The Effects of Flocculant Yeast or Spontaneous Fermentation Strategies Supplemented with an Organic Nitrogen-Rich Additive on the Volatilome and Organoleptic Profile of Wines from a Neutral Grape Variety
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Must and Winemaking Conditions
2.2. Microbiological Analysis
2.3. Chemical Analysis
2.4. Analysis of Wine Volatiles
2.5. Organoleptic Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Kinetics and Microbiota Dynamics During Sampling Stages
3.2. Oenological Parameters
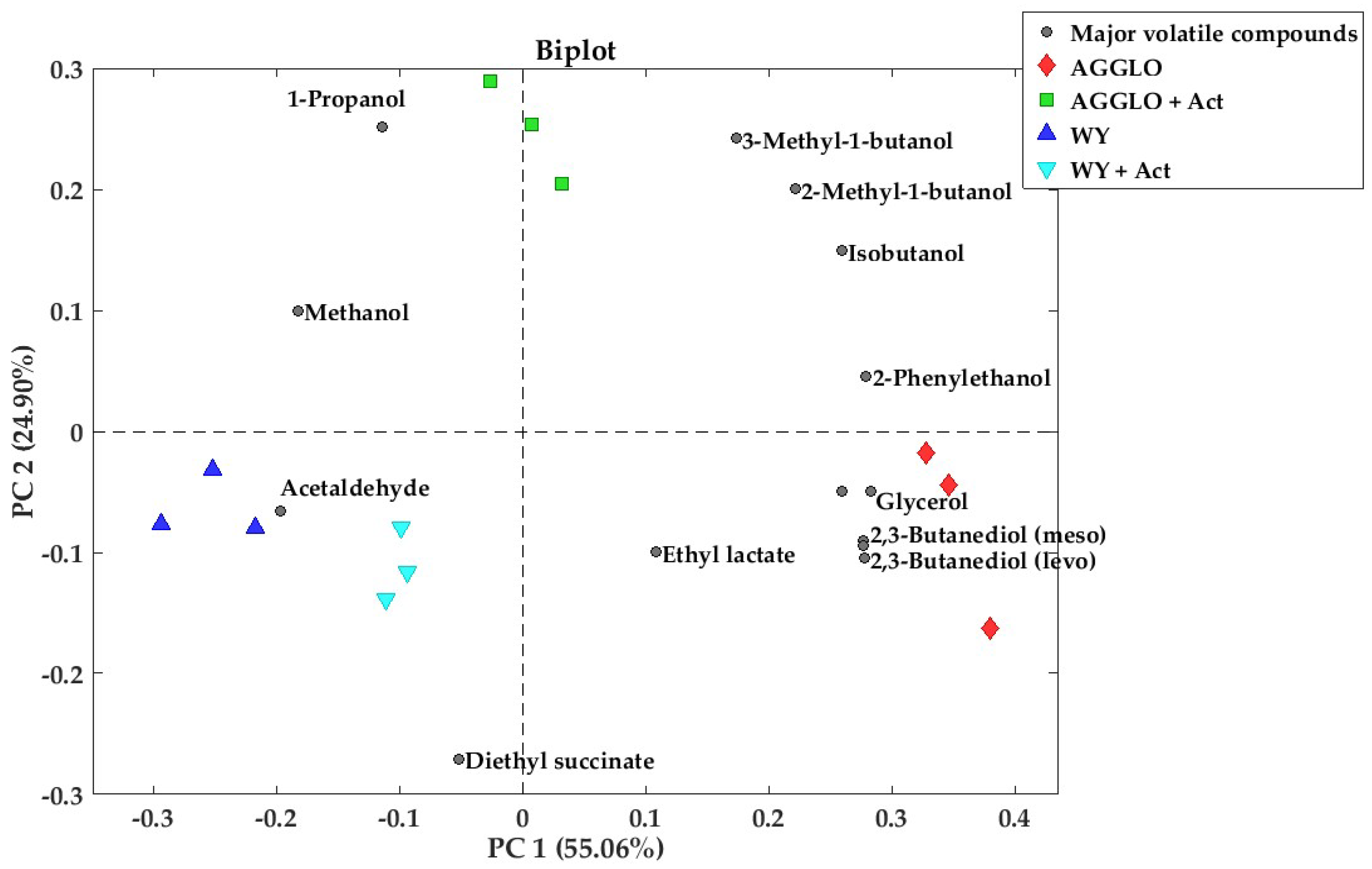
3.3. Major Volatile Compounds and Polyols
| Compounds | CAS | WY | WY + Act | AGGLO | AGGLO + Act | HG | Y | A | OPT | OS | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetaldehyde | 75-07-0 | 86 ± 5 b | 66 ± 3 a | 65 ± 2 a | 66 ± 2 a | 2 | *** | ** | *** | 110 I | 1, 6 |
| Ethyl acetate | 141-78-6 | 28.5 ± 0.1 a | 31.2 ± 0.3 b | 32.5 ± 0.3 c | 30.5 ± 0.8 b | 3 | *** | ns | *** | 7.5 I | 6 |
| Methanol | 67-56-1 | 84 ± 6 a | 84 ± 6 a | 78 ± 3 a | 83 ± 3 a | 1 | ns | ns | ns | 50 I | 6 |
| 1-Propanol | 71-23-8 | 19.9 ± 0.6 b | 18.5 ± 0.5 a | 18.5 ± 0.7 a | 20.9 ± 0.6 b | 2 | ns | ns | *** | 830 I | 6 |
| Isobutanol | 78-83-1 | 26.8 ± 0.4 a | 27.6 ± 0.4 a | 39.6 ± 0.8 c | 37.7 ± 0.5 b | 3 | *** | ns | ** | 40 I | 6 |
| 2-Methyl-1-butanol | 137-32-6 | 38.8 ± 0.5 a | 40.2 ± 0.7 b | 44.7 ± 0.5 c | 45.7 ± 0.5 d | 4 | *** | ** | ns | 30 I | 6 |
| 3-Methyl-1-butanol | 123-51-3 | 214 ± 3 a | 211 ± 5 a | 233 ± 5 b | 244 ± 4 c | 3 | *** | ns | * | 30 I | 6 |
| Acetoin | 513-86-0 | 16 ± 3 a | 21 ± 2 b | 37 ± 4 c | 19.6 ± 0.7 ab | 3 | *** | ** | *** | 150 I | 4 |
| Ethyl lactate | 97-64-3 | 14.7 ± 0.2 a | 14.8 ± 0.3 a | 15.0 ± 0.6 a | 14.5 ± 0.3 a | 1 | ns | ns | ns | 150 I | 4 |
| 2,3-Butanediol (levo) | 24347-58-8 | 258 ± 9 a | 289 ± 8 b | 447 ± 19 c | 268 ± 8 ab | 3 | *** | *** | *** | - | - |
| 2,3-Butanediol (meso) | 5341-95-7 | 36 ± 2 a | 40 ± 3 a | 99 ± 9 b | 39 ± 2 a | 2 | *** | *** | *** | - | - |
| Diethyl succinate | 123-25-7 | 14 ± 2 b | 12.9 ± 0.6 b | 13 ± 1 b | 8 ± 1 a | 2 | ** | ** | * | 100 I | 1 |
| 2-Phenylethanol | 60-12-8 | 27 ± 2 a | 25 ± 1 a | 47 ± 2 c | 34.7 ± 0.6 b | 3 | *** | *** | *** | 10 I | 9 |
| Glycerol | 56-81-5 | 4090 ± 259 a | 4046 ± 118 a | 6946 ± 133 c | 4473 ± 100 b | 3 | *** | *** | *** | - | - |
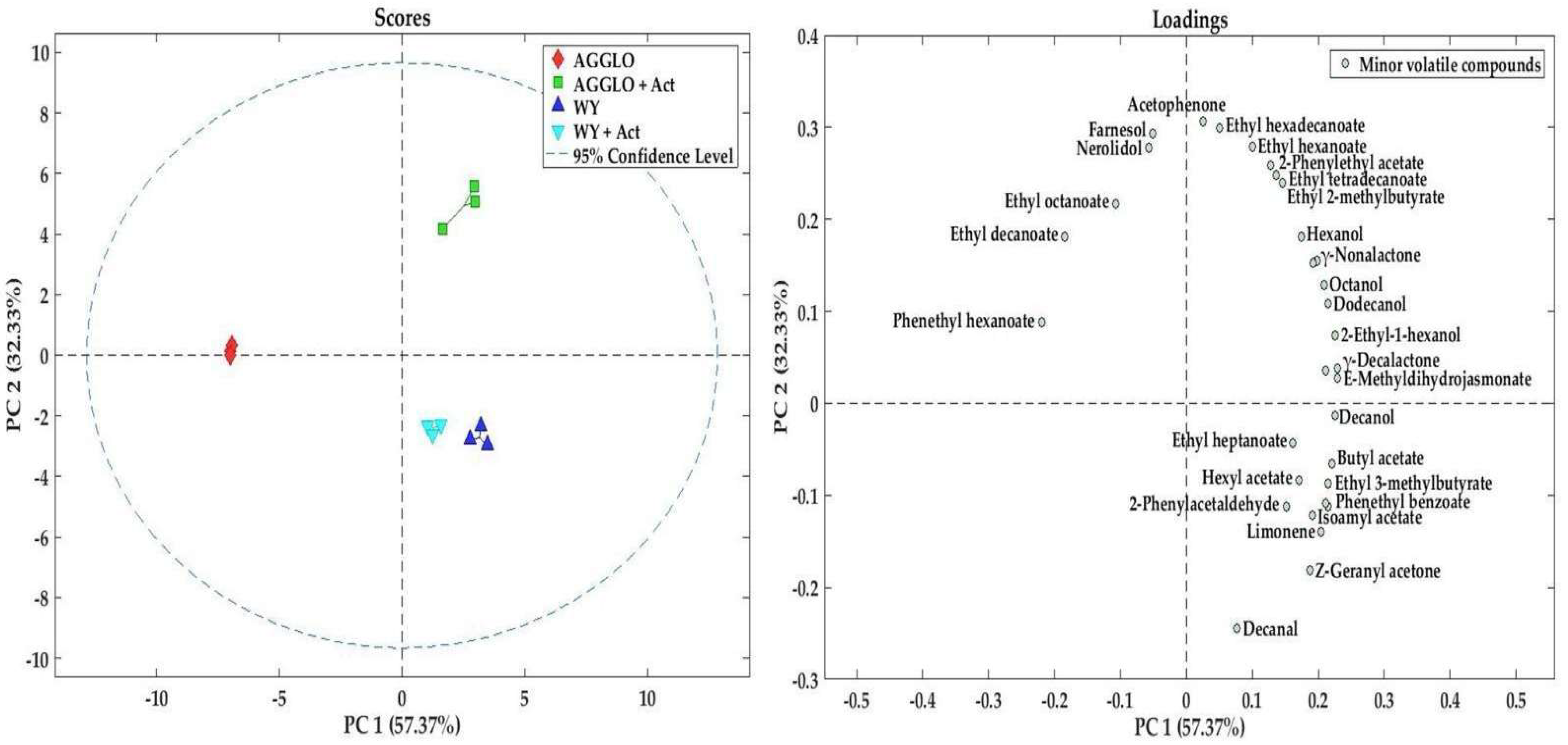
3.4. Minor Volatile Compounds
| CAS | WY | WY + Act | AGGLO | AGGLO + Act | HG | Y | A | OPT | OS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols (5) | |||||||||||
| Hexanol | 111-27-3 | 2272 ± 168 b | 2814 ± 228 b | 1265 ± 502 a | 3973 ± 502 c | 3 | ns | *** | *** | 8000 I | 3 |
| 2-Ethyl-1-hexanol | 104-76-7 | 980 ± 86 c | 855 ± 45 b | 369 ± 81 a | 1097 ± 81 c | 3 | ** | *** | *** | 8000 II | 5 |
| Octanol | 111-87-5 | 266 ± 4 b | 267 ± 6 b | 94 ± 33 a | 374 ± 33 c | 3 | * | *** | *** | 800 III | 8 |
| Decanol | 112-30-1 | 69 ± 1 b | 71 ± 8 b | 24 ± 6 a | 69 ± 6 b | 2 | *** | *** | *** | 400 IV | 8 |
| Dodecanol | 112-53-8 | 149 ± 15 b | 141 ± 10 b | 63 ± 17 a | 187 ± 17 c | 3 | * | *** | *** | 1000 V | 8 |
| Esters (15) | |||||||||||
| Ethyl butyrate | 105-54-4 | 61 ± 2 bc | 57.4 ± 0.3 b | 30 ± 5 a | 64 ± 5 c | 3 | *** | *** | *** | 20 I | 1 |
| Butyl acetate | 123-86-4 | 53.7 ± 0.6 c | 40 ± 3 b | 20.6 ± 0.8 a | 42.2 ± 0.8 b | 3 | *** | ** | *** | 66 VII | 1, 6 |
| Ethyl 2-methylbutyrate | 7452-79-1 | 6.6 ± 0.4 b | 6.4 ± 0.9 b | 3 ± 1 a | 15 ± 1 c | 3 | *** | *** | *** | 18 VIII | 1, 2 |
| Ethyl 3-methylbutyrate | 108-64-5 | 17 ± 1 c | 13.4 ± 0.5 b | 6 ± 1 a | 13 ± 1 b | 3 | *** | * | *** | 3 II | 1, 2 |
| Isoamyl acetate | 123-92-2 | 165 ± 2 c | 201 ± 3 d | 70 ± 12 a | 138 ± 12 b | 4 | *** | *** | ** | 30 I | 1 |
| Ethyl hexanoate | 123-66-0 | 22 ± 0.2 a | 21.7 ± 0.2 a | 21 ± 2 a | 32 ± 2 b | 2 | *** | *** | *** | 14 II | 1, 2 |
| Hexyl acetate | 142-92-7 | 298 ± 12 b | 382 ± 4 c | 211 ± 10 a | 300 ± 10 b | 3 | *** | *** | ns | 670 IX | 1, 2 |
| Ethyl heptanoate | 106-30-9 | 2.2 ± 0.2 b | 2.01 ± 0.04 ab | 1.9 ± 0.2 a | 2.1 ± 0.2 ab | 2 | ns | ns | * | 2.2 VIII | 1, 2 |
| Ethyl octanoate | 106-32-1 | 18.5 ± 0.3 a | 20 ± 0.4 ab | 21 ± 1 b | 21 ± 1 b | 2 | ** | ns | ns | 5 I | 1, 8 |
| 2-Phenylethyl acetate | 103-45-7 | 1094 ± 48 b | 1094 ± 40 b | 902 ± 154 a | 1877 ± 154 c | 3 | *** | *** | *** | 250 I | 7, 9 |
| Ethyl decanoate | 110-38-3 | 48 ± 2 a | 50 ± 2 a | 136 ± 8 c | 99 ± 8 b | 3 | *** | *** | *** | 200 I | 1, 8 |
| Phenethyl hexanoate | 101-60-0 | 0.5 ± 0.07 a | 0.54 ± 0.05 a | 5.7 ± 0.2 c | 1.8 ± 0.2 b | 3 | *** | *** | *** | 250 I | 2, 8, 9 |
| Ethyl tetradecanoate | 124-06-1 | 66 ± 3 c | 57 ± 3 b | 48 ± 6 a | 108 ± 6 d | 4 | *** | *** | *** | 4000 I | 8 |
| Phenethyl benzoate | 94-47-3 | 13 ± 1 c | 9.6 ± 0.3 b | 3.7 ± 0.3 a | 8.6 ± 0.3 b | 3 | *** | * | *** | n.f. | 9 |
| Ethyl hexadecanoate | 628-97-7 | 145 ± 16 a | 182 ± 13 b | 204 ± 6 b | 473 ± 6 c | 3 | *** | *** | *** | 2000 I | 8 |
| Aldehydes (4) | |||||||||||
| Hexanal | 66-25-1 | 7.6 ± 0.1 b | 6.8 ± 0.8 b | 3.9 ± 0.3 a | 10.1 ± 0.3 c | 3 | ns | *** | *** | 5 X | 3 |
| Nonanal | 124-19-6 | 5.7 ± 0.5 c | 5.4 ± 0.2 c | 1.7 ± 0.3 a | 4.2 ± 0.3 b | 3 | *** | *** | *** | 2.5 II | 5 |
| Decanal | 112-31-2 | 5.9 ± 0.6 c | 5.3 ± 0.4 bc | 4.7 ± 0.4 ab | 4.2 ± 0.4 a | 3 | ** | ns | ns | 1.25 II | 5, 8 |
| 2-Phenyl-acetaldehyde | 122-78-1 | 129 ± 9 c | 81 ± 3 ab | 69 ± 9 a | 86 ± 9 b | 3 | *** | ** | *** | 4 XI | 3, 7 |
| Ketones (1) | |||||||||||
| Acetophenone | 98-86-2 | n.d. a | n.d. a | 14 ± 4 b | 56 ± 4 c | 3 | *** | *** | *** | 65 XII | 9 |
| Lactones (2) | |||||||||||
| γ-Nonalactone | 104-61-0 | 69 ± 6 c | 50 ± 3 b | 33 ± 8 a | 84 ± 8 d | 4 | ns | ** | *** | 30 I | 4 |
| γ-Decalactone | 706-14-9 | 44 ± 2 c | 26 ± 5 b | 11 ± 4 a | 40 ± 4 c | 3 | ** | * | *** | 41 I | 1, 4 |
| Terpenes and Norisoprenoids (5) | |||||||||||
| Limonene | 5989-27-5 | 420 ± 48 d | 333 ± 11 c | 31 ± 20 a | 225 ± 20 b | 4 | *** | ** | *** | 10 II | 4, 6 |
| Nerolidol | 7212-44-4 | 11 ± 1 b | 0.8 ± 0.1 a | 25 ± 3 c | 34 ± 3 d | 4 | *** | ns | *** | 700 II | 3, 9 |
| Z-Geranyl acetone | 689-67-8 | 13 ± 1 c | 11.7 ± 0.6 c | 3.1 ± 0.2 a | 6.9 ± 0.2 b | 3 | *** | * | *** | 60 II | 9 |
| Farnesol | 4602-84-0 | 52 ± 5 b | 28 ± 2 a | 126 ± 9 c | 180 ± 9 d | 4 | *** | ** | *** | 20 VI | 9 |
| E-Methyldihydro-jasmonate | 24851-98-7 | 36 ± 3 c | 31 ± 2 b | 11 ± 2 a | 37 ± 2 c | 3 | *** | *** | *** | 70 II | 9 |
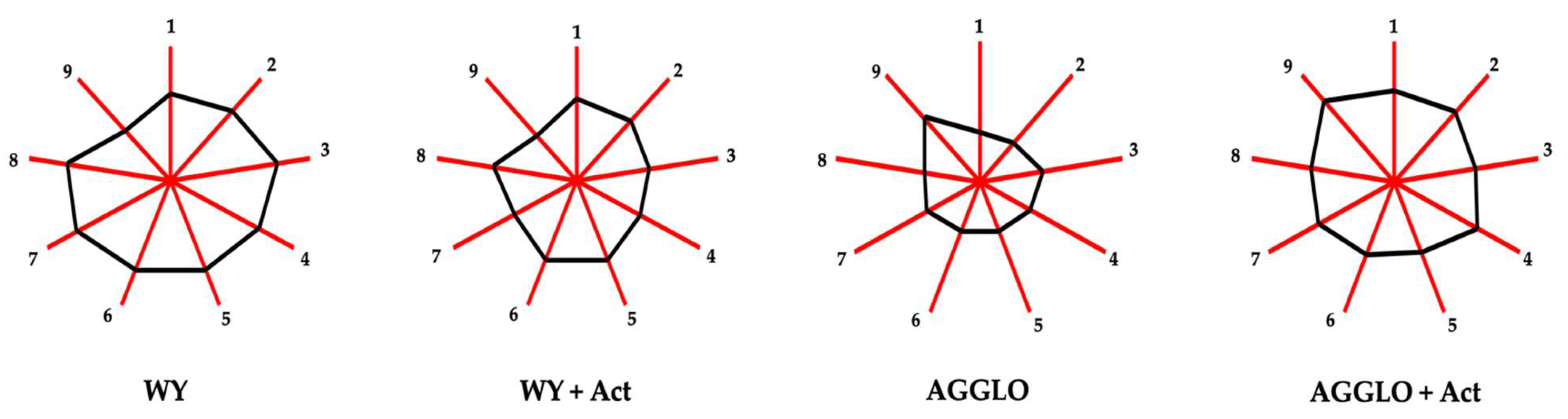
3.5. Odorant Series
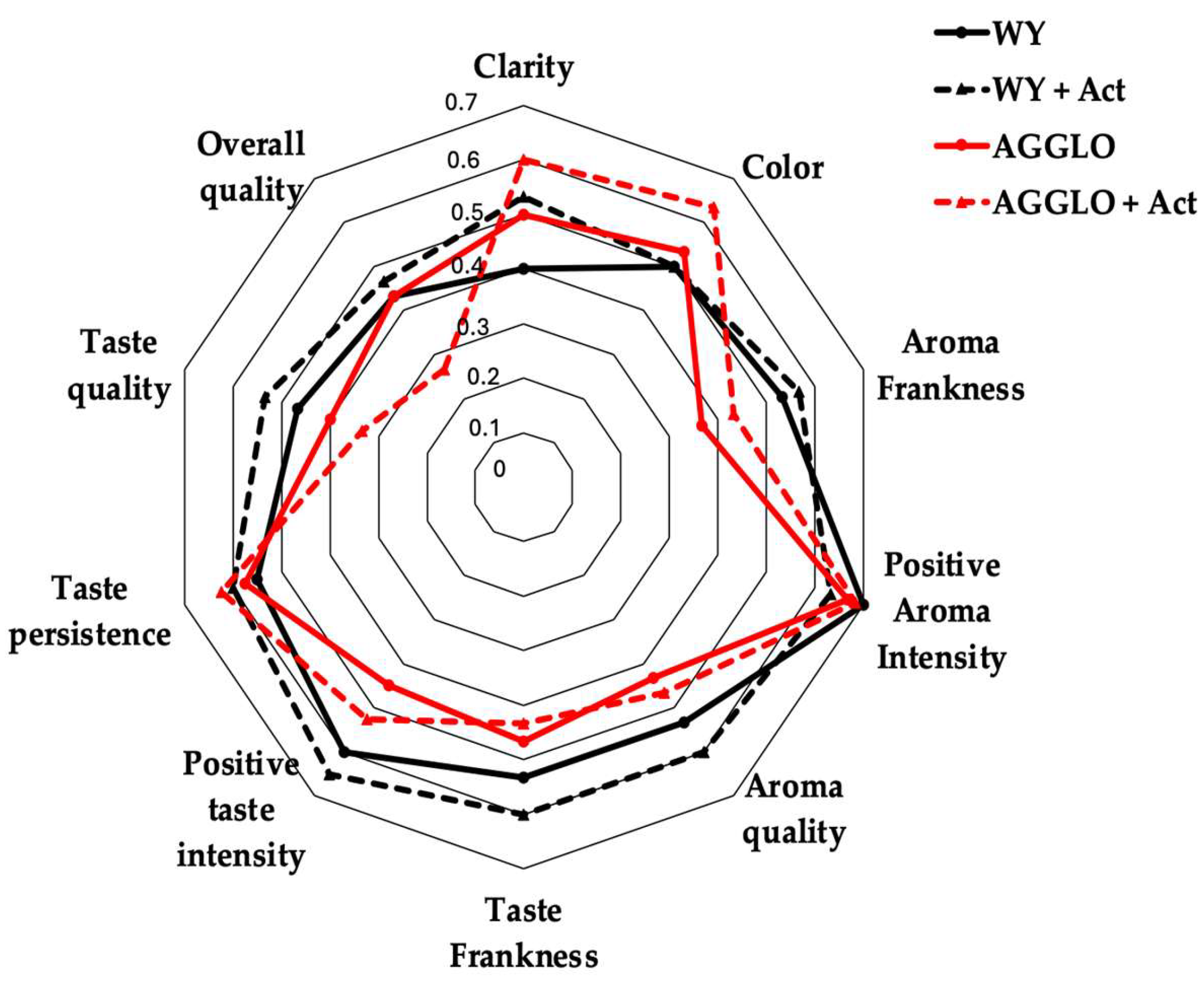
3.6. Organoleptic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADY | active dry yeast |
| ANOVA | analysis of variance |
| CAS | Chemical Abstract Service |
| DAP | diammonium phosphate |
| FID | flame ionization detector |
| GC | gas chromatography |
| HG | homogeneous group |
| LSD | least significant difference |
| MPS | Multi-Purpose Sampler |
| MS | mass spectrometry |
| MVA | Multiple Variant Analysis |
| OAV | Odor Activity Value |
| OIV | International Organisation of Vine and Wine |
| ONC | organic nitrogen compounds |
| OPT | odor perception threshold |
| OS | odorant series |
| PCA | Principal Component Analysis |
| PDMS | polydimethylsiloxane |
| SBSE | stir bar sorptive extraction |
| TDU | Thermal Desorption Unit |
| TPI | Total Polyphenol Index |
| VOCs | volatile organic compounds |
| WY | wild yeast |
| YAN | yeast available nitrogen |
References
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and Composition of Airén Wines Fermented by Sequential Inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Moreno García, J. Proteomic and Metabolomic Study of Wine Yeasts in Free and Immobilized Formats, Subjected to Different Stress Conditions; UCOPress: Cordoba, Spain, 2017; p. 181. [Google Scholar]
- Moreno-García, J.; García-Martinez, T.; Moreno, J.; Mauricio, J.C.; Ogawa, M.; Luong, P.; Bisson, L.F. Impact of Yeast Flocculation and Biofilm Formation on Yeast-Fungus Coadhesion in a Novel Immobilization System. Am. J. Enol. Vitic. 2018, 69, 278–288. [Google Scholar] [CrossRef]
- Wang, X.; Fan, G.; Peng, Y.; Xu, N.; Xie, Y.; Zhou, H.; Liang, H.; Zhan, J.; Huang, W.; You, Y. Mechanisms and Effects of Non-Saccharomyces Yeast Fermentation on the Aromatic Profile of Wine. J. Food Compos. Anal. 2023, 124, 105660. [Google Scholar] [CrossRef]
- Christofi, S.; Papanikolaou, S.; Dimopoulou, M.; Terpou, A.; Cioroiu, I.B.; Cotea, V.; Kallithraka, S. Effect of yeast assimilable nitrogen content on fermentation kinetics, wine chemical composition and sensory character in the production of Assyrtiko wines. Appl. Sci. 2022, 12, 1405. [Google Scholar] [CrossRef]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2008, 11, 242–295. [Google Scholar] [CrossRef]
- López-Lira, C.; Valencia, P.; Urtubia, A.; Landaeta, E.; Tapia, R.A.; Franco, W. Influence of organic nitrogen derived from recycled wine lees and inorganic nitrogen on the chemical composition of Cabernet Sauvignon wines fermented in the presence of non-Saccharomyces yeasts Candida boidinii, C. oleophila, and C. zemplinina. Foods 2024, 13, 4166. [Google Scholar] [CrossRef]
- Almeida, E.L.M.; Moreira e Silva, G.; Mendes-Ferreira, A. Effects of nitrogen supplementation on Saccharomyces cerevisiae JP14 fermentation for mead production. Food Sci. Technol. 2020, 40, 336–343. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, H.; Zhou, X.; Li, H.; Feng, R.; Yuan, F.; Pan, S.; Xu, X. Effect of DAP and glutamine supplementation on sulfur-containing volatiles and sensory properties of Chardonnay wine fermented with Saccharomyces cerevisiae yeast. J. Food Sci. 2023, 88, 1392–1408. [Google Scholar] [CrossRef]
- Andújar-Ortiz, I.; Pozo-Bayón, M.A.; García-Ruiz, A.; Moreno-Arribas, V. Role of specific components from commercial inactive dry yeast winemaking preparations on the growth of wine lactic acid bacteria. J. Agric. Food Chem. 2010, 58, 8392–8399. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; Andújar-Ortiz, I.; Moreno-Arribas, M.V. Volatile profile and potential of inactive dry yeast-based winemaking additives to modify the volatile composition of wines. J. Sci. Food Agric. 2009, 89, 1665–1673. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Ibarz, M.J.; Cacho, J.; Ferreira, V. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005, 89, 163–174. [Google Scholar] [CrossRef]
- Lorenzo, C.; Bordiga, M.; Pérez-Álvarez, E.P.; Travaglia, F.; Arlorio, M.; Salinas, M.R.; Coïsson, J.D.; Garde-Cerdán, T. The impacts of temperature, alcoholic degree and amino acids content on biogenic amines and their precursor amino acids content in red wine. Food Res. Int. 2017, 99, 328–335. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Nitrogen demand of yeasts during alcoholic fermentation. In Handbook of Enology: The Microbiology of Wine and Vinifications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 39–45. [Google Scholar]
- Pérez, D.; Assof, M.; Bolcato, E.; Sari, S.; Fanzone, M. Combined effect of temperature and ammonium addition on fermentation profile and volatile aroma composition of Torrontés Riojano wines. LWT 2018, 87, 488–497. [Google Scholar] [CrossRef]
- Ugliano, M.; Travis, B.; Francias, I.; Henschke, P. Volatile composition and sensory properties of Shiraz wines affected by nitrogen supplementation and yeast species: Rationalizing nitrogen modulation of wine aroma. J. Agric. Food Chem. 2010, 58, 12417–12425. [Google Scholar] [CrossRef]
- Varela, C.; Bartel, C.; Nandorfy, D.E.; Borneman, A.; Schmidt, S.; Curtin, C. Identification of flocculant wine yeast strains with improved filtration-related phenotypes through application of high throughput sedimentation rate assays. Sci. Rep. 2020, 10, 2738. [Google Scholar] [CrossRef]
- Lambrechts, M.; Pretorius, I. Yeast and Its Importance to Wine Aroma. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Godillot, J.; Baconin, C.; Sanchez, I.; Baragatti, M.; Perez, M.; Sire, Y.; Aguera, E.; Sablayrolles, J.-M.; Farines, V.; Mouret, J.R. Analysis of volatile compounds production kinetics: A study of the impact of nitrogen addition and temperature during alcoholic fermentation. Front. Microbiol. 2023, 14, 1124970. [Google Scholar]
- Soares, E.V.; De Coninck, G.; Duarte, F.; Soares, H.M.V. Use of Saccharomyces cerevisiae for Cu2+ removal from solution: The advantages of using a flocculent strain. Biotechnol. Lett. 2002, 24, 663–666. [Google Scholar] [CrossRef]
- Rossouw, D.; Bagheri, B.; Setati, M.E.; Bauer, F.F. Co-flocculation of yeast species, a new mechanism to govern population dynamics in microbial ecosystems. PLoS ONE 2015, 10, e0136249. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.V.Y.; Ielasi, F.S.; Nookaew, I.; Stals, I.; Alonso-Sarduy, L.; Daenen, L.; Van Mulders, S.E.; Stassen, C.; van Eijsden, R.G.E.; Siewers, V.; et al. Molecular mechanism of flocculation self-recognition in yeast and its role in mating and survival. mBio 2015, 6, e00427-15. [Google Scholar] [CrossRef]
- Mill, P.J. The nature of the interactions between flocculent cells in the flocculation of Saccharomyces cerevisiae. J. Gen. Microbiol. 1964, 35, 61–68. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Verachtert, H.; Delvaux, F.R. Yeast flocculation: What brewers should know. Appl. Microbiol. Biotechnol. 2003, 61, 197–205. [Google Scholar] [CrossRef]
- OIV. Available online: https://www.oiv.int/ (accessed on 8 September 2024).
- Palenzuela, M.d.V.; López de Lerma, N.; Sánchez-Suárez, F.; Martínez-García, R.; Peinado, R.A.; Rosal, A. Aroma composition of wines produced from grapes treated with organic amendments. Appl. Sci. 2023, 13, 8001. [Google Scholar] [CrossRef]
- ISO 3591:1977; Sensory Analysis—Apparatus—Wine-Tasting Glass. International Organization for Standardization: Geneva, Switzerland, 1977.
- Somers, J.M.; Bevan, E.A. The inheritance of the killer character in yeast. Gen. Res. 1969, 14, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Vasserot, Y.; Liger-Belair, G.; Marchal, R. Sparkling Wine Production. In Handbook of Enology: Principles, Practices and Recent Innovations; The Asia Tech Publisher: New Delhi, India, 2011; Chapter 31; 52p, ISBN 9788187680253. [Google Scholar]
- Di Gianvito, P.; Perpetuini, G.; Tittarelli, F.; Schirone, M.; Arfelli, G.; Piva, A.; Tofalo, R. Impact of Saccharomyces cerevisiae strains on traditional sparkling wines production. Food Res. Int. 2018, 109, 552–560. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.Á.; Andújar-Ortiz, I.; Moreno-Arribas, M.V. Scientific evidences beyond the application of inactive dry yeast preparations in winemaking. Food Res. Int. 2009, 42, 754–761. [Google Scholar] [CrossRef]
- Vion, C.; Muro, M.; Bernard, M.; Richard, B.; Valentine, F.; Yeramian, N.; Marullo, P. New malic acid producer strains of Saccharomyces cerevisiae for preserving wine acidity during alcoholic fermentation. Food Microbiol. 2023, 112, 104209. [Google Scholar] [CrossRef]
- Vion, C.; Peltier, E.; Bernard, M.; Muro, M.; Marullo, P. Marker-assisted selection of malic-consuming Saccharomyces cerevisiae strains for winemaking. Efficiency and limits of a QTL’s driven breeding program. J. Fungi 2021, 7, 17–24. [Google Scholar]
- Rodriguez-Nogales, J.M.; Fernández-Fernández, E.; Vila-Crespo, J. Effect of the addition of β-glucanases and commercial yeast preparations on the chemical and sensorial characteristics of traditional sparkling wine. Eur. Food Res. Technol. 2012, 235, 729–744. [Google Scholar] [CrossRef]
- Comuzzo, P.; Battistutta, F.; Vendrame, M.; Páez, M.S.; Luisi, G.; Zironi, R. Antioxidant properties of different products and additives in white wine. Food Chem. 2015, 168, 107–114. [Google Scholar] [CrossRef]
- Giacosa, S.; Río Segade, S.; Cagnasso, E.; Caudana, A.; Rolle, L.; Gerbi, V. SO2 in wines: Rational use and possible alternatives. In Red Wine Technology; Academic Press: Cambridge, MA, USA, 2019; pp. 309–321. [Google Scholar]
- Santos, C.V.A.; Pereira, C.; Martins, N.; Cabrita, M.J.; Gomes da Silva, M. Different SO2 doses and the impact on amino acid and volatile profiles of white wines. Beverages 2023, 9, 33. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Sancho-Galán, P.; Jiménez-Cantizano, A.; Palacios, V. A comparative study on volatile compounds and sensory profile of white and red wines elaborated using bee pollen versus commercial activators. Foods 2021, 10, 1082. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Martínez-Lapuente, L.; Bueno-Herrera, M.; Ortega-Heras, M.; Guadalupe, Z.; Ayestarán, B. Use of commercial dry yeast products rich in mannoproteins for white and rosé sparkling wine elaboration. J. Agric. Food Chem. 2015, 63, 5670–5681. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, G.D.; Sánchez-Suárez, F.; Peinado, R.A.; Cotea, V.V.; de Lerma, N.L.; Gabur, I.; Simioniuc, V. Metabolomics of red wines aged traditionally, with chips or staves. Foods 2024, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Caporaso, N.; Moio, L. Influence of yeast strain on odor-active compounds in Fiano wine. Appl. Sci. 2021, 11, 7767. [Google Scholar] [CrossRef]
- Zhang, X.K.; Liu, P.T.; Zheng, X.W.; Li, Z.F.; Sun, J.P.; Fan, J.S.; Ding, Z.Y. The role of indigenous yeasts in shaping the chemical and sensory profiles of wine: Effects of different strains and varieties. Molecules 2024, 29, 4279. [Google Scholar] [CrossRef]
- Comuzzo, P.; Tat, L.; Tonizzo, A.; Battistutta, F. Yeast derivatives (extracts and autolysates) in winemaking: Release of volatile compounds and effects on wine aroma volatility. Food Chem. 2006, 99, 217–230. [Google Scholar] [CrossRef]
- Alexandre, H.; Guilloux-Benatier, M. Yeast autolysis in sparkling wine—A review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Lambert-Royo, M.I.; Ubeda, C.; Del Barrio-Galán, R.; Sieczkowski, N.; Canals, J.M.; Peña-Neira, Á.; i Cortiella, M.G. The diversity of effects of yeast derivatives during sparkling wine aging. Food Chem. 2022, 390, 133174. [Google Scholar] [CrossRef]
- Díaz-Hellín, P.; Úbeda, J.; Briones, A. Improving alcoholic fermentation by activation of Saccharomyces species during the rehydration stage. LWT Food Sci. Technol. 2013, 50, 126–131. [Google Scholar] [CrossRef]
- Muñoz-Castells, R.; Moreno, J.; García-Martínez, T.; Mauricio, J.C.; Moreno-García, J. Assessing the impact of commercial Lachancea thermotolerans immobilized in biocapsules on wine quality: Odor active compounds and organoleptic properties. Fermentation 2024, 10, 303. [Google Scholar] [CrossRef]
- Peinado, R.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- Moreno, J.; Peinado, R.; Mauricio, J.C. Aplicación de levaduras para la disminución de ácido glucónico en procesos de vinificación. In Martínez Bernia y Asociados; Martínez Bernia y Asociados: Córdoba, Spain, 2005; p. 429. ISBN 84-931408-5-6. [Google Scholar]
- Martín-García, F.J.; Palacios-Fernández, S.; López de Lerma, N.; García-Martínez, T.; Mauricio, J.C.; Peinado, R.A. The effect of yeast, sugar and sulfur dioxide on the volatile compounds in wine. Fermentation 2023, 9, 541. [Google Scholar] [CrossRef]
- Zhu, L.X.; Zhang, M.M.; Shi, Y.; Duan, C.Q. Evolution of the aromatic profile of traditional Msalais wine during industrial production. Int. J. Food Prop. 2019, 22, 911–924. [Google Scholar] [CrossRef]
- Peinado-Pardo, I.; Rosa Barbosa, E.M.; Heredia Gutiérrez, A.B.; Escriche Roberto, M.I.; Andrés Grau, A.M. Influence of storage on the volatile profile, mechanical, optical properties and antioxidant activity of strawberry spreads made with isomaltulose. Food Biosci. 2016, 14, 10–20. [Google Scholar] [CrossRef]
- López de Lerma, N.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Influence of two yeast strains in free, bioimmobilized or immobilized with alginate forms on the aromatic profile of long-aged sparkling wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, J.M.; Bastian, S.E.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of Chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Buttery, R.G.; Seifert, R.M.; Guadagni, D.G.; Ling, L.C. Characterization of additional volatile components of tomato. J. Agric. Food Chem. 1971, 19, 524–529. [Google Scholar] [CrossRef]
- Leffingwell & Associates. Available online: http://www.leffingwell.com (accessed on 2 September 2024).
- Boulton, C.A.; Quain, D.E. Brewing Yeast and Fermentation; Blackwell Science: Oxford, UK, 2001; pp. 152–155. ISBN 9780632054756. [Google Scholar]
- Andújar-Ortiz, I.; Chaya, C.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.A. Impact of using new commercial glutathione-enriched inactive dry yeast oenological preparations on the aroma and sensory properties of wines. Int. J. Food Prop. 2014, 17, 987–1001. [Google Scholar] [CrossRef]

| WY | WY + Act | AGGLO | AGGLO + Act | HG | Y | A | ||
|---|---|---|---|---|---|---|---|---|
| pH | 3.22 ± 0.06 a | 3.3 ± 0.1 a | 3.22 ± 0.06 a | 3.26 ± 0.02 a | 1 | ns | ns | ns |
| Titratable acidity (g L−1) | 6.1 ± 0.9 a | 6 ± 1 a | 6 ± 0.3 a | 5.8 ± 0.2 a | 1 | ns | ns | ns |
| Volatile acidity (g L−1) | 0.4 ± 0.1 a | 0.45 ± 0.00 a | 0.48 ± 0.08 a | 0.44 ± 0.06 a | 1 | ns | ns | ns |
| Ethanol (% v/v) | 13.7 ± 0.1 a | 13.7 ± 0.1 a | 13.65 ± 0.05 a | 13.7 ± 0.2 a | 1 | ns | ns | ns |
| Reducing sugars (g L−1) | 3.1 ± 0.5 c | 1.4 ± 0.2 b | 0.9 ± 0.3 a | 0.6 ± 0.4 ab | 3 | *** | ** | * |
| Malic acid (mg L−1) | 653 ± 5 a | 647 ± 30 a | 891 ± 10 b | 907 ± 7 b | 2 | *** | ns | ns |
| Lactic acid (mg L−1) | ≤0.15 | ≤0.15 | ≤0.15 | ≤0.15 | 1 | ns | ns | ns |
| Absorbance 420 nm | 0.247 ± 0.006 b | 0.27 ± 0.01 c | 0.245 ± 0.005 b | 0.227 ± 0.006 a | 3 | *** | ns | *** |
| Absorbance 520 nm | 0.055 ± 0.005 a | 0.0646 ± 0.0005 b | 0.054 ± 0.001 a | 0.051 ± 0.001 a | 2 | ** | ns | ** |
| Absorbance 280 nm (TPI) | 9.71 ± 0.05 c | 8.22 ± 0.03 a | 9.7 ± 0.2 c | 9.10 ± 0.05 b | 3 | *** | *** | *** |
| SO2 free (mg L−1) | 1.4 ± 0.8 a | 1.5 ± 0.9 ab | 4 ± 2 b | 3 ± 1 ab | 2 | * | ns | ns |
| SO2 total (mg L−1) | 40 ± 1 b | 45.1 ± 3 c | 29.2 ± 0.3 a | 30.0 ± 0.2 a | 3 | *** | ** | * |
| Yeast assimilable nitrogen (YAN) | 35 ± 7 b | 42 ± 7 c | 28 ± 1 a | 42 ± 1 c | 3 | ns | *** | ns |
| Odorant Series | WY | WY + Act | AGGLO | AGGLO + Act | HG | Y | A | |
|---|---|---|---|---|---|---|---|---|
| Fruity (1) | 26.7 ± 0.5 b | 25.3 ± 0.1 b | 15.7 ± 0.7 a | 26 ± 1 b | 2 | *** | *** | *** |
| Tropical fruit | 17.9 ± 0.3 b | 17.6 ± 0.2 b | 9.5 ± 0.7 a | 18 ± 1 b | 2 | *** | *** | *** |
| Sweet fruit | 6.6 ± 0.2 c | 5.9 ± 0.1 b | 4.6 ± 0.2 a | 6.6 ± 0.2 c | 3 | *** | *** | *** |
| Forest fruit | 1.2 ± 0.1 b | 1.09 ± 0.03 b | 0.94 ± 0.04 a | 1.4 ± 0.1 c | 3 | ns | ** | *** |
| Green fruit (2) | 9.1 ± 0.6 c | 7.9 ± 0.1 b | 5 ± 0.2 a | 8.8 ± 0.4 c | 3 | *** | *** | *** |
| Green (3) | 34 ± 2 c | 22.1 ± 0.9 b | 18 ± 1 a | 24 ± 2 b | 3 | *** | * | *** |
| Creamy (4) | 12.9 ± 0.3 d | 8.4 ± 0.2 b | 5.8 ± 0.5 a | 11.5 ± 0.4 c | 4 | *** | * | *** |
| Citrus (5) | 49 ± 4 d | 40 ± 1 c | 7.5 ± 0.3 a | 28 ± 2 b | 4 | *** | ** | *** |
| Chemistry (6) | 58 ± 5 d | 49 ± 1 c | 20.2 ± 0.3 a | 40 ± 2 b | 4 | *** | ** | *** |
| Terpenic | 42 ± 5 d | 33 ± 1 c | 3.1 ± 0.5 a | 23 ± 2 b | 4 | *** | ** | *** |
| Ethereal | 4.61 ± 0.02 a | 4.78 ± 0.05 b | 4.64 ± 0.03 a | 4.7 ± 0.09 ab | 2 | ns | ** | ns |
| Alcoholic | 1.7 ± 0.1 a | 1.62 ± 0.05 a | 1.55 ± 0.07 a | 1.66 ± 0.06 a | 1 | ns | ns | ns |
| Fusel | 9.1 ± 0.1 a | 9.1 ± 0.2 a | 10.3 ± 0.2 b | 10.6 ± 0.1 c | 3 | *** | ns | ns |
| Honey (7) | 37 ± 2 d | 24.7 ± 0.8 b | 21 ± 1 a | 29 ± 3 c | 4 | ** | ns | *** |
| Waxy (8) | 9.4 ± 0.4 a | 9.3 ± 0.4 a | 9.1 ± 0.2 a | 9.3 ± 0.7 a | 1 | ns | ns | ns |
| Floral (9) | 10.5 ± 0.6 b | 8.9 ± 0.4 a | 15.1 ± 0.4 c | 21.6 ± 1.2 d | 4 | *** | *** | *** |
| Attributes | WY | WY + Act | AGGLO | AGGLO + Act | HG | p-Value |
|---|---|---|---|---|---|---|
| Sight | 10 ± 2 a | 11 ± 2 a | 11 ± 1 a | 12 ± 1 a | 1 | 0.506 |
| Smell | 24 ± 3 a | 24 ± 2 a | 23 ± 3 a | 23 ± 4 a | 1 | 0.740 |
| Taste | 34 ± 5 a | 36 ± 4 a | 33 ± 5 a | 33 ± 5 a | 1 | 0.544 |
| Overall quality | 9 ± 1 a | 9 ± 1 a | 9 ± 1 a | 9 ± 1 a | 1 | 0.401 |
| Total score | 78 ± 8 a | 80 ± 8 a | 76 ± 8 a | 77 ± 10 a | 1 | 0.708 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Castells, R.; Sánchez-Suárez, F.; Moreno, J.; Álvarez-Gil, J.M.; Moreno-García, J. The Effects of Flocculant Yeast or Spontaneous Fermentation Strategies Supplemented with an Organic Nitrogen-Rich Additive on the Volatilome and Organoleptic Profile of Wines from a Neutral Grape Variety. Appl. Sci. 2025, 15, 4196. https://doi.org/10.3390/app15084196
Muñoz-Castells R, Sánchez-Suárez F, Moreno J, Álvarez-Gil JM, Moreno-García J. The Effects of Flocculant Yeast or Spontaneous Fermentation Strategies Supplemented with an Organic Nitrogen-Rich Additive on the Volatilome and Organoleptic Profile of Wines from a Neutral Grape Variety. Applied Sciences. 2025; 15(8):4196. https://doi.org/10.3390/app15084196
Chicago/Turabian StyleMuñoz-Castells, Raquel, Fernando Sánchez-Suárez, Juan Moreno, José Manuel Álvarez-Gil, and Jaime Moreno-García. 2025. "The Effects of Flocculant Yeast or Spontaneous Fermentation Strategies Supplemented with an Organic Nitrogen-Rich Additive on the Volatilome and Organoleptic Profile of Wines from a Neutral Grape Variety" Applied Sciences 15, no. 8: 4196. https://doi.org/10.3390/app15084196
APA StyleMuñoz-Castells, R., Sánchez-Suárez, F., Moreno, J., Álvarez-Gil, J. M., & Moreno-García, J. (2025). The Effects of Flocculant Yeast or Spontaneous Fermentation Strategies Supplemented with an Organic Nitrogen-Rich Additive on the Volatilome and Organoleptic Profile of Wines from a Neutral Grape Variety. Applied Sciences, 15(8), 4196. https://doi.org/10.3390/app15084196






