Abstract
Aging is associated with cognitive decline, significantly impacting quality of life. Drumming-based cognitive and physical training, a novel intervention, engages motor and cognitive circuits simultaneously, potentially improving executive functions and emotional regulation in older adults. A randomized controlled trial (RCT) was conducted with 40 participants aged 55 years and older, assigned to either an experimental group receiving drumming-based training or a control group undergoing conventional memory exercises. The intervention lasted four weeks, consisting of 30-min training sessions three times per week. Functional near-infrared spectroscopy (fNIRS) was employed to measure brain activity, while cognitive and emotional outcomes were assessed through standardized tests, including the Mini-Mental State Examination (MMSE) and the Geriatric Depression Scale (GDS). Task performance metrics, such as accuracy and success rates, were also recorded. Experimental group exhibited significant improvements in cognitive performance, including a greater number of correct answers (p = 0.0004) and a higher task success rate (p = 0.0001) compared to the control group. fNIRS data revealed increased oxygenated hemoglobin levels in the left orbitofrontal cortex (OFC) (p = 0.028). This study provides compelling evidence that a drumming-based cognitive and physical training program can lead to significant cognitive and emotional benefits in older adults.
1. Introduction
The rising global aging population has led to increased cases of age-related cognitive decline and neurodegenerative diseases such as dementia and Alzheimer’s disease [1]. These conditions not only impair memory and executive functions but also reduce overall cognitive performance, significantly affecting the quality of life of older adults [2]. Given the limitations of pharmacological treatments, which often have variable efficacy and side effects, non-pharmacological interventions have gained traction as viable alternatives to support healthy cognitive aging [3]. Among these, physical and cognitive training programs have been shown to improve brain health and slow cognitive decline by promoting neuroplasticity [4].
Cognitive training has long been recognized for its ability to engage higher-order cognitive functions such as memory, problem-solving, and attention. Interventions that focus on these skills have been shown to improve cognitive task performance and induce structural and functional brain changes in older adults [5]. Additionally, it is still unknown whether a combination of exercise and cognitive training (CECT) is more effective than either intervention alone. The present systematic review and meta-analysis were undertaken to evaluate the effect of CECT on working memory in the elderly [6]. In an fMRI study on dual-task performance, the training group showed increased dorsolateral prefrontal cortex activation, which was correlated with improved performance [7]. The CECT typically involves performing cognitive and motor tasks simultaneously, such as in dual-task walking, exergames, or video-based dancing [8].
Recently, a realistic drumming-based cognitive and physical training was developed specifically to enhance memory in older adults. Virtual Drum Beating, the intervention used in this study, qualifies as an exergame and therefore represents a form of CECT [9]. This intervention, which integrates cognitive exergaming with a virtual drumming component, led to greater brain activation, particularly reflected in increased oxyhemoglobin levels in the dorsolateral prefrontal cortex (DLPFC) and orbitofrontal cortex (OFC) than conventional cognitive training [9]. This form of intervention has the potential to improve brain function by activating the dorsolateral prefrontal cortex (DLPFC) and the orbitofrontal cortex (OFC), areas responsible for cognitive control, decision-making, and emotional regulation in healthy adults [9]. Rhythm-based activities like drumming engage both hemispheres of the brain and stimulate regions involved in motor control, attention, and executive function. Specifically, drumming has been shown to increase connectivity between the motor cortex and prefrontal cortex, enhancing functional integration between cognitive and motor processes [10,11,12].
Building on the existing evidence, this study aims to examine the impact of drumming-based cognitive and physical training on cognitive performance, emotional well-being, and brain activity in older adults. Specifically, this research will focus on changes in Mini-Mental State Examination (MMSE) scores, task performance, and prefrontal cortex activity, using fNIRS to measure brain activation. We hypothesized that the experimental group receiving drumming-based training would show greater improvements in (1) cognitive performance (correct answers, success rate, etc.), (2) emotional well-being (depression scores), and (3) prefrontal cortical activity (HbO concentration) compared to the control group.
2. Materials and Methods
2.1. Study Protocol and Study Design
This study employed a randomized controlled trial (RCT) to investigate the effects of a drumming-based cognitive and physical training program on older adults. The research protocol was approved by the Institutional Review Board (IRB) of Dongguk University Ilsan Hospital (IRB No. DUIH-2023-04-018), and the study was registered at the Clinical Research Information Service (CRIS, KCT0008500. Informed consent was obtained from all participants, and all procedures were performed following the Declaration of Helsinki.
2.2. Participants
A total of 40 participants, aged 55 years or older, were recruited. These individuals were randomly assigned to either an experimental group (n = 20) that received the drumming intervention or a control group (n = 20) that performed conventional memory training. The sample size was determined based on effect size estimates from a prior study using the same Virtual Drum Beating paradigm, which yielded a Cohen’s d of 0.91 for group differences in ∆HbO at the right DLPFC [9]. An a priori power analysis using G*Power (α = 0.05, power = 0.80) indicated that 36 participants would be sufficient, and we increased the sample to 40 to account for potential dropout.
2.3. Materials
Data collection occurred at two time points, both before the intervention and after the intervention. Cognitive performance was assessed using a variety of task-based measures, including the number of correct answers, total training attempts, success rate, and time to highest level. Cognitive and emotional functions were assessed using the Mini-Mental State Examination (MMSE) to evaluate global cognitive function and the Geriatric Depression Scale (GDS) to assess depressive symptoms. Both the experimental and control groups underwent fNIRS (NIRSIT Lite Analysis Tool v3.1.0; OBELAB, Inc., Seoul, Republic of Korea) measurements during their first and final visits to assess the hemodynamic responses of cerebral blood volume during the intervention by detecting oxyhemoglobin levels in the brain. The fNIRS signals were recorded using the NIRSIT Lite system with a sampling rate of 8.138 Hz and dual wavelengths (780 nm and 850 nm). Measurements were performed under controlled indoor LED lighting conditions. The raw data were converted into ∆HbO and ∆HbR concentrations using the modified Beer–Lambert law. A band-pass filter (0.01–0.1 Hz) was applied, and motion artifacts were corrected using the CBSI method available in the NIRSIT Lite software.
The final output of each optode was then reported as mean total oxygenated hemoglobin (HbO μMol). A total of 15 channels were located on both sides of the dorsolateral prefrontal cortex (DLPFC) (channels 2, 3, 12, 14), orbitofrontal cortex (OFC) (channels 1, 4, 7, 10, 13, 15), and frontopolar prefrontal cortex (FPPFL) (channels 5, 6, 8, 9, 11) [13]. Positions of these channels were: channel 1, right lateral OFC; channels 4 and 7, right medial OFC; channels 2 and 3, right DLPFC; channel 15, left lateral OFC; channels 10 and 13, left medial OFC; channels 12 and 14, left DLPFC; channels 5, 6, 8, 9, and 11, frontopolar prefrontal cortex. Figure 1 shows the positions of these channels.
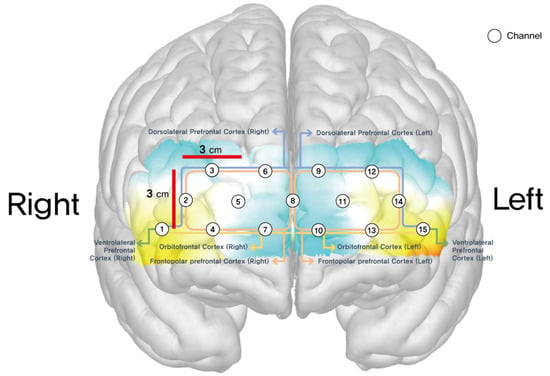
Figure 1.
Schematic layout of the 15 fNIRS channels over the prefrontal cortex regions, including dorsolateral prefrontal cortex (DLPFC), orbitofrontal cortex (OFC), and frontopolar prefrontal cortex (FPPFC).
Channels 1 through 15 corresponded to specific areas of the prefrontal cortex that are known to be involved in these higher-order cognitive processes. Statistical analysis was performed using paired t-tests to compare pre- and post-intervention results within each group and independent t-tests to compare the post-intervention differences between the experimental and control groups. A significance level of p < 0.05 was used to determine statistical significance, and effect sizes were calculated to assess the magnitude of the intervention’s impact.
2.4. Task and Design
The experimental group engaged in memory training using a realistic drumming-based task within an isolated laboratory setting. (Figure 2) Participants stood before a 42-inch LED monitor, where they observed and memorized digits presented on the screen for five seconds. Following this, they were directed to strike virtual drums in the correct sequence using an electronic drumstick held in both hands. This drumstick simulated the tactile sensations and vibrations of actual drumming, while the monitor provided synchronized visual and auditory feedback to enhance the immersive drumming experience. The participants of a control group underwent memory training using conventional paper-and-pencil methods in a dedicated laboratory setting, where digits were visually presented one at a time on a computer screen for five seconds, with the memory span length beginning with three digits and gradually increasing to nine, and after the numbers disappeared from the screen, participants were asked to write them in the correct sequential order on a response sheet [13].

Figure 2.
The virtual drum beating task used for memory training in the experimental group. Participants observed digit sequences and replicated them using a feedback-based drumstick with visual and auditory guidance.
The intervention consisted of 30-min sessions three times per week for a total of 12 sessions over four weeks. Each session was structured to progressively increase in difficulty, beginning with simple rhythmic patterns and gradually incorporating more complex sequences that required higher levels of cognitive engagement and physical coordination. The training aimed to activate cognitive functions through rhythm-based tasks that engaged both the dominant and non-dominant hands. Participants were required to follow rhythmic drumming patterns while also responding to cognitive cues, thus simultaneously stimulating cognitive control, motor coordination, and emotional regulation.
All participants were required to have no severe cognitive impairments that could affect their ability to engage in the intervention. Ethical approval for the study was obtained from the university’s institutional review board, and written informed consent was collected from all participants before their involvement in the research.
2.5. Statistical Analysis
Statistical analysis was performed using paired t-tests to compare pre- and post-intervention results within each group and independent t-tests to compare the post-intervention differences between the experimental and control groups. A significance level of p < 0.05 was used to determine statistical significance, and effect sizes were calculated to assess the magnitude of the intervention’s impact.
3. Results
A total of 40 participants were enrolled in the study, with 20 assigned to the experimental group and 20 to the control group. At baseline, no significant differences were observed between the groups in demographic characteristics, including age (p = 0.6156), weight (p = 0.1930), and gender distribution (p = 0.2357). The only notable difference was in height, with the control group being significantly taller on average (p = 0.0385). Baseline measures of cognitive performance and brain activity were also comparable between the groups, with no significant differences observed in MMSE scores, correct answers, total training attempts, or brain activity across the 15 prefrontal regions (p > 0.05). Therefore, no covariates were included in the analysis (Table 1).

Table 1.
Baseline characteristics of subjects in the experimental group and the control group.
Post-intervention, the experimental group exhibited significant improvements in cognitive performance. The number of correct answers increased by 10.35 points (p = 0.0004), and the success rate of task completions improved by 19.44% (p = 0.0001). Between-group comparisons confirmed the effectiveness of the drumming intervention, with the experimental group outperforming the control group in correct answers (p = 0.0248) and success rate (p = 0.0003) (Table 2).

Table 2.
Difference of measures undergoing cognitive memory training in both groups.
The fNIRS data revealed significant changes in brain activity in the experimental group, particularly in two key regions of the prefrontal cortex. Channel 1, located in the right dorsolateral prefrontal cortex (DLPFC), showed a near-significant increase in brain activity post-intervention (p = 0.074). Channel 15, located in the left orbitofrontal cortex (OFC), exhibited a significant increase in brain activity (p = 0.028) (Table 3).

Table 3.
Differences in HbO when undergoing intervention in both groups (μMol).
A 2 × 2 mixed design ANOVA on correct answers revealed significant main effects of Group (F(1,38) = 10.12, p = 0.003) and Time (F(1,38) = 24.35, p < 0.001), and a significant Group × Time interaction (F(1,38) = 5.67, p = 0.021), indicating a larger pre- to post-improvement in the drumming group. Similarly, for Success Rate, there were main effects of Group (F(1,38) = 12.48, p = 0.001) and Time (F(1,38) = 31.02, p < 0.001), with a significant interaction (F(1,38) = 7.82, p = 0.008). These findings suggest that drumming-based cognitive and physical training effectively enhances cognitive function and prefrontal cortex activity in older adults.
4. Discussion
The findings of this study provide the evidence that a drumming-based cognitive and physical training intervention can lead to significant improvements in cognitive performance, emotional well-being, and brain activity in older adults. Music-based and rhythm-based interventions have received growing attention due to their unique ability to engage multiple cognitive systems simultaneously. A meta-analysis by Pietschnig et al. (2010) analyzed the cognitive effects of music exposure, showing that while Mozart music has been linked to improvements in spatial ability, its overall impact on broader cognitive functions such as attention, memory, and executive function remains inconclusive [14]. Additionally, rhythmic interventions have been found to enhance mood, reduce stress, and improve emotional regulation [15]. Our findings showed a significant increase in the number of correct task answers post-intervention (p = 0.0004) and between-group comparisons (p = 0.0248), supporting the effectiveness of cognitive training in improving accuracy. The mixed-ANOVA results of this study corroborate our t-test findings, demonstrating that rhythm-based multimodal feedback yields specific enhancements in task accuracy both in the number of correct answers and success rates beyond those achieved by conventional memory exercises. This is consistent with Nouchi et al., where tasks like Digit Cancellation, Symbol Search, and Digit Symbol Coding assessed cognitive gains through correct responses, highlighting the role of structured training in enhancing cognitive function in older adults [16]. After applying a 4-week, 12-session drumming-based cognitive and physical training intervention, the significant increase in success rate (19.44%, p = 0.0001) suggests improved task accuracy and learning efficiency. These findings are consistent with previous studies showing that physical activity and multimodal training contribute to cognitive resilience by enhancing executive function, attentional control, and neuroplasticity [17,18]. No significant changes were observed in GDS scores. This may be due to the overall low baseline levels of depressive symptoms (mean ≈ 8.3), which may have limited the potential for measurable emotional improvement. Although the score distribution does not strongly indicate a floor effect across the sample, it may have influenced the results for some individuals.
The final measurements obtained from each optode were expressed as the mean total oxygenated hemoglobin (HbO µm). A total of 15 channels were strategically positioned across both hemispheres, focusing on specific prefrontal regions: the dorsolateral prefrontal cortex (DLPFC) (channels 2, 3, 12, 14), the orbitofrontal cortex (OFC) of channels 1, 4, 7, 10, 13, and 15, and the frontopolar prefrontal cortex (FPPFL) of channels 5, 6, 8, 9, and 11 [19]. Regarding their exact placement, channel 1 was located on the right lateral OFC, while channels 4 and 7 were positioned in the right medial OFC. Channels 2 and 3 were mapped onto the right DLPFC, whereas channel 15 was assigned to the left lateral OFC. Furthermore, channels 10 and 13 corresponded to the left medial OFC, channels 12 and 14 were designated for the left DLPFC, and channels 5, 6, 8, 9, and 11 covered the frontopolar prefrontal cortex. This distribution ensured a comprehensive assessment of hemodynamic activity in these key cognitive regions [19]. In the results, the mean HbO µm channel 15 located on the left OFC of the experimental group, was statistically significant when compared to the control group (p = 0.0285). The orbitofrontal cortex (OFC) integrates sensory information with reward-related and affective processing, linking reinforcement to hedonic experience [20,21]. Additionally, the OFC enables flexible decision-making by updating outcome expectations [22]. In our study, the rhythmic and feedback-rich nature of the drumming task likely engaged OFC-mediated processes by promoting reward expectancy and emotional salience. Furthermore, older adults tend to rely more on OFC-based affective strategies than on dorsolateral prefrontal executive functions [23,24], which may explain the regional specificity observed.
Previous research has also shown that OFC plays a key role in reversal learning, where individuals adjust stimulus-reward associations based on feedback. Lesion studies demonstrate that OFC damage impairs this ability, while other forms of cognitive flexibility are linked to the vlPFC (ventrolateral prefrontal cortex) [25,26]. Functional neuroimaging confirms increased OFC activation during reversal learning, particularly after negative feedback [27]. Moreover, the OFC is involved in emotional regulation via its interaction with the amygdala [28,29]. In this study, after a 4-week, 12-session drumming-based cognitive and physical training intervention, the mean HbO concentration in channel 15, located in the left OFC of the experimental group, was significantly higher compared to the control group (p = 0.0285). This finding contrasts with our previous study, which examined healthy adults and found significant activation in the right dorsolateral prefrontal cortex (DLPFC, Channel 2) and right medial OFC (Channels 7 and 10) during a single drumming session [9]. Given that the OFC is primarily involved in affective and reinforcement learning [30]. Older adults in this study may have relied more on these processes during training. In contrast, younger adults in the previous study likely engaged executive control mechanisms mediated by the DLPFC, as suggested by prior research on age-related differences in cognitive control strategies [23,24].
This prolonged engagement may have facilitated neural adaptation in cognitive and affective processing networks, potentially contributing to increased OFC activation in older adults. While previous research suggests that extended cognitive training enhances prefrontal neuroplasticity [31]., further investigation is needed to determine the specific mechanisms underlying increased OFC involvement in older adults.
This study has several limitations. First, intervention effects were assessed only immediately after the 4-week program, and the long-term retention of cognitive and emotional benefits remains unknown. Second, although fNIRS signals were obtained from multiple channels, the current analysis focused on a pre-defined region of interest, where only a marginal trend was detected, and multiple comparison correction was not applied. This limits the generalizability of the neurophysiological findings. Third, the immersive and rewarding aspects of the drumming-based intervention were intentionally preserved, as they are considered essential therapeutic components rather than mere confounding factors. However, this limits the ability to fully separate engagement effects from specific cognitive mechanisms. Future studies should include a matched-level control group to further clarify these mechanisms. Overall, these findings suggest that drumming-based cognitive-motor training influences distinct neural mechanisms depending on age and training duration. The increased OFC activation observed in this study aligns with its established role in adaptive decision-making and emotional regulation. Future research should further explore how age-related differences, intervention duration, and cognitive demands influence the recruitment of prefrontal regions during cognitive-motor training.
5. Conclusions
In conclusion, this study demonstrates that a 4-week drumming-based cognitive and physical training program can lead to significant improvements in both cognitive performance and brain activity in older adults. The significant increases in correct answers, success rates, and MMSE scores observed in the experimental group, along with the reductions in depressive symptoms, suggest that drumming-based interventions offer a promising approach for enhancing cognitive and emotional health in aging populations. The observed changes in brain activity, particularly in regions of the prefrontal cortex associated with cognitive control and emotional regulation, provide further evidence of the potential neuroplastic effects of this intervention. As such, drumming-based cognitive training may serve as an effective non-pharmacological tool for preventing or mitigating age-related cognitive decline.
Author Contributions
B.-S.K. made substantial contributions to the experimental design, data analysis, and drafting of the manuscript. Y.-G.N. made substantial contributions to the data collection, data analysis, and drafting of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the National Research Foundation of Korea (NRF), grant-funded by the Korean government (MSIT) (No. 2022R1C1C2007812).
Institutional Review Board Statement
This research protocol was approved by the Institutional Review Board (IRB) of Dongguk University Ilsan Hospital. This study was performed following protocols approved by the IRB (No. DUIH-2023-04-018) and included only patients who provided written informed consent. Trial registration: KCT0008500 Clinical Research Information Service (CRIS), Republic of Korea.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors certify that there is no conflicts of interest with any financial organization regarding the material discussed in the manuscript.
References
- Liu, Y.; Tan, Y.; Zhang, Z.; Yi, M.; Zhu, L.; Peng, W. The interaction between ageing and Alzheimer’s disease: Insights from the hallmarks of ageing. Transl. Neurodegener. 2024, 13, 7. [Google Scholar] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- James, C.E.; Müller, D.M.; Müller, C.A.; Van de Looij, Y.; Altenmuller, E.; Kliegel, M.; Van De Ville, D.; Marie, D. Randomized controlled trials of non-pharmacological interventions for healthy seniors: Effects on cognitive decline, brain plasticity and activities of daily living—A 23-year scoping review. Heliyon 2024, 10, e26674. [Google Scholar] [CrossRef]
- Castellote-Caballero, Y.; Carcelén Fraile, M.D.C.; Aibar-Almazán, A.; Afanador-Restrepo, D.F.; González-Martín, A.M. Effect of combined physical–cognitive training on the functional and cognitive capacity of older people with mild cognitive impairment: A randomized controlled trial. BMC Med. 2024, 22, 281. [Google Scholar]
- Lampit, A.; Hallock, H.; Valenzuela, M. Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PLoS Med. 2014, 11, e1001756. [Google Scholar] [CrossRef]
- Wu, Y.; Zang, M.; Wang, B.; Guo, W. Does the combination of exercise and cognitive training improve working memory in older adults? A systematic review and meta-analysis. PeerJ 2023, 11, e15108. [Google Scholar]
- Erickson, K.I.; Colcombe, S.J.; Wadhwa, R.; Bherer, L.; Peterson, M.S.; Scalf, P.E.; Kim, J.S.; Alvarado, M.; Kramer, A.F. Training-induced functional activation changes in dual-task processing: An FMRI study. Cereb. Cortex 2007, 17, 192–204. [Google Scholar]
- Adcock, M.; Fankhauser, M.; Post, J.; Lutz, K.; Zizlsperger, L.; Luft, A.R.; Guimarães, V.; Schättin, A.; de Bruin, E.D. Effects of an in-home multicomponent exergame training on physical functions, cognition, and brain volume of older adults: A randomized controlled trial. Front. Med. 2020, 6, 321. [Google Scholar]
- Nam, Y.-G.; Kwon, B.-S. Prefrontal cortex activation during memory training by virtual drum beating: A randomized controlled trial. Healthcare 2023, 11, 2559. [Google Scholar] [CrossRef]
- Saarman, E. Feeling the beat: Symposium explores the therapeutic effects of rhythmic music. Stanford News, 3 May 2006. [Google Scholar]
- Bengtsson, S.L.; Ullen, F.; Ehrsson, H.H.; Hashimoto, T.; Kito, T.; Naito, E.; Forssberg, H.; Sadato, N. Listening to rhythms activates motor and premotor cortices. Cortex 2009, 45, 62–71. [Google Scholar]
- Deyo, L.J. Cognitive Functioning of Drumming and Rhythm Therapy for Neurological Disorders. 2016. Available online: https://trace.tennessee.edu/utk_chanhonoproj/1983 (accessed on 1 May 2025).
- Moberly, A.C.; Pisoni, D.B.; Harris, M.S. Visual working memory span in adults with cochlear implants: Some preliminary findings. World J. Otorhinolaryngol.-Head Neck Surg. 2017, 3, 224–230. [Google Scholar] [PubMed]
- Pietschnig, J.; Voracek, M.; Formann, A.K. Mozart effect–Shmozart effect: A meta-analysis. Intelligence 2010, 38, 314–323. [Google Scholar]
- Fancourt, D.; Perkins, R.; Ascenso, S.; Carvalho, L.A.; Steptoe, A.; Williamon, A. Effects of group drumming interventions on anxiety, depression, social resilience, and inflammatory immune response among mental health service users. PLoS ONE 2016, 11, e0151136. [Google Scholar] [CrossRef]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Hashizume, H.; Nozawa, T.; Sekiguchi, A.; Nouchi, H.; Kawashima, R. Beneficial effects of short-term combination exercise training on diverse cognitive functions in healthy older people: Study protocol for a randomized controlled trial. Trials 2012, 13, 200. [Google Scholar]
- Kramer, A.F.; Colcombe, S.J.; McAuley, E.; Scalf, P.E.; Erickson, K.I. Fitness, aging and neurocognitive function. Neurobiol. Aging 2005, 26, 124–127. [Google Scholar] [PubMed]
- McAuley, E.; Kramer, A.F.; Colcombe, S.J. Cardiovascular fitness and neurocognitive function in older adults: A brief review. Brain Behav. Immun. 2004, 18, 214–220. [Google Scholar]
- Cho, T.H.; Nah, Y.; Park, S.H.; Han, S. Prefrontal cortical activation in Internet Gaming Disorder Scale high scorers during actual real-time internet gaming: A preliminary study using fNIRS. J. Behav. Addict. 2022, 11, 492–505. [Google Scholar]
- Kringelbach, M.L. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 2005, 6, 691–702. [Google Scholar]
- Kringelbach, M.L.; Rolls, E.T. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004, 72, 341–372. [Google Scholar]
- Schoenbaum, G.; Roesch, M.R.; Stalnaker, T.A. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006, 29, 116–124. [Google Scholar]
- Cabeza, R.; Anderson, N.D.; Locantore, J.K.; McIntosh, A.R. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage 2002, 17, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Nashiro, K.; Sakaki, M.; Braskie, M.N.; Mather, M. Resting-state networks associated with cognitive processing show more age-related decline than those associated with emotional processing. Neurobiol. Aging 2017, 54, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Robbins, T.W.; Roberts, A.C. Dissociation in prefrontal cortex of affective and attentional shifts. Nature 1996, 380, 69–72. [Google Scholar] [CrossRef]
- Keeler, J.F.; Robbins, T.W. Translating cognition from animals to humans. Biochem. Pharm. 2011, 81, 1356–1366. [Google Scholar] [CrossRef]
- Hampshire, A.; Owen, A.M. Fractionating attentional control using event-related fMRI. Cereb. Cortex. 2006, 16, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.J.; Anderson, M.C. Purging of memories from conscious awareness tracked in the human brain. J. Neurosci. 2012, 32, 16785–16794. [Google Scholar] [CrossRef]
- Schmitz, T.W.; Correia, M.M.; Ferreira, C.S.; Prescot, A.P.; Anderson, M.C. Hippocampal GABA enables inhibitory control over unwanted thoughts. Nat. Commun. 2017, 8, 1311. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The functions of the orbitofrontal cortex. Brain Cogn. 2004, 55, 11–29. [Google Scholar] [CrossRef]
- Park, D.C.; Bischof, G.N. The aging mind: Neuroplasticity in response to cognitive training. Dialogues Clin. Neurosci. 2013, 15, 109–119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).