Abstract
Lower limb prosthesis abandonment is a significant challenge, leading to reliance on walking aids, such as wheelchairs, which frequently do not match the patient’s needs and lead to increased morbidity. Prosthesis abandonment is driven by a lack of embodiment, the latter defined as the integration of a prosthetic device into one’s body schema. This review evaluates interventions enhancing embodiment through three dimensions: ownership, agency, and co-location. The aim of this narrative review is to ask what interventions are available to improve embodiment, and what dimensions of embodiment should be included in the standard of care for lower-limb amputation surgery and componentry development. This narrative is constructed through a thorough literature search on how the aforementioned dimensions of embodiment can be optimized. In the studies reviewed, standardization of embodiment metrics and longitudinal data are lacking, hindering clinical translation. Future work must prioritize patient-centered design, integrate multidimensional assessments, and address practical issues to expand eligibility for advanced interventions.
1. Introduction
Each year in the United States, approximately 150,000 patients undergo lower-limb amputation [1]. For these individuals, prosthetic devices serve as vital tools that restore mobility and independence in everyday life. “Prosthesis embodiment” refers to the process by which users mentally integrate these artificial limbs into their body [2,3]. It is the point beyond which a prosthetic device ceases to be perceived merely as an assistive device, but instead as part of a patient’s self and identity. Research indicates that the degree of embodiment is directly related to continued regular use and satisfaction with the prosthesis [4], indicating that the more a user feels that the prosthesis is part of their body, the more likely they are to continue using it. Consequently, loss of embodiment often leads to prosthesis abandonment, as patients choose to discontinue using their devices.
Prosthesis abandonment presents a significant clinical concern. Among lower limb amputees who decide against using their prosthesis, the majority (up to 74%) rely on wheelchairs for mobility, often for decades [5]. When wheelchair use does not meet patient needs and functional capacity, this can be associated with morbidity such as decreased mobility, poor posture, pain, fatigue, pressure ulcers, and increased dependence on personal assistance [6].
It is commonly noted that the clinical standard of care for limb amputation surgery has not changed significantly in the last two centuries, and prosthesis abandonment remains an afterthought. This review builds on previous work on the definition and measurement of embodiment [2,3] and asks the following questions for lower-limb amputees:
- What interventions are available to improve embodiment and reduce abandonment?
- What dimensions of embodiment should be included in the standard of care for lower-limb amputation surgery?
Recent decades have seen rapid advances in lower limb prostheses, becoming sophisticated mechatronic systems that can be directly anchored to the user’s skeletal system and even restore sensation, such as proprioception and cutaneous sensation, to the missing limb. With this new generation of devices, there has been an evolution in goals within the community, moving toward offering integrated devices that users accept as part of themselves as they interact with the external world. This research outlines the landscape of available interventions pertaining to embodiment and explores the mechanisms through which they do so.
However, much is yet to be understood, such as why some users prefer futuristic appearing prosthetics while others prefer natural appearing prosthetics [7]. Furthermore, many interventions are early in development and have not been thoroughly tested. In addition, the lack of a common evaluation framework limits generalizability. Future research should address these concerns in a systematic review by clinicians and clinician-scientists when making formal recommendations on the standard of care for lower-limb amputees.
This review is structured as follows. Section 2 provides an overview of prosthesis embodiment and how to quantify prosthesis embodiment. These ideas inform Section 3, which describes the review’s search strategy. Section 4 describes clinical interventions in development for improving prosthesis embodiment in lower-limb amputees. Finally, Section 5 highlights the future work required for a systematic review.
2. Development of the Dimensions of Embodiment
Surgical amputations of the lower extremity have been reported to have occurred as early as 31,000 years ago [8]. Since then, the role of surgical amputation has grown, and with it, the need for ever-improving prosthetic technology. The first documented example of the use of a lower extremity prosthesis dates back to the 15th century BC, where an amputated right great toe was replaced with a prosthesis made of leather and wood. A significant driver of prosthetic evolution was the introduction of firearms in European warfare in the 16th century, creating novel injury patterns, including blast injuries that created more extensive soft tissue damage, comminuted fractures, and wound contamination. As such, the incidence of lower-limb amputation increased during this time. To meet the needs of his patients with limb loss, French military surgeon Ambrose Paré not only helped refine the technique of lower extremity amputation, but he also hand-crafted artificial limbs for his amputees [8,9].
Although advances have been made in modern surgical techniques and prosthesis technologies, understanding how loss of a limb affects patient quality of life, functional status, and interactions with the prosthetic device continues to be a challenge for clinicians and scientists alike. Numerous patient-reported outcome measures (PROMs) have been used to evaluate the impact of lower-limb amputation and prosthesis use on compartmentalized measures focusing on mental health, chronic pain, and functionality [10,11]. However, there has been a recent push to uncover how a prosthesis affects not only the distinct mental and physical experiences and functions of a limb loss patient, but also a more holistic view of that patient’s psychosocial perception of completeness: prosthesis embodiment.
The rubber hand illusion [12] is the seminal paper that illustrates the powerful nature of embodiment. This paper intuitively identified the following three components of embodiment:
- Co-location, experienced as the volume in space where one feels their body is located.
- Ownership, the experience of something being a part of the body.
- Agency, the experience of authoring the actions of one’s body, and the resulting outcomes.
Coincidentally, Ref.[13] shows that the main factors that contribute to abandonment are
- Lack of control of function.
- Lack of sensation.
- Comfort and fit issues when wearing, including the perceived weight of the device.
And these factors correspond to the lack of prosthesis embodiment.
Throughout the literature, measuring embodiment has been related to the use of upper-limb prostheses [3]. Here, the authors classify the measures as behavioral and physiological. Behavioral measures typically involve conscious judgments or voluntary actions, e.g., PROMs, while physiological measures assess subconscious processes without requiring participant input, e.g., measures of skin conductance, skin temperature, cortical imaging, etc. This distinction is made because behavioral measures capture subjective participant experience or perception, while physiological measures provide objective correlative data of the underpinning neurobiological processes.
Despite the focus on upper limbs, there is one lower-limb specific measure of embodiment: Bekrater–Bodmann’s prosthesis embodiment scale for lower extremity amputees (PEmbS-LLA) [14]. This test quantitatively validates the claim that the concept of the rubber hand can be applied to other regions of the body, including a parallel phenomenon in the lower limb known as the rubber foot illusion [15]. The first part of the test begins with the patient donning the prosthesis and then looking directly at the prosthesis for 60 s. The patient then responds with their level of agreement to the first eight questions in Table 1. In the second part of the test, patients walk for 30 s before answering the last two questions. The total score represents the patient’s overall prosthesis embodiment.

Table 1.
The questions of the PEmbS-LLA test from [16].
From the responses, principal component analysis confirmed that the three dimensions of embodiment also apply to lower-limb amputees [14]. Of the three “dimensions”, ownership explained 54.32% of the variance in the reported embodiment scores, followed by agency at 11.34%, and co-location at 5.44%. In addition, the PEmbS-LLA score and its dimensions are robust [14]. Furthermore, a Bekrater–Bodmann study [16] identified which of the satisfaction indicators identified in a previous systematic review by [18] are closely related to embodiment, such as appearance, properties, fit, gender, etiology, and level of amputation. For functional satisfaction, this study found that the only significant associated factors are residual limb pain and embodiment.
Measurement of embodiment remains an active area of research, and some criticisms of PEmbS-LLA are levied in [2]. However, quantitative validation and its robustness are strong evidence that the three dimensions of embodiment are strong predictors of satisfaction and could lead to reduced prosthesis abandonment. This informs the review’s search strategy of focusing on techniques that address one or more of these dimensions of embodiment. Initially, a literature search was made for the three dimensions directly, but for co-location and agency, two main themes of clinical interventions surfaced:
- Restoring proprioception, or replacing it with osseoperception.
- Restoring cutaneous sensation.
2.1. Proprioception and Osseoperception
Proprioception is the conscious and unconscious intrinsic knowledge of the location of body parts, as well as the sensations of effort, force, torque, and heaviness [19]. In the context of the lower limbs, proprioception is vital to performing activities of daily life. As an example, walking requires coordinated movements of both feet such that when one foot contacts the ground, the contralateral foot must lift from the ground; how this all occurs in a smooth coordinated cycle speaks to the complexity and relative autonomy of these movements, in which proprioception plays a vital role [19].
Studies of subjects with large-fiber sensory neuropathy, which typically leaves motor control intact but removes proprioceptive and tactile afferents, demonstrate the critical role of this sense. These subjects struggle to make coordinated movements in the dark [19]. In a previous study, deafferented subjects exhibited significant deviations compared to control subjects when attempting to draw a line that matches a displayed line, due to unintended motion reversals. These results indicate that compromised proprioception also compromises fine motor control, coordination, and ultimately, agency.
Without proprioception, deafferented subjects who lacked elbow coordination instead simultaneously contracted their elbow flexors and extensors to increase joint stiffness. This resulted in increased energy expenditure and reduced movement efficiency [19]. Furthermore, the absence of proprioceptive feedback contributes to maladaptive cortical plasticity. Preservation of proprioceptive neural pathways has been suggested to minimize neuroma formation, stabilize cortical representations of the limbs, and reduce phantom pain [20,21]. As noted earlier, reduced residual limb pain and ease of movement are significant predictors of embodiment.
Osseointegration (OI) enables a phenomenon known as osseoperception (OP) that is similar to proprioception and that provides similar benefits. Osseoperception refers to the perception of forces and loads experienced by the prosthesis through the direct connection between the prosthesis and residual bone [22]. Amputees using OI prostheses report a greater ability to perceive the type of surface on which they walk [23]. This enhanced sensory feedback allows users to accurately sense their position and posture and make adjustments to improve stability [24]. Furthermore, while vibrations can be transmitted through the socket in a traditional socket prosthesis, Hägström et al. showed that users of OI prostheses were much more sensitive to vibrations [25]. Increased sensitivity activates residual limb mechanoreceptors, further reducing sway and improving postural awareness and control [24].
2.2. Cutaneous Sensation
Cutaneous sensation, which is the topical sensory feedback created by stimulating the cutaneous sensory end organs, is just as critical as proprioception. This allows the individual to sense light touch, pain, pressure, vibration, and temperature. In the context of the lower limb and ambulation, tactile sensations usually signal what forces are exerted on the plantar surface of the foot, which is how posture awareness and maintenance are achieved [26].
When the foot is amputated, cutaneous sensations are lost, and the lack of cutaneous sensation contributes to maladaptive behavior, such as asymmetric gait [27], as well as reliance on visual input [28] and residuum-socket sensations as sensory feedback [29]. As with impaired proprioception, maladaptive behavior correlates with increased phantom pain and decreased embodiment. In particular, the overreliance on the residual limb that causes an asymmetric gait can lead to pain from osteopenia and osteoporosis [30].
Uneven surfaces and perturbations are perceived by cutaneous sensation. This is an important part of the feedback loop for maintaining balance. The CNS makes adjustments that increase sway when cutaneous sensory inputs are missing [30]. The increased sway makes balancing difficult and increases the likelihood of falls, resulting in a loss of confidence, a loss of embodiment, and possibly abandonment of the prosthesis [27,30]. Losing balance produces large counterbalancing movements and other maladaptive behaviors detrimental to embodiment [27].
Weight perception is also influenced by cutaneous sensation. Although much lighter than a normal limb, prostheses are usually reported by the users as uncomfortably heavy [31]. Restoration of cutaneous sensation restores weight perception and enhances embodiment by allowing users to perceive the weight of the prosthesis objectively and cognitively integrate the prosthesis [31].
3. Methods
The literature search was conducted against PubMed with the following queries:
- Prosthesis embodiment AND appearance, targeting ownership.
- Proprioception AND lower extremity prosthesis, targeting proprioception.
- Osseointegration AND lower extremity prosthesis AND embodiment, targeting osseoperception.
- Motor control AND lower extremity prosthesis, targeting agency.
- Sensory feedback AND lower extremity prosthesis, targeting cutaneous sensation.
The selection criteria for inclusion in the review are that the article describes a possible clinical intervention and discusses results indicating an improved embodiment. Due to the nature of this search, many studies have small sample sizes as they are “prosthetic in the loop” studies using bespoke prostheses. It is impractical to de-bias or properly evaluate the effects. Instead, this review aims to provide detailed descriptions of each technique and its qualitative impacts on embodiment. The goal is to create a narrative to provide clinicians with an overview of the available clinical interventions and to provide clinician-scientists with directions for future research.
4. Results
4.1. Appearance and Ownership
The visual appearance of a prosthesis plays a crucial role in promoting embodiment, as recognized by tools such as the PEmbS-LLA scale [14]. Prostheses with natural, anthropomorphic designs enhance embodiment by engaging neural pathways, even with limited training [32]. Visual continuity between the prosthetic limb and the rest of the body is particularly important, as prostheses that closely resemble natural limbs elicit stronger feelings of embodiment compared to less organic designs [33]. Conversely, when prosthetic devices fail to meet aesthetic expectations, users may face significant challenges, including reluctance to use the device, heightened anxiety, and disrupted well-being [34].
The aesthetic design also significantly impacts the acceptance of the prosthesis by shaping the body image and social confidence [7]. Many users report feeling more comfortable in social interactions and personal relationships when their prosthesis appears natural and visually appealing. This sense of comfort often results in reduced anxiety, improved self-esteem, and a stronger sense of integration between the prosthesis and the user’s identity. Positive results encourage users to participate more actively in community life, highlighting the profound influence of prosthesis appearance on social participation and quality of life [7].
Furthermore, the alignment of the prosthetic appearance with the gender identity of the user is essential to promote body image and social comfort [35]. For example, prostheses that do not accommodate the ability to wear high heels can negatively affect the sense of gender identity and self-expression in someone who feels heels are affirming [36]. Furthermore, while some patients prefer prosthetic devices with a realistic appearance that visually blend with their body, others favor a more technological aesthetic, incorporating design elements such as carbon fiber or brushed metal [7]. Since visual realism is a known factor in promoting embodiment during rubber hand illusion experiments, a deeper understanding of the lower limb of the relationship between visual aesthetics, prosthetic integration into one’s identity, and the sense of embodiment remains absent yet critical for providing patients with devices that best meet their individual needs. These findings underscore that the appearance of the prosthesis deeply influences overall well-being and satisfaction. When users feel positive about their prosthesis’ design, they are more likely to embrace it as part of their identity, fostering a sense of ownership and embodiment.
4.2. Bidirectional Devices and Modular Design
Historically, prosthetic devices have been unidirectional devices, focusing primarily on mechanical functional output to replicate the patient’s active range of motion of the joints. These traditional devices have not been designed to transmit sensory feedback from the external environment back to the patient. However, the sense of ownership is a product of the integration of visual and tactile sensory channels [12,37,38,39]. For example, the rubber hand illusion is diminished in cases where visual and tactile stimulation are asynchronous, demonstrating that the congruence of multisensory inputs is vital to the illusion [12]. Therefore, for a lower limb prosthesis to engage one’s sense of ownership, the sensory feedback from that system must be strategically designed and tuned.
Advanced neuroprosthetic technology includes bidirectionality and can communicate sensory feedback to the patient, which is fundamental to enhancing the functionality and user experience of lower limb prostheses, including mobility and balance. Figure 1 summarizes a system diagram for a bidirectional mechanoneural interface (MI) proposed by Herr and Carty in [40]. Their proposed design is modular and identifies the two modules for sensory feedback: a proprioceptive mechanoneural module interface (PMI) and cutaneous mechanoneural interface (CMI). Example PMIs are discussed in Section 4.3 and Section 4.4, and an example of CMIs is discussed in Section 4.5.
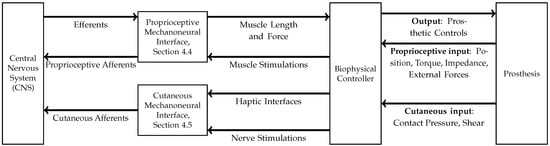
Figure 1.
System diagram for a bidirectional mechanoneural interface (MI) proposed by Herr and Carty in [40]. The MI framework comprises two parts: a proprioceptive mechanoneural interface and a cutaneous mechanoneural interface. The proprioceptive mechanoneural interface provides bidirectional control of the prosthesis through a biophysical controller, whereas the cutaneous mechanoneural interface relays topical sensory feedback afferents to the central nervous system.
The idea of a modular system began in an attempt to represent external forces experienced while walking, such as gravity, in a proprioceptive framework [41]. This resulted in unnatural sensations that could not be characterized solely within a proprioceptive context, motivating the inclusion of cutaneous mechanoneural interfaces in their design to provide natural sensations and improve biomechanical fidelity. However, it should be noted that the intersection between cutaneous sensation and proprioception can be gray. For example, Basla et al. demonstrated that proprioception can be simulated by cutaneous stimulation [42] by using surface skin electrodes to transmit information about the angle of the knee joint to the wearer. Charkhkar et al. were able to generate both perception modalities using the C-FINE devices described in Section 4.5 [43]: Table 2 shows the variety of sensations that can be generated with such devices, some of which are proprioceptive.

Table 2.
The possible elicited sensations from signals generated by C-FINE devices, as perceived by subjects in [43]. Note that the boundary between proprioception and cutaneous sensation can be quite gray, and proprioceptive modalities can also be generated. These descriptions are robust and stable over time.
Prosthesis embodiment is a multifaceted and holistic concept. A modular prosthesis that can integrate both proprioceptive and cutaneous feedback creates a more natural and intuitive sensory experience and reinforces the sense of ownership of the limb. This design is a first step towards that. In addition, studies have not yet been conducted to evaluate a combination of interventions discussed in the review, and could be a direction of future research.
4.3. Proprioceptive Clues Through Haptic Feedback
Muscle vibration can simulate proprioception by stimulating stretch receptors that detect changes in muscle length and tension [44]. One study incorporated vibrational sensory feedback into a myoelectric motor-controlled prosthesis. Although this study had a small sample size, it showed promising results of vibrational haptic feedback improving the satisfaction of lower leg prostheses [45].
Muscle spindles are stretch receptors that are vital for proprioception, but there are a limited number of studies that have investigated how stretch receptors affect prosthesis embodiment [46]. Skin stretch over a vibrated muscle improves proprioceptive signals [19]. One study showed that in people with below-knee amputations (BKAs), stretching the skin along with vibrating the muscle of the remaining portion of the limb produced stronger and more uniform movements [47]. Another study asked participants to move a cursor a certain distance without being able to visualize the cursor. They then analyzed changes in accuracy with and without haptic, vibrational, or skin stretch feedback, and they found fewer errors due to increased proprioception when skin stretch was applied [48].
Although the prospect of skin stretch is promising for improving prosthesis use, there are some limitations to consider. Ref. [48] identifies the following potential constraints:
- The area of skin present to attach a stretch apparatus cannot be too small.
- Different areas of the skin have different properties, such as the presence of hair or elasticity of the skin.
- Different skin characteristics between individuals, such as wrinkles, create variations in sensory perception.
- Shear forces can cause an undesirable tissue breakdown.
The lack of vibrotactile feedback also makes it difficult for users to navigate stairs, a challenging task that many lower limb prosthesis users avoid. Nearly 33% do not use stairs at all, and only 21% can handle stairs without a handrail, both of these low proportions due to complex demands on balance, motor control, limb symmetry, and foot placement awareness [49]. Research has shown promising results using a haptic thigh band that transmits tactile feedback from the prosthetic foot, improving foot placement accuracy during stair climbing by 17.5% in people with BKAs [49]. One user noted that artificial feedback served as “a secondary layer of confirmation, supporting what [they] already suspected about the position of their foot on the stair edge.” Despite providing feedback that differs from natural foot sensations in both location and type, this noninvasive solution helps the prosthesis function more like a natural limb. Although not definitively proven to increase embodiment scores, haptic feedback can enhance proprioception and confidence in patients with BKAs as they use their prosthetic devices when ascending stairs.
These studies highlight how vibrational and skin-stretch feedback can enhance proprioceptive signals, bridging sensory gaps between prosthetic devices and the user’s internal body schema. By simulating natural mechanoreceptor activation, such as muscle spindles or skin stretch, such haptic cues may strengthen the integration of motor intentions with sensory outcomes to bolster embodiment.
4.4. Agonist–Antagonist Myoneural Interface
Traditional amputation techniques achieve a stable residuum through either myodesis, the fixation of muscles to bones, or myoplasty, the fixation of muscles to each other [41,50]. In healthy limbs, the agonist and antagonist muscles function in dynamic opposition: When one muscle contracts, the other stretches. This interplay is vital for proprioception. By leaving the residual muscles in static configurations, traditional amputation techniques disrupt the neuromuscular loops that support proprioception. Mechanoreceptors such as muscle spindles and Golgi tendon organs lose their functionality, severing the feedback to the central nervous system [50].
The agonist–antagonist myoneural interface (AMI) is a surgical construct that was first implemented by the Ewing amputation in [21]. The Ewing amputation technique addresses limitations in traditional amputations by reconstructing the natural agonist–antagonist muscle dynamics. As of 2022, only seven individuals received this procedure [51]. The procedure for BKAs begins with the preparation of the limb, followed by the identification and preservation of key muscle groups [52]. The identified agonist–antagonist pairs are [20] as follows:
- The peroneus longus and the tibialis posterior, for subtalar eversion and inversion.
- The lateral gastrocnemius and the anterior tibialis, for subtalar flexion and dorsiflexion.
The distal tendon of each muscle is transected and reattached to its antagonist’s proximal tendon, forming a closed-loop system that mimics natural agonist–antagonist dynamics [41,52]. Peripheral nerves are carefully redirected to the motor branches of the reconstructed muscles through targeted muscle reinnervation to reduce neuroma formation and facilitate sensory and motor signaling [53]. The residual limb is shaped for prosthesis compatibility and then closed in layers. Although clinical applications of AMI have focused on people with BKAs, the technique can be applied to both above-knee amputations (AKAs) and upper-limb amputations [41].
In the context of the system diagram in Figure 1, the reconstructed muscles form the mechanical part of the proprioceptive mechanoneural interface. As described in [41], the neural part works as follows:
- The central nervous system contracts the agonist muscle using the usual motor nerve efferent control.
- The efferent signal, measured limb positions and forces, and other prosthetic inputs are sent to a biomechanical controller that computes an appropriate proprioceptive afferent signal.
- The nerve innervating the antagonist muscle is stimulated via electrodes to produce an appropriate afferent signal.
AMI improves embodiment by restoring proprioception. Starting in the periphery, muscle spindles and Golgi tendon organs are vital receptors in proprioceptive sensation. When these peripheral receptors are activated, the resulting sensory information regarding limb movement and position is processed through the dorsal columns and the spinocerebellar tract of the spinal cord [54]. This information then activates several areas of the brain, including the parietal cortex [19], the frontal cortex, and the insula [55]. A key region is the Brodmann Area 3a (BA3a) in the somatosensory cortex, which integrates signals from mechanoreceptors in muscles, tendons, and joints, allowing the CNS to coordinate motor planning, movement, and force application [20,56]. In traditional amputations, functional MRI (fMRI) studies reveal significantly lower BA3a activation in amputees with conventional procedures compared to non-amputees while dorsiflexing or plantar flexing their phantom limb. This decreased activity reflects a dependency on compensatory strategies, such as visual or vestibular feedback, which increase cognitive load and reduce movement efficiency [20,50,56]. Furthermore, the BA3a activation of AMI amputees was comparable to that of non-amputees [20].
The restoration of proprioception can also be observed functionally. After calibrating a custom prosthesis capable of performing subtalar joint movements, subjects were asked to generate a specified torque while ascending or descending stairs. Participants with traditional amputations experienced the same motion reversals described in Section 2.1, whereas AMI amputees did not [50,52]. Embodiment was also observed directly. Traditional amputees described the experiment as “playing a video game for the first time” and the prosthetic “behaved in a way that was somewhat surprising,” while AMI amputees felt that the custom prosthesis was their leg and that a conventional prosthetic “doesn’t have the same animation to it.” [52].
AMI also enhances embodiment by enabling superior residual limb motor control compared to traditional amputation techniques that improve ownership and agency. Because AMI has high biomechanical fidelity, coordination between the AMI prosthesis and the contralateral limb is nearly indistinguishable from coordination between non-amputees’ legs [50,56]. In addition, the kinematics of the virtual limb is well-modeled by a model that represents the kinematics of the intact limb [56], indicating that the prosthesis “feels” and can be controlled like the native limb. This model could be used to create more personalized prosthetic devices that take into account scar tissue, other defects, and subject-specific perceptive responses [51]. Finally, for traditional amputees, activating the muscles associated with inversion or eversion of the virtual joint also activates the muscles associated with plantar flexion or dorsiflexion. However, the activation of unwanted muscles is less of a problem in amputees with AMI [21,51]. In fact, amputees with AMI can also activate additional synergetic muscles to create a more natural, efficient, and controlled motion [51].
By reinstating natural agonist–antagonist muscle dynamics, AMI directly improves embodiment by restoring both proprioception and motor control. Enhancing these feedback mechanisms strengthens the user’s sense of ownership and agency, enabling the prosthesis to feel and function like a natural limb.
The AMI approach is still being improved to address notable drawbacks. In [21], patients with peripheral vascular disease were excluded from this study due to concerns about peripheral neuropathy. The graft harvesting process involved in AMI destroys capillaries and risks compromising the revascularization of the muscle [57], which may be a concern in patients with microvascular pathologies such as diabetes. Mild cellulitis was observed in two of the three subjects in [21], but this was resolved with oral antibiotics. A further drawback is that two-step procedures are necessary for proximal amputations, such as AKAs, because surgeons cannot immediately identify which sections of the remaining proximal sciatic nerve in the stump correspond to specific nerves that previously served the muscles of the distal lower leg [41]. As noted in Section 4.2, the main drawback of AMI is that the bandwidth to convey non-proprioceptive feedback is limited. This limitation motivates the cutaneous mechanoneural interfaces discussed in the next section.
4.5. TIME and C-FINE Devices
Technologies such as TIME (Transverse Intrafascicular Multichannel Electrode) and C-FINE (Composite Flat Interface Nerve Electrodes) directly interface with peripheral nerves to provide both cutaneous and proprioceptive sensory feedback. By directly interfacing with the nerve, a more natural sensation can be achieved, and unlike many non-invasive approaches, extensive training is not required. These technologies allow amputees to regain sensations reminiscent of those of a biological limb, significantly improving their ability to control, interact with, and embody the prosthesis.
TIME works by being transversely inserted into the peripheral nerve, enabling it to selectively activate subsets of axons within individual nerve fascicles. This design allows for highly precise stimulation at both the intrafascicular and interfascicular levels for tasks requiring targeted cutaneous sensory perception. Badia et al. demonstrated in [58] the effectiveness of TIME electrodes in the selective activation of target muscles. After insertion, the device is connected to a custom neuroprosthetic system. This custom-designed prosthesis includes a sensorized foot insole. Signals from the insole, an encoder, and TIME electrodes are linked to re-establish cutaneous sensory feedback similar to that of a normal limb [59]. Furthermore, in [27], Petrini et al. showed that the subjects were able to recognize the degree of flexion of the knee at four different angles, as well as the approximate magnitudes and location of the forces exerted. When neural stimulation was removed, the subjects were unable to determine these quantities.
In contrast, C-FINE devices wrap around larger nerves, such as the sciatic nerve. The rectangular cuff-like shape of the C-FINE device applies light pressure to the nerve, deforming it into a flatter geometry. This deformation helps to align the nerve fascicles more closely with the electrodes embedded in the cuff, improving the contact and stimulation efficiency [60]. C-FINE can be used in place of TIME devices in cases where less surgical intervention is desired or to complement them when cutaneous sensory coverage is needed for broader regions of the limb. Using high-density nerve cuffs, C-FINE systems provide consistent cutaneous sensory feedback and allow amputees to regain a natural sensation without the need for precise targeting that TIME electrodes offer. Table 2 from [43] provides a summary of the “feelings” that can be generated by varying the stimulus pulse.
TIME and C-FINE are complementary approaches that can be used together. Zelechowski et al. built a model of how the sciatic nerve reacts to stimulation from TIME and C-FINE devices in [61]. Based on the simulations, a combination of TIME and C-FINE devices was best in terms of the number of fascicles that can be selectively stimulated.
Both TIME and C-FINE devices reduce phantom limb pain for users [43]. For C-FINE devices, subjects felt that the location of the phantom big toe was correlated with the prosthesis [43], suggesting that the subject began to embody the device. Ref. [59] demonstrated that TIME patients reported reduced phantom limb pain with nerve stimulation, as quantified by the Neuropathic Pain Symptom Inventory, compared to without nerve stimulation.
In addition to reducing phantom pain, TIME and C-FINE devices significantly improve functional outcomes for lower limb amputees, though it should be noted that these studies were carried out over small sample sizes. With TIME devices, ref. [59] showed that users were able to walk faster due to improved confidence and embodiment as a result of having restored cutaneous sensations. The link between embodiment and improved functional outcomes was demonstrated through a split-focus activity that involved identifying auditory cues while walking [59]. Finally, the speed was increased despite a decrease in energy expenditure and oxygen uptake. This means that the subject’s walking gait and posture became more efficient [59]. Energy efficiency is crucial for amputees, who often expend more energy than non-amputees when performing basic movements such as basic ambulation [59].
In [27], Petrini et al. designed various physical tasks that demonstrated the ability of TIME devices to overcome challenges when cutaneous sensation is not present, as outlined in Section 2.2.
- Ascending and descending stairs, testing posture on a complex surface.
- Walking on a straight path with obstacles, testing balance on an uneven surface.
- Walking with both feet in a straight line, testing agility.
For C-FINE devices, a study by Charkhkar et al. [30] performed the sensory organization test, a balance test, and calculated the maximum postural sway from the test results. Subjects with C-FINE devices were able to decrease sway and improve balance after interruptions to their visual and somatosensory systems.
Cutaneous sensory feedback also helps users reduce the perceived weight of their prosthetic limb, improving comfort and ease of use during extended periods of activity [31]. Blindfolded study subjects were asked to compare an ankle weight versus the prosthesis’ weight. Amputees who had their cutaneous sensation restored by TIME devices more accurately identified which one was heavier [31]. In addition, this study used a split focus activity similar to the one in [59] to conclude that reduced weight contributed to increased embodiment [31].
In addition to measuring improved embodiment through reduced phantom limb pain and improved functional outcomes, PROMs were used in [27,31,59] to directly assess the impact of restoring cutaneous sensory feedback on embodiment. However, the questionnaire used was their own and was not standardized. Participants noted improvements in naturalness, control, and integration of the prosthesis with their phantom limb. These qualitative insights, combined with behavioral data, provided evidence of increased embodiment facilitated by cutaneous sensory feedback. The findings demonstrated the importance of a holistic approach to the research and development of prostheses and also indicate a lack of universal measurement systems for concepts such as embodiment and proprioception.
4.6. Osseointegration
Osseointegration (OI) is a surgical procedure that can improve the prosthesis-residuum interface by directly anchoring a metal implant into the residual bone and then attaching the prosthesis to the metal implant through a transcutaneous connector through a small opening in the skin [62]. Bone integrates with the metal implant through bony ingrowth, fusing on a cellular level by forming connections between osteocytes and titanium oxide [25]. OI has been used for upper- and lower limb prostheses and compared to traditional socket prostheses has been shown to improve comfort [63], performance [64], use [65], and embodiment [23].
Today, there are three FDA-approved OI implant systems: Osseointegrated prostheses for the Rehabilitation of Amputees (OPRA), Integral Leg Prosthesis (ILP), and Osseointegrated Prosthetic Limb (OPL).
OPRA was the first successful human OI implant system. It is implanted in a two-stage procedure: the first step is the attachment of a threaded titanium alloy bone implant and the second step is the coupling of a transcutaneous abutment to the implant and the creation of a stoma in the skin through which the abutment can protrude, where an external prosthesis can then be attached. Because OPRA is the most established of the OI implant systems, its effectiveness has been extensively studied, and OPRA is cost-effective for highly active amputees and is associated with a higher quality of life [8].
ILP and OPL consist of a stem similar to an intramedullary nail with a texture to promote bone ingrowth and a titanium niobium oxynitride surface to prevent adherence to the skin and promote gliding of the soft tissue. Retention is achieved by press-fit implantation, similar to hip arthroplasty, and the prosthesis is then mounted via a cone and screw system. Like OPRA, two stages are required to implant the ILP for use, while OPL requires only a single stage. With ILP, only 4 to 6 weeks are required between stages, unlike the 6 months required for OPRA. This results in a much shorter time between implantation and full-weight bearing, with only 2 to 3 months required after implantation of ILP and 3 to 18 months for OPRA [62]. Another method similar to the ILP and OPL in development is the Percutaneous Osseointegrated Prosthesis, which is shaped like a hip arthroplasty stem with a texture meant to promote bone ongrowth and features a niobium oxide abutment for soft tissue gliding.
Compressive osseointegration, with the Compress® transcutaneous implant, is currently under investigation through a national FDA-approved clinical trial [66]. Designed into this device are integrated Belleville washers preloaded for 400, 600, or 800 pounds of axial force within the terminus of the residual femur. Through Wolff’s law, it is hypothesized that such axial loading leads to bony hypertrophy of the femur terminus, thereby limiting loosening and bony resorption commonly seen with other non-compressive OI implants secondary to stress shielding. While patients are currently being enrolled, the first custom-designed compressive OI device was implanted at the University of Utah in 2012. Additional custom cases have been performed with published preliminary results [67].
By creating a stable, direct connection between the prosthesis and the user’s skeletal system, OI dramatically enhances the user’s sense of agency and control. This improvement is evidenced through measurements of more stable electromyographic (EMG) signals, indicating more efficient and controlled muscle activation patterns during prosthesis use [68]. Direct skeletal attachment also introduces biomechanical advantages that further enhance the user’s experience and capabilities. Eliminating socket-related problems allows more physiological weight bearing, meaning that users can distribute forces through their residual limb in a way that more closely resembles the function of the natural limb [69]. This improved weight distribution, combined with an improved range of motion unrestricted by traditional socket constraints, contributes to a more intuitive and natural prosthesis control experience [69].
OI patients consistently report a more natural interaction with their prosthetic devices, suggesting that the improved physical connection may contribute to a stronger psychological sense of embodiment [23]. Lundberg et al. used qualitative interviews with 13 patients who had undergone OI implantation and found that OI and OP were associated with dramatic improvements in quality of life and embodiment. Each interview began with the question “How do you experience living with your osseointegrated prosthesis compared to your earlier prostheses suspended with sockets?”. Patients fell into three typical typologies, perceiving their prosthesis as the following: a tool, a “pretend” limb, a part of themselves. Patients in the last typology had sometimes even forgotten about the prosthesis as a whole. One patient stated: “I have a real leg” [23].
Patients from the Lundberg et al. study alluded to embodiment by describing the prosthesis as being “anchored with material from my own body”. Participants described the traditional socket prosthesis as detracting from embodiment: “One part of the body is trapped in this vacuum-packed socket… to be let out of this entrapment, just to feel the sun towards the thigh or the air that surrounds the thigh instead of this heat and the sweating…” All of the participants from this study described a significant functional improvement when compared to a traditional socket prosthesis, expressing positive emotions with being able to walk, bike, and drive, as well as having an increased sense of independence [23]. OP also played a role, with one patient stating, “When I put the foot down, so that I can feel the shock throughout my body… I feel it and it gives me a positive experience of my body as a whole.” Even patients belonging to the other two typologies felt increased embodiment, stating that the prosthesis is “much more integrated than it was with this old prosthesis—it becomes a part of you” [23].
Although OI prostheses offer significant advantages over traditional socket prostheses, there are limitations to their usage. Infection and device failures are notable risks. Low-grade superficial infections can be treated with antibiotics [70], but fracture, implant failure, deeper infections, and osteomyelitis can require implant extraction [71,72]. One study found that 77% of the patients who received the first iterations of IPL required surgical interventions for stoma-associated infections, although 0% of patients with the latest iterations required surgery for infection [73]. The concern about infection, just as noted in AMI Section 4.4, reduces the applicability of this technique in patients with peripheral vascular disease or other chronic disease processes that increase the risks of infection. Another study found that 34% of patients receiving transfemoral OI implantation required revision surgery, due to implant failure at the stem or the dual-cone adapter [74]. The negative experience of extraction can lead to abandonment of the prosthesis, contrary to the goal of improving embodiment.
5. Discussion
With the standard of care and clinical interventions for lower-limb amputees in mind, this review examined the impact of various techniques on three key dimensions of embodiment: ownership, co-location, and agency. In addition, these dimensions are not exclusive and can complement each other in the cases of restoring proprioception and cutaneous sensation.
Recent advances in sensory feedback systems have shown promising results in improving embodiment and functional outcomes. AMI recreates the kinematic sensation of the native limb when the phantom knee is flexed, which aids in restoring proprioception and which then improves embodiment through co-location. Proprioceptive information can also be conveyed through skin stretching and other haptic feedback. Furthermore, osseointegration creates a skeletal connection with the prosthesis, contributes to proprioception, and creates unique sensory feedback in the form of osseoperception. Novel technologies such as TIME and C-FINE electrodes provide targeted sensory feedback through direct nerve interfaces, which allows for finer control of balance and gait. This leads to improved embodiment through increased agency. Finally, outside of improving agency and co-location, this review discusses considerations in prosthetic design, and user-specific preferences play crucial roles in promoting successful embodiment through improved ownership.
5.1. Future Work
First, despite ownership being the main factor in the prosthesis embodiment of the lower limb, very few studies have been conducted on this topic. The apparent contradiction between preferring a natural-looking limb and a brushed metal-looking limb suggests that much is not understood about the topic.
Studies suggest a link between embodiment and prosthesis use, suggesting that studies showing increased embodiment can serve as a proxy for reduced abandonment. However, a direct connection between embodiment and long-term outcomes remains unproven, and the studies included in this review did not comment on abandonment. Further, what differentiates between a prosthesis that is used versus one that is abandoned is multi-factorial and might be measured better by the sum of multiple PROMs, pointing to the addition of a more complete concept of embodiment when assessing overall prosthesis satisfaction. It may be worthwhile to study how PEmbs-LLA is used in conjunction with other PROMs in clinical settings mentioned in [18], such as the Trinity Amputation and Prosthesis Experience Scales-Revised (TAPES-R), the Prosthesis Evaluation Questionnaire (PEQ) and Satisfaction with the Prosthesis Score (SAT-PRO), Beck’s Depression Inventory, and the 36-Item Short Form Survey (SF36).
The multifaceted nature of embodiment demands progress across multiple domains, with standardization of assessment scales emerging as a critical priority. This standardization is essential for a meaningful comparison of possible interventions, especially given the limited pool of patients available to test new developments. In addition to the lack of standardization, as highlighted by [75], few studies have examined how embodiment evolves over time or tracked long-term results, underscoring the need for more longitudinal research. Finally, while a prosthetic device that incorporates all of the technologies surveyed in this review has been designed, it has not yet been built. It remains to be seen how all the parts come together as a whole. These metrics are necessary for systematic reviews to recommend updates to the standard of care.
In addition to addressing embodiment, there are practical limitations of these techniques that can be improved. Future work is underway to reduce the risks for patients with peripheral vascular disease for both the AMI approach and OI. Given that diabetes, peripheral vascular disease, and neuropathy are the leading causes of lower-limb amputation, managing the risk of infection would greatly improve the applicability of such techniques to improve embodiment and reduce abandonment.
5.2. Conclusions
This review presents a clinically based discussion of the techniques and technologies that help augment lower extremity prosthesis embodiment and enhance our understanding of what currently exists that may help reduce lower extremity abandonment rates. Currently, the field is characterized by a diverse array of technologies aimed at enhancing embodiment, yet lacks a systematized understanding of how these interventions combine or translate into meaningful clinical benefit. A more rigorous investigation is needed, moving beyond isolated technological advancements to explore holistic approaches that integrate these tools into a standardized care pathway. Crucially, this pathway must prioritize the individual’s lived experience rather than solely focusing on biomechanical or neurological metrics. A truly effective standard of care will require considering the full spectrum of the user’s experience, incorporating qualitative feedback, and prioritizing patient-centered design to optimize prosthetic integration and quality of life.
Author Contributions
Conceptualization, A.L., C.S., J.S., T.M., L.S., C.P., W.J., R.L.R., D.F., A.W., D.H., S.G., R.S., D.B., R.R., K.M. and A.S.; methodology, T.T.N. and B.W.; data curation, A.L. and J.S.; writing—original draft preparation, T.T.N., B.W., H.A., Q.J., C.C., S.L., S.M., J.G. and Y.T.; writing—review and editing, B.W. and T.T.N.; visualization, B.W.; supervision, A.L.; project administration, A.L, B.W. and T.T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Spencer Greene and Andrew Simpkins were employed by the company Hanger Clinic. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SF-36 | 36-Item Short Form Survey |
| AKA | Above-knee amputation |
| AMI | Agonist–antagonist myoneural interface |
| BKA | Below-knee amputation |
| BA3a | Brodmann area 3a |
| CNS | Central nervous system |
| C-FINE | Composite Flat Interface Nerve Electrodes |
| EMG | Electromyogram |
| FINE | Flat Interface Nerve Electrode |
| fMRI | Functional MRI |
| ILP | Integral Leg Prosthesis |
| ITAP | Intraosseous Transcutaneous Amputation Prosthesis |
| MI | Mechanoneural Interface |
| OPRA | Osseointegrated Prostheses for the Rehabilitation of Amputees |
| OPL | Osseointegrated Prosthetic Limb |
| OI | Osseointegration |
| OP | Osseoperception |
| PROMs | Patient reported outcomes measures |
| POP | Percutaneous Osseointegrated Prosthesis |
| PLP | Phantom limb pain |
| PEmbS-LLA | Prosthesis Embodiment Scale for Lower Limb Amputees |
| PEQ | Prosthetic Evaluation Questionnaire |
| SAT-PRO | Satisfaction with Prosthesis Score |
| TIME | Transverse Intrafascicular Multichannel Electrode |
| TAPES-R | Trinity Amputation and Prosthesis Experience Scales-Revised |
| VA | Veterans Affairs |
References
- Biddiss, E.A.; Chau, T.T. Upper limb prosthesis use and abandonment: A survey of the last 25 years. Prosthetics Orthot. Int. 2007, 31, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, J.; Lendaro, E.; Ortiz-Catalan, M. Prosthetic embodiment: Systematic review on definitions, measures, and experimental paradigms. J. Neuroeng. Rehabil. 2022, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Segil, J.L.; Roldan, L.M.; Graczyk, E.L. Measuring embodiment: A review of methods for prosthetic devices. Front. Neurorobot. 2022, 16, 902162. [Google Scholar] [CrossRef]
- Engdahl, S.M.; Meehan, S.K.; Gates, D.H. Differential experiences of embodiment between body-powered and myoelectric prosthesis users. Sci. Rep. 2020, 10, 15471. [Google Scholar] [CrossRef]
- Gailey, R.; McFarland, L.V.; Cooper, R.A.; Czerniecki, J.; Gambel, J.M.; Hubbard, S.; Maynard, C.; Smith, D.G.; Raya, M.; Reiber, G.E. Unilateral lower-limb loss: Prosthetic device use and functional outcomes in servicemembers from Vietnam war and OIF/OEF conflicts. J. Rehabil. Res. Dev. 2010, 47, 317–332. [Google Scholar] [CrossRef]
- Requejo, P.S.; Furumasu, J.; Mulroy, S.J. Evidence-based strategies for preserving mobility for elderly and aging manual wheelchair users. Top. Geriatr. Rehabil. 2015, 31, 26–41. [Google Scholar] [CrossRef]
- Sansoni, S.; Wodehouse, A.; McFadyen, A.K.; Buis, A. The aesthetic appeal of prosthetic limbs and the uncanny valley: The role of personal characteristics in attraction. Int. J. Des. 2015, 9, 67–81. [Google Scholar]
- Hoyt, B.W.; Walsh, S.A.; Forsberg, J.A. Osseointegrated prostheses for the rehabilitation of amputees (OPRA): Results and clinical perspective. Expert Rev. Med. Devices 2020, 17, 17–25. [Google Scholar] [CrossRef]
- Hernigou, P. Ambroise Paré II: Paré’s contributions to amputation and ligature. Int. Orthop. 2013, 37, 769–772. [Google Scholar] [CrossRef]
- van der Merwe, L.; Birkholtz, F.; Tetsworth, K.; Hohmann, E. Functional and psychological outcomes of delayed lower limb amputation following failed lower limb reconstruction. Injury 2016, 47, 1756–1760. [Google Scholar] [CrossRef]
- Atiç, R.; Aydın, A. Comparison of the demographic and clinical characteristics, functional status and quality of life of lower extremity amputees to identify the reason for undergoing amputation. J. Back Musculoskelet. Rehabil. 2018, 31, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.; Cohen, J. Rubber hands ‘feel’touch that eyes see. Nature 1998, 391, 756. [Google Scholar] [CrossRef] [PubMed]
- Resnik, L.J.; Borgia, M.L.; Clark, M.A. Prevalence and Predictors of Unmet Need for Upper-Limb Prostheses: An Observational Cohort Study. JPO J. Prosthetics Orthot. 2024, 36, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Bekrater-Bodmann, R. Factors associated with prosthesis embodiment and its importance for prosthetic satisfaction in lower limb amputees. Front. Neurorobot. 2021, 14, 604376. [Google Scholar] [CrossRef]
- Crea, S.; D’Alonzo, M.; Vitiello, N.; Cipriani, C. The rubber foot illusion. J. Neuroeng. Rehabil. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Bekrater-Bodmann, R. Perceptual correlates of successful body–prosthesis interaction in lower limb amputees: Psychometric characterisation and development of the Prosthesis Embodiment Scale. Sci. Rep. 2020, 10, 14203. [Google Scholar] [CrossRef]
- Longo, M.R.; Schüür, F.; Kammers, M.P.; Tsakiris, M.; Haggard, P. What is embodiment? A psychometric approach. Cognition 2008, 107, 978–998. [Google Scholar] [CrossRef]
- Baars, E.C.; Schrier, E.; Dijkstra, P.U.; Geertzen, J.H. Prosthesis satisfaction in lower limb amputees: A systematic review of associated factors and questionnaires. J. Med. 2018, 97, e12296. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Srinivasan, S.S.; Tuckute, G.; Zou, J.; Gutierrez-Arango, S.; Song, H.; Barry, R.L.; Herr, H.M. Agonist-antagonist myoneural interface amputation preserves proprioceptive sensorimotor neurophysiology in lower limbs. Sci. Transl. Med. 2020, 12, eabc5926. [Google Scholar] [CrossRef]
- Clites, T.R.; Herr, H.M.; Srinivasan, S.S.; Zorzos, A.N.; Carty, M.J. The Ewing amputation: The first human implementation of the agonist-antagonist myoneural interface. Plast. Reconst. Surg. 2018, 6, e1997. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Chowdhary, R.; Chrcanovic, B.R.; Brånemark, P.I. Osseoperception in dental implants: A systematic review. J. Prosthodont. 2016, 25, 185–195. [Google Scholar] [CrossRef]
- Lundberg, M.; Hagberg, K.; Bullington, J. My prosthesis as a part of me: A qualitative analysis of living with an osseointegrated prosthetic limb. Prosthetics Orthot. Int. 2011, 35, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, B.M.; Davis-Wilson, H.C.; Christiansen, C.L.; Awad, M.E.; Lev, G.; Tracy, J.; Stoneback, J.W. Osseointegrated prostheses improve balance and balance confidence in individuals with unilateral transfemoral limb loss. Gait Posture 2023, 100, 132–138. [Google Scholar] [CrossRef]
- Hägström, E.; Hagberg, K.; Rydevik, B.; Brånemark, R. Vibrotactile evaluation: Osseointegrated versus socket-suspended transfemoral prostheses. JRRD 2013, 50, 1423–1434. [Google Scholar] [CrossRef]
- Shell, C.E.; Christie, B.P.; Marasco, P.D.; Charkhkar, H.; Triolo, R.J. Lower-limb amputees adjust quiet stance in response to manipulations of plantar sensation. Front. Neurosci. 2021, 15, 611926. [Google Scholar] [CrossRef]
- Petrini, F.M.; Valle, G.; Bumbasirevic, M.; Barberi, F.; Bortolotti, D.; Cvancara, P.; Hiairrassary, A.; Mijovic, P.; Sverrisson, A.Ö.; Pedrocchi, A.; et al. Enhancing functional abilities and cognitive integration of the lower limb prosthesis. Sci. Transl. Med. 2019, 11, eaav8939. [Google Scholar] [CrossRef]
- Barnett, C.T.; Vanicek, N.; Polman, R. Postural responses during volitional and perturbed dynamic balance tasks in new lower limb amputees: A longitudinal study. Gait Posture 2013, 37, 319–325. [Google Scholar] [CrossRef]
- Crea, S.; Cipriani, C.; Donati, M.; Carrozza, M.C.; Vitiello, N. Providing time-discrete gait information by wearable feedback apparatus for lower-limb amputees: Usability and functional validation. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 23, 250–257. [Google Scholar] [CrossRef]
- Charkhkar, H.; Christie, B.P.; Triolo, R.J. Sensory neuroprosthesis improves postural stability during Sensory Organization Test in lower-limb amputees. Sci. Rep. 2020, 10, 6984. [Google Scholar] [CrossRef]
- Preatoni, G.; Valle, G.; Petrini, F.M.; Raspopovic, S. Lightening the perceived prosthesis weight with neural embodiment promoted by sensory feedback. Curr. Biol. 2021, 31, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, G.; Romano, D.; Spaccasassi, C.; Mioli, A.; D’Alonzo, M.; Sacchetti, R.; Guglielmelli, E.; Zollo, L.; Di Lazzaro, V.; Denaro, V.; et al. Sensory-and action-oriented embodiment of neurally-interfaced robotic hand prostheses. Front. Neurosci. 2020, 14, 389. [Google Scholar] [CrossRef] [PubMed]
- Tieri, G.; Tidoni, E.; Pavone, E.F.; Aglioti, S.M. Body visual discontinuity affects feeling of ownership and skin conductance responses. Sci. Rep. 2015, 5, 17139. [Google Scholar] [CrossRef]
- Durmus, D.; Safaz, I.; Adıgüzel, E.; Uran, A.; Sarısoy, G.; Goktepe, A.S.; Tan, A.K. The relationship between prosthesis use, phantom pain and psychiatric symptoms in male traumatic limb amputees. Compr. Psychiatry 2015, 59, 45–53. [Google Scholar] [CrossRef]
- Murray, C.D. Embodiment and prosthetics. In Psychoprosthetics; Springer: Berlin/Heidelberg, Germany, 2008; pp. 119–129. [Google Scholar]
- Manz, S.; Valette, R.; Damonte, F.; Avanci Gaudio, L.; Gonzalez-Vargas, J.; Sartori, M.; Dosen, S.; Rietman, J. A review of user needs to drive the development of lower limb prostheses. J. Neuroeng. Rehabil. 2022, 19, 119. [Google Scholar] [CrossRef]
- Kilteni, K.; Maselli, A.; Kording, K.P.; Slater, M. Over my fake body: Body ownership illusions for studying the multisensory basis of own-body perception. Front. Hum. Neurosci. 2015, 9, 119452. [Google Scholar] [CrossRef]
- Braun, N.; Debener, S.; Spychala, N.; Bongartz, E.; Sörös, P.; Müller, H.H.; Philipsen, A. The senses of agency and ownership: A review. Front. Psychol. 2018, 9, 535. [Google Scholar] [CrossRef]
- Schettler, A.; Raja, V.; Anderson, M.L. The embodiment of objects: Review, analysis, and future directions. Front. Neurosci. 2019, 13, 1332. [Google Scholar] [CrossRef]
- Herr, H.; Carty, M.J. The agonist-antagonist myoneural interface. Tech. Orthop. 2021, 36, 337–344. [Google Scholar] [CrossRef]
- Carty, M.J.; Herr, H.M. The agonist-antagonist myoneural interface. Hand Clin. 2021, 37, 435–445. [Google Scholar] [CrossRef]
- Basla, C.; Chee, L.; Valle, G.; Raspopovic, S. A non-invasive wearable sensory leg neuroprosthesis: Mechanical, electrical and functional validation. J. Neural Eng. 2022, 19, 016008. [Google Scholar] [CrossRef] [PubMed]
- Charkhkar, H.; Shell, C.E.; Marasco, P.D.; Pinault, G.J.; Tyler, D.J.; Triolo, R.J. High-density peripheral nerve cuffs restore natural sensation to individuals with lower-limb amputations. J. Neural Eng. 2018, 15, 056002. [Google Scholar] [CrossRef] [PubMed]
- Roll, J.P.; Albert, F.; Thyrion, C.; Ribot-Ciscar, E.; Bergenheim, M.; Mattei, B. Inducing any virtual two-dimensional movement in humans by applying muscle tendon vibration. J. Neurophysiol. 2009, 101, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Feng, Y.; Wang, Q. Combining vibrotactile feedback with volitional myoelectric control for robotic transtibial prostheses. Front. Neurorobotics 2016, 10, 8. [Google Scholar] [CrossRef]
- Papaleo, E.D.; D’Alonzo, M.; Fiori, F.; Piombino, V.; Falato, E.; Pilato, F.; De Liso, A.; Di Lazzaro, V.; Di Pino, G. Integration of proprioception in upper limb prostheses through non-invasive strategies: A review. J. Neuroeng. Rehabil. 2023, 20, 118. [Google Scholar] [CrossRef]
- Shehata, A.W.; Keri, M.I.; Gomez, M.; Marasco, P.D.; Vette, A.H.; Hebert, J.S. Skin stretch enhances illusory movement in persons with lower-limb amputation. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; IEEE: Piscataway Township, NJ, USA, 2019; pp. 1233–1238. [Google Scholar]
- Bark, K.; Wheeler, J.W.; Premakumar, S.; Cutkosky, M.R. Comparison of skin stretch and vibrotactile stimulation for feedback of proprioceptive information. In Proceedings of the 2008 Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems, Reno, NV, USA, 13–14 March 2008; IEEE: Piscataway Township, NJ, USA, 2008; pp. 71–78. [Google Scholar]
- Rokhmanova, N.; Rombokas, E. Vibrotactile feedback improves foot placement perception on stairs for lower-limb prosthesis users. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; IEEE: Piscataway Township, NJ, USA, 2019; pp. 1215–1220. [Google Scholar]
- Srinivasan, S.S.; Gutierrez-Arango, S.; Teng, A.C.E.; Israel, E.; Song, H.; Bailey, Z.K.; Carty, M.J.; Freed, L.E.; Herr, H.M. Neural interfacing architecture enables enhanced motor control and residual limb functionality postamputation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019555118. [Google Scholar] [CrossRef]
- Song, H.; Israel, E.A.; Gutierrez-Arango, S.; Teng, A.C.; Srinivasan, S.S.; Freed, L.E.; Herr, H.M. Agonist-antagonist muscle strain in the residual limb preserves motor control and perception after amputation. Commun. Med. 2022, 2, 97. [Google Scholar] [CrossRef]
- Clites, T.R.; Carty, M.J.; Ullauri, J.B.; Carney, M.E.; Mooney, L.M.; Duval, J.F.; Srinivasan, S.S.; Herr, H.M. Proprioception from a neurally controlled lower-extremity prosthesis. Sci. Transl. Med. 2018, 10, eaap8373. [Google Scholar] [CrossRef]
- Pasquina, P.F.; Bryant, P.R.; Huang, M.E.; Roberts, T.L.; Nelson, V.S.; Flood, K.M. Advances in amputee care. Arch. Phys. Med. Rehabil. 2006, 87, 34–43. [Google Scholar] [CrossRef]
- Pop, I.V.; Espinosa, F.; Blevins, C.J.; Okafor, P.C.; Ogujiofor, O.W.; Goyal, M.; Mona, B.; Landy, M.A.; Dean, K.M.; Gurumurthy, C.B.; et al. Structure of long-range direct and indirect spinocerebellar pathways as well as local spinal circuits mediating proprioception. J. Neurosci. 2022, 42, 581–600. [Google Scholar] [CrossRef]
- Tsakiris, M.; Hesse, M.D.; Boy, C.; Haggard, P.; Fink, G.R. Neural signatures of body ownership: A sensory network for bodily self-consciousness. Cereb. Cortex 2007, 17, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Huang, S.S.; Shallal, C.; Herr, H.M. Restoration of bilateral motor coordination from preserved agonist-antagonist coupling in amputation musculature. J. Neuroeng. Rehabil. 2021, 18, 1–17. [Google Scholar]
- Srinivasan, S.S.; Diaz, M.; Carty, M.; Herr, H.M. Towards functional restoration for persons with limb amputation: A dual-stage implementation of regenerative agonist-antagonist myoneural interfaces. Sci. Rep. 2019, 9, 1981. [Google Scholar] [CrossRef]
- Badia, J.; Boretius, T.; Andreu, D.; Azevedo-Coste, C.; Stieglitz, T.; Navarro, X. Comparative analysis of transverse intrafascicular multichannel, longitudinal intrafascicular and multipolar cuff electrodes for the selective stimulation of nerve fascicles. J. Neuroeng. Rehabil. 2011, 8, 036023. [Google Scholar] [CrossRef] [PubMed]
- Petrini, F.M.; Bumbasirevic, M.; Valle, G.; Ilic, V.; Mijović, P.; Čvančara, P.; Barberi, F.; Katic, N.; Bortolotti, D.; Andreu, D.; et al. Sensory feedback restoration in leg amputees improves walking speed, metabolic cost and phantom pain. Nat. Med. 2019, 25, 1356–1363. [Google Scholar] [CrossRef]
- Yildiz, K.A.; Shin, A.Y.; Kaufman, K.R. Interfaces with the peripheral nervous system for the control of a neuroprosthetic limb: A review. J. Neuroeng. Rehabil. 2020, 17, 1–19. [Google Scholar] [CrossRef]
- Zelechowski, M.; Valle, G.; Raspopovic, S. A computational model to design neural interfaces for lower-limb sensory neuroprostheses. J. Neuroeng. Rehabil. 2020, 17, 24. [Google Scholar] [CrossRef]
- Hoellwarth, J.S.; Tetsworth, K.; Rozbruch, S.R.; Handal, M.B.; Coughlan, A.; Al Muderis, M. Osseointegration for amputees: Current implants, techniques, and future directions. JBJS Rev. 2020, 8, e0043. [Google Scholar] [CrossRef]
- Van de Meent, H.; Hopman, M.T.; Frölke, J.P. Walking ability and quality of life in subjects with transfemoral amputation: A comparison of osseointegration with socket prostheses. Arch. Phys. Med. Rehabil. 2013, 94, 2174–2178. [Google Scholar] [CrossRef]
- Muderis, M.A.; Lu, W.; Glatt, V.; Tetsworth, K. Two-stage osseointegrated reconstruction of post-traumatic unilateral transfemoral amputees. Mil. Med. 2018, 183, 496–502. [Google Scholar] [CrossRef]
- Van Eck, C.F.; McGough, R.L. Clinical outcome of osseointegrated prostheses for lower extremity amputations: A systematic review of the literature. Curr. Orthop. Pract. 2015, 26, 349–357. [Google Scholar] [CrossRef]
- National Library of Medicine. A Study to Evaluate the Safety and Effectiveness Transdermal Compress Device in Participants with Transfemoral Amputations. Available online: https://clinicaltrials.gov/study/NCT06134167 (accessed on 4 August 2024).
- McGough, R.; Goodman, M.; Randall, R.; Forsberg, J.; Potter, B.; Lindsey, B. The Compress® transcutaneous implant for rehabilitation following limb amputation. Der Unfallchirurg 2017, 120, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Catalan, M.; Håkansson, B.; Brånemark, R. An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs. Sci. Transl. Med. 2014, 6, 257re6. [Google Scholar] [CrossRef]
- Li, Y.; Lindeque, B. Percutaneous osseointegrated prostheses for transfemoral amputations. Orthopedics 2018, 41, 75–80. [Google Scholar] [CrossRef]
- Kunutsor, S.; Gillatt, D.; Blom, A. Systematic review of the safety and efficacy of osseointegration prosthesis after limb amputation. J. Br. Surg. 2018, 105, 1731–1741. [Google Scholar] [CrossRef]
- Al Muderis, M.; Khemka, A.; Lord, S.J.; Van de Meent, H.; Frölke, J.P.M. Safety of osseointegrated implants for transfemoral amputees: A two-center prospective cohort study. J. Bone Jt. Surg. 2016, 98, 900–909. [Google Scholar] [CrossRef]
- Tillander, J.; Hagberg, K.; Berlin, Ö.; Hagberg, L.; Brånemark, R. Osteomyelitis risk in patients with transfemoral amputations treated with osseointegration prostheses. Clin. Orthop. Relat. Res. 2017, 475, 3100–3108. [Google Scholar] [CrossRef]
- Juhnke, D.L.; Beck, J.P.; Aschoff, H.H. Fifteen years of experience with Integral-Leg-Prosthesis: Cohort study of artificial limb attachment system. J. Rehabil. Res. Dev. 2015, 52, 407. [Google Scholar] [CrossRef]
- Mohamed, J.; Reetz, D.; van de Meent, H.; Schreuder, H.; Frölke, J.P.; Leijendekkers, R. What are the risk factors for mechanical failure and loosening of a transfemoral osseointegrated implant system in patients with a lower-limb amputation? Clin. Orthop. Relat. Res. 2022, 480, 722–731. [Google Scholar] [CrossRef]
- Balk, E.M.; Gazula, A.; Markozannes, G.; Kimmel, H.J.; Saldanha, I.J.; Resnik, L.J.; Trikalinos, T.A. Lower limb prostheses: Measurement instruments, comparison of component effects by subgroups, and long-term outcomes. Comp. Eff. Rev. 2018, 213, 18-EHC017-EF. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).