Theoretical Prediction of the Color Expression of Malvidin 3-Glucoside by In Silico Tristimulus Colorimetry: Effects of Structure Conformational Changes and Molecular Interactions
Abstract
1. Introduction
2. Materials and Methods
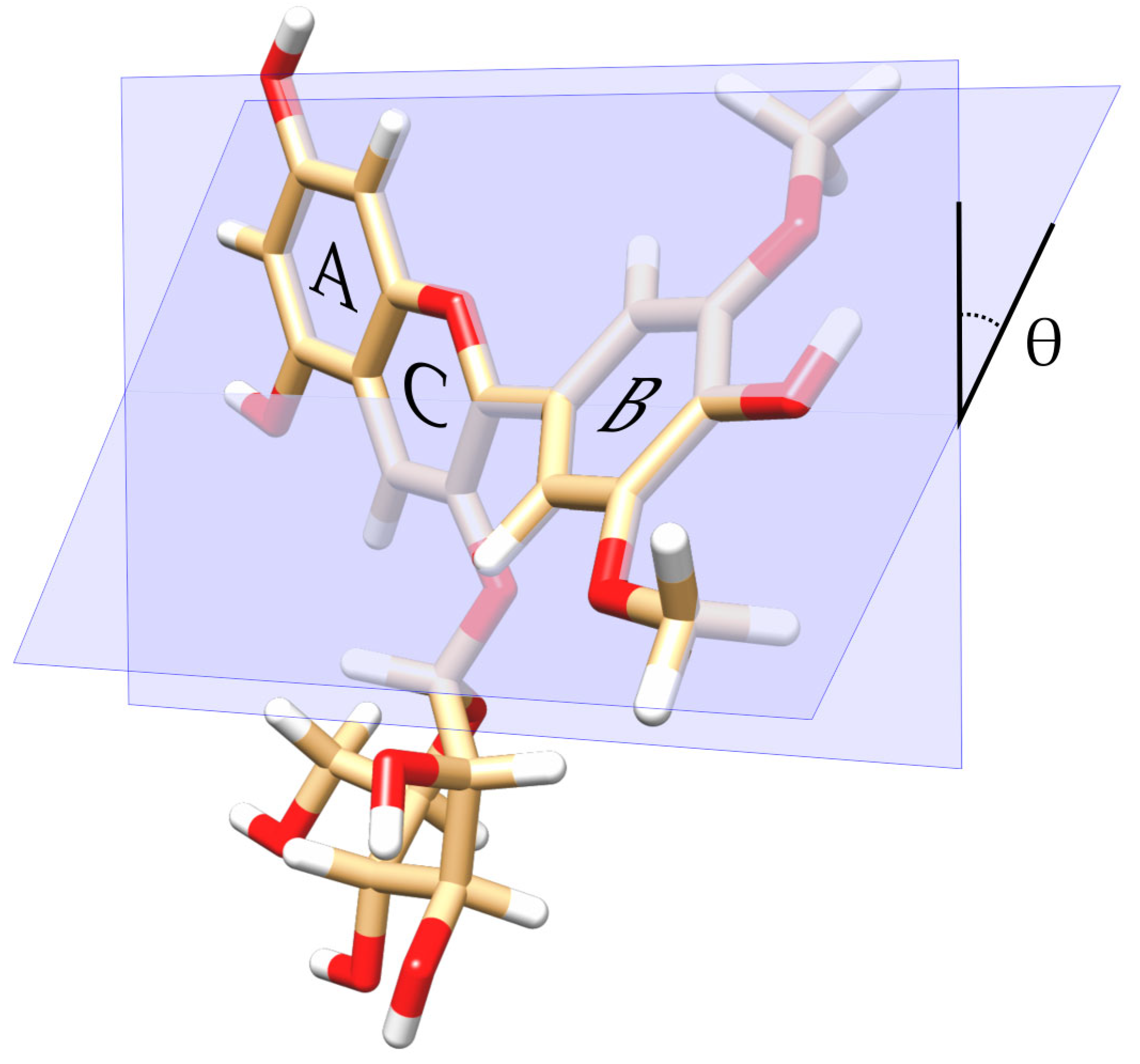
2.1. Electronic Structure Method for Modeling the 3D Structure of Mv3glc Conformers at Different Dihedral Angles
2.2. Modeling of the 3D Structure of the Grape Seed Peptide
2.3. Mv3glc–Grape Seed Peptide Docking Study
2.4. Computing the Theoretical Visible Absorbance Spectrum of Mv3glc Under Different Simulated Environments
2.5. In Silico Colorimetric Methodology in the CIELAB Color Space
- Hue angle (chromatic tonality): hab = arctan (b*/a*);
- Chroma (color intensity or saturation): C*ab = [(a*)2 + (b*)2]1/2.
- Relative contribution (%) of lightness: %ΔL = [(ΔL*)2/(ΔE*ab)2] × 100;
- Relative contribution (%) of chroma: %ΔC = [(ΔC*ab)2/(ΔE*ab)2] × 100;
- Relative contribution (%) of hue: %ΔH = [(ΔH)2/(ΔE*ab)2] × 100, with ΔH being mathematically deduced from ΔH = [(ΔE*ab)2 − (ΔL)2 − (ΔC)2)]1/2.
3. Results and Discussion
3.1. In Silico Analysis of Conformational Effects on Mv3glc Predicted Color
3.2. In Silico Colorimetric Analysis of the Grape Seed Peptide–Mv3glc Interaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIE | Commission Internationale de L’Eclairage |
| DFT | density functional theory |
| GSP | grape seed peptide |
| MD | molecular dynamics |
| mv3glc | malvidin 3-glucoside |
| TD-DFT | time-dependent density functional theory |
References
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Pina, F.; Melo, M.J.; Laia, C.A.T.; Parola, A.J.; Lima, J.C. Chemistry and Applications of Flavylium Compounds: A Handful of Colours. Chem. Soc. Rev. 2012, 41, 869–908. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, V.; Mateus, N. Chemical Transformations of Anthocyanins Yielding a Variety of Colours. Environ. Chem. Lett. 2006, 4, 175–183. [Google Scholar] [CrossRef]
- Basílio, N.; Mendoza, J.; Seco, A.; Oliveira, J.; De Freitas, V.; Pina, F. Strategies Used by Nature to Fix the Red, Purple and Blue Colours in Plants: A Physical Chemistry Approach. Phys. Chem. Chem. Phys. 2021, 23, 24080–24101. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Impact of Location, Type, and Number of Glycosidic Substitutions on the Color Expression of o-Dihydroxylated Anthocyanidins. Food Chem. 2018, 268, 416–423. [Google Scholar] [CrossRef]
- Malcıoğlu, O.B.; Calzolari, A.; Gebauer, R.; Varsano, D.; Baroni, S. Dielectric and Thermal Effects on the Optical Properties of Natural Dyes: A Case Study on Solvated Cyanin. J. Am. Chem. Soc. 2011, 133, 15425–15433. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Fernandes, A.; Brás, N.F.; Oliveira, J.; Mateus, N.; De Freitas, V. Impact of a Pectic Polysaccharide on Oenin Copigmentation Mechanism. Food Chem. 2016, 209, 17–26. [Google Scholar] [CrossRef]
- He, Z.; Zhu, H.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Complexation of Bovine β-Lactoglobulin with Malvidin-3-O-Glucoside and Its Effect on the Stability of Grape Skin Anthocyanin Extracts. Food Chem. 2016, 209, 234–240. [Google Scholar] [CrossRef]
- Buchweitz, M.; Speth, M.; Kammerer, D.R.; Carle, R. Impact of Pectin Type on the Storage Stability of Black Currant (Ribes Nigrum, L.) Anthocyanins in Pectic Model Solutions. Food Chem. 2013, 139, 1168–1178. [Google Scholar] [CrossRef]
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine Fining with Plant Proteins. Molecules 2019, 24, 2186. [Google Scholar] [CrossRef] [PubMed]
- Chamizo-González, F.; Estévez, I.G.; Gordillo, B.; Manjón, E.; Escribano-Bailón, M.T.; Heredia, F.J.; González-Miret, M.L. First Insights into the Binding Mechanism and Colour Effect of the Interaction of Grape Seed 11S Globulin with Malvidin 3-O-Glucoside by Fluorescence Spectroscopy, Differential Colorimetry and Molecular Modelling. Food Chem. 2023, 413, 135591. [Google Scholar] [CrossRef] [PubMed]
- Mora-Garrido, A.B.; Escudero-Gilete, M.L.; González-Miret, M.L.; Heredia, F.J.; Cejudo-Bastante, M.J. Effect of the Addition of Protein Hydrolysates from Grape Seed Meal Residue to Red Wines in Warm Regions in the Stabilization Stage. LWT 2024, 205, 116554. [Google Scholar] [CrossRef]
- Yao, L.; Xu, J.; Zhang, L.; Zheng, T.; Liu, L.; Zhang, L. Physicochemical Stability-Increasing Effects of Anthocyanin via a Co-Assembly Approach with an Amphiphilic Peptide. Food Chem. 2021, 362, 130101. [Google Scholar] [CrossRef]
- López-Molina, M.F.; Rodríguez-Pulido, F.J.; Mora-Garrido, A.B.; González-Miret, M.L.; Heredia, F.J. New Approaches for Screening Grape Seed Peptides as Colourimetric Modulators by Malvidin-3-O-Glucoside Stabilisation. Food Chem. 2025, 464, 141708. [Google Scholar] [CrossRef]
- Gordillo, B.; Rodríguez-Pulido, F.J.; Escudero-Gilete, M.L.; González-Miret, M.L.; Heredia, F.J. Comprehensive Colorimetric Study of Anthocyanic Copigmentation in Model Solutions. Effects of pH and Molar Ratio. J. Agric. Food Chem. 2012, 60, 2896–2905. [Google Scholar] [CrossRef]
- Heredia, F.J.; Francia-Aricha, E.M.; Rivas-Gonzalo, J.C.; Vicario, I.M.; Santos-Buelga, C. Chromatic Characterization of Anthocyanins from Red Grapes—I. pH Effect. Food Chem. 1998, 63, 491–498. [Google Scholar] [CrossRef]
- CIE (Ed.) Colorimetry, 3rd. ed.; Technical report/CIE; CIE, Central Bureau: Vienna, Austria, 2004; ISBN 978-3-901906-33-6. [Google Scholar]
- Chen, Q.; Xiao, Y.; Zhang, W.; Mu, W. Current Methods and Applications in Computational Protein Design for Food Industry. Crit. Rev. Food Sci. Nutr. 2020, 60, 3259–3270. [Google Scholar] [CrossRef]
- Trouillas, P.; Di Meo, F.; Gierschner, J.; Linares, M.; Sancho-Garcia, J.C.; Otyepka, M. Optical Properties of Wine Pigments: Theoretical Guidelines with New Methodological Perspectives. Tetrahedron 2015, 71, 3079–3088. [Google Scholar] [CrossRef]
- Anouar, E.H.; Osman, C.P.; Weber, J.-F.F.; Ismail, N.H. UV/Visible Spectra of a Series of Natural and Synthesised Anthraquinones: Experimental and Quantum Chemical Approaches. SpringerPlus 2014, 3, 233. [Google Scholar] [CrossRef]
- Soto-Rojo, R.; Baldenebro-López, J.; Flores-Holguín, N.; Glossman-Mitnik, D. Comparison of Several Protocols for the Computational Prediction of the Maximum Absorption Wavelength of Chrysanthemin. J. Mol. Model. 2014, 20, 2378. [Google Scholar] [CrossRef] [PubMed]
- Anouar, E.H.; Gierschner, J.; Duroux, J.-L.; Trouillas, P. UV/Visible Spectra of Natural Polyphenols: A Time-Dependent Density Functional Theory Study. Food Chem. 2012, 131, 79–89. [Google Scholar] [CrossRef]
- Sinopoli, A.; Calogero, G.; Bartolotta, A. Computational Aspects of Anthocyanidins and Anthocyanins: A Review. Food Chem. 2019, 297, 124898. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Schwabe, T.; Mück-Lichtenfeld, C. Density Functional Theory with Dispersion Corrections for Supramolecular Structures, Aggregates, and Complexes of (Bio)Organic Molecules. Org Biomol Chem 2007, 5, 741–758. [Google Scholar] [CrossRef]
- Rusishvili, M.; Grisanti, L.; Laporte, S.; Micciarelli, M.; Rosa, M.; Robbins, R.J.; Collins, T.; Magistrato, A.; Baroni, S. Unraveling the Molecular Mechanisms of Color Expression in Anthocyanins. Phys. Chem. Chem. Phys. 2019, 21, 8757–8766. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Li, Y.; Prejanò, M.; Toscano, M.; Russo, N. Oenin and Quercetin Copigmentation: Highlights from Density Functional Theory. Front. Chem. 2018, 6, 245. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Chamizo-González, F.; Heredia, F.J.; Rodríguez-Pulido, F.J.; González-Miret, M.L.; Gordillo, B. Proteomic and Computational Characterisation of 11S Globulins from Grape Seed Flour By-Product and Its Interaction with Malvidin 3-Glucoside by Molecular Docking. Food Chem. 2022, 386, 132842. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Ramirez, A.; Heredia, F.J.; González-Miret, M.; Lourdes, M.; Moro, Á.; Corsino, J. CromaLab, 2.0; Sevilla, Spain, 2004.
- Ge, X.; Calzolari, A.; Baroni, S. Optical Properties of Anthocyanins in the Gas Phase. Chem. Phys. Lett. 2015, 618, 24–29. [Google Scholar] [CrossRef]
- Rustioni, L.; Di Meo, F.; Guillaume, M.; Failla, O.; Trouillas, P. Tuning Color Variation in Grape Anthocyanins at the Molecular Scale. Food Chem. 2013, 141, 4349–4357. [Google Scholar] [CrossRef] [PubMed]
- Deineka, V.I.; Kulchenko, Y.Y. Quantum-Chemical Calculations for the Electronic Absorption Spectra of Certain Anthocyanidins. Russ. J. Phys. Chem. A 2021, 95, 1378–1385. [Google Scholar] [CrossRef]
- Martínez, J.A.; Melgosa, M.; Pérez, M.M.; Hita, E.; Negueruela, A.I. Note. Visual and Instrumental Color Evaluation in Red Wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Di Meo, F.; Sancho Garcia, J.C.; Dangles, O.; Trouillas, P. Highlights on Anthocyanin Pigmentation and Copigmentation: A Matter of Flavonoid π-Stacking Complexation To Be Described by DFT-D. J. Chem. Theory Comput. 2012, 8, 2034–2043. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Prejanò, M.; Marino, T.; Russo, N.; Tao, Y.; Li, Y. Gallic Acid Improves Color Quality and Stability of Red Wine via Physico-Chemical Interaction and Chemical Transformation as Revealed by Thermodynamics, Real Wine Dynamics and Benchmark Quantum Mechanical Calculations. Food Res. Int. 2024, 188, 114510. [Google Scholar] [CrossRef]


| Mv3glc Conformation | Dihedral Angle (θ) | L* | a* | b* | C*ab | hab |
|---|---|---|---|---|---|---|
| 1 | 0 | 50.27 | 50.39 | 1.30 | 50.41 | 1.47° |
| 2 | 10 | 50.24 | 50.73 | −2.72 | 50.80 | 356.94° |
| 3 | 20 | 49.71 | 50.33 | −4.73 | 50.55 | 354.63° |
| 4 | 30 | 49.68 | 50.11 | −12.92 | 51.75 | 345.54° |
| 5 | 40 | 50.83 | 48.33 | −25.89 | 54.83 | 331.82° |
| 6 | 50 | 55.92 | 39.87 | −37.68 | 54.86 | 316.61° |
| 7 | 60 | 67.90 | 20.21 | −35.49 | 40.84 | 299.66° |
| 8 | 70 | 89.80 | 1.68 | −9.91 | 10.05 | 279.63° |
| 9 | 80 | 93.61 | 2.08 | −4.85 | 5.28 | 293.77° |
| 10 | 90 | 97.61 | 2.67 | 0.48 | 2.71 | 10.23° |
| Dihedral Angle (θ) | ΔE*ab | %ΔL | %ΔC | %ΔH |
|---|---|---|---|---|
| 0–10 | 4.03 | 0.01 | 0.94 | 99.05 |
| 0–20 | 6.05 | 0.85 | 0.06 | 99.09 |
| 0–30 | 14.24 | 0.17 | 0.89 | 98.94 |
| 0–40 | 27.27 | 0.04 | 2.62 | 97.34 |
| 0–50 | 40.77 | 1.92 | 1.19 | 96.89 |
| 0–60 | 50.75 | 12.07 | 3.56 | 84.38 |
| 0–70 | 63.72 | 38.47 | 40.12 | 21.41 |
| 0–80 | 65.19 | 44.19 | 47.92 | 7.89 |
| 0–90 | 67.22 | 49.58 | 50.35 | 0.07 |
| Quantum Chemical Calculations | Spectral Parameters | CIELAB Color Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dihedral Angle (θ) | E Docking (kcal/mol) | Molecular Interaction | λmax | A520 | L* | a* | b* | C*ab | hab | |
| 1 | 67.0° | −4.1 | π-π/π-alkyl | 492 | 0.36 | 79.4 | 20.0 | 7.3 | 21.3 | 20.2º |
| 2 | −85.3° | −4.1 | hydrogen bond/π-alkyl | 424 | 0.08 | 94.4 | −2.5 | 28.6 | 28.7 | 94.9º |
| 3 | 66.5° | −4.1 | π-π/π-alkyl | 542 | 0.32 | 77.1 | 10.4 | −5.2 | 11.6 | 333.4º |
| 4 | 71.3° | −4.0 | hydrogen bond/π-alkyl | 476 | 0.29 | 82.5 | 14.2 | 12.3 | 18.8 | 40.9º |
| 5 | 66.5° | −4.0 | π-π/π-alkyl | 512 | 0.45 | 72.8 | 22.8 | −7.3 | 23.9 | 342.2° |
| 6 | 58.0° | −4.0 | π-π/π-alkyl | 472 | 0.43 | 77.3 | 21.7 | 22.4 | 31.2 | 45.9º |
| 7 | 60.0° | −4.0 | hydrogen bond/π-alkyl | 484 | 0.54 | 74.4 | 30.8 | 16.4 | 34.9 | 28.1º |
| 8 | −83.7° | −4.0 | π-π/π-alkyl | 428 | 0.08 | 93.4 | −1.0 | 15.2 | 15.6 | 93.9º |
| 9 | −85.1° | −3.9 | π-π/π-alkyl | 428 | 0.08 | 93.9 | −1.1 | 16.2 | 16.2 | 93.9º |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamizo-González, F.; Heredia, F.J.; López-Molina, M.F.; Rodríguez-Pulido, F.J.; González-Miret, M.L.; Gordillo, B. Theoretical Prediction of the Color Expression of Malvidin 3-Glucoside by In Silico Tristimulus Colorimetry: Effects of Structure Conformational Changes and Molecular Interactions. Appl. Sci. 2025, 15, 4238. https://doi.org/10.3390/app15084238
Chamizo-González F, Heredia FJ, López-Molina MF, Rodríguez-Pulido FJ, González-Miret ML, Gordillo B. Theoretical Prediction of the Color Expression of Malvidin 3-Glucoside by In Silico Tristimulus Colorimetry: Effects of Structure Conformational Changes and Molecular Interactions. Applied Sciences. 2025; 15(8):4238. https://doi.org/10.3390/app15084238
Chicago/Turabian StyleChamizo-González, Francisco, Francisco J. Heredia, María Fernanda López-Molina, Francisco J. Rodríguez-Pulido, M. Lourdes González-Miret, and Belén Gordillo. 2025. "Theoretical Prediction of the Color Expression of Malvidin 3-Glucoside by In Silico Tristimulus Colorimetry: Effects of Structure Conformational Changes and Molecular Interactions" Applied Sciences 15, no. 8: 4238. https://doi.org/10.3390/app15084238
APA StyleChamizo-González, F., Heredia, F. J., López-Molina, M. F., Rodríguez-Pulido, F. J., González-Miret, M. L., & Gordillo, B. (2025). Theoretical Prediction of the Color Expression of Malvidin 3-Glucoside by In Silico Tristimulus Colorimetry: Effects of Structure Conformational Changes and Molecular Interactions. Applied Sciences, 15(8), 4238. https://doi.org/10.3390/app15084238







