. Opin. Electrochem. 2021, 26, 100677. [Google Scholar] [CrossRef]
- Jain, M.; Khan, S.A.; Sharma, K.; Jadhao, P.R.; Pant, K.K.; Ziora, Z.M.; Blaskovich, M.A. Current perspective of innovative strategies for bioremediation of organic pollutants from wastewater. Bioresour. Technol. 2022, 344, 126305. [Google Scholar] [CrossRef]
- Gonçalves, C.R.; da Silva Delabona, P. Strategies for bioremediation of pesticides: Challenges and perspectives of the Brazilian scenario for global application—A review. Environ. Adv. 2022, 8, 100220. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Chen, F.; Zhang, Y.; Liao, X. Chlorine dioxide treatment for the removal of pesticide residues on fresh lettuce and in aqueous solution. J. Food Control. 2014, 40, 106–112. [Google Scholar] [CrossRef]
- Gan, W.; Ge, Y.; Zhong, Y.; Yang, X. The reactions of chlorine dioxide with inorganic and organic compounds in water treatment: Kinetics and mechanisms. Environ. Sci. Water Res. Technol. 2020, 6, 2287–2312. [Google Scholar] [CrossRef]
- Tian, F.; Qiang, Z.; Liu, C.; Zhang, T.; Dong, B. Kinetics and mechanism for methiocarb degradation by chlorine dioxide in aqueous solution. Chemosphere 2010, 79, 646–651. [Google Scholar] [CrossRef]
- Pergal, M.V.; Kodranov, I.D.; Dojčinović, B.; Avdin, V.V.; Stanković, D.M.; Petković, B.B.; Manojlović, D.D. Evaluation of azamethiphos and dimethoate degradation using chlorine dioxide during water treatment. Environ. Sci. Pollut. Res. 2020, 27, 27147–27160. [Google Scholar] [CrossRef]
- Mathon, B.; Coquery, M.; Liu, Z.; Penru, Y.; Guillon, A.; Esperanza, M.; Choubert, J.M. Ozonation of 47 organic micropollutants in secondary treated municipal effluents: Direct and indirect kinetic reaction rates and modelling. Chemosphere 2021, 262, 127969. [Google Scholar] [CrossRef] [PubMed]
- Acero, J.L.; Benitez, F.J.; Real, F.J.; Maya, C. Oxidation of acetamide herbicides in natural waters by ozone and by the combination of ozone/hydrogen peroxide: Kinetic study and process modeling. Ind. Eng. Chem. Res. 2003, 42, 5762–5769. [Google Scholar] [CrossRef]
- Cruz-Alcalde, A.; Sans, C.; Esplugas, S. Priority pesticide dichlorvos removal from water by ozonation process: Reactivity, transformation products and associated toxicity. Sep. Purif. Technol. 2018, 192, 123–129. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Hedberg, K. A Reinvestigation of the Structures of Chlorine Monoxide and Chlorine Dioxide by Electron Diffraction. J. Am. Chem. Soc. 1950, 72, 3108–3112. [Google Scholar] [CrossRef]
- Chlorine Dioxide, Chlorite and Chlorate in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality. Available online: https://www.who.int/docs/default-source/wash-documents/wash-chemicals/chlorine-dioxide-chlorite-chlorate-background-document.pdf (accessed on 25 January 2025).
- Xu, M.Y.; Lin, Y.L.; Zhang, T.Y.; Hu, C.Y.; Tang, Y.L.; Deng, J.; Xu, B. Chlorine dioxide-based oxidation processes for water purification: A review. J. Hazard. Mater. 2022, 436, 129195. [Google Scholar] [CrossRef]
- Ma, X.; Chen, H.; Chen, R.; Hu, X. Direct and activated chlorine dioxide oxidation for micropollutant abatement: A review on kinetics, reactive sites, and degradation pathway. Water 2022, 14, 2028. [Google Scholar] [CrossRef]
- Trambarulo, R.; Ghosh, S.N.; Burrus, C.A., Jr.; Gordy, W. The molecular structure, dipole moment, and g factor of ozone from its microwave spectrum. J. Chem. Phys. 1953, 21, 851–855. [Google Scholar] [CrossRef]
- Kalemos, A.; Mavridis, A. Electronic structure and bonding of ozone. J. Chem. Phys. 2008, 129, 054312. [Google Scholar] [CrossRef]
- Harcourt, R.D.; Skrezenek, F.L.; Wilson, R.M.; Flegg, R.H. Ab initio valence bond calculations for the ground state of ozone. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1986, 82, 495–509. [Google Scholar] [CrossRef]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, M.K.; Gunten, U.V. Mechanisms of phenol ozonation—Kinetics of formation of primary and secondary reaction products. Ozone Sci. Eng. 2009, 31, 201–215. [Google Scholar] [CrossRef]
- Champ, T.B.; Jang, J.H.; Lee, J.L.; Wu, G.; Reynolds, M.A.; Abu-Omar, M.M. Lignin-derived non-heme iron and manganese complexes: Catalysts for the on-demand production of chlorine dioxide in water under mild conditions. Inorg. Chem. 2021, 60, 2905–2913. [Google Scholar] [CrossRef]
- Taube, H.; Dodgen, H. Applications of radioactive chlorine to the study of the mechanisms of reactions involving changes in the oxidation state of chlorine. J. Am. Chem. Soc. 1949, 71, 3330–3336. [Google Scholar] [CrossRef]
- Tran, T.V.; Dobosz, L.M.; Argasinski, J.K. The Effect of Highly Concentrated Chlorine Dioxide on Physical Properties of Fluoropolymers. In Proceedings of the CORROSION 2008, New Orleans, LA, USA, 16–20 March 2008. [Google Scholar]
- Odeh, I.N.; Francisco, J.S.; Margerum, D.W. New pathways for chlorine dioxide decomposition in basic solution. Inorg. Chem. 2002, 41, 6500–6506. [Google Scholar] [CrossRef]
- Couri, D.; Abdel-Rahman, M.S.; Bull, R.J. Toxicological effects of chlorine dioxide, chlorite and chlorate. Environ. Health Perspect. 1982, 46, 13–17. [Google Scholar] [CrossRef]
- Yang, B.; Fang, H.; Chen, B.; Yang, S.; Ye, Z.; Yu, J. Effects of reductive inorganics and NOM on the formation of chlorite in the chlorine dioxide disinfection of drinking water. J. Environ. Sci. 2021, 104, 225–232. [Google Scholar] [CrossRef]
- Gordon, G.; Kieffer, R.G.; Rosenblatt, D.H. The chemistry of chlorine dioxide. Prog. Inorg. Chem. 1972, 15, 201–286. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Greene, A.K.; Seydim, A.C. Use of ozone in the food industry. LWT Food Sci. Technol. 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Sarron, E.; Gadonna-Widehem, P.; Aussenac, T. Ozone treatments for preserving fresh vegetables quality: A critical review. Foods 2021, 10, 605. [Google Scholar] [CrossRef]
- Gerrity, D.; Rosario-Ortiz, F.L.; Wert, E.C. Application of ozone in water and waste water treatment. In Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; Stefan, M.I., Ed.; IWA Publishing: London, UK, 2017. [Google Scholar]
- Wei, C.; Zhang, F.; Hu, Y.; Feng, C.; Wu, H. Ozonation in water treatment: The generation, basic properties of ozone and its practical application. Rev. Chem. Eng. 2017, 33, 49–89. [Google Scholar] [CrossRef]
- Greene, A.K.; Güzel-Seydim, Z.B.; Seydim, A.C. Chemical and physical properties of ozone. In Ozone in Food Processing; O’Donnell, C., Tiwari, B.K., Cullen, P.J., Rice, R.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Rougé, V.; Allard, S.; Croué, J.P.; von Gunten, U. In situ formation of free chlorine during ClO2 treatment: Implications on the formation of disinfection byproducts. Environ. Sci. Technol. 2018, 52, 13421–13429. [Google Scholar] [CrossRef]
- Ben, W.; Shi, Y.; Li, W.; Zhang, Y.; Qiang, Z. Oxidation of sulfonamide antibiotics by chlorine dioxide in water: Kinetics and reaction pathways. Chem. Eng. J. 2017, 327, 743–750. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. Rate constants of reactions of ozone with organic and inorganic compounds in water—II: Dissociating organic compounds. Water Res. 1983, 17, 185–194. [Google Scholar] [CrossRef]
- Tizaoui, C.; Grima, N. Kinetics of the ozone oxidation of Reactive Orange 16 azo-dye in aqueous solution. Chem. Eng. J. 2011, 173, 463–473. [Google Scholar] [CrossRef]
- Ochir, D.; Lee, Y.; Shin, J.; Kim, S.; Kwak, J.; Chon, K. Oxidative treatments of pesticides in rainwater runoff by HOCl, O3, and O3/H2O2: Effects of pH, humic acids and inorganic matters. Separations 2021, 8, 101. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Liu, M.X.; Sang, Y.X.; Kang, C.Y.; Wang, X.H. Degradation of prometryn in Ruditapes philippinarum using ozonation: Influencing factors, degradation mechanism, pathway and toxicity assessment. Chemosphere 2020, 248, 126018. [Google Scholar] [CrossRef]
- Pergal, M.V.; Kodranov, I.D.; Pergal, M.M.; Dojčinović, B.P.; Stanković, D.M.; Petković, B.B.; Manojlović, D.D. Assessment of degradation of sulfonylurea herbicides in water by chlorine dioxide. Water Air Soil Pollut. 2018, 229, 287. [Google Scholar] [CrossRef]
- Sorlini, S.; Gialdini, F.; Biasibetti, M.; Collivignarelli, C. Influence of drinking water treatments on chlorine dioxide consumption and chlorite/chlorate formation. Water Res. 2014, 54, 44–52. [Google Scholar] [CrossRef]
- Gordon, G.; Rosenblatt, A.A. Chlorine dioxide: The current state of the art. Ozone Sci. Eng. 2005, 27, 203–207. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giuggioli, N.; Maghenzani, M.; Peano, C.; Giacalone, G. Improving storability of strawberries with gaseous chlorine dioxide in perforated clamshell packaging. Pol. J. Food Nutr. Sci. 2018, 68, 141–148. [Google Scholar] [CrossRef]
- Malka, S.K.; Park, M.H. Fresh produce safety and quality: Chlorine dioxide’s role. Front. Plant Sci. 2022, 12, 775629. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lin, Y.J.; Kuo, W.C. Pesticide residue removal from vegetables by ozonation. J. Food Eng. 2013, 114, 404–411. [Google Scholar] [CrossRef]
- de Souza, L.P.; Faroni, L.R.D.A.; Heleno, F.F.; Pinto, F.G.; de Queiroz, M.E.L.R.; Prates, L.H.F. Ozone treatment for pesticide removal from carrots: Optimization by response surface methodology. Food Chem. 2018, 243, 435–441. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Kaavya, R.; Jayanath, Y.; Veenuttranon, K.; Lueprasitsakul, P.; Divya, V.; Ramesh, S.V. Ozone as a novel emerging technology for the dissipation of pesticide residues in foods—A review. Trends Food Sci. Technol. 2020, 97, 38–54. [Google Scholar] [CrossRef]
- Aslam, R.; Alam, M.S.; Saeed, P.A. Sanitization potential of ozone and its role in postharvest quality management of fruits and vegetables. Food Eng. Rev. 2020, 12, 48–67. [Google Scholar] [CrossRef]
- Korn, C.; Andrews, R.C.; Escobar, M.D. Development of chlorine dioxide-related by-product models for drinking water treatment. Water Res. 2002, 36, 330–342. [Google Scholar] [CrossRef]
- Cosson, H.; Ernst, W.R. Photodecomposition of chlorine dioxide and sodium chlorite in aqueous solution by irradiation with ultraviolet light. Ind. Eng. Chem. Res. 1994, 33, 1468–1475. [Google Scholar] [CrossRef]
- Kong, Q.; Fan, M.; Yin, R.; Zhang, X.; Lei, Y.; Shang, C.; Yang, X. Micropollutant abatement and byproduct formation during the co-exposure of chlorine dioxide (ClO2) and UVC radiation. J. Hazard. Mater. 2021, 419, 126424. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.H.; Wu, K.L.; Lin, W.C.; Shi, H.J. Photolysis of chlorine dioxide under UVA irradiation: Radical formation, application in treating micropollutants, formation of disinfection byproducts, and toxicity under scenarios relevant to potable reuse and drinking water. Environ. Sci. Technol. 2022, 56, 2593–2604. [Google Scholar] [CrossRef]
- Chen, H.; Lin, T.; Wang, P.; Wang, Y.; Wei, W.; Zhu, S. A novel solar-activated chlorine dioxide process for atrazine degradation in drinking water. Water Res. 2023, 239, 120056. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.; Bérubé, P.R. Removal of disinfection by-product precursors with ozone-UV advanced oxidation process. Water Res. 2005, 39, 2136–2144. [Google Scholar] [CrossRef]
- Peyton, G.R.; Glaze, W.H. Destruction of pollutants in water with ozone in combination with ultraviolet radiation. 3. Photolysis of aqueous ozone. Environ. Sci. Technol. 1988, 22, 761–767. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Benitez, F.J.; Acero, J.L.; Real, F.J. Degradation of carbofuran by using ozone, UV radiation and advanced oxidation processes. J. Hazard. Mater. 2002, 89, 51–65. [Google Scholar] [CrossRef]
- Rajeswari, R.; Kanmani, S. Comparative study on photocatalytic oxidation and photolytic ozonation for the degradation of pesticide wastewaters. Desalination Water Treat. 2010, 19, 301–306. [Google Scholar] [CrossRef]
- Misra, N.N. The contribution of non-thermal and advanced oxidation technologies towards dissipation of pesticide residues. Trends Food Sci. Technol. 2015, 45, 229–244. [Google Scholar] [CrossRef]
- Ikeura, H.; Kobayashi, F.; Tamaki, M. Ozone microbubble treatment at various water temperatures for the removal of residual pesticides with negligible effects on the physical properties of lettuce and cherry tomatoes. J. Food Sci. 2013, 78, 350–355. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. Kinetics of reactions of chlorine dioxide (OClO) in water—I. Rate constants for inorganic and organic compounds. Water Res. 1994, 28, 45–55. [Google Scholar] [CrossRef]
- Pang, R.; Li, N.; Hou, Z.; Huang, J.; Yue, C.; Cai, Y.; Song, J. Degradation of sulfonamide antibiotics and a structurally related compound by chlorine dioxide: Efficiency, kinetics, potential products and pathways. Chem. Eng. J. 2023, 451, 138502. [Google Scholar] [CrossRef]
- He, G.; Zhang, T.; Li, Y.; Li, J.; Chen, F.; Hu, J.; Dong, F. Comparison of fleroxacin oxidation by chlorine and chlorine dioxide: Kinetics, mechanism and halogenated DBPs formation. Chemosphere 2022, 286, 131585. [Google Scholar] [CrossRef]
- Benitez, F.J.; Beltran-Heredia, J.; Gonzalez, T.; Lara, P. Oxidation of Fenuron by chlorine dioxide. J. Environ. Sci. Health Part A 1992, 27, 643–662. [Google Scholar] [CrossRef]
- UNFAO (Food and Agriculture Organization of the United Nations); WHO (World Health Organization); Methiocarb (132). Pesticide residues in food—1999: Toxicological evaluations. In Proceedings of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group, Rome, Italy, 20–29 September 1999; pp. 531–601. [Google Scholar]
- Wang, H.L.; Dong, J.; Jiang, W.F. Study on the treatment of 2-sec-butyl-4, 6-dinitrophenol (DNBP) wastewater by ClO2 in the presence of aluminum oxide as catalyst. J. Hazard. Mater. 2010, 183, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Hoigne, J.; Bader, H. The role of hydroxyl radical reactions in ozonation processes in aqueous solutions. Water Res. 1976, 10, 377–386. [Google Scholar] [CrossRef]
- Chelme-Ayala, P.; El-Din, M.G.; Smith, D.W. Kinetics and mechanism of the degradation of two pesticides in aqueous solutions by ozonation. Chemosphere 2010, 78, 557–562. [Google Scholar] [CrossRef]
- Broséus, R.; Vincent, S.; Aboulfadl, K.; Daneshvar, A.; Sauvé, S.; Barbeau, B.; Prévost, M. Ozone oxidation of pharmaceuticals, endocrine disruptors and pesticides during drinking water treatment. Water Res. 2009, 43, 4707–4717. [Google Scholar] [CrossRef]
- Sintuya, P.; Narkprasom, K.; Jaturonglumlert, S.; Whangchai, N.; Peng-Ont, D.; Varith, J. Effect of gaseous ozone fumigation on organophosphate pesticide degradation of dried chilies. Ozone Sci. Eng. 2018, 40, 473–481. [Google Scholar] [CrossRef]
- de Freitas, R.D.S.; Faroni, L.R.D.A.; de Queiroz, M.E.L.R.; Heleno, F.F.; Prates, L.H.F. Degradation kinetics of pirimiphos-methyl residues in maize grains exposed to ozone gas. J. Stored Prod. Res. 2017, 74, 1–5. [Google Scholar] [CrossRef]
- Fan, X.D.; Zhang, W.L.; Xiao, H.Y.; Qiu, T.Q.; Jiang, J.G. Effects of ultrasound combined with ozone on the degradation of organophosphorus pesticide residues on lettuce. RSC Adv. 2015, 5, 45622–45630. [Google Scholar] [CrossRef]
- Wang, H.; Zhan, J.; Gao, L.; Yu, G.; Komarneni, S.; Wang, Y. Kinetics and mechanism of thiamethoxam abatement by ozonation and ozone-based advanced oxidation processes. J. Hazard. Mater. 2020, 390, 122180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Yang, H.; Wang, X.; Xie, Y. Oxidation of isoprothiolane by ozone and chlorine: Reaction kinetics and mechanism. Chemosphere 2019, 232, 516–525. [Google Scholar] [CrossRef]
- Sales-Alba, A.; Cruz-Alcalde, A.; López-Vinent, N.; Cruz, L.; Sans, C. Removal of neonicotinoid insecticide clothianidin from water by ozone-based oxidation: Kinetics and transformation products. Sep. Purif. Technol. 2023, 316, 123735. [Google Scholar] [CrossRef]
- Li, C.; Yuan, S.; Jiang, F.; Xie, Y.; Guo, Y.; Yu, H.; Yao, W. Degradation of fluopyram in water under ozone enhanced microbubbles: Kinetics, degradation products, reaction mechanism, and toxicity evaluation. Chemosphere 2020, 258, 127216. [Google Scholar] [CrossRef] [PubMed]
- Phan Dang, C.T.; Truong, D.H.; Nguyen, T.L.A.; Taamalli, S.; El Bakali, A.; Louis, F.; Dao, D.Q. Oxidation of metazachlor herbicide by ozone in the gas and aqueous phases: Mechanistic, kinetics, and ecotoxicity studies. Environ. Sci. Pollut. Res. 2024, 31, 49427–49439. [Google Scholar] [CrossRef]
- Beltrán, F.J.; González, M.; Rivas, F.J.; Acedo, B. Determination of kinetic parameters of ozone during oxidations of alachlor in water. Water Environ. Res. 2000, 72, 689–697. [Google Scholar] [CrossRef]
- Tawk, A.; Deborde, M.; Labanowski, J.; Thibaudeau, S.; Gallard, H. Transformation of the Β-triketone pesticides tembotrione and sulcotrione by reactions with ozone: Kinetic study, transformation products, toxicity and biodegradability. Ozone Sci. Eng. 2017, 39, 3–13. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, H.; Peng, P.; Bo, H. Degradation and transformation of atrazine under catalyzed ozonation process with TiO2 as catalyst. J. Hazard. Mater. 2014, 279, 444–451. [Google Scholar] [CrossRef]
- Tian, F.X.; Xu, B.; Zhang, T.Y.; Gao, N.Y. Degradation of phenylurea herbicides by chlorine dioxide and formation of disinfection by-products during subsequent chlor (am) ination. Chem. Eng. J. 2014, 258, 210–217. [Google Scholar] [CrossRef]
- Lopez, A.; James, H.; Fielding, M. By-products formation during degradation of isoproturon in aqueous solution. I: Ozonation. Water Res. 2001, 35, 1695–1704. [Google Scholar] [CrossRef]
- Lopez, A.; Mascolo, G.; Tiravanti, G.; Passino, R. Degradation of herbicides (ametryn and isoproturon) during water disinfection by means of two oxidants (hypochlorite and chlorine dioxide). Water Sci. Technol. 1997, 35, 129–136. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Z.; He, Y.; Dong, S.; Dong, F.; He, Z.; Ma, J. Metribuzin and metamitron degradation using catalytic ozonation over tourmaline: Kinetics, degradation pathway, and toxicity. Sep. Purif. Technol. 2023, 309, 123028. [Google Scholar] [CrossRef]
- Pergal, M.V.; Kodranov, I.D.; Pergal, M.M.; Gašić, U.; Stanković, D.M.; Petković, B.B.; Manojlović, D.D. Degradation products, mineralization, and toxicity assessment of pesticides malathion and fenitrothion. Water Air Soil Pollut. 2020, 231, 433. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Wang, J.; Wu, Z.; Bai, B.; Tian, J.; Wu, Z. Degradation of malathion and carbosulfan by ozone water and analysis of their by-products. J. Sci. Food Agric. 2022, 102, 7072–7078. [Google Scholar] [CrossRef]
- Cruz-Alcalde, A.; Sans, C.; Esplugas, S. Exploring ozonation as treatment alternative for methiocarb and formed transformation products abatement. Chemosphere 2017, 186, 725–732. [Google Scholar] [CrossRef]
- Savi, G.D.; Piacentini, K.C.; Bortolotto, T.; Scussel, V.M. Degradation of bifenthrin and pirimiphos-methyl residues in stored wheat grains (Triticum aestivum L.) by ozonation. Food Chem. 2016, 203, 246–251. [Google Scholar] [CrossRef]
- Begum, A.; Gautam, S.K. Endosulfan and lindane degradation using ozonation. Environ. Technol. 2012, 33, 943–949. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Tiemur, A.; Wang, J.; Wu, B. Degradation of pesticide residues by gaseous chlorine dioxide on table grapes. Postharvest Biol. Technol. 2018, 137, 142–148. [Google Scholar] [CrossRef]
- Bourgin, M.; Violleau, F.; Debrauwer, L.; Albet, J. Kinetic aspects and identification of by-products during the ozonation of bitertanol in agricultural wastewaters. Chemosphere 2013, 90, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhu, M.; Lu, G. Oxidant-mediated radical reactions of the azole fungicide TEB in aquatic media: Degradation mechanism and toxicity evolution. Chemosphere 2024, 351, 141263. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tang, C.; Liu, Y.; Chen, J. Mechanism and kinetic analysis of the degradation of atrazine by O3/H2O2. Water 2022, 14, 1412. [Google Scholar] [CrossRef]
- Bavasso, I.; Montanaro, D.; Di Palma, L.; Petrucci, E. Electrochemically assisted decomposition of ozone for degradation and mineralization of Diuron. Electrochim. Acta 2020, 331, 135423. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, L.; Ma, J.; Ye, F. Ozonation Degradation Organophosphorus Pesticides in Water. In Proceedings of the 2011 International Conference on Computer Distributed Control and Intelligent Environmental Monitoring, Changsha, China, 19–20 February 2011; IEEE: New York, NY, USA; pp. 2130–2132. [Google Scholar]
- Rokbani, O.; Fattouch, S.; Chakir, A.; Roth, E. Heterogeneous oxidation of two triazole pesticides (diniconazole and tebuconazole) by OH-radicals and ozone. Sci. Total Environ. 2019, 694, 133745. [Google Scholar] [CrossRef]

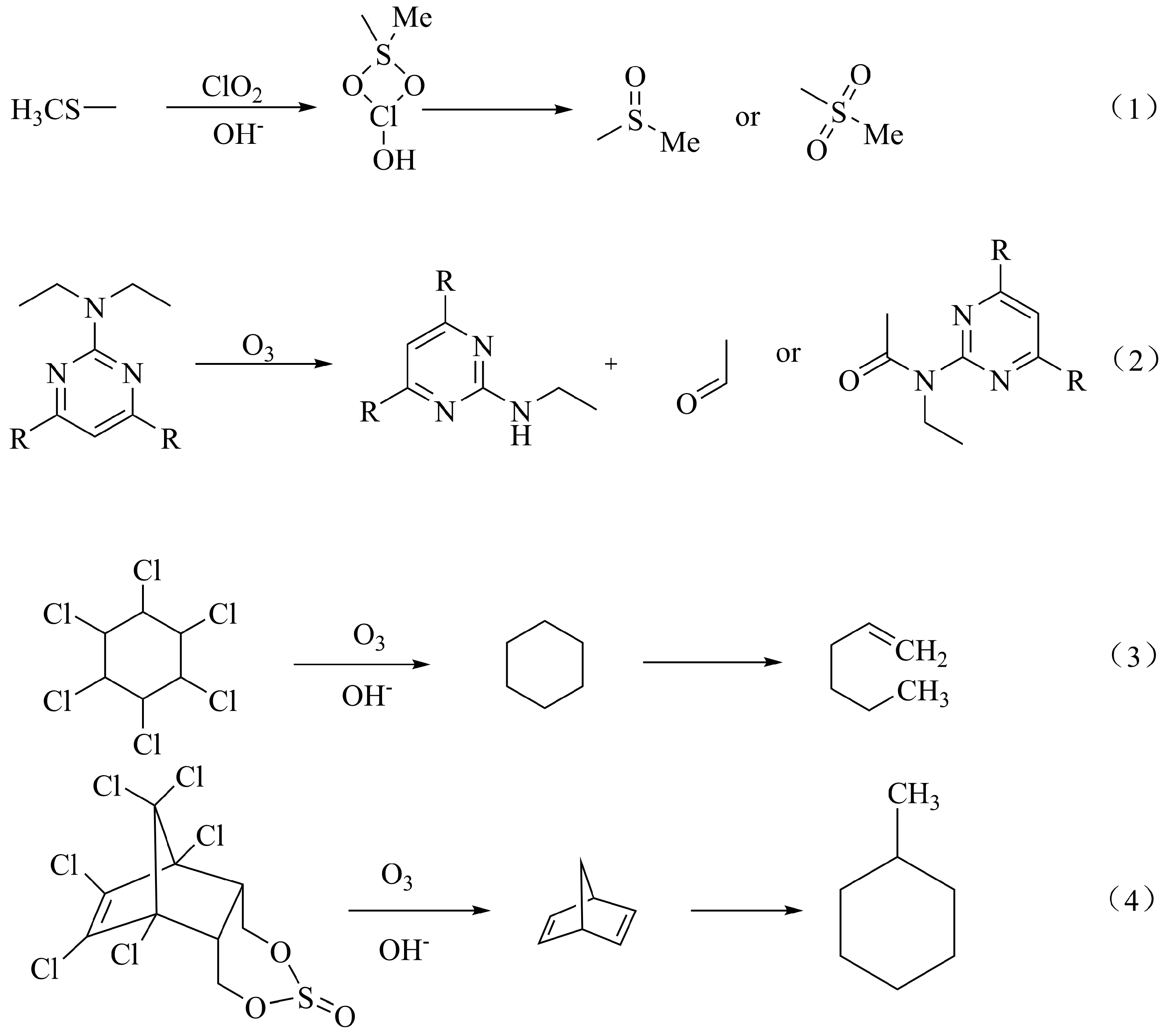
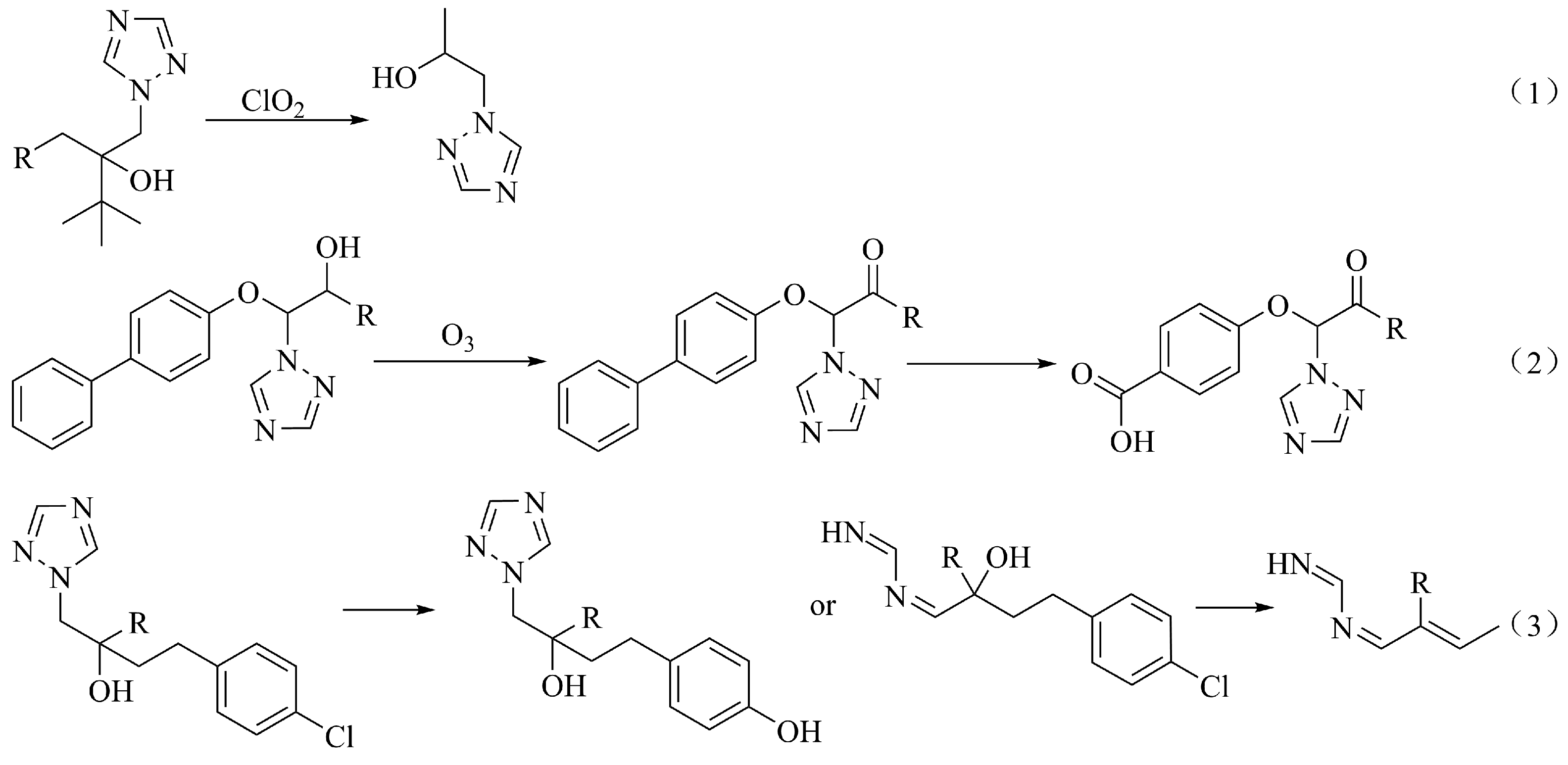
| Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| cold plasma | high efficiency, non-thermal nature, and wide range of applicability | high cost of equipment and immature technology | [24] |
| ultrasound | fast, efficient, and non-polluting | device immaturity, energy consumption, and noise hazard | [25] |
| irradiation | non-polluting and highly efficient | high capital investment and high facility requirements | [26] |
| pulsed electric field | short processing time | high costs and inability to handle solids | [27] |
| Fenton | low energy consumption, low cost, and high efficiency | iron sludge production and catalyst wastage | [28] |
| photocatalysis | non-toxic and chemically stable | catalyst selectivity and high cost of efficient photocatalysts | [29] |
| electrochemical | wide degradation range and no toxic by-products | electric power systems are not practical for large-scale application | [30] |
| bioremediation | green, non-toxic, and completely degradable | microorganisms promote growth under harsh conditions; immature process for large-scale use | [31,32] |
| Pesticide | Chlorine Dioxide | Ozone | Reference |
|---|---|---|---|
| atrazine | C(ClO2) = 100 μM, solar, 30 min, degradation 100% | C(O3/H2O2) = 20 mol/L pH = 7, degradation 92.59% | [80,120] |
| diuron | C(ClO2) = 94 μM, pH = 4, 2 min, degradation 97.8% | C(O3) = 34.8 mg/L pH = 3, 2 h, degradation 20% | [108,121] |
| nicosulfuron | C(ClO2) = 10 mg/L, pH = 3, 6 h, degradation 92.77% | none | [67] |
| dimethoate | C(ClO2) = 10 mg/L, 6 h, light, degradation 98.22% | C(O3) = 10mg/L, 15 min, degradation 60.3% | [36,122] |
| methiocarb | C(ClO2) = 63 μM, pH = 7.4, 6 h, 22 °C degradation 78.21% | C(O3) = 2.8 mg/L, pH = 7, 22 °C, degradation 100% | [35,114] |
| tebuconazole | 0 °C, 24 d, degradation 31.29% | C(O3) = 0.4 ppm, 25 °C small degradation rate | [117,123] |
| lindane | none | 57 mg/minO3, pH = 12, degradation 82% | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Jin, R.; Qiao, Y.; Liu, J.; He, Z.; Jia, M.; Jiang, Y. Influencing Factors, Kinetics, and Pathways of Pesticide Degradation by Chlorine Dioxide and Ozone: A Comparative Review. Appl. Sci. 2025, 15, 5154. https://doi.org/10.3390/app15095154
Liu Z, Jin R, Qiao Y, Liu J, He Z, Jia M, Jiang Y. Influencing Factors, Kinetics, and Pathways of Pesticide Degradation by Chlorine Dioxide and Ozone: A Comparative Review. Applied Sciences. 2025; 15(9):5154. https://doi.org/10.3390/app15095154
Chicago/Turabian StyleLiu, Zhaoguo, Riya Jin, Yina Qiao, Jiaoqin Liu, Zengdi He, Mengye Jia, and Yu Jiang. 2025. "Influencing Factors, Kinetics, and Pathways of Pesticide Degradation by Chlorine Dioxide and Ozone: A Comparative Review" Applied Sciences 15, no. 9: 5154. https://doi.org/10.3390/app15095154
APA StyleLiu, Z., Jin, R., Qiao, Y., Liu, J., He, Z., Jia, M., & Jiang, Y. (2025). Influencing Factors, Kinetics, and Pathways of Pesticide Degradation by Chlorine Dioxide and Ozone: A Comparative Review. Applied Sciences, 15(9), 5154. https://doi.org/10.3390/app15095154





