Effect of Asparaginase Treatment on Biscuit Volatile Compounds
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Biscuits
2.2. Determination of Levels of Asparagine in the Dough
2.3. Measurement of Quality Attributes of Biscuits
2.3.1. Colour
2.3.2. Volatile Organic Compounds (VOCs)
2.4. Statistical Analysis
3. Results
3.1. Asparagine Content in the Dough
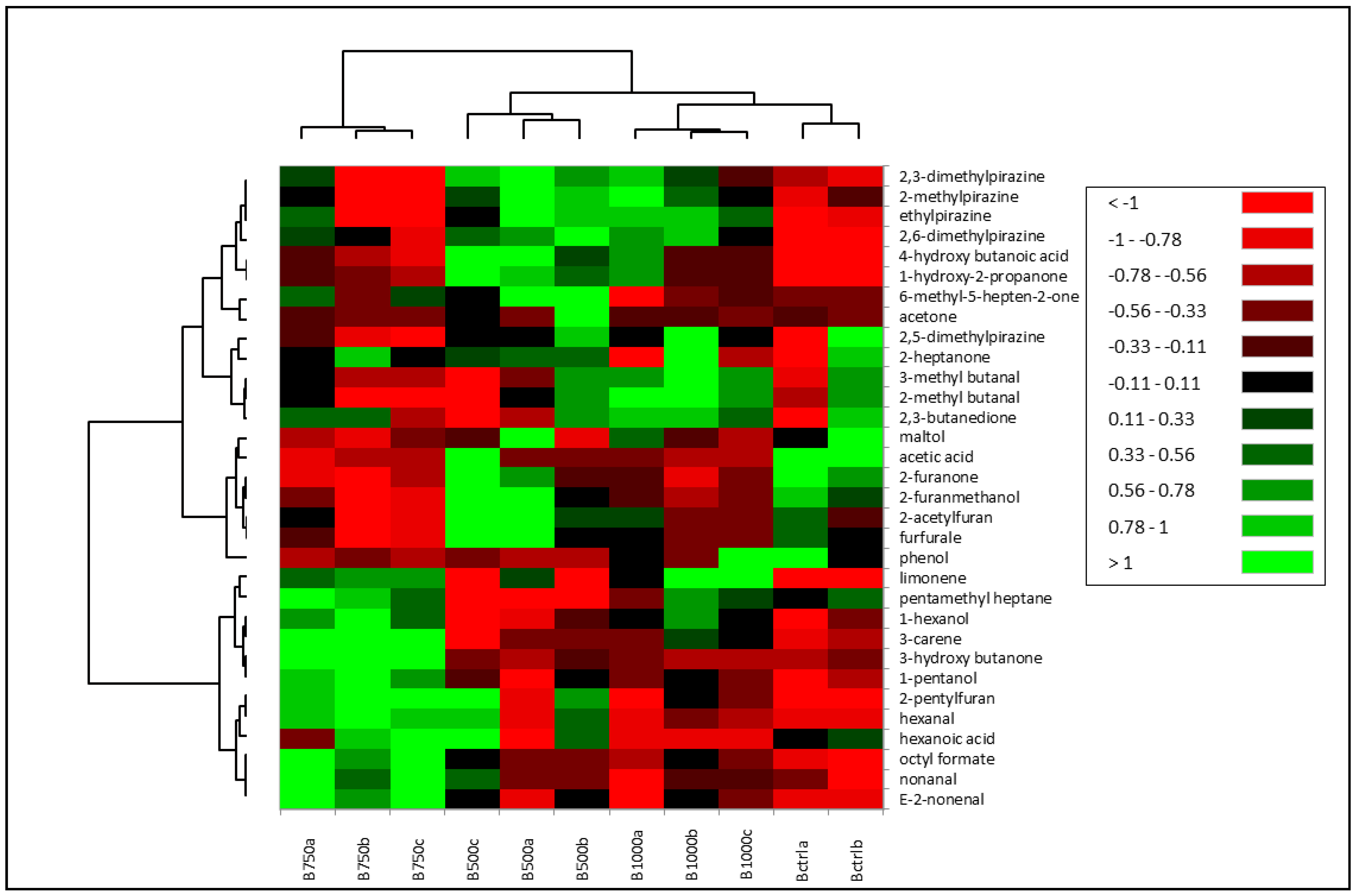
3.2. VOCs
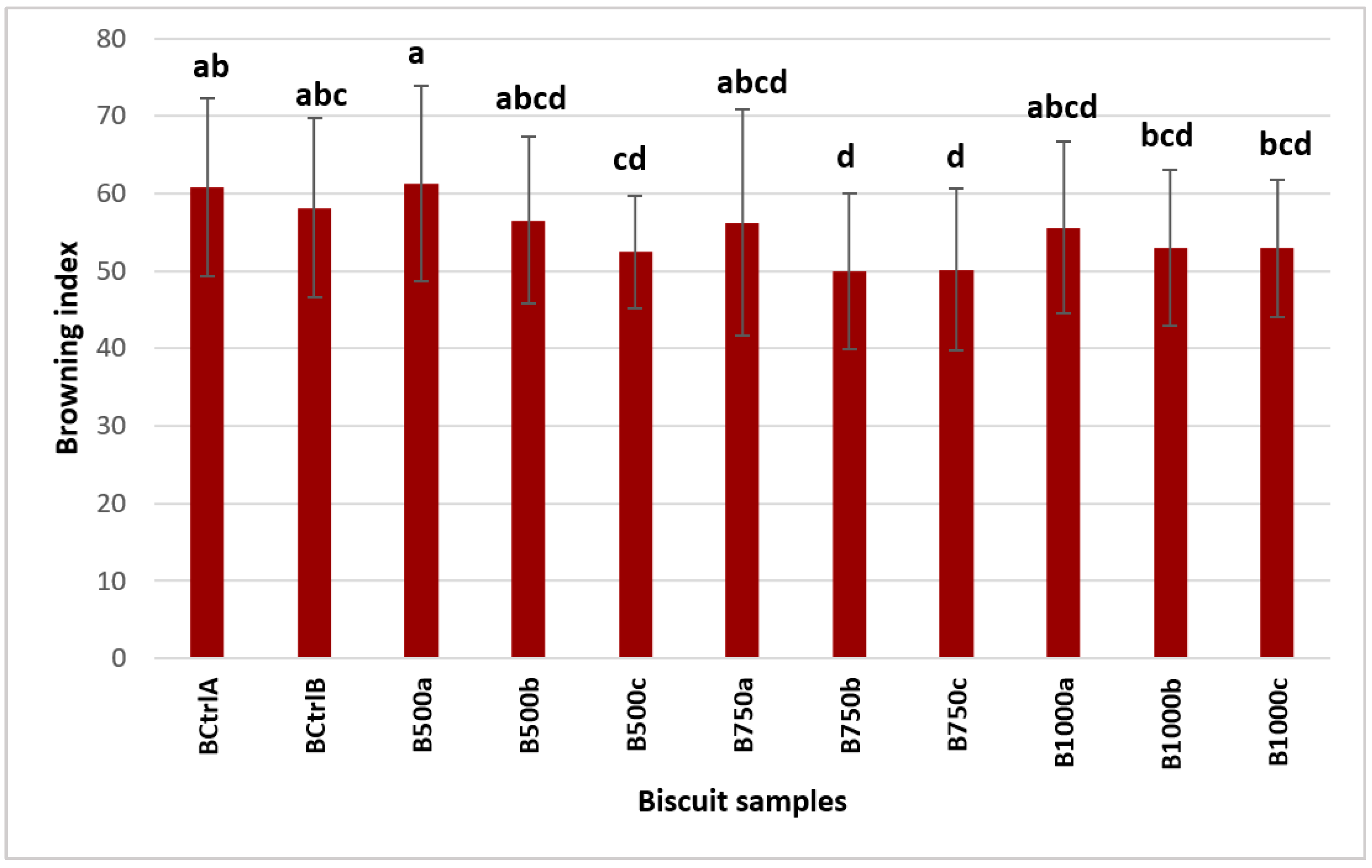
3.3. Colour
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, J.K. Thermal Generation or Aroma. In Flavour Development, Analysis and Perception in Food and Beverages; Woodhead Publishing, Elsevier: Cambridge, UK, 2015; pp. 151–185. ISBN 978-1-78242-103-0. [Google Scholar]
- van Boekel, M.A.J.S. Kinetic Modeling of Reactions in Foods; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-429-11375-8. [Google Scholar]
- van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S.; Somoza, V.; Knorr, D.; Jasti, P.R.; Eisenbrand, G. A Review on the Beneficial Aspects of Food Processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Fogliano, V. Acrylamide and 5-Hydroxymethylfurfural (HMF): A Review on Metabolism, Toxicity, Occurrence in Food and Mitigation Strategies. LWT-Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Regulation-2017/2158-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2017/2158/oj (accessed on 8 November 2024).
- Xu, F.; Oruna-Concha, M.-J.; Elmore, J.S. The Use of Asparaginase to Reduce Acrylamide Levels in Cooked Food. Food Chem. 2016, 210, 163–171. [Google Scholar] [CrossRef]
- Žilić, S.; Aktağ, I.G.; Dodig, D.; Filipović, M.; Gökmen, V. Acrylamide Formation in Biscuits Made of Different Wholegrain Flours Depending on Their Free Asparagine Content and Baking Conditions. Food Res. Int. 2020, 132, 109109. [Google Scholar] [CrossRef]
- Muneer, F.; Siddique, M.H.; Azeem, F.; Rasul, I.; Muzammil, S.; Zubair, M.; Afzal, M.; Nadeem, H. Microbial L-Asparaginase: Purification, Characterization and Applications. Arch. Microbiol. 2020, 202, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, H.V.; Kornbrust, B.A.; Østergaard, P.R.; Stringer, M.A. Evaluating the Potential for Enzymatic Acrylamide Mitigation in a Range of Food Products Using an Asparaginase from Aspergillus Oryzae. J. Agric. Food Chem. 2009, 57, 4168–4176. [Google Scholar] [CrossRef] [PubMed]
- Haase, N.U.; Grothe, K.-H.; Matthäus, B.; Vosmann, K.; Lindhauer, M.G. Acrylamide Formation and Antioxidant Level in Biscuits Related to Recipe and Baking. Food Addit. Contam. Part A 2012, 29, 1230–1238. [Google Scholar] [CrossRef]
- Anese, M.; Quarta, B.; Peloux, L.; Calligaris, S. Effect of Formulation on the Capacity of L-Asparaginase to Minimize Acrylamide Formation in Short Dough Biscuits. Food Res. Int. 2011, 44, 2837–2842. [Google Scholar] [CrossRef]
- Kukurová, K.; Ciesarová, Z.; Mogol, B.A.; Açar, Ö.Ç.; Gökmen, V. Raising Agents Strongly Influence Acrylamide and HMF Formation in Cookies and Conditions for Asparaginase Activity in Dough. Eur. Food Res. Technol. 2013, 237, 1–8. [Google Scholar] [CrossRef]
- Musa, S.; Becker, L.; Oellig, C.; Scherf, K.A. Asparaginase Treatment to Mitigate Acrylamide Formation in Wheat and Rye Cookies. LWT 2024, 203, 116365. [Google Scholar] [CrossRef]
- Garvey, E.C.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Factors Influencing the Sensory Perception of Reformulated Baked Confectionary Products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1160–1188. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Ferrigno, A.; Acampa, I.; Serpen, A.; Açar, Ö.Ç.; Gökmen, V.; Fogliano, V. Effect of Flour Type on Maillard Reaction and Acrylamide Formation during Toasting of Bread Crisp Model Systems and Mitigation Strategies. Food Res. Int. 2009, 42, 1295–1302. [Google Scholar] [CrossRef]
- Schouten, M.A.; Tappi, S.; Rocculi, P.; Romani, S. Mitigation Strategies to Reduce Acrylamide in Cookies: Effect of Formulation. Food Rev. Int. 2023, 39, 4793–4834. [Google Scholar] [CrossRef]
- Gazi, S.; Göncüoğlu Taş, N.; Görgülü, A.; Gökmen, V. Effectiveness of Asparaginase on Reducing Acrylamide Formation in Bakery Products According to Their Dough Type and Properties. Food Chem. 2023, 402, 134224. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Raffo, A.; Carcea, M.; Castagna, C.; Magrì, A. Improvement of a Headspace Solid Phase Microextraction-Gas Chromatography/Mass Spectrometry Method for the Analysis of Wheat Bread Volatile Compounds. J. Chromatogr. A 2015, 1406, 266–278. [Google Scholar] [CrossRef]
- Vieira-Porto, A.C.; Cunha, S.C.; Rosa, E.C.; DePaula, J.; Cruz, A.G.; Freitas-Silva, O.; Fernandes, J.O.; Farah, A. Chemical Composition and Sensory Profiling of Coffees Treated with Asparaginase to Decrease Acrylamide Formation during Roasting. Food Res. Int. 2024, 186, 114333. [Google Scholar] [CrossRef]
- Cho, H.; Park, M.K.; Kim, Y.-S. Study on Volatile Compounds Formed from the Thermal Interaction of Hydrolyzed Vegetable Proteins with Reducing Sugars. Food Sci. Biotechnol. 2023, 32, 283–298. [Google Scholar] [CrossRef]
- Luo, C.; Yin, Q.; Zeng, L.; Zhang, Q.; Wang, B.; Yu, G.; Shen, S.; Xie, W. Insight into Co-Pyrolysis Behavior of Asparagine/Fructose Mixtures Using on-Line Pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2025, 186, 106969. [Google Scholar] [CrossRef]
- Luo, Y.; Li, R.; Zhu, S.; Peng, J.; Huang, Q.; Zhao, T.; Ho, C.-T. Formation of Volatile Pyrazinones in the Asparagine Maillard Reaction Systems and Novel Pyrazinone Formation Pathways in the Amidated-Alanine Maillard Reaction Systems. J. Agric. Food Chem. 2024, 72, 11153–11163. [Google Scholar] [CrossRef]
- Maire, M.; Rega, B.; Cuvelier, M.-E.; Soto, P.; Giampaoli, P. Lipid Oxidation in Baked Products: Impact of Formula and Process on the Generation of Volatile Compounds. Food Chem. 2013, 141, 3510–3518. [Google Scholar] [CrossRef] [PubMed]
- Purlis, E. Browning Development in Bakery Products—A Review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Anese, M.; Quarta, B.; Frias, J. Modelling the Effect of Asparaginase in Reducing Acrylamide Formation in Biscuits. Food Chem. 2011, 126, 435–440. [Google Scholar] [CrossRef]
- Musa, S.; Becker, L.; Oellig, C.; Scherf, K.A. Influence of Asparaginase on Acrylamide Content, Color, and Texture in Oat, Corn, and Rice Cookies. J. Agric. Food Chem. 2024, 72, 22875–22882. [Google Scholar] [CrossRef] [PubMed]


| Sample Code | Asparaginase (ASNU/kg Flour) | Resting Time (min) | Dough T (°C) |
|---|---|---|---|
| B500a | 500 | 0 | - |
| B500b | 500 | 15 | 20 °C |
| B500c | 500 | 15 | 50 °C |
| B750a | 750 | 0 | - |
| B750b | 750 | 15 | 20 °C |
| B750c | 750 | 15 | 50 °C |
| B1000a | 1000 | 0 | - |
| B1000b | 1000 | 15 | 20 °C |
| B1000c | 1000 | 15 | 50 °C |
| BCtrlA | 0 | 0 | - |
| BCtrlB | 0 | 15 | 20 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masciola, F.; Baiamonte, I.; Marconi, E.; Melloni, S.; Nardo, N.; Narducci, V.; Sanchez del Pulgar, J.; Turfani, V.; Raffo, A. Effect of Asparaginase Treatment on Biscuit Volatile Compounds. Appl. Sci. 2025, 15, 3779. https://doi.org/10.3390/app15073779
Masciola F, Baiamonte I, Marconi E, Melloni S, Nardo N, Narducci V, Sanchez del Pulgar J, Turfani V, Raffo A. Effect of Asparaginase Treatment on Biscuit Volatile Compounds. Applied Sciences. 2025; 15(7):3779. https://doi.org/10.3390/app15073779
Chicago/Turabian StyleMasciola, Francesca, Irene Baiamonte, Emanuele Marconi, Sahara Melloni, Nicoletta Nardo, Valentina Narducci, Jose Sanchez del Pulgar, Valeria Turfani, and Antonio Raffo. 2025. "Effect of Asparaginase Treatment on Biscuit Volatile Compounds" Applied Sciences 15, no. 7: 3779. https://doi.org/10.3390/app15073779
APA StyleMasciola, F., Baiamonte, I., Marconi, E., Melloni, S., Nardo, N., Narducci, V., Sanchez del Pulgar, J., Turfani, V., & Raffo, A. (2025). Effect of Asparaginase Treatment on Biscuit Volatile Compounds. Applied Sciences, 15(7), 3779. https://doi.org/10.3390/app15073779







