Alkali-Treated, Nanostructured-Micro-Porous Titanium Surfaces Enhance Osteogenic Differentiation of Adipose Derived Stem Cells
Abstract
1. Introduction
2. Materials and Methods
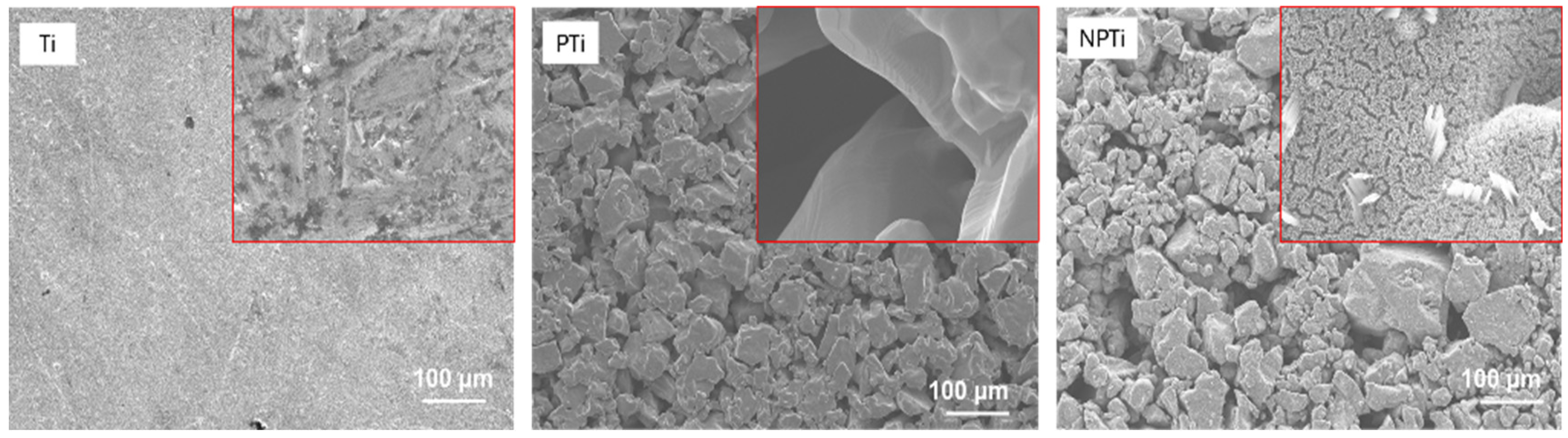
2.1. Fabrication of Nanostructured-Micro-Porous Titanium Surfaces
2.2. Characterization of Nanostructured-Micro-Porous Titanium Surfaces
2.3. Adipose Derived Stem Cell (ADSC) Culture
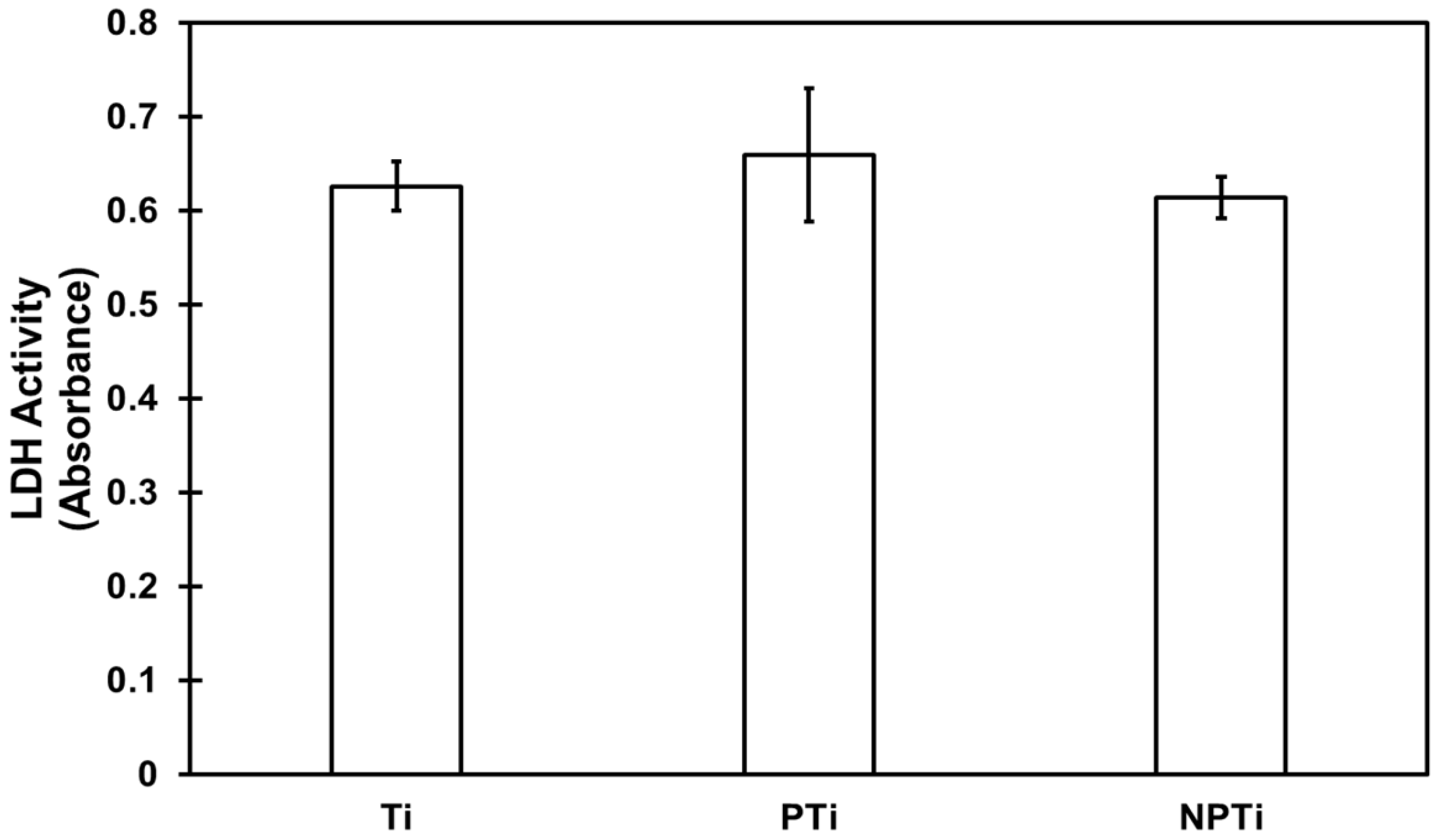
2.4. Cytotoxicity of Different Surfaces
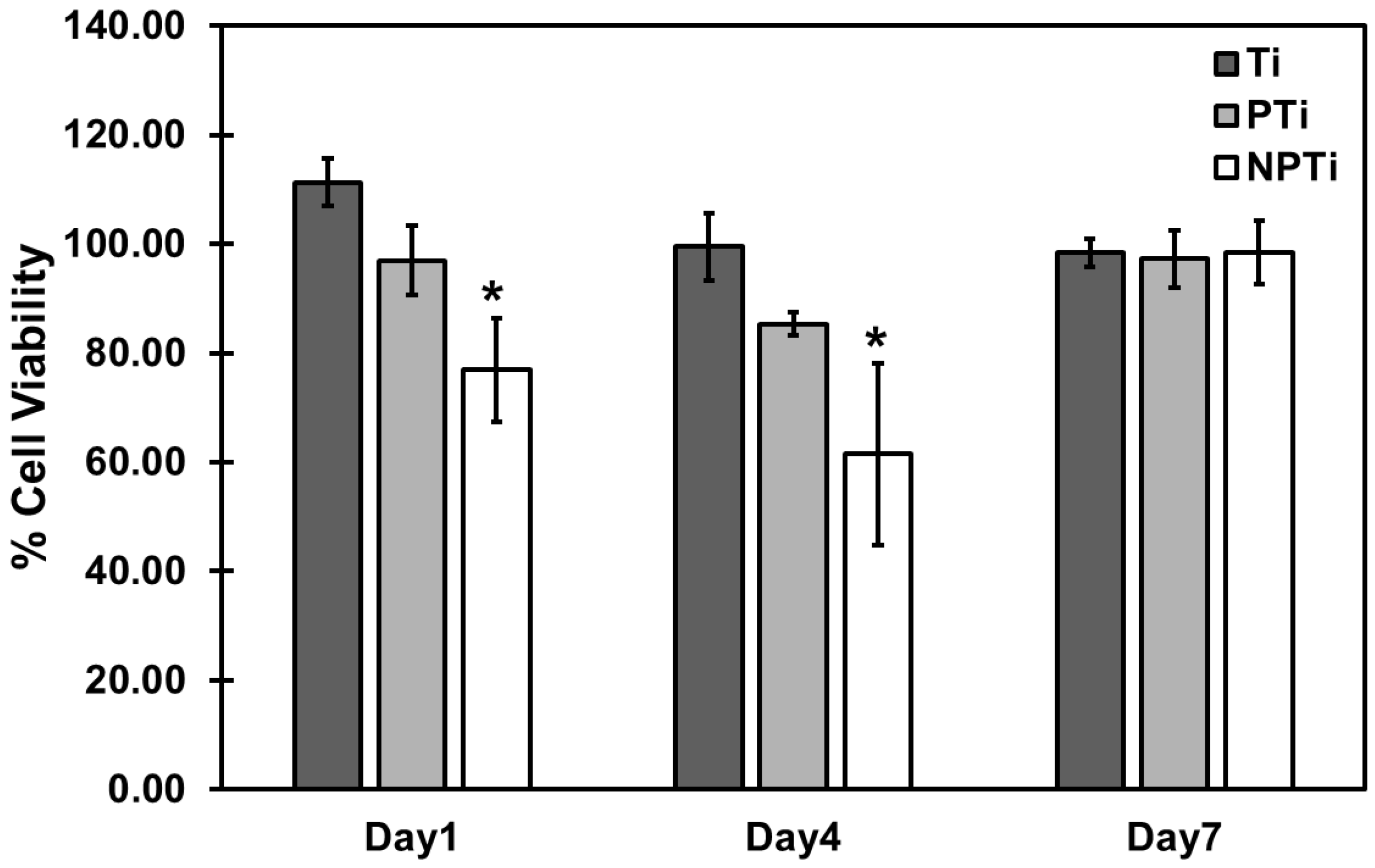
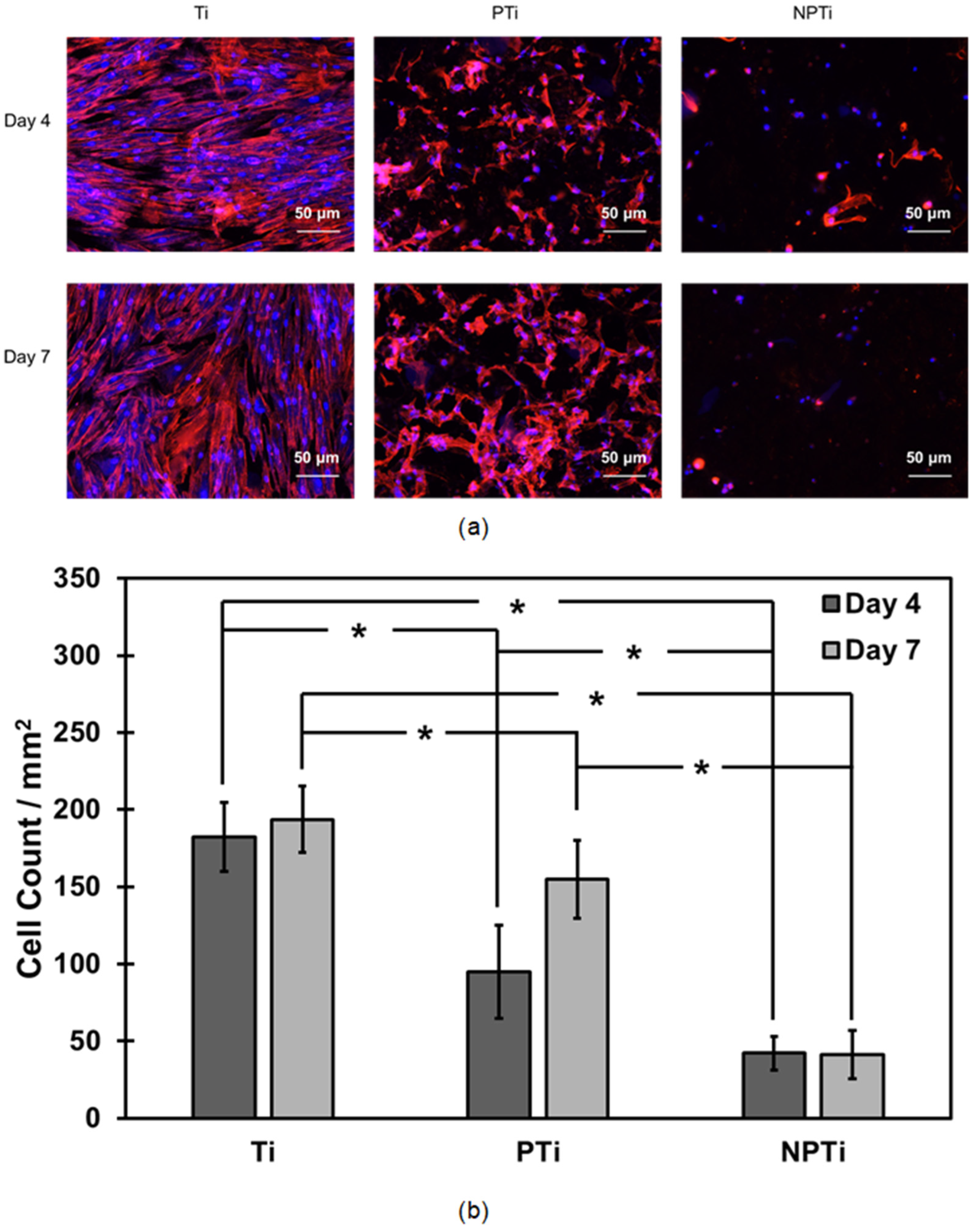
2.5. ADSC Adhesion and Proliferation on Different Surfaces
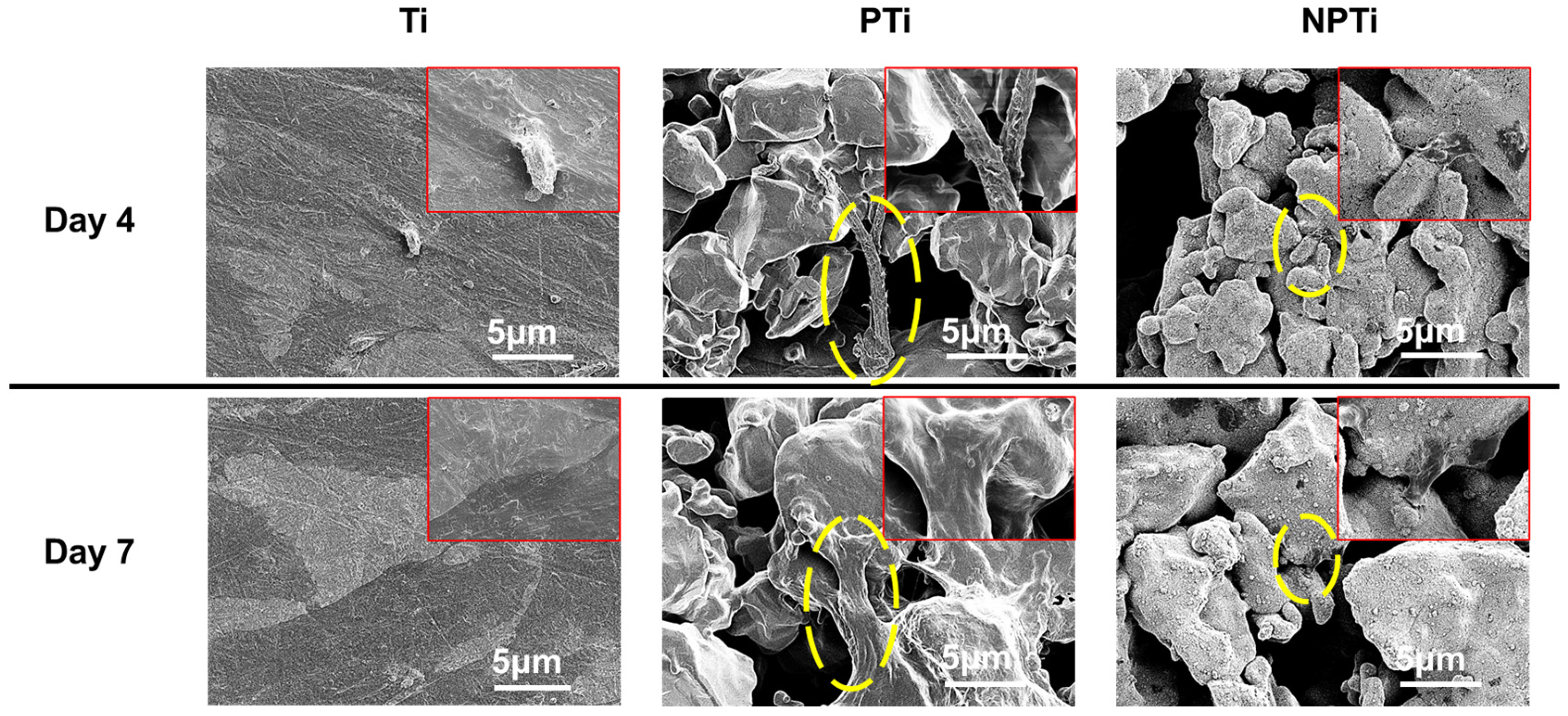
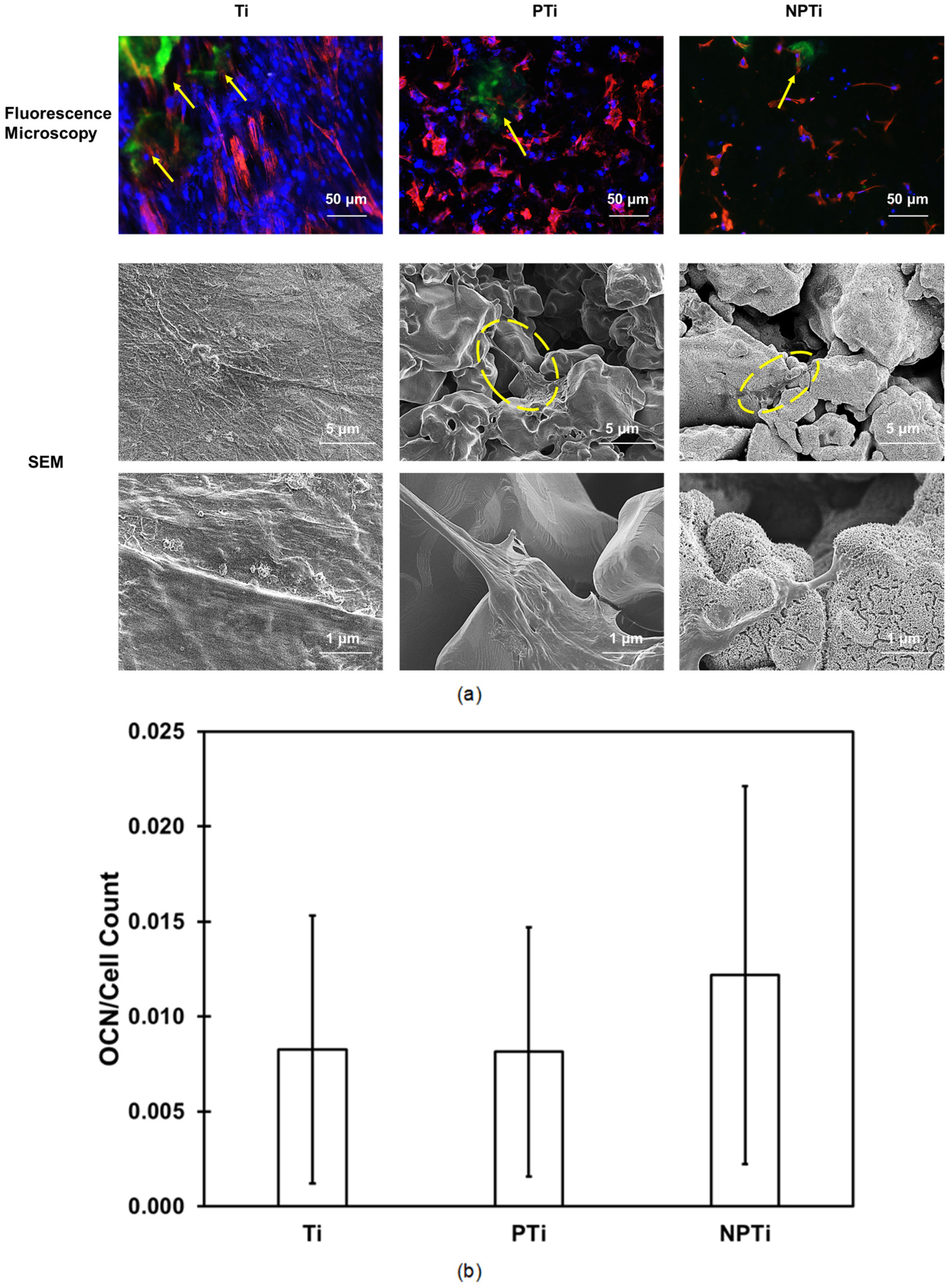
2.6. ADSC Differentiation on Different Surfaces
- The total protein content was determined using a commercially available microBCA assay. In total, 150 μL of working reagent were generated from the assay and 150 μL of protein supernatant were added to a 96-well plate and incubated for 2 h at 37 °C and 5% CO2. After the incubation period, the absorbance was read at 562 nm. The total protein concentration was determined from a standard absorbance curve versus the known albumin standard provided by the manufacturer.
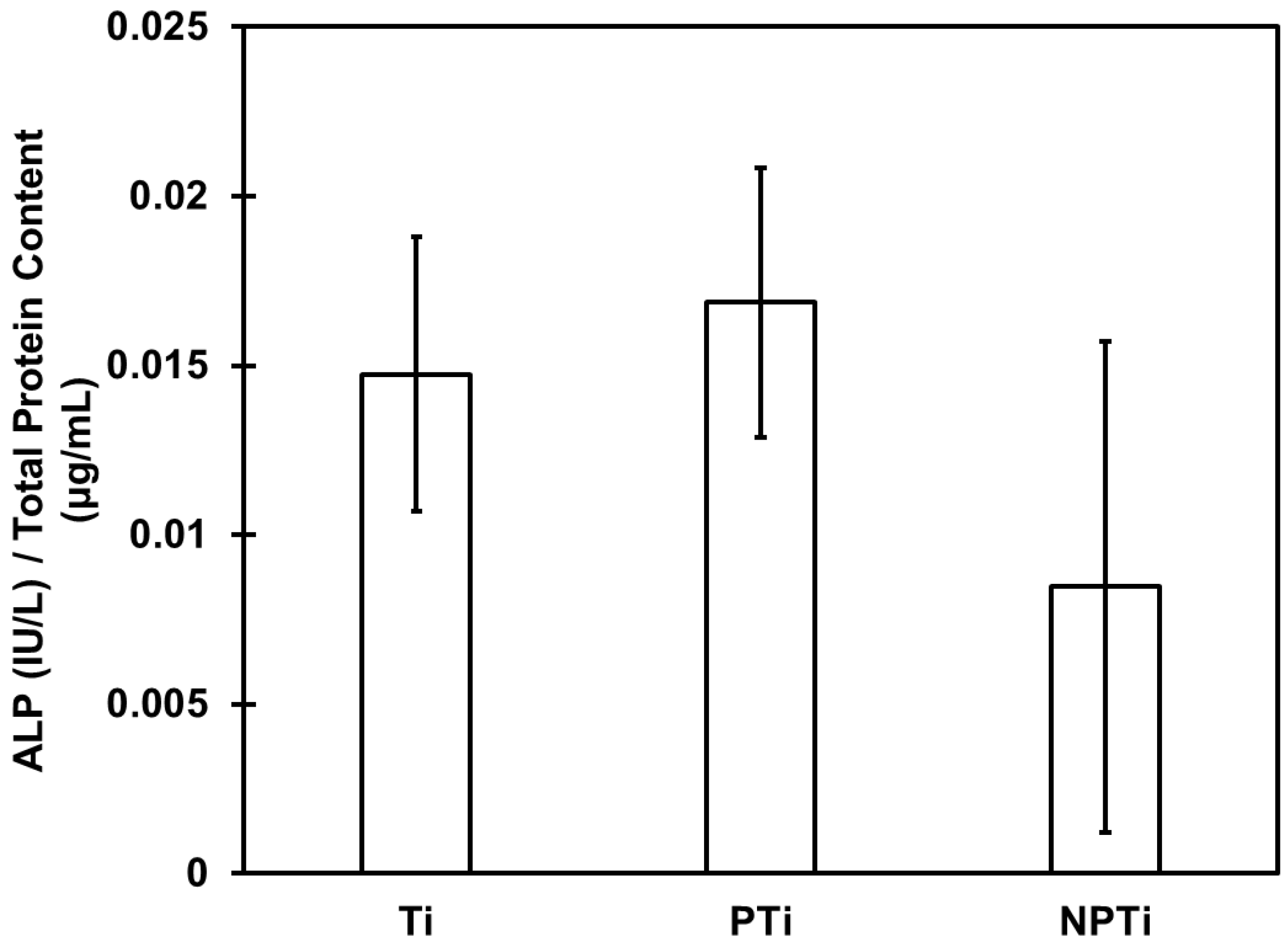
- ALP activity was determined using a commercially available ALP assay kit from QuantiChromTM. In total, 150 μL of working reagent were prepared from the assay kit, and 50 μL of protein supernatant were added to a 96-well plate. The absorbance was read at 405 nm and repeated after 4 min. Absorbance was converted to concentration using an ALP standard, and data were normalized using the total protein content.
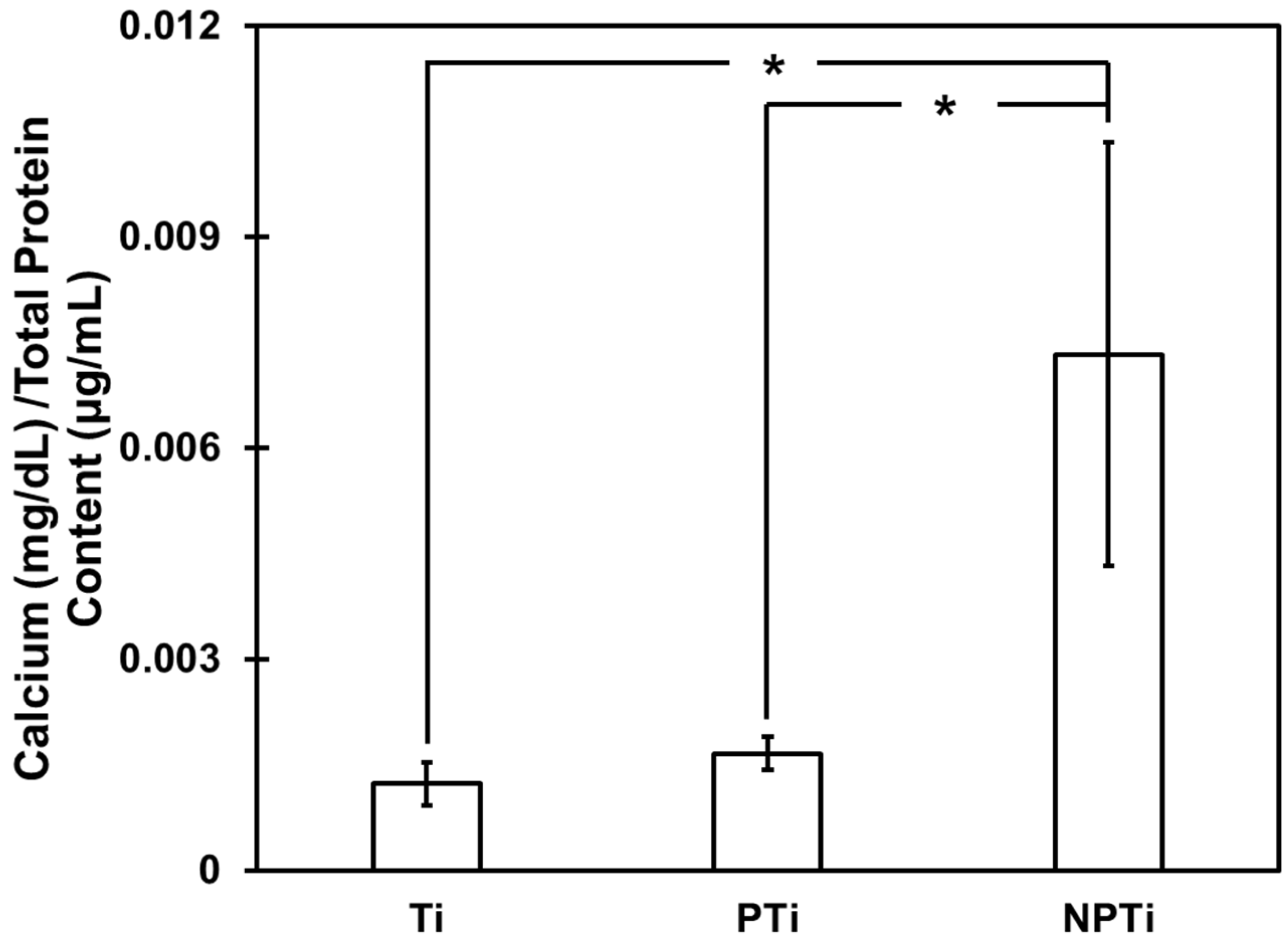
- Calcium deposition was determined using a commercially available calcium reagent test from Teco Diagnostics (Anaheim, CA, USA). The protein supernatant was removed, and surfaces were rinsed with DI water. In total, 6 M HCl (Hydrochloric acid) solution were added to the wells, and the surfaces were placed in a shaker for 12 h at 100 rpm to ensure all the calcium dissolved in the solution. After 12 h, 1 mL of working reagent was prepared from the test kit, and 20 μL of HCl–calcium solution were added to a 24-well plate. The absorbance was read at 570 nm and was converted to concentration using the calcium standard provided by the manufacturer and the data was normalized using total protein content.
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarraf, M.; Ghomi, E.R.; Alipour, S.; Ramakrishna, S.; Sukiman, N.L. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio-Des. Manuf. 2021, 5, 371–395. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.-W.; Zhong, Y.; Wang, L. Multi-Scale Surface Treatments of Titanium Implants for Rapid Osseointegration: A Review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, G.; Mendonça, D.B.S.; Aragão, F.J.L.; Cooper, L.F. Advancing dental implant surface technology—From micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gao, G.; Wu, G. Self-assembled three-dimensional hierarchical porous V2O5/graphene hybrid aerogels for supercapacitors with high energy density and long cycle life. J. Mater. Chem. A Mater. 2015, 3, 1828–1832. [Google Scholar] [CrossRef]
- Maleki, H.; Shahbazi, M.-A.; Montes, S.; Hosseini, S.H.; Eskandari, M.R.; Zaunschirm, S.; Verwanger, T.; Mathur, S.; Milow, B.; Krammer, B.; et al. Mechanically Strong Silica-Silk Fibroin Bioaerogel: A Hybrid Scaffold with Ordered Honeycomb Micromorphology and Multiscale Porosity for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 17256–17269. [Google Scholar] [CrossRef]
- Kenar, H.; Köse, G.T.; Hasirci, V. Tissue engineering of bone on micropatterned biodegradable polyester films. Biomaterials 2006, 27, 885–895. [Google Scholar] [CrossRef]
- Wilkinson, A.; Hewitt, R.N.; McNamara, L.E.; McCloy, D.; Meek, R.D.; Dalby, M.J. Biomimetic microtopography to enhance osteogenesis in vitro. Acta Biomater. 2011, 7, 2919–2925. [Google Scholar] [CrossRef]
- Yu, T.T.; Cui, F.Z.; Meng, Q.Y.; Wang, J.; Wu, D.C.; Zhang, J.; Kou, X.X.; Yang, R.L.; Liu, Y.; Zhang, Y.S.; et al. Influence of Surface Chemistry on Adhesion and Osteo/Odontogenic Differentiation of Dental Pulp Stem Cells. ACS Biomater. Sci. Eng. 2017, 3, 1119–1128. [Google Scholar] [CrossRef]
- Lu, R.J.; Wang, X.; He, H.X.; E, L.-L.; Li, Y.; Zhang, G.L.; Li, C.J.; Ning, C.Y.; Liu, H.C. Tantalum-incorporated hydroxyapatite coating on titanium implants: Its mechanical and in vitro osteogenic properties. J. Mater. Sci. Mater. Med. 2019, 30, 111. [Google Scholar] [CrossRef]
- Rajendran, A.; Kapoor, U.; Jothinarayanan, N.; Lenka, N.; Pattanayak, D.K. Effect of Silver-Containing Titania Layers for Bioactivity, Antibacterial Activity, and Osteogenic Differentiation of Human Mesenchymal Stem Cells on Ti Metal. ACS Appl. Bio Mater. 2019, 2, 3808–3819. [Google Scholar] [CrossRef]
- Savargaonkar, A.V.; Munshi, A.H.; Soares, P.; Popat, K.C. Antifouling Behavior of Copper-Modified Titania Nanotube Surfaces. J. Funct. Biomater. 2023, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Li, J.; Pan, H.; Zhang, W.; Zeng, D.; Xu, L.; Wu, Q.; Zhang, X.; Liu, X.; Jiang, X. Strontium delivery on topographical titanium to enhance bioactivity and osseointegration in osteoporotic rats. J. Mater. Chem. B 2015, 3, 4790–4804. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Brandstetter, C.; Beutner, R.; Hess, R.; Bierbaum, S.; Wagner, K.; Scharnweber, D.; Gbureck, U.; Moseke, C. Multifunctional calcium phosphate based coatings on titanium implants with integrated trace elements. Biomed. Mater. 2020, 15, 025006. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Slamovich, E.B.; Webster, T.J. Enhanced osteoblast functions on anodized titanium with nanotube-like structures. J. Biomed. Mater. Res. A 2008, 85A, 157–166. [Google Scholar] [CrossRef]
- Porter, J.R.; Henson, A.; Popat, K.C. Biodegradable poly(ε-caprolactone) nanowires for bone tissue engineering applications. Biomaterials 2009, 30, 780–788. [Google Scholar] [CrossRef]
- Park, J.K.; Kim, Y.; Yeom, J.; Jeon, J.H.; Yi, G.; Je, J.H.; Hahn, S.K. The Topographic Effect of Zinc Oxide Nanoflowers on Osteoblast Growth and Osseointegration. Adv. Mater. 2010, 22, 4857–4861. [Google Scholar] [CrossRef]
- Oliveira, F.C.; Carvalho, J.O.; Gusmão, S.B.S.; Gonçalves, L.d.S.; Mendes, L.M.S.; Freitas, S.A.P.; Gusmão, G.O.d.M.; Viana, B.C.; Marciano, F.R.; Lobo, A.O. High loads of nano-hydroxyapatite/graphene nanoribbon composites guided bone regeneration using an osteoporotic animal model. Int. J. Nanomed. 2019, 14, 865–874. [Google Scholar] [CrossRef]
- Wen, C.E.; Yamada, Y.; Hodgson, P.D. Fabrication of novel TiZr alloy foams for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1439–1444. [Google Scholar] [CrossRef]
- Barbas, A.; Bonnet, A.-S.; Lipinski, P.; Pesci, R.; Dubois, G. Development and mechanical characterization of porous titanium bone substitutes. J. Mech. Behav. Biomed. Mater. 2012, 9, 34–44. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Yuan, T.; Zhu, X.D.; Xiang, Z.; Fan, Y.J.; Zhang, X.D. The enhanced effect of surface microstructured porous titanium on adhesion and osteoblastic differentiation of mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2013, 24, 2235–2246. [Google Scholar] [CrossRef]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef]
- Habibovic, P.; Li, J.; van der Valk, C.M.; Meijer, G.; Layrolle, P.; van Blitterswijk, C.A.; de Groot, K. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials 2005, 26, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, S.; Neo, M.; Kim, H.-M.; Kokubo, T.; Nakamura, T. Osteoinduction of porous bioactive titanium metal. Biomaterials 2004, 25, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.V.D.; Manzoor, K.; Menon, D.; Selvamurugan, N.; Nair, S.V. The design of novel nanostructures on titanium by solution chemistry for an improvedosteoblast response. Nanotechnology 2009, 20, 195101. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, J.; Manivasagam, V.K.; Gopal, V.; Garcia, C.B.; Hameed, P.; Manivasagam, G.; Webster, T.J. Hydrothermal treatment of etched titanium: A potential surface nano-modification technique for enhanced biocompatibility. Nanomedicine 2019, 20, 102016. [Google Scholar] [CrossRef]
- Sabino, R.M.; Mondini, G.; Kipper, M.J.; Martins, A.F.; Popat, K.C. Tanfloc/heparin polyelectrolyte multilayers improve osteogenic differentiation of adipose-derived stem cells on titania nanotube surfaces. Carbohydr. Polym. 2021, 251, 117079. [Google Scholar] [CrossRef]
- CellTiter-Blue® Cell Viability Assay. Available online: https://www.promega.com/products/cell-health-assays/cell-viability-and-cytotoxicity-assays/celltiter_blue-cell-viability-assay/?catNum=G8080 (accessed on 26 October 2023).
- Savargaonkar, A.V.; Holloway, E.; Madruga, L.Y.C.; Pereira, B.L.; Soares, P.; Popat, K.C. Anti-Bacterial Properties and Hemocompatibility of Alkali Treated Nano-Structured Micro-Porous Titanium Surfaces. Biomimetics 2025, 10, 115. [Google Scholar] [CrossRef]
- Manivasagam, V.K.; Popat, K.C. Endothelial and smooth muscle cell interaction with hydrothermally treated titanium surfaces. Vitr. Models 2024, 3, 109–123. [Google Scholar] [CrossRef]
- Madruga, L.Y.; Sabino, R.M.; Santos, E.C.; Popat, K.C.; Balaban, R.d.C.; Kipper, M.J. Carboxymethyl-kappa-carrageenan: A study of biocompatibility, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2020, 152, 483–491. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R. Porous Titanium Implants: A Review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Chang, B.; Song, W.; Han, T.; Yan, J.; Li, F.; Zhao, L.; Kou, H.; Zhang, Y. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and bone ingrowth. Acta Biomater. 2016, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Dias-Netipanyj, M.F.; Cowden, K.; Sopchenski, L.; Cogo, S.C.; Elifio-Esposito, S.; Popat, K.C.; Soares, P. Effect of crystalline phases of titania nanotube arrays on adipose derived stem cell adhesion and proliferation. Mater. Sci. Eng. C 2019, 103, 109850. [Google Scholar] [CrossRef] [PubMed]
- Albuschies, J.; Vogel, V. The role of filopodia in the recognition of nanotopographies. Sci. Rep. 2013, 3, srep01658. [Google Scholar] [CrossRef]
- McNamara, L.E.; Sjöström, T.; Seunarine, K.; Meek, R.D.; Su, B.; Dalby, M.J. Investigation of the limits of nanoscale filopodial interactions. J. Tissue Eng. 2014, 5, 2041731414536177. [Google Scholar] [CrossRef]
- Watchrarat, K.; Korchunjit, W.; Buranasinsup, S.; Taylor, J.; Ritruechai, P.; Wongtawan, T. MEM α Promotes Cell Proliferation and Expression of Bone Marrow Derived Equine Mesenchymal Stem Cell Gene Markers but Depresses Differentiation Gene Markers. J. Equine Veter. Sci. 2017, 50, 8–14. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- de Souza, R.F.B.; de Souza, F.C.B.; Thorpe, A.; Mantovani, D.; Popat, K.C.; Moraes, A.M. Phosphorylation of chitosan to improve osteoinduction of chitosan/xanthan-based scaffolds for periosteal tissue engineering. Int. J. Biol. Macromol. 2020, 143, 619–632. [Google Scholar] [CrossRef]
- Huang, Z.; Nelson, E.R.; Smith, R.L.; Goodman, S.B. The Sequential Expression Profiles of Growth Factors from Osteroprogenitors to Osteoblasts In Vitro. Tissue Eng. 2007, 13, 2311–2320. [Google Scholar] [CrossRef]
- Cowden, K.; Dias-Netipanyj, M.F.; Popat, K.C. Effects of titania nanotube surfaces on osteogenic differentiation of human adipose-derived stem cells. Nanomedicine 2019, 17, 380–390. [Google Scholar] [CrossRef]
- Huang, Y.Z.; He, S.K.; Guo, Z.J.; Pi, J.K.; Deng, L.; Dong, L.; Zhang, Y.; Su, B.; Da, L.C.; Zhang, L.; et al. Nanostructured titanium surfaces fabricated by hydrothermal method: Influence of alkali conditions on the osteogenic performance of implants. Mater. Sci. Eng. C 2019, 94, 1–10. [Google Scholar] [CrossRef]
- Manivasagam, V.K.; Popat, K.C. Hydrothermally treated titanium surfaces for enhanced osteogenic differentiation of adipose derived stem cells. Mater. Sci. Eng. C 2021, 128, 112315. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Taguchi, Y.; Komasa, S.; Sekino, T.; Tanaka, M. Cell Differentiation on Nanoscale Features of a Titanium Surface: Effects of Deposition Time in NaOH Solution. J. Hard Tissue Biol. 2014, 23, 63–70. [Google Scholar] [CrossRef]
| %Ti 2p3/2 | %C 1s | %O 1s | %Na 1s | |
|---|---|---|---|---|
| Ti | 7.38 | 57.43 | 35.20 | - |
| PTi | 14.15 | 38.56 | 47.30 | - |
| NPTi | 3.62 | 27.53 | 44.78 | 24.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savargaonkar, A.V.; Holloway, E.; Popat, K.C. Alkali-Treated, Nanostructured-Micro-Porous Titanium Surfaces Enhance Osteogenic Differentiation of Adipose Derived Stem Cells. Appl. Sci. 2025, 15, 5061. https://doi.org/10.3390/app15095061
Savargaonkar AV, Holloway E, Popat KC. Alkali-Treated, Nanostructured-Micro-Porous Titanium Surfaces Enhance Osteogenic Differentiation of Adipose Derived Stem Cells. Applied Sciences. 2025; 15(9):5061. https://doi.org/10.3390/app15095061
Chicago/Turabian StyleSavargaonkar, Aniruddha Vijay, Emma Holloway, and Ketul C. Popat. 2025. "Alkali-Treated, Nanostructured-Micro-Porous Titanium Surfaces Enhance Osteogenic Differentiation of Adipose Derived Stem Cells" Applied Sciences 15, no. 9: 5061. https://doi.org/10.3390/app15095061
APA StyleSavargaonkar, A. V., Holloway, E., & Popat, K. C. (2025). Alkali-Treated, Nanostructured-Micro-Porous Titanium Surfaces Enhance Osteogenic Differentiation of Adipose Derived Stem Cells. Applied Sciences, 15(9), 5061. https://doi.org/10.3390/app15095061








