Abstract
This study investigates the valorization of tomato by-products—peels, seeds, and juice—through innovative extraction and bioprocessing techniques. Lycopene recovery from tomato peels was optimized using ultrasound- and microwave-assisted extraction (UAE-MAE) with ethyl lactate as the solvent. The optimal conditions (0.03 g/mL, 500 W microwave power, 600 W ultrasound power) yielded a lycopene content of 37.08 mg/100 g of peels and an extraction yield (EY) of 91.20%. For tomato seeds, oil extraction methods, including conventional stirring, UAE-MAE, Soxhlet extraction, and pressurized liquid extraction (PLE), were evaluated. Conventional stirring achieved the highest oil yield (19.66%), followed closely by UAE-MAE (19.53%). However, PLE produced the highest lycopene content (44.0 mg/100 g oil) and significant levels of linoleic acid (544.7 mg/g oil), though Soxhlet extraction yielded slightly more (608.9 mg/g oil). Tomato juice was processed into high-nutritional value vinegar via a two-stage fermentation process. The final product had 5.42% acidity, a pH of 2.85, and retained a high lycopene content (9.19 mg/100 g). This study underscores the potential of innovative extraction and bioprocessing strategies for the valorization of tomato by-products, promoting waste reduction and the development of high-value functional products in alignment with principles of the circular bioeconomy.
1. Introduction
Tomatoes (Solanum lycopersicum) are one of the most popular vegetables worldwide, with an estimated annual production of approximately 192 million tons in 2023 according to FAOSTAT [1]. The tomato processing industry plays a crucial role in producing various food products like sauces, canned tomatoes, ketchup, and juice. However, this industry generates substantial amounts of by-products, accounting for approximately 25% of the tomato mass [2,3]. These by-products, commonly referred to as tomato pomace, consist of three primary components derived from the whole fruit: pulp (40%), peels (27%), and seeds (33%) [4].
The accumulation of large amounts of tomato processing waste negatively impacts both the economy and the environment. To address this, a circular economy approach promotes the conversion and valorization of these by-products into value-added products [5]. Research has demonstrated that tomato processing waste is a rich source of dietary fiber, polyphenols, carotenoids (including lycopene and β-carotene), oil, and proteins, all of which have notable nutritional and health benefits [6]. Practical solutions for utilizing these by-products include bioactive compound extraction, particularly from tomato peels, which are rich in carotenoids [7], and conversion into biofuels or electricity [8]. Additionally, separating seeds from the remaining pulp allows their processing into edible tomato oil, adding commercial value.
Tomato peels are an abundant source of carotenoids, which are the main bioactive compounds found in tomato processing by-products. Among these, lycopene is the most prevalent, followed by β-carotene and lutein. Since the human body is unable to synthesize these compounds, they must be obtained through dietary sources, particularly from carotenoid-rich foods and supplements [9]. Carotenoids are essential for human health, offering numerous benefits such as cancer prevention, cardiovascular support, and immune system regulation [10,11]. Lycopene, the natural red pigment responsible for the deep-red color of tomatoes and tomato-based products, is commonly incorporated as a food additive for its coloring and antioxidant properties [12].
Extracting lycopene from tomato peels presents a significant challenge, as it is intricately bound within the peel structure. Therefore, an effective method is required for its efficient extraction. In recent years, considerable research has been conducted to enhance lycopene extraction techniques. Various solvent extraction systems have been investigated, including ethanol/hexane, acetone/hexane, and pure ethyl acetate [13,14]. However, some of these organic solvents pose toxicity concerns. Moreover, advancements in extraction technology have introduced novel approaches that optimize lycopene recovery from tomato by-products. Techniques such as microwave-assisted extraction and ultrasound-assisted extraction have demonstrated the ability to substantially reduce the extraction time and solvent usage while enhancing the lycopene yield compared to conventional methods [15,16,17,18,19]. Despite these innovations, limited investigations have studied the synergistic application of these advanced extractions methods to further improve lycopene recovery from tomato peels.
Tomato seeds contain 20–36% oil, predominantly composed of unsaturated fatty acids, with oleic and linoleic acids being the most abundant. As a result, extracting oil from tomato seeds has become a valuable method for managing waste in the tomato processing industry. Research has shown that tomato seed oil exhibits superior antioxidant properties compared to other commonly consumed edible oils, such as soybean and olive oil [4,20].
The most commonly used methods for tomato seed oil extraction are Soxhlet extraction and conventional stirring, typically using hexane as a solvent [4]. Emerging technologies such as UAE-MAE and PLE aim to improve efficiency. These advanced methods remain underexplored regarding their effectiveness in extracting tomato seed oil and their impacts on its bioactive composition. Kumar et al. (2023) used UAE-MAE with ethanol and ethyl acetate, enhancing the extraction efficiency and yielding 23.07% oil [20]. Eller et al. (2010) used accelerated solvent extraction with hexane, obtaining a 20% oil yield, though without providing data on the resulting oil’s fatty acid content [21].
Overripe and damaged tomatoes, along with tomato juice by-products from processing, can be repurposed into high-value functional products. Vinegar has demonstrated beneficial effects on hyperglycemia and hypertension, as well as reducing body weight, fat mass, and triglyceride levels [22]. It is traditionally produced from sugar- or starch-rich raw materials such as rice, malt, apples, and grapes via a two-stage fermentation process, where ethanol is produced by yeast and then converted into acetic acid by acetic acid bacteria [23]. Growing health concerns have increased the demand for vinegar from diverse fruits and vegetables, leading to novel vinegar varieties made from onion, pineapple, strawberry, and sweet potato [24] Perumpuli et al. (2022) studied tomato vinegar production, achieving an acetic acid concentration of 4.12% (w/v) within 3–5 days of fermentation at 36 °C [24].
This study aimed to valorize tomato by-products through innovative extraction methods and bioprocessing strategies. The synergistic application of UAE-MAE was employed to recover lycopene from tomato peels using ethyl lactate as the solvent. Extraction parameters, including solid/liquid ratio, microwave power, and ultrasound power were optimized using the response surface methodology (RSM) to maximize the extraction yield. The recovery of tomato seed oil was also investigated through various extraction methods, including Soxhlet extraction, conventional stirring, UAE-MAE, and PLE. The final oil product was analyzed for yield, carotenoid content, and unsaturated fatty acid composition using HPLC and GC analyses, respectively. Lastly, the conversion of tomato juice into vinegar through a two-stage fermentation process was studied. The final tomato vinegar product was characterized for its lycopene content, acetic acid concentration, ethanol concentration, and pH. This comprehensive approach highlights the potential of innovative extraction and bioprocessing techniques to enhance the value of tomato by-products, promoting sustainability in the food industry.
2. Materials and Methods
2.1. Materials and Chemicals
Tomato by-products were provided by KYKNOS (Athens, Greece). The solvents ethyl L-lactate (≥98%), n-hexane, acetone, tertiary butyl hydroquinone (TBHC), methylene chlorine, and methanol were purchased from Sigma-Aldrich (St. Louis, MO, USA) and were of analytical grade. Acetonitrile, 1-butanol, and dichloromethane of HPLC grade were also purchased from Sigma-Aldrich (St. Louis, MO, USA), while n-heptane (HPLC grade) was obtained from Carlo Erba (Emmendingen, Germany). For the preparation of nutrient media, the reagents tryptone, yeast extract, NaCl, agar, peptone from meat, and glucose (D-(+)-glucose monohydrate) were supplied by Sigma-Aldrich (St. Louis, MO, USA) and Fluka Analytical. The yeast Saccharomyces cerevisiae, a commercially available product named JOTIS Instant Dry Yeast, was supplied by Jotis (Greece, Athens), while the bacterium Acetobacter pasteurianus DSM 3509 was obtained from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany). The reagents potassium hydroxide and sodium hydroxide were purchased from Fisher Chemicals (Leicestershire, UK) and the phenolphthalein indicator from Sigma-Aldrich (St. Louis, MO, USA). Distilled water was used in all experiments.
2.2. Valorization of Tomato Peels
2.2.1. Soxhlet Extraction for the Recovery of Lycopene
Soxhlet extraction was employed as an exhaustive extraction method to determine the maximum achievable lycopene concentration, serving as a reference for assessing the efficiency of alternative extraction techniques. The Soxhlet extraction system consisted of a spherical flask placed on a heating source, with a horizontally attached extraction chamber and a condenser at the top. The condenser was connected to a water supply as a refrigerant. The sample was placed in a container of pressed paper in the special chamber of the extraction device. A mixture of hexane and acetone at a ratio of 1:1 (v/v) was used as the extraction solvent at a solid/liquid ratio of 0.05 g/mL. The extraction process lasted for 4 h, during which 10 siphon cycles were completed. Following extraction, the mixture was centrifuged at 2500 rpm for 10 min, and the resulting supernatant was collected for solvent evaporation and concentration of the final extract. Solvent evaporation was carried out using a Büchi Rotavapor R-200 vacuum rotary evaporator with an integrated Büchi Heating Bath B-490 (Büchi Laboratories Technik AG, Flawil, Switzerland) set at 40–45 °C. Once evaporation was complete, the extract was redissolved in 1 mL of methylene chlorine. To prevent lycopene oxidation, a small amount of tertiary butylhydroquinone (TBHC) was added.
2.2.2. Synergistic Application of UAE-MAE for the Recovery of Lycopene
The recovery of lycopene from the dried peels was performed using a synergistic application of the UAE-MAE method. The extraction was carried out using the XO-SM50 Ultrasonic Microwave Reaction System (Nanjing Xianou Instruments Manufacture Co., Ltd., Nanjing City, China). In each experiment, the dry powder of the peels was mixed with ethyl acetate, which was the extraction solvent. The extraction process was conducted at 45 °C for 8 min, with the ultrasonicator operating in a pulse mode of 1 s on and 1 s off. To investigate the effects of extraction parameters on the lycopene content (mg/100 g of tomato peels) and extraction yield (EY, %), response surface methodology with a Central Composite Design (CCD) was employed. The independent variables—solid/liquid ratio (g/mL), microwave power (W), and ultrasound power (W)—were optimized within the ranges of 0.03–0.1 g/mL, 0–500 W and 0–600 W, respectively (Table 1). After the extraction was complete, the lycopene extracts were obtained following the procedure outlined in Section 2.2.1. Each experiment was conducted in triplicate.

Table 1.
Lycopene content (mg/100 g of tomato peels) and extraction yield (EY, %) (variables) of the tomato peel extracts based on Central Composite Design.
2.3. Characterization of Tomato Peel Extracts
2.3.1. Extraction Yield of Lycopene from Tomato Peels
The extraction yield of lycopene from tomato peels using the UAE-MAE method was assessed by comparing it to the Soxhlet extraction method, which was employed as an exhaustive extraction technique to determine the maximum achievable lycopene concentration. The yield was calculated by comparing the lycopene content of extracts obtained under various UAE-MAE extraction conditions to that of the Soxhlet extract, which served as the reference for maximum recovery. The extraction yield was determined using the following equation:
2.3.2. Analysis of Lycopene Content in Peel Extracts
The lycopene content in peel extracts obtained from UAE-MAE and Soxhlet extraction was determined using high-performance liquid chromatography (HPLC). An HPLC Shimadzu HP 1100 Series (Columbia, MD, USA) equipped with a diode array detector and an automatic Agilent 1200 Series injector was used. Lycopene was analyzed with a YMC C30 analytical column (5 μm, 250 × 4.6 mm I.D.) at ambient temperature, according to the experimental procedure described by Drosou and Krokida (2024) [25].
2.4. Valorization of Tomato Seeds—Extraction Methods for the Recovery of Tomato Seed Oil
The seeds were separated from the peels by sedimentation in water using a glass cylinder, due to the lower specific gravity; the tomato peels floated on the surface of water while the fraction of seeds settled to the bottom. Finally, the pomace mass was dissolved in the water. The recovered seeds were then freeze-dried in a vacuum freeze-dryer (Biobase Biodustry, Co., Ltd., Shandong, China). After the drying process, the dried seeds were used to develop a powder of 500 μm–2000 μm using a grinder. After this treatment the produced powder of the seeds, was obtained and stored at 4 °C for further treatment for the recovery of oil.
2.4.1. Conventional Extraction
Conventional extraction with simple stirring was used to obtain tomato seed oil. The extraction process was conducted using food-grade hexane as the solvent at a solid/liquid ratio of 0.1 g/mL for 4 h at room temperature. After extraction, the sample containing the treated seeds and the extracted oil with the solvent was subjected to centrifugation at 2500 rpm for 10 min, where the oil fraction with the solvent was separated. The resulting fraction was then processed in a rotary evaporator under vacuum at 38 °C to remove the hexane and obtain the oil for further characterization. Each experiment was conducted in triplicate.
2.4.2. Soxhlet Extraction
The recovery of tomato seed oil using Soxhlet extraction was performed using the setup described in Section 2.2.1. The sample was placed in a pressed paper container within the specialized chamber of the extraction device. Food-grade hexane was used as the extraction solvent at a solid/liquid ratio of 0.05 g/mL. The extraction process lasted for 3 h, during which 20 siphon cycles were completed. After extraction, oil collection was carried out through centrifugation and solvent evaporation, following the procedure outlined in Section 2.4.1. Each experiment was conducted in triplicate.
2.4.3. Synergistic Application of UAE-MAE
The synergistic application of UAE-MAE was used for the recovery of tomato seed oil. The extraction was performed using the XO-SM50 Ultrasonic Microwave Reaction System (Nanjing Xianou Instruments Manufacture Co., Ltd., Nanjing City, China). In each experiment, the dry powder of the seeds was mixed with hexane, which was the extraction solvent, at a solid/liquid ratio of 0.05 g/mL. The extraction process was conducted at 40 °C for 10 min, with the microwave power set to 500 W and ultrasound power set to 450 W. After extraction, oil collection was carried out through centrifugation and solvent evaporation, following the procedure outlined in Section 2.4.1. Each experiment was conducted in triplicate.
2.4.4. Pressurized Liquid Extraction (PLE)
PLE was employed for the recovery of tomato seed oil, using the PLE® Pressurized Liquid Extraction System (Fluid Management Systems, Watertown, NY, USA). The sample was placed in a 100 mL stainless steel extraction cell at a solid/liquid ratio of 0.05 g/mL, with food-grade hexane as the extraction solvent. The extraction process was conducted at a pressure of 1750 psi and a temperature of 100 °C for 6 min. After extraction, oil collection was carried out through centrifugation and solvent evaporation, following the procedure outlined in Section 2.4.1. Each experiment was conducted in triplicate.
2.5. Characterization of Tomato Seed Oil
2.5.1. Determination of Oil Yield
The extraction yield of tomato oil obtained from different extraction methods was calculated with the following equation:
2.5.2. Analysis of Lycopene Content in Seed Oil
The tomato seed oil obtained through different extraction methods was analyzed for its lycopene content using HPLC, as described in Section 2.3.2.
2.5.3. Analysis of Fatty Acids
The fatty acid profile of the tomato seed oil was analyzed using gas chromatography with a mass detector (GC-MS) on a Shimadzu Nexis GC-2030 device equipped with an SP-2340 column from Supelco (30 m × 0.25 mm × 0.2 μm). The carrier gas was helium, with a flow rate of 1.3 mL/min. Before the analysis, free fatty acids were converted into fatty acid methyl esters. In brief, 0.5 g of oil was mixed with 5 mL of n-heptane (HPLC grade) and 1 mL of a 2 M methanolic KOH solution. The mixture was shaken for 30 s and incubated for 15 min. Then, an aliquot from the upper organic phase was taken and filtered using syringe filters with a pore size of 0.45 μm and a filter size of 25 mm. The esterified samples were injected at a temperature of 250 °C, following a thermal profile starting at 150 °C (for 8 min) and increasing to 250 °C within 24 min at a constant rate of 5 °C/min. The fatty acid methyl esters were quantified using the F.A.M.E. Mix C4-C24 as an external standard and fatty acid 19:0 as an internal standard for quantification.
2.6. Valorization of Tomato Juice Derived from Defective Fruits and By-Products
2.6.1. Pre-Culture and Microorganism Maintenance
The bacterial strain A. pasteurianus 3509, which was stored at −80 °C in a glycerol solution for preservation, was first cultured on an agar plate and subsequently incubated in a conical flask containing an appropriate nutrient medium. The nutrient media used in both cases were prepared in the laboratory and sterilized at 121 °C for 20 min. For the agar plates, LB medium was used and was composed of (g/L of deionized water) tryptone (10 g), yeast extract (5 g), NaCl (10 g), and agar (15 g). For the conical flasks, YPG medium was used and was composed of (g/L of deionized water) peptone (3 g), yeast extract (5 g), and glucose (20 g). The LB plates were inoculated with the bacterial strain and incubated at 27 °C for 24 h. After sufficient bacterial growth, the culture was transferred into conical flasks, each containing 100 mL of YPG medium. The flasks were then placed in a thermostatic incubator under aerobic conditions with continuous agitation at 180 rpm and a temperature of 27 °C. The incubation period lasted 72 h.
2.6.2. Ethanol Production—First Fermentation Stage (Alcoholic Fermentation)
Before initiating the fermentation process, the bioreactor (Bioengineering AG, Wald, Switzerland) used for acetic acid production was appropriately prepared. This included calibrating the pH meter in the system and sterilizing the bioreactor to prevent contamination by unwanted microorganisms. During the sterilization process, deionized water was added to the bioreactor and initially heated to 50 °C with stirring at 100 rpm to lubricate the mechanical stirring equipment. The temperature was then increased to 85 °C with stirring at 150 rpm for 20 min. Subsequently, the stirring speed was raised to 1000 rpm, and sterilization was carried out at 121 °C for approximately 30 min. The sampling and outlet valves were also sterilized simultaneously.
To convert the sugars present in tomato juice into ethanol, the yeast S. cerevisiae (0.4% w/v) was introduced into the bioreactor. The tomato juice was supplemented with sugar to reach a concentration of 13 °Brix. This mixture was transferred to the bioreactor, and fermentation was conducted at 30 °C with stirring at 200 rpm under anaerobic conditions for three days. During this period, samples were collected to monitor the sugar content and pH, ensuring that the pH remained below 5.
After completing the first fermentation stage, the produced tomato wine was removed from the bioreactor and centrifuged to separate the solids and yeast cells. Subsequently, total solids, total acidity, pH, and the lycopene content were measured. The clarified tomato wine was then returned to the bioreactor for the second stage of fermentation.
2.6.3. Acetic Acid Production—Second Fermentation Stage (Acetic Fermentation)
In the second stage of fermentation, the ethanol produced during the first stage and present in the tomato wine was converted into acetic acid, yielding the final product. The tomato wine in the bioreactor was inoculated with the bacterium A. pasteurianus 3509 (10% v/v), which had been previously cultivated under optimal conditions. The temperature was maintained at 30°C, and the stirring speed was set to 200 rpm.
Since acetic fermentation is an aerobic process, the oxygen supply was maintained at 1 vvm (1 L air/L medium/min) to ensure adequate aeration throughout the fermentation period. The experiment lasted for nine days, during which daily samples were collected to determine total acidity and monitor pH levels.
2.7. Characterization of Tomato Vinegar
2.7.1. Lycopene Content in Tomato Vinegar
The lycopene content in the tomato juice, the intermediate product of alcoholic fermentation and the final vinegar product was determined by HPLC analysis, as described in Section 2.3.2.
2.7.2. Sugar and Alcohol Contents
The sugar concentration was measured at regular intervals using samples collected from the bioreactor during the alcoholic fermentation of tomato juice. The alcohol content was estimated stoichiometrically based on the reaction equation. The sugar measurement was performed by determining the refractive index of the centrifuged sample using an AR2008 digital refractometer (A. KRUSS Optronic, Hamburg, Germany). The refractometer readings were converted to °Brix using a calibration curve and tomato juice samples with known sugar contents. To construct the calibration curve, the total solids of the tomato juice used were initially measured, assuming that they corresponded to the total sugar content in this case. Each experiment was conducted in triplicate.
2.7.3. pH Measurement
The pH measurement was conducted using a Multiparameter Bench Meter (Mi 180, MARTINI Instruments). Initially, the instrument was calibrated according to the manufacturer’s instructions using buffer solutions of known pH values. For each measurement, an adequate amount of sample was placed in a beaker and the pH value was recorded once the reading stabilized. The sample temperature was maintained between 20 and 25 °C. Each experiment was conducted in triplicate.
2.7.4. Total Acidity
The total acidity of vinegar was determined by the sum of free carboxyl groups present in both molecular and anionic forms. The measurement was performed via titration, based on the neutralization of acidic groups in the sample using a standardized alkaline solution in the presence of an indicator. For this analysis, a 5 mL centrifuged sample was transferred into a conical flask, followed by the addition of 45 mL of distilled water and 2–3 drops of phenolphthalein. The sample was titrated with a 0.1 N NaOH solution, which was added dropwise while stirring until a persistent pink color appeared. The endpoint was reached when the pink color remained stable for 30 s. The sample was diluted with water to facilitate easier observation of the color change. Each experiment was conducted in triplicate. The percentage of acetic acid in the titrated samples was calculated using Equation (3):
where N is the normality of NaOH solution (N), V is the volume of NaOH consumed (mL), M is the molecular weight of the dominant acid in the solution divided by the number of hydrogen ions neutralized per acid molecule (60 for acetic acid), and S is the sample volume (mL or g). The percentage acidity was expressed as the quantity of the dominant acid in g per 100 g or 100 mL of the sample.
2.7.5. Total Dissolved Solids (TDSs)
Total dissolved solids (TDSs) refer to the soluble components of juices. In tomato juice, around 98% of the TDSs is assumed to be sugars. Based on this assumption, a TDS measurement is considered an indirect method for estimating the sugar content in tomato juice samples. For the determination of TDSs, the samples were centrifuged and then filtered through a 0.45 μm membrane filter. A 10 g portion of the filtrate was placed in a pre-weighed crystallizing dish and dried at 105 °C for 24 h in a drying oven (Heraeus, Hanau, Germany). The remaining residue was weighed and expressed in mg per 100 g of sample. Each experiment was conducted in triplicate.
2.8. Experimental Design and Statistical Analysis
RSM was used to analyze the experimental data, enabling the optimization of the UAE-MAE process for lycopene recovery from tomato peels. The analysis was performed with StatSoft STATISTICA 12.0 software (Hamburg, Germany). A Central Composite Design (CCD) was employed within the RSM framework to determine the optimal conditions for the independent variables. Each experiment was conducted in triplicate. The independent variables examined were the solid/liquid ratio (g/mL, X1), microwave power (W, X2), and ultrasound power (W, X3), with each variable coded at three levels: −1, 0, and 1. The dependent variables in the model included the lycopene content (mg/100 g of tomato peels) and extraction yield (EY, %). The responses from 16 experimental runs are presented in Table 1. The generalized second-order quadratic equation, which describes the relationship between the response and the independent variables, is as follows:
where Y is the dependent variable (lycopene content, EY); b0 is the intercept; b1, b2, and b3 are linear coefficients; b11, b22, and b33 are quadratic coefficients; and b12, b13, and b23 represent the interaction effects between variables. The independent variables X1, X2, and X3 correspond to the solid/liquid ratio, microwave power, and ultrasound power, respectively.
To evaluate the significance of the effects of the independent variables on the lycopene content and EY, an analysis of variance (ANOVA) was performed, with a significance threshold set at p < 0.05. The independent variable with the lowest p-value was considered to have the most influence on the response, while variables with a p-value > 0.05 were excluded from the model, unless their quadratic or interaction effects were significant [26]. The model was then simplified by removing these non-significant factors, and the resulting reduced model was used for further data interpretation.
A 95% confidence level was used for the error assessment. ANOVA was also used to determine the significance level for each response, while a lack-of-fit test was conducted to evaluate the adequacy of the mathematical model. The F-value was employed to determine how well the model fit the data, and the model’s predictive accuracy was assessed using the R² value. To better visualize the relationships and interactions between the independent variables, three-dimensional surface plots were used. For enhanced clarity, only the interaction effects of the most significant variables on the dependent variables are shown.
Additionally, one-way ANOVA was performed to compare differences between the samples, and Tukey’s post hoc test (α = 0.05) was applied to identify any statistically significant differences. All statistical analyses were carried out using StatSoft STATISTICA 12.0 software (Hamburg, Germany).
3. Results and Discussion
3.1. Recovery of Lycopene from Tomato Peels
The extraction of lycopene from tomato peels was investigated using the UAE-MAE method, while Soxhlet extraction was conducted as a reference method, serving as an exhaustive extraction technique to determine the maximum achievable lycopene yield. The lycopene content of the extract obtained from Soxhlet extraction was 40.66 ± 3.08 mg/100 g of tomato peels. Various conditions, including the solid/liquid ratio, microwave power, and ultrasound power, were tested during the UAE-MAE process. The solid/liquid ratio ranged from 0.03 to 0.10 g/mL, microwave power from 0 to 500 W, and ultrasound power from 0 to 600 W. The optimal extraction conditions for lycopene recovery were found at a solid/liquid ratio of 0.03 g/mL with 500 W microwave power and 600 W ultrasound power, resulting in the highest lycopene content (37.08 ± 2.09 mg/100 g of tomato peels) and extraction yield (91.20 ± 2.90%). Although Soxhlet extraction provided slightly higher recovery, UAE-MAE offered several key advantages, particularly in terms of processing time and energy efficiency. Soxhlet extraction required 4 h of continuous heating, leading to higher energy consumption and an increased risk of the thermal degradation of bioactive compounds. In contrast, UAE-MAE significantly reduced the extraction time to 8 min, minimizing exposure to heat and preserving the structural integrity of lycopene. Furthermore, UAE-MAE has demonstrated greater industrial scalability potential. Unlike Soxhlet extraction, which is primarily a batch-based laboratory technique and not inherently designed for large-scale continuous operations, UAE-MAE offers a more adaptable framework for industrial applications. The extensive processing time and high solvent consumption associated with Soxhlet extraction, without achieving any considerable increase in lycopene recovery, limit its feasibility for large-scale extraction setups. Studies have shown that UAE-MAE can be integrated into continuous extraction systems, further enhancing its feasibility for industrial production. Future research should focus on optimizing large-scale UAE-MAE equipment and evaluating its long-term economic viability.
3.1.1. Model Fitting
The analysis of the variance for the response surface model of the lycopene content (mg/100 g of tomato peels) and EY (%) of tomato peel extracts is presented in Table 2.

Table 2.
Analysis of variance for the response surface model of the lycopene content (mg/100 g of tomato peels) and EY (%) of tomato peel extracts.
Lycopene Content
The results from fitting the experimental data to the response surface model for the lycopene content in the extracted samples are presented in Table 2. The model showed a coefficient of determination (R2) value 0.92, suggesting a strong correlation between the experimental data and the model’s predictions. The F-values and p-values indicate the significance of each coefficient for the various terms, with higher F-values and lower p-values reflecting more impactful factors [27]. The response surface model that describes the lycopene content is represented by Equation (5):
Among the model terms, the linear effect of microwave power (X2) was found to be the most influential factor on the lycopene content, as indicated by its F-value of 236.13 (p < 0.001). This was followed by the interaction between the solid/liquid ratio and ultrasound power (X1X3). The interaction between microwave power and ultrasound power (X2X3) and the linear terms of the solid/liquid ratio (X1) and ultrasound power (X3) showed smaller but still statistically significant impacts. Non-significant factors included the quadratic terms for the solid/liquid ratio (X12), microwave power (X22), and ultrasound power (X32), as well as the interaction between the solid/liquid ratio and microwave power (X1X2).
In summary, the most critical factors affecting the lycopene content were the linear terms for the solid/liquid ratio, microwave power, and ultrasound power, and the interaction terms of X1X3 and X2X3. Based on these results, a new regression analysis was carried out, excluding the non-significant terms. The revised fitted surface model for predicting lycopene content is expressed as follows:
The updated model, with an F-value of 809.02 and a p-value of 0.00, demonstrated a highly significant ability to predict the lycopene content. The coefficient of determination (R2) was 0.91, indicating that the model explained the majority of the variation in the data, which suggested a very strong fit. Figure 1 presents a plot of the predicted versus observed values for the lycopene content, showing a strong correlation between them. This close agreement further validates the model’s accuracy and its ability to predict the lycopene content based on the specified extraction parameters.
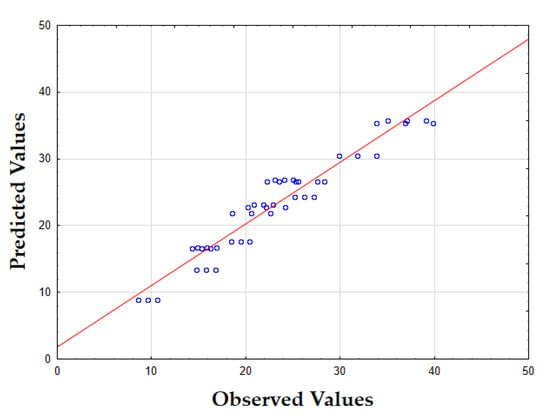
Figure 1.
Plots of predicted versus actual values for the lycopene content (mg/100 g of tomato peels) of tomato peel extracts using UAE-MAE. The red line represents the line of perfect prediction (predicted = actual), and the blue circles indicate the individual experimental data points.
Extraction Yield (EY)
The response surface model for EY also resulted in a high R2 value of 0.95 (Table 2). The corresponding mathematical model for EY is given by Equation (7):
The linear term for microwave power (X2) was the most significant factor (F-value = 440.40, p-value < 0.001) affecting EY, followed by the interaction between the solid/liquid ratio and ultrasound power (X1X3). The interaction between microwave power and ultrasound power (X2X3), along with the linear effects of the solid/liquid ratio (X1) and ultrasound power (X3) were also found to be significant. In contrast, the quadratic terms for the solid/liquid ratio (X12), microwave power (X22), and ultrasound power (X32) and the interaction between the solid/liquid ratio and microwave power (X1X2) were not significant in influencing EY.
The results indicate that the linear terms for the solid/liquid ratio, microwave power, and ultrasound power, and the interaction terms of X1X3 and X2X3 are critical factors influencing EY, similar to the findings for the lycopene content. To refine the model, a new regression analysis was performed by excluding the non-significant variables, resulting in the following updated surface model:
The F-value of 1454.97 and p-value below 0.00 indicate that the model is highly significant in predicting the EY. The R2 value of 0.95 confirms that the model accounts for most of the variability in the data. As shown in Figure 2, the data points are tightly clustered around the diagonal, which suggests minimal discrepancy between the predicted and observed EY values. This indicates that the model consistently predicts the EY with only small errors across the entire range of values tested, demonstrating its accuracy and reliability.
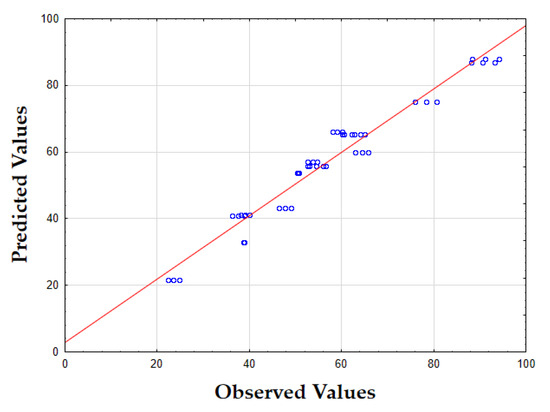
Figure 2.
Plots of predicted versus actual values for the EY (%) of tomato peel extracts using UAE-MAE. The red line represents the line of perfect prediction (predicted = actual), and the blue circles indicate the individual experimental data points.
Research on lycopene extraction from tomato peels using combined UAE-MAE method remains limited. A study by Lianfu et al. (2008) applied the UAE-MAE technique for lycopene recovery from tomatoes, revealing that microwave power and the solid/liquid ratio were key factors influencing lycopene yield. Their findings demonstrated that increasing the microwave power and decreasing the solid/liquid ratio led to a maximized lycopene yield [28]. These results align with the present study, which also identified microwave power and the solid/liquid ratio as critical determinants of the lycopene content and extraction yield. Given the scarcity of research exploring the combined impacts of microwave and ultrasound, the findings of this study are primarily compared with previous studies that utilized either UAE or MAE techniques independently. For instance, Kumcuoglu et al. (2014) utilized UAE for lycopene extraction from tomato waste and found that ultrasound power increases both the lycopene content and yield, particularly when lower solid/liquid ratios are used [17]. Similarly, Ho et al. (2015) employed MAE for lycopene recovery from tomato peels and reported that this method efficiently disrupted the cell structure, thereby improving the lycopene yield. The optimal lycopene extract obtained through MAE had an extraction yield of 10.362 mg/100 g [29]. The highest lycopene content reported in this study is likely due to the combined effects of ultrasound and microwave power.
3.1.2. Interpretation of the Response Surface Model and Contour Plot
The response surface plots demonstrate the effects of the solid/liquid ratio (g/mL), microwave power (W), and ultrasound power (W) on the lycopene content and extraction yield (EY) of extracts obtained from tomato peels.
Figure 3 shows three-dimensional (3D) response surface and contour plots that visually represent the relationship between the independent variables (solid/liquid ratio, microwave power, and ultrasound power) and the dependent variables (lycopene content and EY). These plots provide a clearer understanding of how variations in the independent variables impact the results.
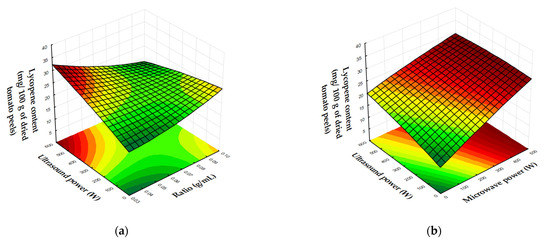
Figure 3.
Response surface and contour plots showing the effects of MW power, US power, and the solid/liquid ratio on the lycopene content (mg/100 g of tomato peels). (a) US power vs. ratio (MW power: 200 W); (b) US power vs. MW power (ratio: 0.03 g/mL).
Figure 3a illustrates that increasing ultrasound power and reducing the solid/liquid ratio significantly increase the lycopene content. The highest lycopene content is observed when ultrasound power is in the range of 500–600 W and solid/liquid ratio is less than 0.05 g/mL. Figure 3b illustrates the influences of microwave and ultrasound power on the lycopene content. As microwave power increases from 300 to 500 W, the lycopene content of the extracts is maximized, regardless of the ultrasound power is applied.
Figure 4a,b depict the interaction effects between ultrasound power and the solid/liquid ratio, as well as microwave and ultrasound power on EY. A similar trend to the lycopene content is observed for EY, as it is directly influenced by the lycopene content of the extracts obtained under various extraction conditions, since the Soxhlet method provides a constant lycopene content. Consequently, EY is maximized when the solid/liquid ratio is minimized and ultrasound power is above 500 W (Figure 4a). Regarding the influence of microwave and ultrasound power, the EY is maximum when the microwave power ranges between 300 and 500 W, regardless of the ultrasound power applied (Figure 4b).
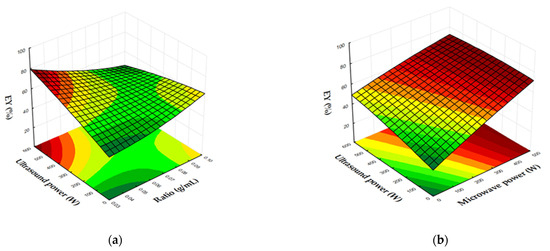
Figure 4.
Response surface and contour plots showing the effects of MW power, US power, and the solid/liquid ratio on EY (%). (a) US power vs. ratio (MW power: 200 W); (b) US power vs. MW power (ratio: 0.03 g/mL).
Based on these plots, the optimal extraction conditions for maximizing the lycopene content and EY through the synergistic application of UAE-MAE involve low solid/liquid ratios (below 0.05 g/mL) and moderate to high levels of microwave and ultrasound power (between 300 and 500 W for microwave power and between 500 and 600 W for ultrasound power). These findings from the response surface plots align with the optimum experimental conditions for lycopene extraction from tomato peels. The ideal parameters identified through experiments were a solid/liquid ratio of 0.03 g/mL, microwave power of 500 W, and ultrasound power of 600 W, resulting in a high lycopene content (37.08 mg/100 g of tomato peels) and an EY of 91.20%. Previous research on tomato waste analysis has shown that the lycopene content can range from 13.40 to 81.54 mg/100 g [30]. However, Strati and Oreopoulou (2014) noted that extraction yields vary widely due to factors such as the tomato variety, processing methods, by-product fraction, and extraction techniques, among others [31].
3.2. Recovery of Tomato Seed Oil
3.2.1. Oil Yield
The impacts of different extraction methods on the tomato seed oil yield are shown in Figure 5. Conventional (Con) stirring, Soxhlet extraction, UAE-MAE, and PLE were used to extract oil from tomato seeds, with hexane as the solvent. Overall, no statistically significant differences were observed between conventional stirring, Soxhlet extraction, and the UAE-MAE method. The highest oil yield (19.66 ± 0.71%) was obtained through conventional stirring, followed closely by UAE-MAE (19.53 ± 0.83%) and Soxhlet extraction (19.18 ± 0.62%) The PLE method yielded the lowest amount of oil (17.17 ± 0.63%). These results are consistent with previous research on tomato seed oil extraction, although data for the UAE-MAE and PLE methods are still limited. For instance, Szabo et al. (2021) reported a yield of 19.1 ± 0.2% for tomato seed oil extraction using continuous stirring for 2 h at room temperature [32]. In another study, Ouatmani et al. (2021) found a higher yield of 21.6 ± 1.5% using Soxhlet extraction with n-hexane for 8 h [33]. Kumar et al. (2023) used the UAE-MAE method with a solvent system of ethanol and ethyl acetate, yielding 23.07% oil [20]. Lastly, Eller et al. (2010) employed accelerated solvent extraction (PLE) with hexane, obtaining a 20% oil yield., which is slightly higher than the result from this study [21].
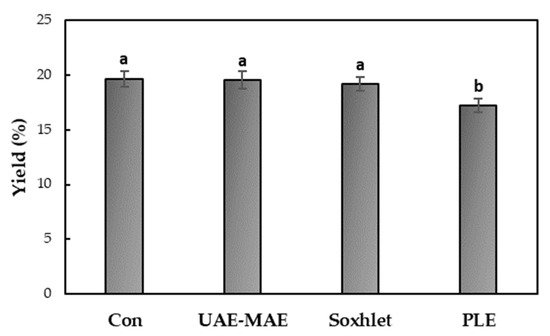
Figure 5.
Effect of the extraction method on the tomato seed oil yield. Different lowercase letters above the bars indicate statistically significant differences (p < 0.05).
In conclusion, both Soxhlet extraction and conventional stirring are well-established methods for extracting oil from seeds; however, they are time-consuming techniques. The UAE-MAE method produced a comparable oil yield to these traditional methods, but in a significantly shorter time—10 min. Although the PLE method resulted in the lowest yield, it was still efficient, achieving oil extraction in only 6 min.
3.2.2. Lycopene Content of Tomato Seed Oil
The lycopene content of the tomato seed oil obtained from different extraction methods was analyzed using HPLC-DAD, and the results are presented in Figure 6. The findings indicate significant variations in the lycopene content, depending on the extraction method. Among the methods tested, PLE proved to be the most efficient, yielding the highest lycopene concentration (44.0 ± 2.0 mg/100 g of oil), followed by UAE-MAE, with a lycopene content of 38.0 ± 2.0 mg/100 g of oil. Soxhlet extraction and conventional stirring were less efficient, resulting in a lycopene content of 28.0 ± 1.0 mg/100 g of oil and 13.0 ± 2.0 mg/100 g of oil, respectively.
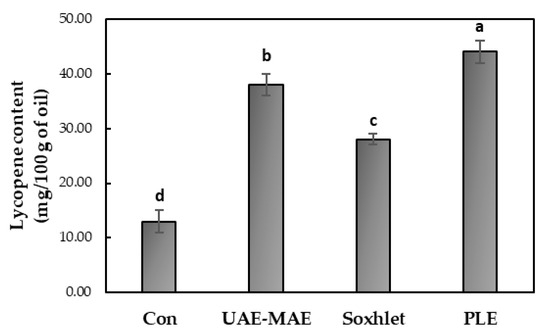
Figure 6.
Effect of the extraction method on the lycopene content of tomato seed oil. Different lowercase letters above the bars indicate statistically significant differences (p < 0.05).
PLE emerged as the most effective method for lycopene recovery, combining a high yield, short extraction time, and preservation of lycopene stability. The use of pressurized conditions facilitated enhanced solvent penetration due to increased diffusion from the solid matrix to the bulk solvent and the reduction of solvent viscosity, improving extraction efficiency while minimizing the risks of oxidative and thermal degradation [34]. UAE-MAE also proved to be an efficient technique, achieving high lycopene yields while requiring less time and energy than traditional Soxhlet extraction. The ultrasound cavitation and microwave heating effectively disrupted the plant cell matrix, enhancing mass transfer and, as a consequence, lycopene extraction [20]. While Soxhlet extraction is known for its ability to extract lipophilic compounds, its extended heating period led to lower lycopene yields than both PLE and UAE-MAE, likely due to thermal degradation of the compound [35]. Conventional stirring, on the other hand, was the least efficient method, yielding the lowest lycopene content. Its limited ability to break down cellular structures and enhance the solvent interaction resulted in poor lycopene recovery.
3.2.3. Fatty Acid Content of Tomato Seed Oil
The fatty acid composition of tomato seed oil extracted using different methods is presented in Table 3. Linoleic acid (ω-6) was the most abundant fatty acid across all extraction methods, confirming that tomato seed oil is rich in polyunsaturated fatty acids, particularly omega-6, which is known for its nutritional benefits. Oleic acid (ω-9) was the second most prevalent fatty acid. Among the saturated fatty acids, palmitic acid was the most abundant, while stearic acid was consistently present in lower amounts. Linolenic acid (ω-3), an essential polyunsaturated fatty acid, was the least abundant across all methods. These results align with previous studies on the fatty acid composition of tomato seed oil, confirming linoleic acid as the primary fatty acid, with oleic and palmitic acids also present in significant amounts [36].

Table 3.
Fatty acid composition (mg/g oil) of tomato seed oil extracted using different methods.
Soxhlet extraction resulted in the highest concentrations of oleic acid (258.5 ± 4.2 mg/g), linoleic acid (608.9 ± 4.6 mg/g), and linolenic acid (23.2 ± 2.2 mg/g), indicating its effectiveness in extracting unsaturated fatty acids. The PLE method also resulted in high levels of linoleic (544.7 ± 4.8 mg/g) and oleic acid (231.7 ± 4.3 mg/g), while UAE-MAE yielded comparable amounts of these fatty acids (530.5 ± 4.7 mg/g and 217.2 ± 4.1 mg/g, respectively). In contrast, conventional stirring extracted the lowest fatty acid concentrations, particularly for oleic (178.0 ± 4.0 mg/g) and linoleic acid (416.3 ± 4.6 mg/g), suggesting that this method is less effective at extracting unsaturated fatty acids. Among all methods, Soxhlet extraction achieved the highest overall extraction efficiency, while UAE-MAE and PLE provided competitive yields with significantly shorter processing times. These findings highlight the potential of UAE-MAE and PLE as efficient and environmentally friendly extraction methods for tomato seed oil.
The findings of this study demonstrate that UAE-MAE and PLE are promising alternative extraction methods for tomato seed oil, offering comparable yields but increased lycopene and fatty acid recoveries compared to conventional techniques. While Soxhlet extraction remains an effective method for exhaustive oil recovery, its extended processing time and batch-based nature make it unsuitable for large-scale industrial production. Its inability to be integrated into continuous extraction systems further reduces its feasibility for high-throughput applications. Similarly, conventional stirring, while simple and widely used, is limited by long processing times and inefficient mass transfer, making it less efficient for maximizing compound recovery compared to advanced extraction techniques like UAE-MAE and PLE. UAE-MAE, in particular, provides significant reductions in extraction time and energy consumption while maintaining high oil and lycopene yields, suggesting its suitability for large-scale applications. Moreover, PLE exhibits superior lycopene and fatty acid recoveries due to its pressurized environment. However, the high initial investment and operational costs associated with the moderate and high pressures utilized in PLE systems may pose challenges for its widespread adoption at an industrial scale. These advantages position UAE-MAE and PLE as sustainable extraction approaches, aligning with modern demands for environmentally friendly and efficient processing methods. Future research should focus on optimizing process parameters for large-scale industrial application and evaluating the economic feasibility of integrating these techniques into existing production lines.
3.3. Fermentation Process and Product Characterization
Tomato juice was converted into acetic acid through a two-step fermentation process. To determine the lycopene content in the tomato juice, an HPLC analysis was performed, showing an initial concentration of 12.15 ± 0.75 mg/100 g before fermentation. The results from each fermentation stage, along with the characterization of the products, are presented in the following sections.
3.3.1. First Fermentation Stage—Alcoholic Fermentation
During alcoholic fermentation, samples were taken from the reactor and analyzed for their sugar and alcohol contents. The results, presented in Figure 7, illustrate the changes observed throughout the process. As depicted, the sugars in the tomato juice were gradually converted into alcohol (ethanol), leading to a decrease in the sugar content and an increase in the alcohol concentration. The initial sugar content was 12.21 ± 1.05 °Brix which decreased to 3.73 ± 0.91 °Brix after 72 h. The final alcohol content in the wine produced from tomato juice was 5.58 ± 0.21%. This result was similar to previous findings in which tomato wine showed a maximum ethanol production rate in medium of 5.8% after 3 days [22]. However, the alcohol level in the reactor appeared to have reached its final value as early as the beginning of the second day (after 25–28 h). This suggests that the alcoholic fermentation process could have been terminated earlier than the day three, reducing both the processing time and fermentation costs.
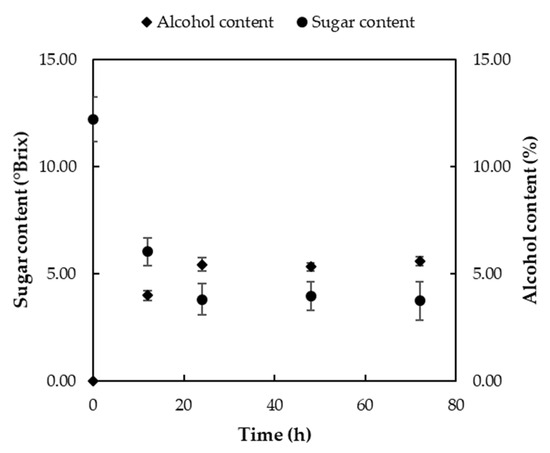
Figure 7.
Changes in the sugar (°Brix) and alcohol (%) contents during the alcoholic fermentation stage.
The final product of alcoholic fermentation served as an intermediate stage in the production of acetic acid. However, measurements were performed to characterize it, ensuring comprehensive monitoring of the fermentation process (Table 4). An analysis of the intermediate product’s properties indicated that it retained a significant portion of its lycopene content (10.02 ± 0.64 mg/100 g) while also achieving the required ethanol concentration for acetic acid production. According to this specification, the alcohol content in the wine used for acetic acid production should range between 5.5 and 7%. This ethanol percentage was achieved by adjusting the initial sugar concentration to 12.21 ± 1.05 °Brix. As shown in Table 4, the final sugar content and TDSs exhibited minimal variation, confirming the accuracy of the refractive index measurement of the tomato wine.

Table 4.
Properties of the alcoholic fermentation product.
3.3.2. Second Fermentation Stage—Acetic Fermentation
During acetic fermentation, samples were collected from the reactor and analyzed for their total acidity and alcohol content. The results are presented in Figure 8.
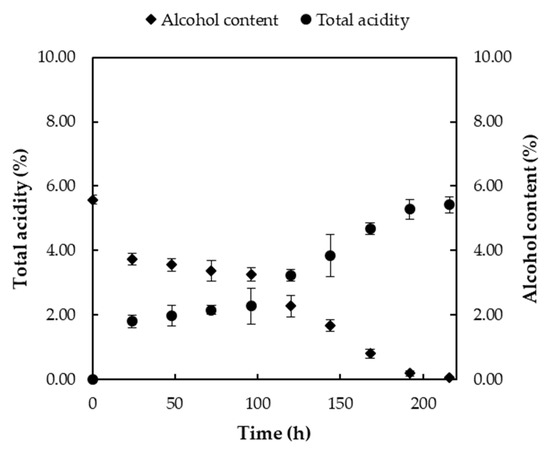
Figure 8.
Changes in total acidity (%) and the alcohol (%) content during the acetic fermentation stage.
As observed in Figure 8, during the final stage of fermentation, the alcohol produced in the alcoholic fermentation step was converted into acetic acid. As a result, the alcohol content decreased while the total acidity increased. The process was considered complete when the total acidity reached the desired level, which in this case was 5.42 ± 0.24%. The total acidity began to rise sharply after 72 h and reached its final value after 200 h (on the ninth day) of fermentation. This finding was consistent with the results from Lee et al. (2013), who reported an acetic acid concentration of 5.6% in the final tomato vinegar after the acetic acid fermentation process [22]. Similarly, the alcohol content declined significantly after 72 h and continued to decrease gradually until the ninth and final day, when it was nearly depleted. The final total acidity value of 5.42 ± 0.24%—the maximum achievable under these conditions—was attained by adjusting the initial sugar concentration to 12–13 °Brix and was reached after a total fermentation period of 13 days.
Measurements were conducted on the final product of acetic fermentation to characterize its properties, with the results summarized in Table 5. Based on the lycopene content of the acetic acid product (9.19 ± 0.70 mg/100 g) and considering that the initial lycopene concentration in the tomato juice before fermentation was 12.15 ± 0.75 mg/100 g, it was concluded that the final product retained most of the lycopene from the raw material. This means that the product also preserves its beneficial antioxidant properties. Furthermore, since the minimum acidity required for a product to be legally classified as vinegar is 5.0%, the tomato vinegar produced using this method met this standard and could be marketed accordingly [37]. Comparing the acidity (5.42 ± 0.24%) and pH (2.85 ± 0.10) values of the final product with those of commercial white vinegar (6% acidity and pH 3), a high degree of similarity is observed. Cejudo-Bastante et al. (2017) developed a tomato vinegar through a two-stage fermentation process, achieving a pH value of 3.08 and a low alcohol content, which aligns with the results of the present study [38]. Finally, the final total acidity fell within the expected range, as it was indirectly adjusted at the beginning of the process through the initial sugar content of the juice (12.2 °Brix).

Table 5.
Properties of the acetic fermentation product.
4. Conclusions
This study demonstrates that tomato by-products, including peels, seeds, and juice, are valuable sources of bioactive compounds that can be effectively utilized through innovative extraction and bioprocessing methods. UAE-MAE proved to be an efficient technique for lycopene extraction from tomato peels, with optimal conditions (0.03 g/mL solid/liquid ratio, 500 W microwave power, and 600 W ultrasound power) yielding a lycopene content of 37.08 mg/100 g and an extraction efficiency of 91.20%. This method provides a sustainable alternative to conventional extraction techniques, reducing the processing time and solvent consumption while maintaining high recovery yields. Future research should focus on optimizing UAE-MAE for large-scale applications and ensuring the stability of extracted lycopene in food formulations.
In the case of tomato seeds, UAE-MAE and PLE were identified as promising green extraction techniques for obtaining oil rich in lycopene and fatty acids. The extracted oil products were analyzed for lycopene and fatty acid contents, revealing that PLE produced the highest lycopene concentration (44.0 mg/100 g oil), with UAE-MAE also achieving notable levels. These methods demonstrated comparable efficiency to conventional techniques such as Soxhlet extraction and conventional stirring but with significantly reduced processing times and solvent usage. While Soxhlet extraction remains widely used, its extended processing time and high energy requirements limit its feasibility for large-scale applications. The adoption of UAE-MAE and PLE could lead to more sustainable and cost-effective oil extraction processes in the food and nutraceutical industries, contributing to resource efficiency and environmental sustainability.
Fermentation of tomato juice into vinegar was also confirmed as an effective bioprocessing approach that retains lycopene and maintains acidity levels, which could offer functional benefits. The high acidity (5.42%) and lycopene content (9.19 mg/100 g) of the final vinegar product suggest its potential as a functional food ingredient with antioxidant properties. Future studies should explore ways to enhance the stability and sensory attributes of tomato-based vinegar while assessing its bioavailability and potential health benefits in real-world applications.
While this study provides valuable insights into the valorization of tomato by-products, certain challenges remain that necessitate further research. The extraction and bioprocessing methods were developed and tested at a laboratory scale, and their scalability and economic feasibility must be evaluated for industrial implementation. Additionally, although the functional potential of the extracted bioactive compounds has been demonstrated, their stability and bioavailability in real-world food and supplement formulations need to be thoroughly assessed. Further investigations should focus on how effectively these compounds are absorbed, metabolized, and retained in the human body, as well as their degradation behavior during processing and storage. Stability studies should also explore environmental factors such as temperature, light exposure, and packaging conditions to optimize shelf life. Furthermore, advancements in encapsulation techniques and carrier systems could be explored to improve the controlled release and bioavailability of these compounds in functional foods and nutraceuticals. Future research should also explore alternative green solvents, advanced extraction techniques, and life-cycle assessments to improve both efficiency and sustainability. By addressing these aspects, the valorization of tomato by-products can be optimized for large-scale applications, contributing to the development of functional foods and nutraceuticals within a circular bioeconomy framework.
Author Contributions
Conceptualization, P.M.E., C.J.B. and M.K.; methodology, P.M.E. and C.J.B.; software, C.D., K.T.L., C.J.B. and M.K.; validation, C.D., K.T.L., P.M.E. and C.J.B.; formal analysis, C.D., K.T.L., P.M.E. and C.J.B.; investigation, P.M.E., C.J.B., E.T. and M.K.; resources, M.K.; data curation, C.D., K.T.L., P.M.E. and C.J.B.; writing—original draft preparation, C.D., K.T.L., M.D., P.M.E. and C.J.B.; writing—review and editing, C.D., K.T.L., P.M.E., C.J.B., E.T. and M.K.; visualization, C.D., K.T.L. and C.J.B.; supervision, M.K.; project administration, M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this research was funded by ERA-NET MANUNET-II (Project name: TOMATOCYCLE), with the support of Operational Program Western Greece 2012–2020 (MIS 5021385).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author (privacy reasons).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAOSTAT. 2023. Available online: https://www.fao.org/faostat/en/#data/ (accessed on 26 February 2025).
- Szabo, K.; Diaconeasa, Z.; Cătoi, A.F.; Vodnar, D.C. Screening of Ten Tomato Varieties Processing Waste for Bioactive Components and Their Related Antioxidant and Antimicrobial Activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of Lycopene from Industrially Derived Tomato Processing By-Products by Pulsed Electric Fields-Assisted Extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- Sangeetha, K.; Ramyaa, R.B.; Mousavi Khaneghah, A.; Radhakrishnan, M. Extraction, Characterization, and Application of Tomato Seed Oil in the Food Industry: An Updated Review. J. Agric. Food Res. 2023, 11, 100529. [Google Scholar] [CrossRef]
- Terenzi, C.; Bermudez, G.; Medri, F.; Davani, L.; Tumiatti, V.; Andrisano, V.; Montanari, S.; De Simone, A. Phenolic and Antioxidant Characterization of Fruit By-Products for Their Nutraceuticals and Dietary Supplements Valorization under a Circular Bio-Economy Approach. Antioxidants 2024, 13, 604. [Google Scholar] [CrossRef]
- Eslami, E.; Carpentieri, S.; Pataro, G.; Ferrari, G. A Comprehensive Overview of Tomato Processing By-Product Valorization by Conventional Methods versus Emerging Technologies. Foods 2023, 12, 166. [Google Scholar]
- Jiménez Bolaño, D.C.; Insuasty, D.; Rodríguez Macías, J.D.; Grande-Tovar, C.D. Potential Use of Tomato Peel, a Rich Source of Lycopene, for Cancer Treatment. Molecules 2024, 29, 3079. [Google Scholar] [CrossRef]
- Sabio, E.; Álvarez-Murillo, A.; Román, S.; Ledesma, B. Conversion of Tomato-Peel Waste into Solid Fuel by Hydrothermal Carbonization: Influence of the Processing Variables. Waste Manag. 2016, 47, 122–132. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.E.; Ranga, F.; Roman, I.; Khaoula, H.; Boudaya, E.; Ltaief, A.B.; Aouani, W.; Thiamrat, M.; Vodnar, D.C. Carotenoid Recovery from Tomato Processing By-Products through Green Chemistry. Molecules 2022, 27, 3771. [Google Scholar] [CrossRef]
- Singh, P.; Goyal, G.K. Dietary Lycopene: Its Properties and Anticarcinogenic Effects. Compr. Rev. Food Sci. Food Saf. 2008, 7, 255–270. [Google Scholar]
- Drosou, C.; Krokida, M. A Comparative Study of Encapsulation of β-Carotene via Spray-Drying and Freeze-Drying Techniques Using Pullulan and Whey Protein Isolate as Wall Material. Foods 2024, 13, 1933. [Google Scholar] [CrossRef]
- MacHmudah, S.; Zakaria; Winardi, S.; Sasaki, M.; Goto, M.; Kusumoto, N.; Hayakawa, K. Lycopene Extraction from Tomato Peel By-Product Containing Tomato Seed Using Supercritical Carbon Dioxide. J. Food Eng. 2012, 108, 290–296. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, B.H. Determination of Carotenoids in Tomato Juice by Liquid Chromatography. J. Chromatogr. A 2003, 1012, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Periago, M.J.; Rincón, F.; Agüera, M.D.; Ros, G. Mixture Approach for Optimizing Lycopene Extraction from Tomato and Tomato Products. J. Agric. Food Chem. 2004, 52, 5796–5802. [Google Scholar] [CrossRef]
- Lasunon, P.; Phonkerd, N.; Tettawong, P.; Sengkhamparn, N. Effect of Microwave-Assisted Extraction on Bioactive Compounds from Industrial Tomato Waste and Its Antioxidant Activity. Food Res. 2021, 5, 468–474. [Google Scholar] [CrossRef]
- Silva, Y.P.A.; Ferreira, T.A.P.C.; Celli, G.B.; Brooks, M.S. Optimization of Lycopene Extraction from Tomato Processing Waste Using an Eco-Friendly Ethyl Lactate–Ethyl Acetate Solvent: A Green Valorization Approach. Waste Biomass Valorization 2019, 10, 2851–2861. [Google Scholar] [CrossRef]
- Kumcuoglu, S.; Yilmaz, T.; Tavman, S. Ultrasound Assisted Extraction of Lycopene from Tomato Processing Wastes. J. Food Sci. Technol 2014, 51, 4102–4107. [Google Scholar] [CrossRef]
- Laina, K.T.; Drosou, C.; Stergiopoulos, C.; Eleni, P.M.; Krokida, M. Optimization of Combined Ultrasound and Microwave-Assisted Extraction for Enhanced Bioactive Compounds Recovery from Four Medicinal Plants: Oregano, Rosemary, Hypericum, and Chamomile. Molecules 2024, 29, 5773. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Laina, K.T.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. Revolutionizing Wine Waste: Advanced Techniques for Polyphenol Recovery from White Wine Byproducts. Agriculture 2025, 15, 648. [Google Scholar] [CrossRef]
- Kumar, S.; Nirmal Thirunavookarasu, S.; Sunil, C.K.; Vignesh, S.; Venkatachalapathy, N.; Rawson, A. Mass Transfer Kinetics and Quality Evaluation of Tomato Seed Oil Extracted Using Emerging Technologies. Innov. Food Sci. Emerg. Technol. 2023, 83, 103203. [Google Scholar] [CrossRef]
- Eller, F.J.; Moser, J.K.; Kenar, J.A.; Taylor, S.L. Extraction and Analysis of Tomato Seed Oil. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 755–762. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.D.; Jeong, J.H.; Lee, M.K.; Jeong, Y.K.; Shim, K.H.; Seo, K. Il New Vinegar Produced by Tomato Suppresses Adipocyte Differentiation and Fat Accumulation in 3T3-L1 Cells and Obese Rat Model. Food Chem. 2013, 141, 3241–3249. [Google Scholar] [CrossRef]
- Vegas, C.; Mateo, E.; González, Á.; Jara, C.; Guillamón, J.M.; Poblet, M.; Torija, M.J.; Mas, A. Population Dynamics of Acetic Acid Bacteria during Traditional Wine Vinegar Production. Int. J. Food Microbiol. 2010, 138, 130–136. [Google Scholar] [CrossRef]
- Perumpuli, P.A.B.N.; Buddhika, M.A.A.; Kaumal, M.N. Production of Antioxidant Rich Tomato Vinegar: An Alternative to Coconut Vinegar in Culinary Production. Curr. Appl. Sci. Technol. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M. Enrichment of White Chocolate with Microencapsulated β-Carotene: Impact on Quality Characteristics and β-Carotene Stability during Storage. Foods 2024, 13, 2699. [Google Scholar] [CrossRef]
- Mari, A.; Andriotis, P.; Drosou, C.; Laina, K.T.; Panagiotou, N.; Krokida, M. Enhancing Shelf-Life Stability of Refrigerated Potatoes through Osmotic Dehydration and Ohmic Heating Optimization: A Strategy to Mitigate Enzymatic Browning. Potato Res. 2024. [Google Scholar] [CrossRef]
- Tsakiri-Mantzorou, Z.; Drosou, C.; Mari, A.; Stramarkou, M.; Laina, K.T.; Krokida, M. Edible Coating with Encapsulated Antimicrobial and Antibrowning Agents via the Emerging Electrospinning Process and the Conventional Spray Drying: Effect on Quality and Shelf Life of Fresh-Cut Potatoes. Potato Res. 2024. [Google Scholar] [CrossRef]
- Lianfu, Z.; Zelong, L. Optimization and Comparison of Ultrasound/Microwave Assisted Extraction (UMAE) and Ultrasonic Assisted Extraction (UAE) of Lycopene from Tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Liceaga, A.M.; San Martín-González, M.F. Microwave-Assisted Extraction of Lycopene in Tomato Peels: Effect of Extraction Conditions on All-Trans and Cis-Isomer Yields. LWT 2015, 62, 160–168. [Google Scholar] [CrossRef]
- Stajčić, S.; Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Mandić, A.; Četojević-Simin, D. Tomato Waste: Carotenoids Content, Antioxidant and Cell Growth Activities. Food Chem. 2015, 172, 225–232. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Recovery of Carotenoids from Tomato Processing By-Products—A Review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Szabo, K.; Vasile Dulf, F.; Oke Teleky, B.-E.; Eleni, P.; Boukouvalas, C.; Krokida, M.; Kapsalis, N.; Rusu, A.V.; Socol, C.T.; Cristian Vodnar, D. Evaluation of the Bioactive Compounds Found in Tomato Seed Oil and Tomato Peels Influenced by Industrial Heat Treatments. Foods 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Ouatmani, T.; Haddadi-Guemghar, H.; Boulekbache-Makhlouf, L.; Mehidi-Terki, D.; Maouche, A.; Madani, K. A Sustainable Valorization of Industrial Tomato Seeds (Cv Rio Grande): Sequential Recovery of a Valuable Oil and Optimized Extraction of Antioxidants by Microwaves. J. Food Process Preserv. 2021, 46, e16123. [Google Scholar] [CrossRef]
- Cardenas-Toro, F.P.; Alcázar-Alay, S.C.; Coutinho, J.P.; Godoy, H.T.; Forster-Carneiro, T.; Meireles, M.A.A. Pressurized Liquid Extraction and Low-Pressure Solvent Extraction of Carotenoids from Pressed Palm Fiber: Experimental and Economical Evaluation. Food Bioprod. Process. 2015, 94, 90–100. [Google Scholar] [CrossRef]
- Calvo, M.M.; Dado, D.; Santa-María, G. Influence of Extraction with Ethanol or Ethyl Acetate on the Yield of Lycopene, β-Carotene, Phytoene and Phytofluene from Tomato Peel Powder. Eur. Food Res. Technol. 2007, 224, 567–571. [Google Scholar] [CrossRef]
- Botineștean, C.; Hădărugă, N.G.; Hădărugă, D.I.; Jianu, I. Fatty Acids Composition by Gas Chromatography-Mass Spectrometry (GC-MS) and Most Important Physical-Chemicals Parameters of Tomato Seed Oil. J. Agroaliment. Process. Technol. 2012, 18, 89–94. [Google Scholar]
- Silva, V.; Mehrpour, G.; Soares, V.; Santo, D.; Nunes, P.; Quintas, C. Quality and Biological Properties of Vinegar Processed from Non-Valorized Fruits in Southern Portugal. Future Foods 2024, 9, 100337. [Google Scholar] [CrossRef]
- Cejudo-Bastante, C.; Durán-Guerrero, E.; García-Barroso, C.; Castro-Mejías, R.; Castro, R. Volatile Compounds, Polyphenols and Sensory Quality in the Production of Tomato Vinegar. J. Food Nutr. Res. 2017, 5, 391–398. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).