Effect of Cassava Flour and Ginger Powder Addition on Physicochemical and Antioxidant Properties of Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
Bread Making
2.3. Moisture Content (MC)
2.4. Water Activity (aw)
2.5. Firmness
2.6. Crumb Structure
2.7. Volscan Measurement
2.8. Colour Measurements
2.9. Chemical Analysis
2.9.1. Extraction of Antioxidants
2.9.2. Total Phenolic Content (TPC) Analysis
2.9.3. Determination of Antioxidant Capacity (AC)
2.10. Statistical Analysis
3. Results
3.1. Moisture Content and Water Activity
3.2. Crumb Structure
3.3. Firmness
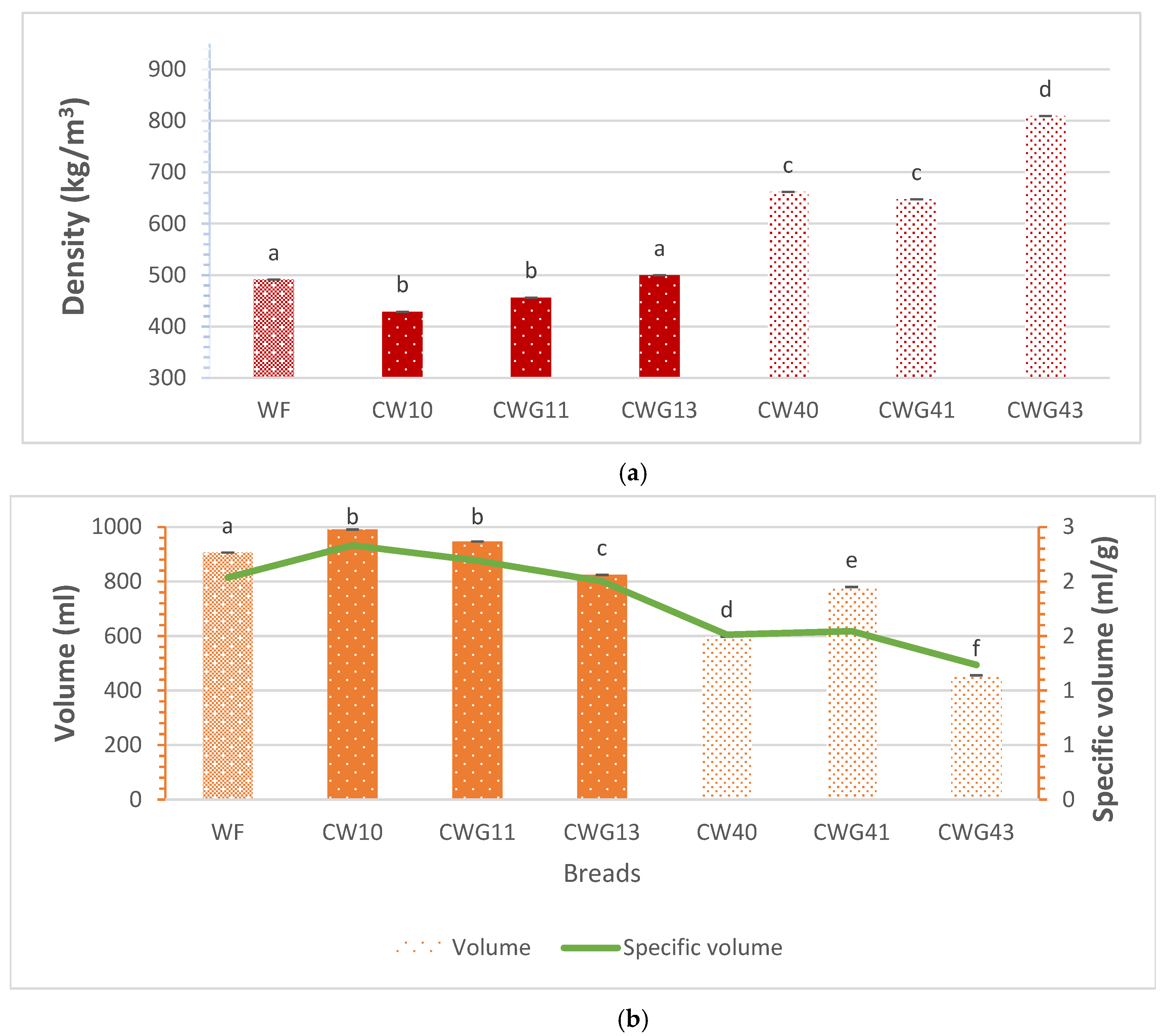
3.4. Density, Volume, and Specific Volume
3.5. Colour Measurements
3.6. Total Phenolic Content and Antioxidant Capacity
4. Discussion
4.1. Moisture Content and Water Activity
4.2. Firmness
4.3. Crumb Structure
4.4. Density, Volume, and Specific Volume
4.5. Colour Measurements
4.6. Total Phenolic Content (TPC)
4.7. Antioxidant Capacity (AC)
5. Conclusions
Author Contributions
Funding
Institutional Review Board statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Aghalari, Z.; Dahms, H.U.; Sillanpaa, M. Evaluation of nutrients in bread: A systematic review. J. Health Popul. Nutr. 2022, 41, 50. [Google Scholar] [PubMed]
- Irge, D.D. Chemical Composition and Nutritional Quality of Wheat, Teff (Eragrostis tef (Zucc) Trotter), Barley (Hordeum vulgare L.) and Rice (Oryza sativa)—A Review. Food Sci. Qual. Manag. 2017, 59, 6–12. [Google Scholar]
- Kumar, P.; Yadava, R.K.; Gollen, B.; Kumar, S.; Verma, R.K.; Yadav, S. Nutritional Contents and Medicinal Properties of Wheat: A Review. Life Sci. Med. Res. 2011, 22, 1–10. [Google Scholar]
- Wheat Flour, White, all-Purpose, Unenriched [Internet]. 2019. Available online: https://fdc.nal.usda.gov/food-details/789951/nutrients (accessed on 17 June 2024).
- Ribeiroa, M.; Nunesc, F.M.; Rodriguez-Quijanod, M.; Carrillod, J.M.; Branlarde, G.; Igrejas, G. Next-generation therapies for celiac disease: The gluten-targeted approaches. Treands Food Sci. Technol. 2018, 75, 56–71. [Google Scholar] [CrossRef]
- Oguntuase, S.O.; Ijarotimi, O.S.; Oluwajuyitan, T.D.; Oboh, G. Nutritional, antioxidant, carbohydrate hydrolyzing enzyme inhibitory activities, and glyceamic index of wheat bread as influence by bambara groundnut substitution. A Springer Nat. J. 2022, 4, 121. [Google Scholar] [CrossRef]
- Junejo, S.A.; Rashid, A.; Yang, L.; Xu, Y.; Kraithong, S.; Zhou, Y. Effects of spinach powder on the physicochemical and antioxidant properties of durum wheat bread. Food Sci. Technol. 2021, 150, 112058. [Google Scholar]
- Sigüenza-Andres, T.; Gallego, C.; Gomez, M. Can cassava improve the quality of gluten free breads? Food Sci. Technol. 2021, 149, 111923. [Google Scholar]
- Shahbandeh, M. Wheat Consumption Worldwide in 2023/2024, by Country (in 1000 Metric Tons). 2024. Available online: https://www.statista.com/statistics/1094065/total-global-wheat-consumption-by-country/ (accessed on 12 June 2024).
- Reynolds, M.P.; Braun, H. Wheat Improvement Food Security in a Changing Climate; Springer Nature: Cham, Switzerland, 2023; p. 624. [Google Scholar]
- Bayata, A. Review on Nutritional Value of Cassava for Use as a Staple Food. Sci. J. Anal. Chem. 2019, 7, 83–91. [Google Scholar] [CrossRef]
- EL-Sharkawy, M.A. Cassava biology and physiology. Plant Mol. Biol. 2003, 53, 621–641. [Google Scholar] [CrossRef]
- Gbadegesin, M.A.; Wills, M.A.; Beeching, J.R. Diversity of LTR-retrotransposons and Enhancer/Suppressor Mutator-like transposons in cassava (Manihot esculenta Crantz). Mol Genet Genom. 2008, 280, 305–317. [Google Scholar]
- Bamikole, A.; Tahirou, A.; Richardson, O. An Assessment of Cassava Post-Harvest Losses (PHL) in South-West Nigeria: A Case Study of Oyo-State, Nigeria. Acta Sci. Nutr. Health 2022, 6, 144–158. [Google Scholar]
- Obi, C.N.; Eze, P.C.; Ukoha, P.C. Microbiological and Nutritional Compositions of Garri Produced using Traditional Fermentation and Instant Mechanical Methods with and without added Palm Oil. Niger. J. Microbiol. 2022, 36, 6214–6228. [Google Scholar]
- da Cruz, E.P.; Pires, J.B.; dos Santos, F.N.; Fonseca, L.M.; Radünz, M.; Magro, J.D.; Gandra, E.A.; Zavareze, E.d.R.; Dias, A.R.G. Encapsulation of lemongrass essential oil into cassava starch fibers for application as antifungal agents in bread. Food Hydrocoll. 2023, 145, 109105. [Google Scholar] [CrossRef]
- Chijioke, U.; Madu, T.; Okoye, B.; Ogunka, A.P.; Ejechi, M.; Ofoeze, M.; Ogbete, C.; Njoku, D.; Ewuziem, J.; Kalu, C.; et al. Quality attributes of fufu in South-East Nigeria: Guide for cassava breeders. Int. J. Food Sci. Technol. 2021, 56, 1247–1257. [Google Scholar] [CrossRef]
- Ukwuru, M.U.; Egbonu, S.E. Recent development in cassava-based products research. Acad. J. Food Res. 2013, 1, 001–013. [Google Scholar]
- Etong, D.I.; Mustapha, A.O.; Lawrence, I.G.; Jacob, A.G.; Oladimeji, M.O. Nutritional and Physicochemical Properties of Wheat (Triticum vulgare), Cassava (Manihot esculenta) and Sweet Potato (Ipomoea batatas) Flours. Pak. J. Nutr. 2014, 13, 439–445. [Google Scholar]
- Sapirstein, H.; Wu, Y.; Koksel, F.; Graf, R. A study of factors influencing the water absorption capacity of Canadian hard red winter wheat flour. J. Cereal Sci. 2018, 81, 52–59. [Google Scholar]
- Adewole, S.; Omowole, A. Cassava value addition: A case study of cassava -based bread produced in Ondo state, Nigeria. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2018, 18, 31–36. [Google Scholar]
- Nilusha, R.A.T.; Jayasinghe, J.M.J.K.; Perera, O.D.A.N.; Perera, P.I.P. Proximate composition, physicochemical. functional properties of flours from cassava. Int. J. Food Sci. 2021, 2021, 6064545. [Google Scholar]
- Li, M.; Zhang, Y.; You, X.; Wang, Y.; Zhou, K.; Wei, P.; Wei, L. Assessment of Functional Properties of Wheat–Cassava Composite Flour. Food Nutr. 2023, 12, 3585. [Google Scholar] [CrossRef]
- Adeniyi, O.O.; Ariwoola, O.S. Comparative Proximate Composition of Maize (Zea mays L.) Varieties Grown in South-western Nigeria. Int. Ann. Sci. 2019, 7, 1–5. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Siroha, A.K. Difference in protein content of wheat (Triticum aestivum L.): Effect on functional, pasting, color and antioxidant properties. J. Saudi Soc. Agric. Sci. 2019, 18, 378–384. [Google Scholar] [CrossRef]
- Eleazu, C.; Eleazu, K.; Aniedu, C.; Amajor, J.; Ikpeama, A.; Ebenzer, I. Effect of Partial Replacement of Wheat Flour with High Quality Cassava Flour on the Chemical Composition, Antioxidant Activity, Sensory Quality, and Microbial Quality of Bread. Prev. Nutr. Food Sci. 2015, 19, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Olugbuyi, A.O.; Adepeju, A.B.; Ayodele, B.O.; Oluwajuyitan, T.D. Mature green plantain-amaranth flour inclusion improved wheat bread nutrients, antioxidant activities, glycemic index/load and carbohydrate hydrolyzing enzyme inhibitory activities. Food Chem. Adv. 2023, 3, 100455. [Google Scholar] [CrossRef]
- Ozcan, M.M. The effect of ginger (Zingiber officinale) powders at different concentrations on bioactive compounds, antioxidant activity, phenolic constituents, nutrients and sensory characteristics of wheat bread. Int. J. Gastron. Food Sci. 2022, 28, 100532. [Google Scholar] [CrossRef]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Alimi, B.A.; Siwela, M. Dough rheology and loaf quality of wheat-cassava bread using different cassava varieties and wheat substitution levels. Food Biosci. 2020, 34, 100529. [Google Scholar] [CrossRef]
- Aryee, F.N.A.; Oduro, I.; Ellis, W.O.; Afuakwa, J.J. The physicochemical properties of flour samples from the roots of 31 varieties of cassava. Food Control 2006, 17, 916–922. [Google Scholar] [CrossRef]
- Chisenga, S.M.; Workneh, S.T.; Bultosa, G.; Alimi, B.A. Progress in research and applications of cassava flour and starch: A review. J. Food Sci. Technol 2019, 56, 2799–2813. [Google Scholar] [CrossRef]
- Olatidoye, O.P.; Shittu, A.; Sobowale, S.S.; Olayemi, W.A.; Adeluka, I.F. Influence of hydrocolloids addition (carboxymethylcellulose and guargum) on some quality attributes of wheat and high quality cassava flour and its bread making potentials. Croat. J. Food Technol. Biotechnol. Nutr. 2020, 15, 46–54. [Google Scholar] [CrossRef]
- Jensen, S.; Skibset, L.H.; Kidmose, U.; Thybo, A.K. Addition of cassava flours in bread-making: Sensory and textural evaluation. LWT-Food Sci. Technol. 2015, 60, 292–299. [Google Scholar] [CrossRef]
- Lu, H.; Guo, L.; Zhang, L.; Xie, C.; Li, W.; Li, K. Study on quality characteristics of cassava flour and cassava flour short biscuits. Food Nutr. 2020, 8, 521–533. [Google Scholar]
- Bala, A.; Gul, K.; Riar, C.S. Functional and sensory properties of cookies prepared from wheat flour supplemented with cassava and water chestnut flours. Cogent Food Agric. 2015, 1, 1019815. [Google Scholar]
- Dewettinck, K.; Van Bockstaele, F.; Kühne, B.; Van de Walle, D.; Courtens, T.M.; Gellynck, X. Nutritional value of bread: Influence of processing, food interaction and consumer perception. J. Cereal Sci. 2008, 48, 243–257. [Google Scholar] [CrossRef]
- Aprodu, L.; Serban, L.; Banu, L. Influence of ginger powder on dough rheological properties and bread quality. AgroLife Sci. J. 2019, 8, 9–15. [Google Scholar]
- Azmat, S.; Gulzar, B.; Fatima, T. Ginger and its health benefits: A review. Int. J. Unani Integr. Med. 2019, 3, 64–69. [Google Scholar]
- Malu, S.P.; Obochi, G.O.; Tawo, E.N.; Nyong, B.E. Antibacterial activity and medicinal properties of ginger (Zingiber officinale). Glob. J. Pure Appl. Sci. 2009, 15, 365–368. [Google Scholar]
- Baptista, B.G.; Ribeiro, M.; Cardozo, L.F.; Leal, V.d.O.; Regis, B.; Mafra, D. Nutritional benefits of ginger for patients with non-communicable diseases. Clin. Nutr. ESPEN 2022, 49, 1–16. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar]
- Indiarto, R.; Subroto, E. Ginger rhizomes (Zingiber officinale) functionality in food and health perspective: A review. Food Res. Int. 2021, 5, 497–505. [Google Scholar]
- Ozola, B.; Augspole, I.; Duma, M.; Kreicbergs, V. Bioactive compounds in fresh and dried ginger root (Zingiber officinale). Food Belt 2019, 50, 265–268. [Google Scholar]
- Balestra, F.; Cocci, E.; Pinnavaia, G.; Romani, S. Evaluation of antioxidant, rheological and sensorial properties of wheat flour dough and bread containing ginger powder. LWT-Food Sci. Technol. 2011, 44, 700–705. [Google Scholar] [CrossRef]
- Bakers, F.o. How bread is made. Factsheet 2017, 7, 1–5. [Google Scholar]
- AACC. Measurement of Bread Firmness by Universal Testing Machine. Staleness/Texture. 1999. Available online: https://www.google.com.hk/url?sa=t&source=web&rct=j&opi=89978449&url=https://img67.chem17.com/1/20170312/636249351441623177119.pdf&ved=2ahUKEwigyKu63q6MAxV9_rsIHTmKE7MQFnoECBoQAQ&usg=AOvVaw2FLgisZnqabv4PEsjB7BRn (accessed on 25 March 2025).
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Amercan J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Moore, S. Why is Moisture Content Analysis of Food Important? 2020. Available online: https://www.news-medical.net/life-sciences/Why-is-Moisture-Content-Analysis-of-Food-Important.aspx (accessed on 28 July 2024).
- Ayub, M.; Wahab, S.; Durrani, Y. Effect of Water Activity (Aw) Moisture Content and Total Microbial Count on the Overall Quality of Bread. Int. J. Agric. Biol. 2003, 5, 274–278. [Google Scholar]
- Ibrahim, U.K.; Rahman, N.A.A.; Suzihaque, M.U.H.; Hashib, S.A.; Aziz, R.A.A. Effect of baking conditions on the physical properties of bread incorporated with green coffee beans (GCB). Mater. Sci. Eng. 2020, 736, 062019. [Google Scholar] [CrossRef]
- Ishida, P.M.G.; Steel, C.J. Physicochemical and sensory characteristics of pan bread samples available in the Brazilian market. Food Sci. Technol. 2014, 34, 746–754. [Google Scholar] [CrossRef]
- Osella, A.; Sanchez, H.D.; Carrara, C.R.; Torre, M.A.d.l.; Buera, M.P. Water redistribution and structural changes of starch during storage of gluten-free bread. Starch-Starke 2005, 57, 208–216. [Google Scholar] [CrossRef]
- Every, D.; Gerrard, J.A.; Gilpin, M.J.; Ross, M.; Newberry, M.P. Staling in starch bread: The effect of gluten addition on specific loaf volume and firming rate. Starch/Starke 1998, 50, 443–446. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Gluten-free dough’s: Rheological properties, testing procedures-Methods and potential problems. In Free Food Science and Technology; Gallagher, E., Ed.; Wiley-Blackwell: Ames, IA, USA, 2009; pp. 52–82. [Google Scholar]
- Defloor, R.; Leijskens, R.; Bokanga, J.; Delcour, J.A. Delcour, Impact of genotype, crop age and planting season on the breadmaking and gelatinisation properties of cassava (Manihot esculenta Crantz) Flour. J. Sci. Food Agric. 1995, 68, 167–174. [Google Scholar] [CrossRef]
- Eriksson, E.; Koch, K.; Tortoe, C.; Akonor, P.T.; Oduro-Yeboah, C. Evaluation of the Physical and Sensory Characteristics of Bread Produced from Three Varieties of Cassava and Wheat Composite Flours. Food Public Health 2014, 4, 214–222. [Google Scholar]
- Manano, J.; Ogwok, P.; Byarugaba-Bazirake, G.W.; Mugampoza, E. Rheological, Baking and Sensory Characteristics of Bread from Wheat-Cassava Composite Dough. J. Food Res. 2021, 10, 18. [Google Scholar] [CrossRef]
- Abdelghafor, R.F.; Mustafa, A.I.; Ibrahim, A.M.H.; Krishnan, P.G. Quality of bread from composite flour of sorghum and hard white winter wheat. Food Bioprocess Technol. 2011, 3, 9–15. [Google Scholar]
- Grenier, D.; Rondeau-Mouro, C.; Dedey, K.B.; Morel, M.; Lucas, T. Gas cell opening in bread dough during baking. Trends Food Sci. Technol. 2021, 109, 482–498. [Google Scholar] [CrossRef]
- Shittu, T.A.; Rajil, A.O.; Sanni, O. Bread from composite cassava-wheat flour: Effect of baking time and temperature on some physical properties of bread loaf. Food Res. Int. 2007, 40, 280–290. [Google Scholar] [CrossRef]
- Sroan, B.S.; Bean, S.R.; MacRitchie, F. Mechanism of gas cell stabilization in bread making. I. The primary gluten–starch matrix. J. Cereal Sci. 2009, 49, 32–40. [Google Scholar] [CrossRef]
- Oladunmoye, O.O.; Akinoso, R.; Olapade, A.A. Evaluation of Some Physical–Chemical Properties of Wheat, Cassava, Maize and Cowpea Flours For Bread Making. J. Food Qual. 2010, 33, 693–708. [Google Scholar] [CrossRef]
- Shittu, T.A.; Dixon, A.; Awonorin, S.O.; Sanni, L.O.; Maziya-Dixon, B. Bread from composite cassava–wheat flour: Effect of cassava genotype and nitrogen fertilizer on bread quality. Food Res. Intern 2008, 41, 569–578. [Google Scholar] [CrossRef]
- Aboaba, O.; Obakpolor, E. The leavening ability of bakers yeast on dough prepared with composite flour (wheat/cassava). Afr. J. Food Sci. 2010, 4, 325–329. [Google Scholar]
- Eleazu, C.O.; Eleazu, K.C. Determination of proximate composition, total carotenoid, reducing sugars and residual cyanide levels of flours 6 new yellow and white cassava (Manihot esculenta Crantz) varieties. Am. J. Food Technol. 2012, 7, 642–649. [Google Scholar]
- Ghafoor, K.; Juhaimi, F.A.; Özcan, M.M.; Uslu, N.; Babikera, E.E.; Ahmeda, I.A.M. Total phenolics, total carotenoids, individual phenolics and antioxidant activity of ginger (Zingiber officinale) rhizome as affected by drying methods. LWT-Food Sci. Technol. 2020, 126, 109354. [Google Scholar]
- Zhai, S.; Liu, J.; Wan, Y.; Cao, S.; He, Z. A Genome-Wide Association Study Reveals a Rich Genetic Architecture of Flour Color-Related Traits in Bread Wheat. Front. Plant Sci. 2018, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; joh, A. Pigment Composition Responsible for the Pale Yellow Color of Ginger (Zingiber officinale) Rhizomes. Food Sci. Technol. Res. 2014, 20, 971–978. [Google Scholar] [CrossRef]
- Shao, X.; Lishuang, L.V.; Parks, T.; Wu, H.; Ho, C.; Sang, S. Quantitative Analysis of Ginger Components in Commercial Products Using Liquid Chromatography with Electrochemical Array Detection. J. Agric. Food Chem. 2010, 58, 12608–12614. [Google Scholar]
- Lim, H.S.; Park, S.H.; Ghafoor, K.; Hwang, S.Y.; Park, J. Quality and antioxidant properties of bread containing turmeric (Curcuma longa L.) cultivated in South Korea. Food Chem. 2011, 124, 1577–1582. [Google Scholar] [CrossRef]
- Yi, B.; Hu, L.; Mei, W.; Zhou, K.; Wang, H.; Luo, Y.; Wei, X.; Dai, H. Antioxidant phenolic compound in cassava. Molecules 2010, 16, 10157–10167. [Google Scholar]
- Kumar, H.; Choudhary, N.; Naveen, V.; Suman, K.; Seth, R. Phenolic compounds and their health benefits: A review. J. Food Res. Technol. 2014, 2, 46–59. [Google Scholar]
- Zagórska, J.; Czernicka-Bos, L.; Kukula-Koch, W.; Szalak, R.; Koch, W. Impact of Thermal Processing on the Composition of Secondary Metabolites of Ginger Rhizome—A Review. Foods 2022, 11, 3484. [Google Scholar] [CrossRef]
- Gopi, S.; Varma, K.; Jude, S. Study on temperature dependent conversion of active components of ginger. Int. J. Pharma Sci. 2016, 6, 1344–1347. [Google Scholar]
- Zielinski, H.; Amigo-Benavent, M.; Castillo, M.D.D.; Horszwald, A.; Zielinska, D. Formulation and baking process affect Maillard reaction development and antioxidant capacity of ginger cakes. J. Food Nutr. Res. 2010, 49, 140–148. [Google Scholar]
- Li, H.; Tsao, R.; Deng, Z. Factors affecting the antioxidant potential and health benefits of plant foods. Can. J. Plant Sci. 2012, 90, 1101–1111. [Google Scholar]
- McCance and Widdowson’s ‘Composition of Foods Integrated Dataset’ on the Nutrient Content of the UK Food Supply [Internet]. Public Health England. 2021. Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid#full-publication-update-history (accessed on 15 March 2025).
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; RahmanMir, M. A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci. World J. 2015, 2015, 816364. [Google Scholar]
- Çubukçu, H.C.; Kılıçaslan, N.S.D.; Durak, I. Different effects of heating and freezing treatments on the antioxidant properties of broccoli, cauliflower, garlic and onion. An experimental in vitro study. Sao Paulo Med. J. 2019, 137, 407–413. [Google Scholar] [PubMed]
| Nutritional Composition (%) | ||||
|---|---|---|---|---|
| Carbohydrate | Starch | Crude Fibre | Protein | |
| Cassava flour | 79 | 73 | 6 | 7 |
| Wheat flour | 73 | 70 | 3 | 21 |
| Corn flour | 71 | 69 | 2 | 11 |
| Samples | |||||||
|---|---|---|---|---|---|---|---|
| Ingredients (g) | WF | CW10 | CWG11 | CWG13 | CW40 | CWG41 | CWG43 |
| Wheat flour | 600 | 540 | 534 | 522 | 360 | 354 | 342 |
| Cassava flour | 0 | 60 | 60 | 60 | 240 | 240 | 240 |
| Ginger powder | 0 | 0 | 6 | 18 | 0 | 6 | 18 |
| Sugar | 60 | ||||||
| Salt | 6 | ||||||
| Diamond improver | 12 | ||||||
| Margarine | 60 | ||||||
| Yeast | 12 | ||||||
| Water | 220 | ||||||
| Parameters | WF | CW10 | CWG11 | CWG13 | CW40 | CWG41 | CWG43 |
|---|---|---|---|---|---|---|---|
| Moisture content (%) | 28.60 ± 0.14 a | 25.04 ± 0.38 b | 26.37 ± 0.15 c | 28.20 ± 0.47 a | 24.57 ± 0.07 d | 26.61 ± 0.11 e | 25.19 ± 0.19 b |
| Water activity | 0.843 ± 0.006 c | 0.866 ± 0.007 d | 0.878 ± 0.004 e | 0.868 ± 0.001 d | 0.870 ± 0.001 d | 0.867 ± 0.001 d | 0.867 ± 0.001 d |
| Cell volume | 23.67 ± 1.15 e | 29.00 ± 1.00 e,f | 31.33 ± 1.15 f | 29.00 ± 5.00 e,f | 30.33 ± 2.31 f | 32.00 ± 1.00 f | 30.67 ± 5.03 f |
| Area of holes | 0.52 ± 0.79 | 0.91 ± 1.57 g | 0.72 ± 0.87 g | 0.37 ± 0.65 g | 1.74 ± 1.45 | 0.04 ± 0.06 g | 1.89 ± 1.64 g |
| Number of holes | 1.14 ± 1.74 h | 1.03 ± 1.78 h | 0.75 ± 0.84 h | 0.34 ± 0.58 h | 1.48 ± 0.06 h | 0.04 ± 0.06 h | 1.14 ± 1.02 h |
| Firmness (g) | 1518 ± 381 i | 1644 ± 163 i | 1912 ± 161 i | 2098 ± 141 i | 4538 ± 388 j | 4381 ± 417 j | 6676 ± 482 k |
| Colour Value | WF | CW10 | CWG11 | CWG13 | CW40 | CWG41 | CWG43 |
|---|---|---|---|---|---|---|---|
| L* | 66.09 ± 1.00 a | 57.02 ± 1.72 a | 76.53 ± 0.52 a | 72.34 ± 1.38 a | 71.89 ± 1.02 a | 71.59 ± 1.93 a | 68.35 ± 0.50 a |
| a* | 1.61 ± 0.05 b | 1.92 ± 0.06 b | 2.41 ± 0.07 c | 3.72 ± 0.31 d | 4.90 ± 0.31 e | 4.20 ± 0.31 f | 5.76 ± 0.23 g |
| b* | 9.87 ± 0.50 h | 15.47 ± 0.20 i | 16.65 ± 0.18 j | 18.79 ± 0.66 k,m | 18.18 ± 0.25 k | 15.71 ± 0.60 l | 19.27 ± 0.53 m |
| C* | 10.00 ± 0.49 n | 15.59 ± 0.20 o | 16.83 ± 0.18 p | 19.16 ± 0.63 q | 18.83 ± 0.31 q | 16.26 ± 0.63 o,p | 20.11 ± 0.56 r |
| H° | 81.16 ± 0.09 s | 81.63 ± 0.03 t | 81.37 ± 0.07 t | 80.65 ± 0.25 u | 79.71 ± 0.19 v | 78.89 ± 0.19 v | 79.26 ± 0.11 s |
| E* | 1.03 ± 1.10 t | 14.51 ± 0.44 u | 13.03 ± 0.45 u,v | 11.32 ± 1.34 v,w | 10.89 ± 0.53 w | 8.81 ± 1.79 x | 10.39 ± 0.67 w,x |
| Parameters | WF | CW10 | CWG11 | CWG13 | CW40 | CWG41 | CWG43 |
|---|---|---|---|---|---|---|---|
| TPC (GAE in mg/100 g dwb) | 3.42 ± 0.19 a | 3.04 ± 0.02 b | 3.58 ± 0.03 a | 4.54 ± 0.35 c | 3.04 ± 0.05 b | 3.21 ± 0.08 a | 3.95 ± 0.18 d |
| FRAP (mmol/100 g dwb) | 2.04 ± 0.26 e | 5.07 ± 0.61 f | 5.48 ± 0.24 f | 4.68 ± 0.50 f | 3.07 ± 0.24 g | 7.94 ± 0.79 h | 4.74 ± 0.24 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, I.E.; Tas, A.A. Effect of Cassava Flour and Ginger Powder Addition on Physicochemical and Antioxidant Properties of Bread. Appl. Sci. 2025, 15, 3762. https://doi.org/10.3390/app15073762
Robinson IE, Tas AA. Effect of Cassava Flour and Ginger Powder Addition on Physicochemical and Antioxidant Properties of Bread. Applied Sciences. 2025; 15(7):3762. https://doi.org/10.3390/app15073762
Chicago/Turabian StyleRobinson, Iberedem E., and Ayten A. Tas. 2025. "Effect of Cassava Flour and Ginger Powder Addition on Physicochemical and Antioxidant Properties of Bread" Applied Sciences 15, no. 7: 3762. https://doi.org/10.3390/app15073762
APA StyleRobinson, I. E., & Tas, A. A. (2025). Effect of Cassava Flour and Ginger Powder Addition on Physicochemical and Antioxidant Properties of Bread. Applied Sciences, 15(7), 3762. https://doi.org/10.3390/app15073762






